Abstract
A new genus of Hypopopomatinae armored catfish is described from the northern portions of South America, namely the Amazon, Orinoco and Guianan coastal drainages. The new genus is diagnosed from all remaining hypoptopomatines by having the canal cheek plate on the ventral surface of the head posteriorly elongated and contacting the cleithrum, in addition to other features that distinguish the new genus from specific genera. Five new species are described and 18 species currently allocated in Parotocinclus, Hisonotus, and Curculionichthys are transferred to the new genus and rediagnosed. Parotocinclus amazonensis and P. aripuanensis are considered junior synonyms of P. britskii. The secondary sexual dimorphism of the members of the new genus is detailed and illustrated. Morphological characters are used to delimit four phenotypic groups of species that might have phylogenetic significance, which still have to be properly tested. A key to the species is offered and diagnoses, illustrations, and distribution maps are provided for all species.
Keywords:
Biodiversity; Identification key; South America; Systematics; Taxonomy
Resumo
Um novo gênero de cascudo da subfamília Hypopopomatinae é descrito das porções do norte da América do Sul, a saber, as drenagens costeiras da Amazônia, Orinoco e Guiana. O novo gênero é diagnosticado de todos os demais hypoptopomatineos por possuir a placa com canal da bochecha na superfície ventral da cabeça, alongada posteriormente e em contato com o cleitro, além de outras características que distinguem o novo gênero de gêneros específicos. Cinco novas espécies são descritas e 18 espécies atualmente alocadas em Parotocinclus, Hisonotus e Curculionichthys são transferidas para o novo gênero e rediagnosticadas. Parotocinclus amazonensis e P. aripuanensis são considerados sinônimos juniores de P. britskii. O dimorfismo sexual secundário dos membros do novo gênero é detalhado e ilustrado. Caracteres morfológicos são usados para delimitar quatro grupos fenotípicos de espécies que podem ter significado filogenético, que ainda precisam ser devidamente testados. Uma chave para as espécies é apresentada e diagnoses, ilustrações e mapas de distribuição são fornecidos para todas as espécies.
Palavras-chave:
América do Sul; Biodiversidade; Chave de identificação; Sistemática; Taxonomia
INTRODUCTION
The northern portion of the cis-Andean South American continent encompassing the Amazon, Orinoco, and coastal river basins of the Guianas is an immense, historically connected hydrographic complex, termed the Amazon-Orinoco-Guianas (AOG) Core by Albert et al., (2011)Albert JS, Petry P, Reis RE. Major biogeographic and phylogenetic patterns. In: Albert JS, Reis RE, editors. Historical biogeography of Neotropical freshwater fishes. California: University of California Press; 2011. p.21–57. https://doi.org/10.1525/california/9780520268685.001.0001
https://doi.org/10.1525/california/97805...
and the Greater Amazon by Van der Sleen, Albert, (2017)Van der Sleen P, Albert JS. Field guide to the fishes of the Amazon, Orinoco, and Guianas. New Jersey: Princeton University Press; 2017.. Loricariids inhabiting this region represent an amazing assemblage of species, containing members of all subfamilies except Delturinae, which is endemic to coastal rivers of eastern Brazil. This group includes an astonishing diversity of shapes, sizes, and behaviors, from which Hypoptopomatinae stands out as small sized, plant or bottom dweller species popularly known as “otos” worldwide or “cascudinhos” in Brazil. Two groups of hypoptopomatines have been traditionally described from the Greater Amazon, those belonging to the tribe Hypoptopomatini and Parotocinclus Eigenmann & Eigenmann, 1889. More recently, three other genera have been assigned to species of cascudinhos inhabiting this region, Corumbataia Britski, 1997, Curculionichthys Roxo, Silva, Ochoa & Oliveira, 2015 and Hisonotus Eigenmann & Eigenmann, 1889.
Parotocinclus was originally described as a subgenus of Hisonotus to separate Otocinclus maculicauda Steindachner, 1877 from remaining species based on its possession of an adipose fin. Subsequently, Miranda Ribeiro, (1939)Miranda Ribeiro P. Um Paraotocinclus [sic] do Nordeste Brasileiro (Peixes-Larocaridae [sic]-Hypoptopomatinae). Bol Biol. 1939; 4(3):364–66. elevated Parotocinclus to genus and added P. cesarpintoiMiranda Ribeiro, 1939Miranda Ribeiro P. Um Paraotocinclus [sic] do Nordeste Brasileiro (Peixes-Larocaridae [sic]-Hypoptopomatinae). Bol Biol. 1939; 4(3):364–66. and Boeseman, (1974)Boeseman M. On two Surinam species of Hypoptopomatinae, both new to science (Loricariidae, Siluriformes, Ostariophysi). Proc Konin Nederl Akad Wetensch. Ser. C, Biol Med Scienc. 1974; 77(3):251–71. described P. britskii Boeseman, 1974. After 88 years have passed since the description of Parotocinclus, Garavello, (1977)Garavello JC. Systematics and geographical distribution of the genus Parotocinclus Eigenmann & Eigenmann, 1889 (Ostariophysii, Loricariidae). Arq Zool. 1977; 28(4):1–37. https://doi.org/10.11606/issn.2176-7793.v28i4p1-37
https://doi.org/10.11606/issn.2176-7793....
