ABSTRACT
The reproductive potential of red porgy in coastal waters of Buenos Aires Province (Argentina) and Uruguay (34º-39ºS) was studied by means of a macroscopic and histological analysis of the gonads. Length and age at first maturity were determined, and fecundity, spawning frequency and egg quality were estimated. The spawning season extended from spring through summer between October and January with a peak of spawning in December. Length and age at maturity for sexes combined was 24.5 cm TL and 1.54 years, respectively. Batch fecundity ranged from 6,974 (25 cm TL) to 110,725 (39 cm TL) hydrated oocytes and showed significant linear relationships with total length and ovary-free weight. Relative fecundity ranged from 16 to 172 oocytes per female gram (ovary free). Spawning frequency was 58.5% during January 2011, indicating that females spawned once every 1-2 days at the peak of the spawning season. The life history of red porgy in Argentina and Uruguay was characterized by a young age at first maturity and partial, almost daily, spawning in a bounded time period, a life history strategy that would facilitate population expansion or colonization of new areas, and high population recovery rates or growth (r).
Keywords:
Bonaerense Coastal Ecosystem; Fecundity; Length at first maturity; Red porgy; Spawning frequency
RESUMEN
Se analizó el potencial reproductivo del besugo en aguas costeras de la provincia de Buenos Aires (Argentina) y Uruguay sobre la base del análisis macroscópico e histológico de las gónadas. Se determinó la talla y edad de primera madurez sexual y se estimaron la fecundidad, frecuencia de puesta y calidad ovocitaria. Se pudo establecer que la actividad reproductiva del besugo acontece durante primavera-verano, entre octubre y enero, con un pico de desove principal en diciembre. La estimación de la talla y edad de primera madurez para ambos sexos fue de 24,5 cm LT y 1,54 años respectivamente. La fecundidad parcial presentó un ajuste lineal con la talla y el peso de la hembra y varió entre 6.974 (25 cm LT) y 110.725 (39 cm LT) ovocitos hidratados. La fecundidad relativa osciló entre 16 y 172 ovocitos hidratados g-1. Los valores de ambos parámetros presentaron diferencias interanuales. La frecuencia reproductiva, determinada mediante el uso de los porcentajes de hembras con folículos post ovulatorios, fue de 58,5% durante enero de 2011, lo que indica que los desoves ocurren una vez cada 1-2 días. Los resultados hallados indican que el besugo se caracteriza por presentar una edad de primera madurez baja (entre 1 y 2 años), desoves parciales casi diarios pero en un período de tiempo acotado. Este tipo de estrategia podría explicar tasas de recuperación o de crecimiento poblacional (r) altas como así también la ampliación de su área de distribución o la colonización de nuevas áreas.
Palabras clave:
Ecosistema Costero Bonarense; Fecundidad; Frequencia de desove; Longitud de primera madurez; Pargo
Introduction
The red porgy Pagrus pagrus (Linnaeus, 1758) (Perciformes: Sparidae) inhabits warm temperate to subtropical waters on both sides of the North Atlantic, including the northern Gulf of Mexico and Mediterranean Sea, and in the southwest Atlantic from Venezuela to Argentina (Manooch, Hassler, 1978Manooch CS, Hassler WW. Synopsis of Biological data on the red porgy, Pagrus pagrus, Linnaeus. Washington: NOAA/NMFS; 1978. (FAO fisheries synopsis; 116).; Menezes, Figueiredo, 1985Menezes NA, Figueiredo JL. Manual de peixes marinhos do sudeste do Brasil. V. Teleostei (4). São Paulo: Museu de Zoologia da Universidade de São Paulo. 1985.; Cotrina, 1989Cotrina CP. Estudio biológico del besugo (Pagrus pagrus) del Ecosistema Costero Bonaerense. [Ph.D. Thesis]. Buenos Aires: Universidad Nacional de Buenos Aires; 1989., Bauchot, Hureau, 1990Bauchot ML, Hureau JC. Sparidae. In: Quéro JC, Hureau JC, Karrer C, Post A, Saldanha L, editors. Check-list of the fishes of the eastern tropical Atlantic (CLOFETA) Vol. 2. Paris: JNICT/SEI/UNESCO; 1990. p.790-812; Vaughan et al., 1992Vaughan DS, Huntsman GR, Manooch CS, Rohde FC, Ulrich GF. Population characteristics of the red porgy, Pagrus pagrus, stock off the Carolinas. Bull Mari Sci. 1992; 50(1):1-20.; Haimovici, 1998Haimovici M. Present state and perspectives for the southern Brazil shelf demersal fisheries. Fisheries Manag Ecol. 1998; 5:277-89.; Labroupoulou et al., 1999Labroupoulou M, Machias A, Tsimenides N. Habitat selection and diet of juvenile red porgy, Pagrus pagrus (Linnaeus, 1758). Fish Bull . 1999; 97(3):495-507.; Galván et al., 2009Galván DE, Venerus LA, Irigoyen AJ. 2009. The reef-fish fauna from the Northern Patagonian gulfs of Argentina, South-western Atlantic. Open Fish Sci J. 2009; 2(1):90-98.; Menezes et al., 2006Menezes GM, Sigler MF, Silva HM, Pinho MR. Structure and zonation of demersal fish assemblages off the Azores Archipielago (mid-Atlantic). Mar Ecol Prog Ser. 2006; 324: 241-60.). Adults are typically associated with low-profile hard (live) bottom, rocky, or gravel habitats (Manooch, Hassler, 1978Manooch CS, Hassler WW. Synopsis of Biological data on the red porgy, Pagrus pagrus, Linnaeus. Washington: NOAA/NMFS; 1978. (FAO fisheries synopsis; 116).; Grimes et al., 1982Grimes CB, Manooch CS, Huntsman GR. Reef and rock outcropping fishes of the outer continental shelf of North Carolina and South Carolina, and ecological notes on the red porgy and vermillon snapper. Bull Mar Sci. 1982; 32(1):277-89.; Alekseev, 1982Alekseev FE. Hermaphroditism in sparid fishes (Perciformes, Sparidae). I. Protogyny in porgies, Pagrus pagrus, P. orphus, P. ehrenbergi and P. auriga, from West Africa. J Ichthyol. 1982; 22(5):85-94.). In Argentina and Uruguay, P. pagrus can be found in coastal waters no deeper than 75 m, mainly from 34° to 41° 25’S. This species also occurs seasonally, mainly November to March, in the San Matías Gulf (Rio Negro) on Playa Dorada shores (41°39’S), and in the San Jose Gulf (42°15’S) and Nuevo Gulf (42°57’S) in Península Valdés (Chubut) (Galván et al., 2009Galván DE, Venerus LA, Irigoyen AJ. 2009. The reef-fish fauna from the Northern Patagonian gulfs of Argentina, South-western Atlantic. Open Fish Sci J. 2009; 2(1):90-98.; García, Molinari, 2013García S, Molinari G. El besugo (Pagrus pagrus) en el Atlántico Sudoccidental. Distribución y densidades. Informe de Investigación 28. Mar de Plata: INIDEP ; 2013.). Ninety-four marine and freshwater fish species belonging to 49 families cohabit in the Buenos Aires Coastal Zone, also known as the Bonaerense Coastal Ecosystem (BCE). The BCE supports a multispecies demersal fishery, known as “variado costero”, with different commercial fleet types (craft, bay or creek, coastal and off-shore). During 2015, red porgy ranked 7th in total total landings among 35 species included in the “variado costero” (Prosdocimi et al., 2016Prosdocimi L, Monsalvo M, Bernasconi F, Martinez-Puljak G. Informe anual variado costero, 2015. Coordinación Gestión de Pesquerías - DNPP, Subsecretaría de Pesca y Acuicultura, Ministerio de Agroindustria, Presidencia de la Nación. INFORME GP. 2016; 02. 19 p. Available from: http://www.agroindustria.gob.ar/
http://www.agroindustria.gob.ar/...
). The current scheme of extraction includes an important participation of the fleet with greater fishing power, operating with non-selective bottom trawls in the months and areas where the resource is concentrated during the reproductive activity (Lagos et al., 2013Lagos N, García S, Fernández Aráoz N. Análisis de la pesquería de besugo (Pagrus pagrus) en el área norte del Ecosistema Costero Bonaerense-Uruguayo. Período 2000-2010. Publ Com Téc Mix Fr Mar. 2013; 23:155-76.). This situation, along with an increasing trend in catches from 2003 onwards, together with the economic and fishing importance of the species, determined the need for a fishery diagnosis. For this reason from 2006, biological and fishing studies were taken up and prioritized, aiming to obtain the necessary information to do recommendations for a sustainable management of the resource. In this context, between 2009 and 2012, was established a maximum allowable catch of 6,500 t per year applicable to the Argentine - Uruguayan Common Fishing Zone (ZCPAU), which was suggested with a precautionary approach. From 2003 increased landings of this species to reach peak levels in 2008 and 2009 (close to 6500 t) followed by a decline, with fluctuating around 3,000t in 2011-2015. However, biomass estimates show a growing trend with a risk less than 10% declining below 40% virgin biomass in the medium term (10 years), maintaining current levels of exploitation (Lagos et al., 2015Lagos NA, García S, Cordo H. 2015. Análisis del estado de explotación y recomendaciones de manejo de besugo (Pagrus pagrus) en la ZCPAU. Año 2013. Informe Técnico de Investigación 20. Mar de Plata: INIDEP ; 2015.).
As regards the reproductive characteristics, Pagrus pagrus is a protogynous species and gonadal development proceeds through three different ways. In the first way, immature fish (less than 3 years-old) develop the testicular tissue and the ovaries degenerate before sexual maturity (“primary males” type). In the second way, the development of the gonads is completed with maturation of the ovarian zone, and the fish function as females. In the last way, after a single, or possibly repeated spawnings, females change sex and function as males (“secondary males” type) (Fostier et al., 2000Fostier A, Kokokiris L, LeMenn F, Mourot B, Pavlidis M, Divanach P, Kentori M. Recent advances in reproductional aspects of Pagrus pagrus. Cah Options Méditerr. 47; 2000:181-92.). Both in captivity and in the wild, red porgy first mature at 2 years old (Cotrina, Christiansen, 1994Cotrina CP, Christiansen HE. El comportamiento reproductivo del besugo (Pagrus pagrus) en el ecosistema costero bonaerense. Rev Invest Des Pesq. 1994; 9:25-58.; Kokokiris et al., 1999Kokokiris L, Bruslé S, Kentouri M, Fostier A. Sexual maturity and hermaphroditism of the red porgy Pagrus pagrus (Teleostei: Sparidae). Mar Biol. 1999; 134(4):621-29.). Macroscopic staging and gonadosomatic index data suggest that the main spawning season for red porgy in the BCE occurs between late spring and summer (Ciechomski, Cassia, 1974Ciechomski JD, Cassia MC. Reproducción y fecundidad del besugo Pagrus pagrus (Linne) en el Mar Argentino (Pisces, Sparidae). Physis (A). 1974; 33(87):443-52.). Fecundity of this species has been shown to be indeterminate (Daniel, 2003Daniel EA. Sexual maturity, spawning dynamics, and fecundity of red porgy, Pagrus pagrus, off the southeastern United States. [Master’s Thesis]. Charleston: University of Charleston; 2003.). Estimation of fecundity is often difficult since most fish produce large numbers of small eggs; but it can be particularly challenging for indeterminate spawners like red porgy because fecundity is not fixed prior to the start of spawning, so oocyte counts for individual females do not accurately reflect annual fecundity. To estimate this variable in indeterminate species, the number of batches of eggs produced by a female during the reproductive season is multiplied by batch fecundity, which is the number of oocytes spawned in a single batch (Kjesbu, 2009Kjesbu OS. Applied fish reproductive biology: contribution of individual reproductive potential to recruitment and fisheries management. In: Jakobsen T, Fogarty MJ, Megrey BA, Moksness E, editors. Fish reproductive biology: implications for assessment and management. Oxford: Wiley-Blackwell; 2009. p.293-332.). Typically, the number of batches is calculated as the product of spawning season duration in days and the spawning fraction (number of days between spawning events), which is estimated as the proportion of mature females that are found to possess spawning indicators, such as hydrated oocytes or postovulatory follicles, when caught during the spawning season (Murua et al., 2003Murua H, Kraus G, Saborido-Rey F, Witthames PR, Thorsen A, Junquera S. Procedures to estimate fecundity of marine fish species in relation to their reproductive strategy. J Northw Atl Fish Sci. 2003; 33:33-54.).
Information on reproductive biology of exploited fish stocks is essential for several reasons. Age or size at sexual maturity is a parameter used in most stock assessment models, directly affecting the estimation of spawning biomass and productivity. Also, the reproductive effort measured by the size or age of a species is essential in Stock-Recruitment models to understand the variability in the strength of recruitment. Other reproductive traits such as fecundity, spawning frequency and duration of the spawning season are necessary to properly estimate egg and larvae production, especially in species with indeterminate fecundity (Hunter, Macewicz, 1985Hunter JR, Macewicz BJ. 1985. Measurement of spawning frequency in multiple spawning fishes. In: Lasker R, editor. An egg production method for estimating spawning biomass of pelagic fish: application to the northern anchovy, Engraulis mordax. La Jolla: NOAA/NMFS ; 1985. p.79-94.). Annual changes in these variables could affect the stock productivity and produce variability in fish recruitment (Macchi et al., 2004Macchi GJ, Pájaro M, Ehrlich M. Seasonal egg production pattern of the Patagonian stock of Argentine hake (Merluccius hubbsi). Fish Res. 2004; 67(1):25-38.). On the other hand, some reproductive variables may be associated with changes in the population structure, such as size or age at first maturity, which may decline over the years as a consequence of over-exploitation (Hubold, 1978Hubold G. Variations in growth rate and maturity of herring in the Northern North Sea in the years 1955-1973. Rapp Proces- Verb Réun Cos Int Explor Mer. 1978; 172:154-63.; Beacham, 1983Beacham TD. Variability in median size and age at sexual maturity of Atlantic cod, Gadus morhua, on the Scotian shelf in the Northwest Atlantic Ocean. Fish Bull.1983; 81(2):303-21.; Trippel, 1995Trippel EA. Age at maturity as a stress indicator in fisheries. BioScience. 1995; 45(11):759-71.).
We provide data on the reproductive potential of red porgy at the southern edge of their range in the western Atlantic based on macroscopic and histological gonad analysis. Because red porgy is intensely exploited in Argentina and Uruguay, the need is urgent to better understand its biology. The aim of this study was to determine size and age at sexual maturity in order to compare with previously estimations obtained in the 80’. Moreover, fecundity, spawning frequency and egg quality were estimated for first time for BCE. This information is critical for assessment and management of red porgy, and the estimated reproductive parameters may have direct application in models used to assess the status and dynamics of their populations.
Material and Methods
Sample collections and histological analysis. Specimens of Pagrus pagrus and oceanographic data were obtained during six research trawl surveys of the Instituto Nacional de Investigación y Desarrollo Pesquero (INIDEP) carried out in coastal waters of Argentina and Uruguay, 2008-2013 (Fig. 1).
Locations where Pagrus pagrus were collected during the research surveys carried out in coastal waters of Argentina and Uruguay between 2008 and 2013.