revised the genus and transferred Microlepidogaster doceanus Miranda Ribeiro, 1918, Microlepidogaster bahiensis Miranda Ribeiro, 1918, Plecostomus spilosoma Fowler, 1941, and P. spilurus Fowler, 1941 to Parotocinclus. In addition, Garavello, (1977)Garavello JC. Systematics and geographical distribution of the genus Parotocinclus Eigenmann & Eigenmann, 1889 (Ostariophysii, Loricariidae). Arq Zool. 1977; 28(4):1–37. https://doi.org/10.11606/issn.2176-7793.v28i4p1-37
https://doi.org/10.11606/issn.2176-7793....
described the species P. cristatusGaravello, 1977Garavello JC. Systematics and geographical distribution of the genus Parotocinclus Eigenmann & Eigenmann, 1889 (Ostariophysii, Loricariidae). Arq Zool. 1977; 28(4):1–37. https://doi.org/10.11606/issn.2176-7793.v28i4p1-37
https://doi.org/10.11606/issn.2176-7793....
, P. amazonensisGaravello, 1977Garavello JC. Systematics and geographical distribution of the genus Parotocinclus Eigenmann & Eigenmann, 1889 (Ostariophysii, Loricariidae). Arq Zool. 1977; 28(4):1–37. https://doi.org/10.11606/issn.2176-7793.v28i4p1-37
https://doi.org/10.11606/issn.2176-7793....
, P. jimiGaravello, 1977Garavello JC. Systematics and geographical distribution of the genus Parotocinclus Eigenmann & Eigenmann, 1889 (Ostariophysii, Loricariidae). Arq Zool. 1977; 28(4):1–37. https://doi.org/10.11606/issn.2176-7793.v28i4p1-37
https://doi.org/10.11606/issn.2176-7793....
, P. cearensisGaravello, 1977Garavello JC. Systematics and geographical distribution of the genus Parotocinclus Eigenmann & Eigenmann, 1889 (Ostariophysii, Loricariidae). Arq Zool. 1977; 28(4):1–37. https://doi.org/10.11606/issn.2176-7793.v28i4p1-37
https://doi.org/10.11606/issn.2176-7793....
, and P. minutusGaravello, 1977Garavello JC. Systematics and geographical distribution of the genus Parotocinclus Eigenmann & Eigenmann, 1889 (Ostariophysii, Loricariidae). Arq Zool. 1977; 28(4):1–37. https://doi.org/10.11606/issn.2176-7793.v28i4p1-37
https://doi.org/10.11606/issn.2176-7793....
, elevating to 11 the number of valid species. After the revision of Garavello, (1977)Garavello JC. Systematics and geographical distribution of the genus Parotocinclus Eigenmann & Eigenmann, 1889 (Ostariophysii, Loricariidae). Arq Zool. 1977; 28(4):1–37. https://doi.org/10.11606/issn.2176-7793.v28i4p1-37
https://doi.org/10.11606/issn.2176-7793....
, species of Parotocinclus were described in a more steady pace, reaching to a total of 39 species and as a result comprising the most diverse genus of the Hypoptopomatinae.
The distribution of Parotocinclus includes two wide areas in South America which encompass two geographically isolated and phenotypically distinct groups of species. There is a morphologically variable group of species, in which the canal cheek plate on the ventral surface of the head is rounded and not expanded posteriorly to contact the pectoral girdle, varies in size from less that 30 to above 60 mm SL, and that occupies the coastal drainages of the Brazilian Shield from Santa Catarina State of Brazil, in the south, to Piauí State, in the north, including the rio São Francisco. These are the typical Parotocinclus and include the type-species P. maculicauda along with P. adamanteus Pereira, Santos, de Pinna & Reis, 2019, P. arandai Sarmento-Soares, Lehmann & Martins-Pinheiro, 2009, P. bahiensis, P. bidentatus Gauger & Buckup, 2005, P. cabessadecuia Ramos, Lima & Ramos, 2017, P. cearensis, P. cesarpintoi, P. cristatus, P. doceanus, P. fluminense Roxo, Melo, Silva & Oliveira, 2017, P. haroldoi Garavello, 1988, P. jacumirim Silva-Junior, Ramos & Zanata, 2020, P. jequi Lehmann, Braun, Pereira & Reis, 2013, P. jimi, P. jumbo Britskii & Garavello, 2002, P. minutus, P. muriaensis Gauger & Buckup, 2005, P. nandae Lehmann, Camelier & Zanata, 2020, P. planicauda Garavello & Britski, 2003, P. prata Ribeiro, Melo & Pereira, 2002, P. robustus Lehmann & Reis, 2012, P. seridoensis Ramos, Barros-Neto, Britski & Lima, 2013, P. spilosoma, and P. spilurus.
The other group of species, entirely allopatric to the former, inhabit the Greater Amazon. These species belong to a clade diagnosed by the canal cheek plate on the ventral surface of the head being posteriorly elongated and contacting the cleithrum. Species in this clade are small (up to 33 mm SL), usually have a long, pointed snout, are often sharply barred with dark colors, and many have a dark, triangular spot at the dorsal-fin origin. This clade includes Parotocinclus amazonensis, P. aripuanensis Garavello, 1988, P. britskii, P. collinsaeSchmidt & Ferraris, 1985Schmidt RE, Ferraris Jr. CJ. A new species of Parotocinclus (Pisces: Loricariidae) from Guyana. Proc Biol Soc Wash. 1985; 98(2):341–46. Available from: https://www.biodiversitylibrary.org/page/34648620#page/365/mode/1up
https://www.biodiversitylibrary.org/page...
, P. dani Roxo, Silva & Oliveira, 2016, P. eppleyi Schaefer & Provenzano, 1993, P. halbothi Lehmann, Lazzarotto & Reis, 2014, P. hardmani Lehmann, Lujan & Reis, 2022, P. kwarup Lehmann & Reis, 2021, P. longirostrisGaravello, 1988Garavello JC. Three new species of Parotocinclus Eigenmann & Eigenmann, 1889 with comments on their geographical distribution (Pisces, Loricariidae). Naturalia. 1988; 13:117–28., P. pentakelis Roxo, Messias & Silva, 2019, P. polyochrusSchaefer, 1988Schaefer SA. A new species of the loricariid genus Parotocinclus from southern Venezuela (Pisces: Siluroidei). Copeia. 1988; 1988(1):182–88. https://doi.org/10.2307/1445936
https://doi.org/10.2307/1445936...
, P. variola Lehmann, Schvambach & Reis, 2015, and P. yaka Lehmann, Lima & Reis, 2018.
In addition to the above Parotocinclus species, six other Amazon cascudinhos share the characters listed above and belong in this clade. These are Hisonotus acuen Silva, Roxo & Oliveira, 2014, H. bockmanniCarvalho & Datovo, 2012Carvalho M, Datovo A. A new species of cascudinho of the genus Hisonotus (Siluriformes: Loricariidae: Hypoptopomatinae) from the upper Rio Tapajós basin, Brazil. Copeia. 2012; 2012(2):266–75. https://doi.org/10.1643/CI-11-016
https://doi.org/10.1643/CI-11-016...
, H. chromodontus Britski & Garavello, 2007, H. dinizaeRibeiro-Silva, Silva, Venere, Silva & Roxo, 2020Ribeiro-Silva LR, Silva GSC, Venere PC, Silva HP, Roxo FF. Description of a new species of Hisonotus (Loricariidae: Siluriformes) from rio Araguaia basin. Zootaxa. 2020; 4860(4):553–62. https://doi.org/10.11646/zootaxa.4860.4.5
https://doi.org/10.11646/zootaxa.4860.4....
, H. jumaorum Dias, Silva, Oliveira & Roxo, 2018, and Curculionichthys hera Gamarra, Calegari & Reis, 2019.
Phylogenetic studies containing species of this clade have so far included few species. A clade containing C. hera (treated as Curculionichthys sp. n.), Hisonotus acuen, H. bockmanni, H. chromodontus, and four species of Amazonian
Parotocinclus, P. collinsae, P. amazonensis, P. britskii, and P. eppleyi has already been recovered in a combined molecular and morphological phylogenetic analysis of the
Hypoptopomatinae (Reis et al., 2017Reis RE, Calegari BB, Carvalho TP, Cramer CA, Delapieve MLS, Lehmann A. P, Pereira EHL. A phylogeny of the armored catfishes, with emphasis on the Neoplecostominae-Hypoptopomatinae clade (Siluriformes: Loricariidae): Integrating phenotypical and molecular data. Londrina: II International Symposium on Phylogeny and Classification of Neotropical Fishes; 2017.). In addition, Roxo et al. (2019)Roxo FF, Ochoa LE, Sabaj MH, Lujan NK, Covain R, Silva GSC, Melo BF, Albert JS, Chang J, Foresti F, Alfaro ME, Oliveira C. Phylogenomic reappraisal of the Neotropical catfish family Loricariidae (Teleostei: Siluriformes) using ultraconserved elements. Mol Phyl Evol. 2019; 135:148–65. https://doi.org/10.1016/j.ympev.2019.02.017
https://doi.org/10.1016/j.ympev.2019.02....