In some years additional samples were obtained from commercial landings from Mar del Plata port. Tab. 1 shows dates, sample sizes, and size ranges of red porgy from each trawl survey and annual summaries of the same data from commercial landings samples. Total length (TL) in centimeters and total weight in grams (TW) were recorded for each sampled fish. Individuals were sexed (males, females and hermaphrodites) and maturity stage was determined macroscopically only for males and females, using the following key: (1) immature; (2) developing; (3) spawning capable; (4) regressing; and (5) regenerating (Macchi, Pájaro, 2003Macchi GJ, Pájaro M. Comparative reproductive biology of some commercial marine fishes from Argentina. Fisken og havet. 2003; 12:69-75.; Brown-Peterson et al., 2011Brown-Peterson NJ, Wyanski DM, Saborido-Rey F, Macewicz BJ, Lowerre-Barbieri SK. Standardized terminology for describing reproductive development in fishes. Mar Coast Fish. 2011; 3(1):52-70.). In addition, during 2011 research survey, sagittal otoliths were collected for age determination following (García, Déspos, 2015García S, Déspos J. Crecimiento y mortalidad natural del besugo (Pagrus pagrus) en aguas del Atlántico Sudoccidental (34° a 42°S). Informe de Investigación 96. Mar de Plata: INIDEP; 2015.).
Date, number and length range of Pagrus pagrus sampled during six research surveys and five years commercial landings carried out between 2008 and 2013.
For histological examinations, gonads were preserved in 10% neutral buffered formalin (n=138 males, n=821 females and n=141 hermaphrodites). Fixed gonads were weighed (GW) to the nearest 0.1 g and a portion of tissue was removed from the centre of each gonad, dehydrated in ethanol, cleared in xylol and embedded in paraffin. Tissues were sectioned at approximately 4 μm thick and stained with Harris’s haematoxylin followed by eosin counterstain (García del Moral, 1993García del Moral R. Manual de laboratorio de anatomía patológica. México: McGraw-Hill /Interamericana de España S.A; 1993.).
To determine the seasonal pattern of the reproductive cycle, mean monthly gonadosomatic index (GSI), calculated as GW/TW*100 for each individual, and the mean estimated by month and sex, in order to analyze the general pattern of the reproductive cycle from commercial landings samples during 2011-2012.
Estimation of reproductive variables. Duration of the breeding season was estimated based on diagnosis of the maturity stages obtained by visual information and histological analysis. The variation in the monthly composition of the maturity stages was used to describe the reproductive cycle. Females were considered as reproductively active when they were capable of spawning at the time of capture or in the near future (stages 2 or 3 of the macroscopic scale) (Hunter et al., 1992Hunter JR, Macewicz BJ, Lo NCH, Kimbrell CA. Fecundity, spawning, and maturity of female dover sole Microstomus pacificus, with an evaluation of assumptions and precision. Fish Bull . 1992; 90(1):101-28.).
Description of the stages of postovulatory follicles (POF) degeneration was adapted from that reported for Micropogonias furnieri and Macrodon ancylodon of the Río de la Plata area (Macchi et al., 2003Macchi GJ, Pájaro M. Comparative reproductive biology of some commercial marine fishes from Argentina. Fisken og havet. 2003; 12:69-75.; Militelli, Macchi, 2004Militelli MI, Macchi GJ. Spawning and fecundity of king weakfish, Macrodon ancylodon, in the Río de la Plata estuary, Argentina - Uruguay. J Mar Biol Assoc UK . 2004; 84(2):443-47.). In these species the degenerative process of the POFs was faster than that observed for other species (Hunter, Goldberg, 1980Hunter JR, Goldberg SR. Spawning incidence and batch fecundity in northern anchovy, Engraulis mordax. Fish Bull . 1980; 77(3):641-52.) because of high water temperatures in the Río de la Plata area during summer (Macchi et al., 2003Macchi GJ, Acha EM, Militelli MI. Seasonal egg production of whitemouth croaker (Micropogonias furnieri) in the Río de la Plata estuary, Argentina-Uruguay. Fish Bull . 2003; 101(2):332-42.), so it was considered the most adequate scale for the purposes of the present study (new POFs: between 0 and 6 h after spawning; POF1: 12-h-old).
The size and age at first maturity (L50 and A50, respectively) were estimated by a logistic model by length/age class using the maximum likelihood method: S(L)=1/(1+exp(-c*(L-L50))) (Kendall, Stuart, 1967Kendall MG, Stuart A. Advanced theory of statistics. 2nd edition. London: Charles Griffin; 1967.). All individuals with gonad stages other than immature (1) were considered mature or adult (based on macroscopic and microscopic examination), and their frequency was used as a response variable and total length was the explanatory variable. This analysis was performed during the reproductive season, to avoid biases in maturity determination, and only for those years containing samples of both immature and mature individuals (Tab. 2). Coefficients of the regressions obtained for from the different sampled years were compared using a Chi-square test (Aubone, Wöhler, 2000Aubone A, Wöhler OC. Aplicación del método de máxima verosimilitud a la estimación de parámetros y comparación de curvas de crecimiento de Von Bertalanffy. Informe Técnico 37. Mar de Plata: Secretaría de Agricultura, Ganadería, Pesca y Alimentación, INDEP; 2000.). Due to the low number of juvenile male and hermaphrodite individuals in samples and to avoid problems in the maturation stage assignment in hermaphrodites, L50 and A50 were estimated with combined males and females and the hermaphrodites were not included.
Number of Pagrus pagrus individuals per year used for estimations of length at first maturity (L50), batch and relative fecundity (BF/RF), hydrated oocytes dry weight (DW) and diameter (OD).
Spawning frequency was estimated from the incidence of females with postovulatory follicles (POFs 1), following Hunter, Goldberg (1980Hunter JR, Goldberg SR. Spawning incidence and batch fecundity in northern anchovy, Engraulis mordax. Fish Bull . 1980; 77(3):641-52.), and only from samples collected during the January 2011 research survey, the only cruise that covered the entire distribution area of the species. To calculate the mean and coefficient of variation of this variable we used the equations developed by Picquelle, Stauffer (1985Picquelle S, Stauffer G. Parameter estimation for an egg production method of anchovy biomass assessment. In: Lasker R, editor. An egg production method for estimating spawning biomass of pelagic fish: application to the northern anchovy, Engraulis mordax. La Jolla: NOAA/NMFS ; 1985. p.7-16.).
Batch fecundity (BF, number of oocytes released per spawning) was estimated by using the hydrated oocyte method (Hunter et al., 1985Hunter JR, Macewicz BJ. 1985. Measurement of spawning frequency in multiple spawning fishes. In: Lasker R, editor. An egg production method for estimating spawning biomass of pelagic fish: application to the northern anchovy, Engraulis mordax. La Jolla: NOAA/NMFS ; 1985. p.79-94.). Samples were examined histologically to determine the presence of postovulatory follicles (POFs) and hydrated oocytes. To avoid biases when estimating batch fecundity, only ovaries with hydrated oocytes and without resent POF (less than 6h) were used, because of this only samples collected during January 2011 and December 2013 can used (Tab. 2). Three pieces of ovary of approximately 0.1 g each were sampled from the anterior, middle and posterior section of each gonad, weighed (0.1 mg) and the number of hydrated oocytes counted. Batch fecundity was the product of the mean number of hydrated oocytes per unit ovarian weight and total ovarian weight (GW).
Relative fecundity (RF, number of hydrated oocytes per gram of ovary-free body weight) was estimated as the batch fecundity divided by female weight (without ovaries). The nutritional condition was obtained for each female using the Fulton´s condition factor (K= (TW/TL3)*100). The relationships between BF and the variables TL, TW (ovary-free) and K, were described using simple standard regression (Draper, Smith, 1981Draper NR, Smith H. Applied regression analysis. 2nd ed. New York: Wiley and Sons; 1981 (Wiley series in probability and mathematical statistics).). Comparisons between the relationships BF versus TL obtained from different years were based on the overlapping length ranges of females applying an analysis of covariance (Draper, Smith, 1981Draper NR, Smith H. Applied regression analysis. 2nd ed. New York: Wiley and Sons; 1981 (Wiley series in probability and mathematical statistics).).
The size and weight of unfertilized eggs can tentatively be used to evaluate or estimate the overall developmental potential of eggs after fertilization (Hinckley, 1990Hinckley S. Variation of egg size of walleye pollock Theragra chalcogramma with a preliminary examination of the effect of egg size on larval size. Fish Bull . 1990; 88(3):471-83.; Wootton, 1994Wootton RJ. Life histories as sampling devices: optimum egg size in pelagic fishes. J Fish Biol. 1994; 45(6):1067-77.; Trippel, 1998Trippel EA. Egg size and viability and seasonal offspring production of young Atlantic cod. Trans Am Fish Soc. 1998; 127(3):339-59.). For instance, the size of egg and oil drop was sometimes considered to be beneficial for the future development of the embryo. To obtain an estimate of the quality of spawning in different years, the diameter (OD), oil droplet diameter (OdD) and dry weight (DW) of hydrated oocytes from P. pagrus collected in 2010, 2011, 2012 and 2013 (Tab. 2). Samples of 100 hydrated oocytes were removed from each individual, rinsed in distilled water, dried for 24h at 60ºC and weighed (0.1 mg). The oocyte diameter and oil droplet diameter were measured only for fully hydrated oocytes using an optical microscope at 4x magnification and an image analyzer program.
Results
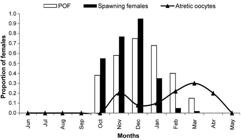
Spawning period. Red porgy in coastal waters of the Buenos Aires Province (Argentina) and Uruguay were reproductively active between October and March, with a principal peak in December. Reproductively active females (stage 2 or 3) were observed between October and May (males October-April), with the highest percentages of spawning activity from October to January, coinciding with the highest values of GSI (Fig. 2). In addition, actively spawning females (those with hydrated oocytes and/or with post-ovulatory follicles) were observed between October and March, with the highest proportion in December (Fig. 3). Atretic oocytes were observed November through April; their incidence increased from December to a peak in March, toward the end of the reproductive season, indicating the end of spawning activity (Fig. 3).
Mean monthly gonadosomatic index (GSI) and monthly percent frequency of active males (M) and females (F) of Pagrus pagrus.
Monthly proportions of Pagrus pagrus females with hydrated oocytes, post-ovulatory follicles (POF) and atretic oocytes.
Length and age at first maturity. The estimates of length at first maturity (L50) of P. pagrus collected during 2010 and 2013 didn’t show significant differences (P<0.05) between years and was 24.9 cm TL (c=0.45, n=235) and 24.3 cm TL respectively (c=0.74; n=593). However, highly significant differences were observed in the logistic curve slope (P <0.01). Age at first maturity (A50), only estimated for 2011, was 1.54 years (c=1.22, n = 419). The youngest mature individuals (both male and female) were estimated at 1 year old.
Spawning frequency. Females with POFs were found in the 91% of fishing hauls containing red porgy. The mean daily percentage of mature females with day 1 POFs was 58.5% (n=189; CV=0.6), equivalent to a spawning frequency of about 1-2 days for January 2011.
Fecundity. Batch fecundity (BF) estimated for P. pagrus collected in January 2011(n=30) ranged between 6974 (25 cm TL) and 110725 (39 cm TL) hydrated oocytes with a mean of 37315 (±8371). BF showed significant linear relationships (P<0.05) with total length (TL), total weight (TW, ovary free) and age but no relationship with condition factor (Fig. 4). In December 2013, BF showed no relationship with female size, weight or age but a significant linear relationship (P<0.05) with condition factor; however, sample size was small (n=7) (Fig. 4). 2013 BF ranged from 7606 to 28552 hydrated oocytes, with a mean of 13071 (± 5360). Relative fecundity (RF) estimated with data of the two years ranged from 16 to 172 hydrated oocytes/g of ovary-free body weight, was highly variable, and showed no relationship to female size and age. The mean RF estimated for 2011 (90± 15 oocytes/g) differed significantly (P<0.05) from that for 2013 (32 ±12 oocytes/g).
Relationships of batch fecundity (BF) to: (a) total length, (b) total weight (without ovary), (c) age, and (d) condition factor for Pagrus pagrus collected in 2011 (cross) and 2013 (circles).
Size and weight of the oocytes. DW of 100 hydrated oocytes for the 221 red porgy we examined ranged between 1.2 and 3.6 mg. Mean DW was 2.55 ± 0.071 mg for 2010, 2.23 ± 0.075 mg for 2011, 1.51 ± 0.095 mg for 2012 and 2.02 ± 0.235 mg for 2013, and these values differed significantly (P<0.05) from each other. DW showed no significant relationship with size or condition (K) for 2011, 2012 and 2013; however in 2010 it did show a significant linear relationship (P<0.05) with total length (TL), age and weight (TW) (Fig. 5). A significant linear relationship (P<0.05) was also observed between DW and TW in 2011.
OD for the 43 red porgy with completely hydrated oocytes we examined ranged between 806 and 979 μm with a mean of 905 ± 26 μm. We found no significant relationship (P<0.05) between OD and TL, TW or K. The diameter of the oil droplet (OdD) ranged from 192 to 243 μm and averaged 215 ± 6 μm. As with OD there were no significant relationships (P<0.05) between OdD and TL, TW, or K.
Relationships between oocyte dry weight and: (a) total length; (b) total weight (without ovary); (c) age in years; and (d) condition factor for Pagrus pagrus. Gray squares: 2010; cross: 2011; black triangles: 2012 and circles: 2013.Gray line: 2010 and black line: 2011.
Discussion
Our findings that the main spawning activity of red porgy in coastal waters of Buenos Aires Province (Argentina) and Uruguay (34º-39ºS) occurred between October and January, with a principal peak in December, are coincident to those reported by Ciechomski, Cassia (1974Ciechomski JD, Cassia MC. Reproducción y fecundidad del besugo Pagrus pagrus (Linne) en el Mar Argentino (Pisces, Sparidae). Physis (A). 1974; 33(87):443-52.) and Cotrina, Christiansen (1994Cotrina CP, Christiansen HE. El comportamiento reproductivo del besugo (Pagrus pagrus) en el ecosistema costero bonaerense. Rev Invest Des Pesq. 1994; 9:25-58.). Atretic oocytes were observed from November through April with a peak in March was quite similar to that of Fostier et al. (2000Fostier A, Kokokiris L, LeMenn F, Mourot B, Pavlidis M, Divanach P, Kentori M. Recent advances in reproductional aspects of Pagrus pagrus. Cah Options Méditerr. 47; 2000:181-92.) for cultured P. pagrus in Greece.
Length at first maturity (L50) estimated for P. pagrus in 2010 and 2013 not differed significantly between years. L50 estimates for sexes combined of 24.9 and 24.3 cm TL in 2010 and 2013, respectively, were very similar to the only previous estimate for red porgy in the Argentine Sea, ~25 cm TL, reported by Cotrina, Christiansen (1994Cotrina CP, Christiansen HE. El comportamiento reproductivo del besugo (Pagrus pagrus) en el ecosistema costero bonaerense. Rev Invest Des Pesq. 1994; 9:25-58.) and based on samples collected during 1976-1982. However, were lower than those obtained in the northern hemisphere by Daniel (2003Daniel EA. Sexual maturity, spawning dynamics, and fecundity of red porgy, Pagrus pagrus, off the southeastern United States. [Master’s Thesis]. Charleston: University of Charleston; 2003.) (28.9 cm TL) but similar to those estimated by Klibansky, Scharf (2013Klibansky N, Scharf FS. Size-dependent and temporal variability in batch number and fecundity of red porgy, a protogynous, indeterminate spawner, in the U.S. South Atlantic. Mar Coast Fish . 2013; 5(1):39-52.) (25.5 cm TL), and higher than obtained in the northeastern Gulf of Mexico by DeVries (2006DeVries AD. The life history, reproductive ecology and demography of the red porgy, Pagrus pagrus, in the northestem Gulf of Mexico. [Ph. D. Dissertation]. Tallahassee: Florida State University; 2006.) (21.1 cm TL).
Our estimated age at first maturity (A50) of 1.54 years for sexes combined was lower than the 2-3 years reported by Cotrina, Christiansen (1994Cotrina CP, Christiansen HE. El comportamiento reproductivo del besugo (Pagrus pagrus) en el ecosistema costero bonaerense. Rev Invest Des Pesq. 1994; 9:25-58.) for red porgy in the BCE and the 4 years in the Mediterranean reported by Kokokiris et al. (1999Kokokiris L, Bruslé S, Kentouri M, Fostier A. Sexual maturity and hermaphroditism of the red porgy Pagrus pagrus (Teleostei: Sparidae). Mar Biol. 1999; 134(4):621-29.). However, it was similar to estimates from the southeastern US (Harris, McGovern, 1997Harris PJ, McGovern JC. Changes in the life history of red porgy, Pagrus pagrus, from the southeastern United States, 1972-1994. Fish Bull . 1997; 95(4):732-47.) and the Gulf of Mexico (Hood, Johnson, 2000Hood PB, Johnson AK. Age, growth, mortality, and reproduction of red porgy, Pagrus pagrus, from the eastern Gulf of Mexico. Fish Bull . 2000; 98(4):723-35.; DeVries, 2006DeVries AD. The life history, reproductive ecology and demography of the red porgy, Pagrus pagrus, in the northestem Gulf of Mexico. [Ph. D. Dissertation]. Tallahassee: Florida State University; 2006.). A50 estimated for red porgy, was lower than that of other species in the BCE, such as whitemouth croaker (Micropogonias furnieri) (2.5 years, Arena, Hertl, 1983Arena GJ, Hertl E. Aspectos referentes al ciclo reproductor de la corvina blanca (Micropogon opercularis) de la subárea platense. Una primera evaluación de las informaciones disponibles desde 1976 a 1979. Informe Técnico 36. Montevideo: INAPE; 1983.), striped weakfish (Cynoscion guatucupa) (4 years, Vieira, Haimovici, 1997Vieira PC, Haimovici M. Reproduςão da pescada olhuda Cynoscion guatucupa, sin. C. striatus (Scianidae, Teleosteii) no Sul do Brasil. Atlântica. 1997; 19:133-44.) and Brazilian flathead (Percophis brasiliensis) (2 years, Barretto et al., 2011Barretto AC, Sáez MB, Rico MR, Jaureguizar AJ. Age determination, validation, and growth of Brazilian flathead (Percophis brasiliensis) from the southwest Atlantic coastal waters (34º-41ºS). Lat Am J Aquat Res. 2011; 39(2):297-305.).
Our estimated spawning frequency of about once every 1-2 days, with a daily proportion of spawning females estimated to be as high as 0.58, was consistent with the every 1-2 days reported for red porgy in the Mediterranean Sea by Mylonas et al. (2004Mylonas CC, Papadaki M, Pavlidis M, Divanach P. Evaluation of egg production and quality in the Mediterranean red porgy (Pagrus pagrus) during two consecutive spawning seasons. Aquaculture . 2004; 232(1-4):637-49.) and in the U.S. South Atlantic Bight (SAB) by Klibansky, Scharf (2013Klibansky N, Scharf FS. Size-dependent and temporal variability in batch number and fecundity of red porgy, a protogynous, indeterminate spawner, in the U.S. South Atlantic. Mar Coast Fish . 2013; 5(1):39-52.). Other sparids exhibit daily spawning include the Bluespotted Seabream Pagrus caeruleostictus (Stepkina, 1973Stepkina MV. Some biological characteristics of Pagrus ehrenbergii Val. J Ichthyol . 1973; 13:641-49.), Gilthead Bream Sparus auratus (Zohar, Gordin, 1979Zohar Y, Gordin H. Spawning kinetics in the gilthead sea-bream, Sparus aurata L. after low doses of human chronic gonadotropin. J Fish Biol . 1979; 15(6):665-70.), Squirefish Chrysophrys auratus (Scott et al., 1993Scott SG, Zeldis JR, Pankhurst NW. Evidence of daily spawning in natural populations of the New Zealand snapper Pagrus auratus (Sparidae). Environ Biol Fishes. 1993; 36(2):149-56.), and Yellow Seabream Dentex hypselosomus (Yoda, Yoneda, 2009Yoda M, Yoneda M. Assessment of reproductive potential in multiple-spawning fish with indeterminate fecundity: a case study of yellow sea bream Dentex hypselosomus in the East China Sea. J Fish Biol . 2009; 74(10):2338-54.). Our spawning frequency estimates were higher than those reported for other species of the BCE like as M. furnieri (3-5 days; Macchi et al., 2003Macchi GJ, Pájaro M. Comparative reproductive biology of some commercial marine fishes from Argentina. Fisken og havet. 2003; 12:69-75.), Cynoscion guatucupa (6-8 days; Militelli et al., 2013Militelli MI, Macchi GJ, Rodrigues KA. Comparative reproductive biology of Sciaenidae family species in the Río de la Plata and Buenos Aires Coastal Zone, Argentina. J Mar Biol Assoc UK . 2013; 93(2):413-23.) and P. brasiliensis (6 days; Militelli, Macchi, 2001Militelli MI, Macchi GJ. Preliminary estimate of spawning frequency and batch fecundity of Brazilian flathead, Percophis brasiliensis, in coastal waters off Buenos Aires Province. Sci Mar. 2001; 65(2):169-72.).
Batch fecundity (BF) obtained for 2011 was a linear function of total length and ovary-free body weight, contrary to that reported by Ciechomski, Cassia (1974Ciechomski JD, Cassia MC. Reproducción y fecundidad del besugo Pagrus pagrus (Linne) en el Mar Argentino (Pisces, Sparidae). Physis (A). 1974; 33(87):443-52.), which observed a potential relationship in both cases. This may be due to the fact that our estimates only included females smaller than 40 cm TL. BF estimated for 2011 showed a linear function with age, this is the first description of this relationship for the BCE. Few studies have estimated batch fecundity of P. pagrus, primarily because hydrated females are rarely encountered and the diary spawning frequency makes difficult to encountered females without recent POF. Some previous studies on red porgy fecundity were made with females in advanced maturation stage (with yolked oocytes), prior to oocyte hydration (Ciechomski, Cassia, 1974Ciechomski JD, Cassia MC. Reproducción y fecundidad del besugo Pagrus pagrus (Linne) en el Mar Argentino (Pisces, Sparidae). Physis (A). 1974; 33(87):443-52.; Manooch, 1976Manooch CS. Reproductive cycle, fecundity, and sex ratios of the red porgy, Pagrus pagrus (Pisces: Sparidae) in North Carolina. Fish Bull . 1976; 74(4):775-81.; Klibansky, Scharf, 2013Klibansky N, Scharf FS. Size-dependent and temporal variability in batch number and fecundity of red porgy, a protogynous, indeterminate spawner, in the U.S. South Atlantic. Mar Coast Fish . 2013; 5(1):39-52.). Ciechomski, Cassia (1974Ciechomski JD, Cassia MC. Reproducción y fecundidad del besugo Pagrus pagrus (Linne) en el Mar Argentino (Pisces, Sparidae). Physis (A). 1974; 33(87):443-52.) and Manooch (1976Manooch CS. Reproductive cycle, fecundity, and sex ratios of the red porgy, Pagrus pagrus (Pisces: Sparidae) in North Carolina. Fish Bull . 1976; 74(4):775-81.) considered the estimation of the absolute or potential fecundity as the number of total yolked oocytes early in the spawning season; because of this the estimations obtained are not comparable with those of the present paper. However, Klibansky, Scharf (2013Klibansky N, Scharf FS. Size-dependent and temporal variability in batch number and fecundity of red porgy, a protogynous, indeterminate spawner, in the U.S. South Atlantic. Mar Coast Fish . 2013; 5(1):39-52.) estimated the BF by counting oocytes of the most advanced cohort, the results obtained during 2009-2010 were similar for the smaller females but lower for larger ones (~65000 for 40 cm TL).
Relative fecundity ranged from 16 to 172 hydrated oocytes/g of ovary-free body weight, and showed no significant relationships with female size, weight, age or condition factor (K). These values are similar to that reported by Fostier et al. (2000Fostier A, Kokokiris L, LeMenn F, Mourot B, Pavlidis M, Divanach P, Kentori M. Recent advances in reproductional aspects of Pagrus pagrus. Cah Options Méditerr. 47; 2000:181-92.) but lower than obtained for others sparids like Sparus macrophtalamus (Nguyen-Xuan, Wojciechowski, 1973Nguyen-Xuan L, Wojciechowski J. Maturity and fecundity of Dentex macrophthalmus (Sparidae) from north-west African coast. Acta Ichtyol Pisc. 1973; 3(1):49-59.), Dentex dentex (Loir et al., 2001Loir L, Le Gac F, Somarakis S, Pavlidis M. Sexuality and gonadal cycle of the common dentex (Dentex dentex) in intensive culture. Aquaculture . 2001; 194(3-4):363-81.), Dentex gibbosus (Grubisic et al., 2007Grubisic L, Mrcelic GJ, Skakelja N, Katavic I, Ticina V, Sliskovic M. Reproductive biology of pink dentex Dentex gibbosus (Rafinesque) from the Adriatic Sea, Croatia. Aquacult Res. 2007; 38(9):991-1001.) and Diplodus vulgaris (Gonçalves, Erzini, 2000Gonçalves JMS, Erzini K. The reproductive biology of the two-banded sea bream (Diplodus vulgaris) from the southwest coast of Portugal. J Appl Ichthyol. 2000; 16(3):110-16.). Mean values of RF were similar to those reported for M. furnieri, C. guatucupa and Umbrina canosai (Militelli et al., 2013Militelli MI, Macchi GJ, Rodrigues KA. Comparative reproductive biology of Sciaenidae family species in the Río de la Plata and Buenos Aires Coastal Zone, Argentina. J Mar Biol Assoc UK . 2013; 93(2):413-23.) and lower than those for P. brasiliensis (319 ± 129 oocytes/ g for 1999; Militelli, Macchi, 2001Militelli MI, Macchi GJ. Preliminary estimate of spawning frequency and batch fecundity of Brazilian flathead, Percophis brasiliensis, in coastal waters off Buenos Aires Province. Sci Mar. 2001; 65(2):169-72.).
Values of OD and OdD were coincident with those previously reported for both wild and captive red porgy (Machinandiarena et al., 2003Machinandiarena L, Müller M, López A. Early life stages of development of the red porgy Pagrus pagrus (Pisces, Sparidae) in captivity, Argentina. Invest Mar, Valparaíso. 2003; 31(1):5-13.; Radonic et al., 2005Radonic M, López A, Oka M, Aristizabal EO. Effect of the incubation temperature on the embryonic development and hatching time of eggs of the red porgy Pagrus pagrus (Linne, 1758) (Pisces: Sparidae). Rev Biol Mar Oceanogr. 2005; 40(2):91-99.). Our results suggest that hydrated oocytes sampled in 2010 had a greater amount of reserves, suggesting an egg quality higher than in other years analyzed. Nonetheless, this conclusion is based on limited data; hence this possibility should be further investigated. In general, the size of hydrated oocytes in red porgy was similar to that found in other sparids that range 850-1090 µm (Glamuzina et al., 1989Glamuzina B, Jug-Dujaković J, Katavić I. Preliminary studies on reproduction and larval rearing of common dentex, Dentex dentex (Linnaeus 1758). Aquaculture. 1989;77(1):75-84.; Greco et al., 1993Greco S, Lo Paro G, Caridi D, Perdichizzi F, Cammaroto S, Micale V, Genovese L. Controlled spawning and larval development in the sharpsnout sea bream (Diplodus puntazzo, Sparidae). In: Barnabé G, Kestemont P, editors. Production, environment and quality: Proceedings of the International Conference Bordeaux Aquaculture ‘92 (EAS Special Publication, 18). Ghent: European Aquaculture Society; 1993. p.185-188.) and higher than Pagellus erythrinus (Güner et al., 2004Güner Y, Özden O, Altunok M, Koru E, Kızak V. Spawning and larvae production of common pandora, Pagellus erythrinus L. Isr J Aquacult-Bamid. 2004; 56(3):209-17.). Pagrus pagrus OD and OdD were also similar to other species of BCE like M. furnieri, C. guatucupa, U. canosai (Militelli et al., 2013Militelli MI, Macchi GJ, Rodrigues KA. Comparative reproductive biology of Sciaenidae family species in the Río de la Plata and Buenos Aires Coastal Zone, Argentina. J Mar Biol Assoc UK . 2013; 93(2):413-23.) and P. brasiliensis (Militelli, 1999), but the oocyte dry weight estimated for the last one was lower (1.70 ± 0.12 mg to 100 oocytes for 2003) (Rodrigues et al., 2007Rodrigues KA, Militelli MI, Macchi GJ. Área de puesta, fecundidad y calidad ovocitaria del pez palo (Percophis brasiliensis) en aguas costeras de la provincia de Buenos Aires. Resultados de campañas de investigación realizadas por el INIDEP durante el periodo 1998-2003. Informe Técnico 26. Mar de Plata: INIDEP ; 2007.).
A species’ life history characteristics are important factors in its population dynamics. Population growth rate depends directly upon fecundity, survivorship, and timing of reproduction. Averaged over many generations, the three parameters must balance, or the population eventually will decline to extinction or grow exponentially (Winemiller, 2006Winemiller KO. Life history strategies, population regulation, and implications for fisheries management. Can J Fish Aquat Sci. 2006; 62(4):872-85.). Winemiller (2006Winemiller KO. Life history strategies, population regulation, and implications for fisheries management. Can J Fish Aquat Sci. 2006; 62(4):872-85.) proposed three endpoint strategies that result from trade-offs among age of maturation (positively correlated with mean generation time), fecundity, and survivorship. The periodic strategy corresponds to high fecundity and older age at maturity (the latter a correlate of population turnover rate) and low juvenile survivorship. The opportunistic strategy of high r (= maximum population growth rate) via rapid maturation corresponds to low values of all three parameters. The equilibrium strategy corresponds to species with low fecundity, older age at maturity, and high juvenile survivorship. Red porgy’s early age at first maturity (<2 years) and almost daily spawning, although only during a limited (spring - summer) period of time, most closely corresponds to an opportunistic life history strategy, which would explain its high recovery rates of population growth (r) as well as its expanding range and colonization of new areas (García, Molinari, 2013García S, Molinari G. El besugo (Pagrus pagrus) en el Atlántico Sudoccidental. Distribución y densidades. Informe de Investigación 28. Mar de Plata: INIDEP ; 2013.).
Our results provide new estimations of reproductive parameters of P. pagrus in the BCE after 30 years, which will help to fill a literature gap about these topics with the aim to collaborate in the specie assessment and management.
Acknowledgments
This research was conducted within the INIDEP’s Coastal Project. We express our gratitude to Hugo Brachetta and Marta Estrada for the preparation of histological sections. INIDEP contribution N. 2060.
References
- Alekseev FE. Hermaphroditism in sparid fishes (Perciformes, Sparidae). I. Protogyny in porgies, Pagrus pagrus, P. orphus, P. ehrenbergi and P. auriga, from West Africa. J Ichthyol. 1982; 22(5):85-94.
- Arena GJ, Hertl E. Aspectos referentes al ciclo reproductor de la corvina blanca (Micropogon opercularis) de la subárea platense. Una primera evaluación de las informaciones disponibles desde 1976 a 1979. Informe Técnico 36. Montevideo: INAPE; 1983.
- Aubone A, Wöhler OC. Aplicación del método de máxima verosimilitud a la estimación de parámetros y comparación de curvas de crecimiento de Von Bertalanffy. Informe Técnico 37. Mar de Plata: Secretaría de Agricultura, Ganadería, Pesca y Alimentación, INDEP; 2000.
- Barretto AC, Sáez MB, Rico MR, Jaureguizar AJ. Age determination, validation, and growth of Brazilian flathead (Percophis brasiliensis) from the southwest Atlantic coastal waters (34º-41ºS). Lat Am J Aquat Res. 2011; 39(2):297-305.
- Bauchot ML, Hureau JC. Sparidae. In: Quéro JC, Hureau JC, Karrer C, Post A, Saldanha L, editors. Check-list of the fishes of the eastern tropical Atlantic (CLOFETA) Vol. 2. Paris: JNICT/SEI/UNESCO; 1990. p.790-812
- Beacham TD. Variability in median size and age at sexual maturity of Atlantic cod, Gadus morhua, on the Scotian shelf in the Northwest Atlantic Ocean. Fish Bull.1983; 81(2):303-21.
- Brown-Peterson NJ, Wyanski DM, Saborido-Rey F, Macewicz BJ, Lowerre-Barbieri SK. Standardized terminology for describing reproductive development in fishes. Mar Coast Fish. 2011; 3(1):52-70.
- Ciechomski JD, Cassia MC. Reproducción y fecundidad del besugo Pagrus pagrus (Linne) en el Mar Argentino (Pisces, Sparidae). Physis (A). 1974; 33(87):443-52.
- Cotrina CP. Estudio biológico del besugo (Pagrus pagrus) del Ecosistema Costero Bonaerense. [Ph.D. Thesis]. Buenos Aires: Universidad Nacional de Buenos Aires; 1989.
- Cotrina CP, Christiansen HE. El comportamiento reproductivo del besugo (Pagrus pagrus) en el ecosistema costero bonaerense. Rev Invest Des Pesq. 1994; 9:25-58.
- Daniel EA. Sexual maturity, spawning dynamics, and fecundity of red porgy, Pagrus pagrus, off the southeastern United States. [Master’s Thesis]. Charleston: University of Charleston; 2003.
- DeVries AD. The life history, reproductive ecology and demography of the red porgy, Pagrus pagrus, in the northestem Gulf of Mexico. [Ph. D. Dissertation]. Tallahassee: Florida State University; 2006.
- Draper NR, Smith H. Applied regression analysis. 2nd ed. New York: Wiley and Sons; 1981 (Wiley series in probability and mathematical statistics).
- Fostier A, Kokokiris L, LeMenn F, Mourot B, Pavlidis M, Divanach P, Kentori M. Recent advances in reproductional aspects of Pagrus pagrus Cah Options Méditerr. 47; 2000:181-92.
- Galván DE, Venerus LA, Irigoyen AJ. 2009. The reef-fish fauna from the Northern Patagonian gulfs of Argentina, South-western Atlantic. Open Fish Sci J. 2009; 2(1):90-98.
- García del Moral R. Manual de laboratorio de anatomía patológica. México: McGraw-Hill /Interamericana de España S.A; 1993.
- García S, Déspos J. Crecimiento y mortalidad natural del besugo (Pagrus pagrus) en aguas del Atlántico Sudoccidental (34° a 42°S). Informe de Investigación 96. Mar de Plata: INIDEP; 2015.
- García S, Molinari G. El besugo (Pagrus pagrus) en el Atlántico Sudoccidental. Distribución y densidades. Informe de Investigación 28. Mar de Plata: INIDEP ; 2013.
- Glamuzina B, Jug-Dujaković J, Katavić I. Preliminary studies on reproduction and larval rearing of common dentex, Dentex dentex (Linnaeus 1758). Aquaculture. 1989;77(1):75-84.
- Gonçalves JMS, Erzini K. The reproductive biology of the two-banded sea bream (Diplodus vulgaris) from the southwest coast of Portugal. J Appl Ichthyol. 2000; 16(3):110-16.
- Greco S, Lo Paro G, Caridi D, Perdichizzi F, Cammaroto S, Micale V, Genovese L. Controlled spawning and larval development in the sharpsnout sea bream (Diplodus puntazzo, Sparidae). In: Barnabé G, Kestemont P, editors. Production, environment and quality: Proceedings of the International Conference Bordeaux Aquaculture ‘92 (EAS Special Publication, 18). Ghent: European Aquaculture Society; 1993. p.185-188.
- Grimes CB, Manooch CS, Huntsman GR. Reef and rock outcropping fishes of the outer continental shelf of North Carolina and South Carolina, and ecological notes on the red porgy and vermillon snapper. Bull Mar Sci. 1982; 32(1):277-89.
- Grubisic L, Mrcelic GJ, Skakelja N, Katavic I, Ticina V, Sliskovic M. Reproductive biology of pink dentex Dentex gibbosus (Rafinesque) from the Adriatic Sea, Croatia. Aquacult Res. 2007; 38(9):991-1001.
- Güner Y, Özden O, Altunok M, Koru E, Kızak V. Spawning and larvae production of common pandora, Pagellus erythrinus L. Isr J Aquacult-Bamid. 2004; 56(3):209-17.
- Haimovici M. Present state and perspectives for the southern Brazil shelf demersal fisheries. Fisheries Manag Ecol. 1998; 5:277-89.
- Harris PJ, McGovern JC. Changes in the life history of red porgy, Pagrus pagrus, from the southeastern United States, 1972-1994. Fish Bull . 1997; 95(4):732-47.
- Hinckley S. Variation of egg size of walleye pollock Theragra chalcogramma with a preliminary examination of the effect of egg size on larval size. Fish Bull . 1990; 88(3):471-83.
- Hood PB, Johnson AK. Age, growth, mortality, and reproduction of red porgy, Pagrus pagrus, from the eastern Gulf of Mexico. Fish Bull . 2000; 98(4):723-35.
- Hubold G. Variations in growth rate and maturity of herring in the Northern North Sea in the years 1955-1973. Rapp Proces- Verb Réun Cos Int Explor Mer. 1978; 172:154-63.
- Hunter JR, Goldberg SR. Spawning incidence and batch fecundity in northern anchovy, Engraulis mordax Fish Bull . 1980; 77(3):641-52.
- Hunter JR, Lo NCH, Leong RJH. Batch fecundity in multiple spawning fishes. In Lasker R, editor. An egg production method for estimating spawning biomass of pelagic fish: application to the northern anchovy, Engraulis mordax La Jolla: NOAA/NMFS; 1985. p.67-77.
- Hunter JR, Macewicz BJ. 1985. Measurement of spawning frequency in multiple spawning fishes. In: Lasker R, editor. An egg production method for estimating spawning biomass of pelagic fish: application to the northern anchovy, Engraulis mordax La Jolla: NOAA/NMFS ; 1985. p.79-94.
- Hunter JR, Macewicz BJ, Lo NCH, Kimbrell CA. Fecundity, spawning, and maturity of female dover sole Microstomus pacificus, with an evaluation of assumptions and precision. Fish Bull . 1992; 90(1):101-28.
- Kendall MG, Stuart A. Advanced theory of statistics. 2nd edition. London: Charles Griffin; 1967.
- Kjesbu OS. Applied fish reproductive biology: contribution of individual reproductive potential to recruitment and fisheries management. In: Jakobsen T, Fogarty MJ, Megrey BA, Moksness E, editors. Fish reproductive biology: implications for assessment and management. Oxford: Wiley-Blackwell; 2009. p.293-332.
- Klibansky N, Scharf FS. Size-dependent and temporal variability in batch number and fecundity of red porgy, a protogynous, indeterminate spawner, in the U.S. South Atlantic. Mar Coast Fish . 2013; 5(1):39-52.
- Kokokiris L, Bruslé S, Kentouri M, Fostier A. Sexual maturity and hermaphroditism of the red porgy Pagrus pagrus (Teleostei: Sparidae). Mar Biol. 1999; 134(4):621-29.
- Labroupoulou M, Machias A, Tsimenides N. Habitat selection and diet of juvenile red porgy, Pagrus pagrus (Linnaeus, 1758). Fish Bull . 1999; 97(3):495-507.
- Lagos NA, García S, Cordo H. 2015. Análisis del estado de explotación y recomendaciones de manejo de besugo (Pagrus pagrus) en la ZCPAU. Año 2013. Informe Técnico de Investigación 20. Mar de Plata: INIDEP ; 2015.
- Lagos N, García S, Fernández Aráoz N. Análisis de la pesquería de besugo (Pagrus pagrus) en el área norte del Ecosistema Costero Bonaerense-Uruguayo. Período 2000-2010. Publ Com Téc Mix Fr Mar. 2013; 23:155-76.
- Loir L, Le Gac F, Somarakis S, Pavlidis M. Sexuality and gonadal cycle of the common dentex (Dentex dentex) in intensive culture. Aquaculture . 2001; 194(3-4):363-81.
- Macchi GJ, Acha EM, Militelli MI. Seasonal egg production of whitemouth croaker (Micropogonias furnieri) in the Río de la Plata estuary, Argentina-Uruguay. Fish Bull . 2003; 101(2):332-42.
- Macchi GJ, Pájaro M. Comparative reproductive biology of some commercial marine fishes from Argentina. Fisken og havet. 2003; 12:69-75.
- Macchi GJ, Pájaro M, Ehrlich M. Seasonal egg production pattern of the Patagonian stock of Argentine hake (Merluccius hubbsi). Fish Res. 2004; 67(1):25-38.
- Machinandiarena L, Müller M, López A. Early life stages of development of the red porgy Pagrus pagrus (Pisces, Sparidae) in captivity, Argentina. Invest Mar, Valparaíso. 2003; 31(1):5-13.
- Manooch CS. Reproductive cycle, fecundity, and sex ratios of the red porgy, Pagrus pagrus (Pisces: Sparidae) in North Carolina. Fish Bull . 1976; 74(4):775-81.
- Manooch CS, Hassler WW. Synopsis of Biological data on the red porgy, Pagrus pagrus, Linnaeus. Washington: NOAA/NMFS; 1978. (FAO fisheries synopsis; 116).
- Menezes GM, Sigler MF, Silva HM, Pinho MR. Structure and zonation of demersal fish assemblages off the Azores Archipielago (mid-Atlantic). Mar Ecol Prog Ser. 2006; 324: 241-60.
- Menezes NA, Figueiredo JL. Manual de peixes marinhos do sudeste do Brasil. V. Teleostei (4). São Paulo: Museu de Zoologia da Universidade de São Paulo. 1985.
- Militelli MI. 1999. Biología reproductiva del pez palo, Percophis brasiliensis, (Quoy et Gaimard, 1824) del área bonaerense. [Undergraduate monograph]. Mar del Plata: Universidad Nacional de Mar del Plata; 1999.
- Militelli MI, Macchi GJ. Preliminary estimate of spawning frequency and batch fecundity of Brazilian flathead, Percophis brasiliensis, in coastal waters off Buenos Aires Province. Sci Mar. 2001; 65(2):169-72.
- Militelli MI, Macchi GJ. Spawning and fecundity of king weakfish, Macrodon ancylodon, in the Río de la Plata estuary, Argentina - Uruguay. J Mar Biol Assoc UK . 2004; 84(2):443-47.
- Militelli MI, Macchi GJ, Rodrigues KA. Comparative reproductive biology of Sciaenidae family species in the Río de la Plata and Buenos Aires Coastal Zone, Argentina. J Mar Biol Assoc UK . 2013; 93(2):413-23.
- Murua H, Kraus G, Saborido-Rey F, Witthames PR, Thorsen A, Junquera S. Procedures to estimate fecundity of marine fish species in relation to their reproductive strategy. J Northw Atl Fish Sci. 2003; 33:33-54.
- Mylonas CC, Papadaki M, Pavlidis M, Divanach P. Evaluation of egg production and quality in the Mediterranean red porgy (Pagrus pagrus) during two consecutive spawning seasons. Aquaculture . 2004; 232(1-4):637-49.
- Nguyen-Xuan L, Wojciechowski J. Maturity and fecundity of Dentex macrophthalmus (Sparidae) from north-west African coast. Acta Ichtyol Pisc. 1973; 3(1):49-59.
- Picquelle S, Stauffer G. Parameter estimation for an egg production method of anchovy biomass assessment. In: Lasker R, editor. An egg production method for estimating spawning biomass of pelagic fish: application to the northern anchovy, Engraulis mordax La Jolla: NOAA/NMFS ; 1985. p.7-16.
- Prosdocimi L, Monsalvo M, Bernasconi F, Martinez-Puljak G. Informe anual variado costero, 2015. Coordinación Gestión de Pesquerías - DNPP, Subsecretaría de Pesca y Acuicultura, Ministerio de Agroindustria, Presidencia de la Nación. INFORME GP. 2016; 02. 19 p. Available from: http://www.agroindustria.gob.ar/
» http://www.agroindustria.gob.ar/ - Radonic M, López A, Oka M, Aristizabal EO. Effect of the incubation temperature on the embryonic development and hatching time of eggs of the red porgy Pagrus pagrus (Linne, 1758) (Pisces: Sparidae). Rev Biol Mar Oceanogr. 2005; 40(2):91-99.
- Rodrigues KA, Militelli MI, Macchi GJ. Área de puesta, fecundidad y calidad ovocitaria del pez palo (Percophis brasiliensis) en aguas costeras de la provincia de Buenos Aires. Resultados de campañas de investigación realizadas por el INIDEP durante el periodo 1998-2003. Informe Técnico 26. Mar de Plata: INIDEP ; 2007.
- Scott SG, Zeldis JR, Pankhurst NW. Evidence of daily spawning in natural populations of the New Zealand snapper Pagrus auratus (Sparidae). Environ Biol Fishes. 1993; 36(2):149-56.
- Stepkina MV. Some biological characteristics of Pagrus ehrenbergii Val. J Ichthyol . 1973; 13:641-49.
- Trippel EA. Age at maturity as a stress indicator in fisheries. BioScience. 1995; 45(11):759-71.
- Trippel EA. Egg size and viability and seasonal offspring production of young Atlantic cod. Trans Am Fish Soc. 1998; 127(3):339-59.
- Vaughan DS, Huntsman GR, Manooch CS, Rohde FC, Ulrich GF. Population characteristics of the red porgy, Pagrus pagrus, stock off the Carolinas. Bull Mari Sci. 1992; 50(1):1-20.
- Vieira PC, Haimovici M. Reproduςão da pescada olhuda Cynoscion guatucupa, sin. C. striatus (Scianidae, Teleosteii) no Sul do Brasil. Atlântica. 1997; 19:133-44.
- Winemiller KO. Life history strategies, population regulation, and implications for fisheries management. Can J Fish Aquat Sci. 2006; 62(4):872-85.
- Wootton RJ. Life histories as sampling devices: optimum egg size in pelagic fishes. J Fish Biol. 1994; 45(6):1067-77.
- Yoda M, Yoneda M. Assessment of reproductive potential in multiple-spawning fish with indeterminate fecundity: a case study of yellow sea bream Dentex hypselosomus in the East China Sea. J Fish Biol . 2009; 74(10):2338-54.
- Zohar Y, Gordin H. Spawning kinetics in the gilthead sea-bream, Sparus aurata L. after low doses of human chronic gonadotropin. J Fish Biol . 1979; 15(6):665-70.
Publication Dates
-
Publication in this collection
2017
History
-
Received
08 Sept 2016 -
Accepted
13 June 2017