, in a genomic phylogenetic analysis of the loricariids, found H. acuen, H. chromodontus, Parotocinclus aripuanensis, and an undescribed Parotocinclus from the Amazon to cluster in a clade, which they termed “New Genus 2”.
The possession of an adipose fin by some members of this clade has been misleading for many years, causing those species to be described in Parotocinclus. Other species in this group that lack an adipose fin may have a few platelets in the dorsal midline at the typical adipose-fin position and some of those were also described in Parotocinclus. Other species without an adipose fin, however, were described in other genera with which they share some superficial similarity, like Hisonotus acuen, H. bockmanni, H. chromodontus, H. dinizae, H. jumaorum, and Curculionichthys hera. By reassigning all these species to the new genus being described herein, both Hisonotus and Curculionichthys become more clearly defined and the former becomes restricted to waterways of south and southeastern Brazil.
MATERIAL AND METHODS
Body measurements of the left side of individuals were taken as point-to-point linear distances with digital calipers under a steromicroscope and recorded to the 0.01 mm, following the measurements described mainly by Boeseman, (1968)Boeseman M. The genus Hypostomus Lacépède, 1803, and its Surinam representatives (Siluriformes, Loricariidae). Zool Verhandel. 1968; 99:1–89. and Schaefer, (1997)Schaefer SA. The Neotropical cascudinhos: systematics and biogeography of the Otocinclus catfishes (Siluriformes: Loricariidae). Proc Acad Nat Sci Phila. 1997; 148:1–120. https://www.jstor.org/stable/4065046
https://www.jstor.org/stable/4065046...
. Despite measurements being presented with one significant decimal number, they were recorded and calculations were performed with two decimals because some body structures are very small. More difficult measurement, or those of smaller structures where repeated two to four times and then recorded as the average, to ensure accuracy. Standard length (SL) is expressed in millimeters and other body measurements are given as percent of SL or those that represent subunits of the head, as percent of head length (HL). In order to standardize measurements across species and make them fully comparable, all measurements were taken again. Measurements in the original descriptions of species included in this study were performed by different people, even for those described by ourselves, and show significant differences with those presented here. Specimens were cleared and double stained (cs) for inspection of bones and cartilages following the technique of Taylor, Van Dyke, (1985)Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985; 9(2):107–19.. Dermal plates and vertebral centra were counted from cs specimens only. Identification and counts of dermal plates follow the serial homology scheme proposed by Schaefer, (1997)Schaefer SA. The Neotropical cascudinhos: systematics and biogeography of the Otocinclus catfishes (Siluriformes: Loricariidae). Proc Acad Nat Sci Phila. 1997; 148:1–120. https://www.jstor.org/stable/4065046
https://www.jstor.org/stable/4065046...
. Vertebral counts include five centra modified into the Weberian apparatus and one compound caudal centrum (PU1+U1). Premaxillary and dentary teeth were counted in both sides and the higher value was always recorded. Measurements and counts were compiled and processed as MS-Excel spreadsheets. Data were transferred to PAST v4.03 (Hammer et al., 2001Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol Electron. 2001; 4(1):1–9. Available from: https://palaeo-electronica.org/2001_1/past/past.pdf
https://palaeo-electronica.org/2001_1/pa...
) for statistical analyses, which include Principal Components (PCA) and Linear Discriminant (LDA) analyses. In the description of color, bar is used for transverse marks on body, band is used for transverse marks on fins, and stripe is used for longitudinal marks.
In the species account, type-localities are written as in the original description, with occasional corrections or additions made between square brackets. Geographic descriptors are written in the language of the country of origin, to avoid translating error. Accordingly, we use “rio” for Brazilian localities, “Río” for localities in Spanish speaking countries, and “River” for Guyana. For simplicity, we also use English for the geographic descriptors in Suriname. Specimens examined belong to fish collections whose acronyms are given in Sabaj, (2020)Sabaj MH. Codes for natural history collections in ichthyology and herpetology. Copeia. 2020; 108(3):593–669. https://doi.org/10.1643/ASIHCODONS2020
https://doi.org/10.1643/ASIHCODONS2020...
. In the lists of examined material, museum abbreviation and catalog number come first, followed by the number of specimens and locality data. For the new species, type-material includes catalog number followed by the number and SL range of specimens in that lot, the number and SL range of specimens measured for the morphometric comparisons, in parentheses, locality, date of collection, and collectors. Conservation status of the new species were assessed following the categories and criteria of the International Union for Conservation of Nature (IUCN Standards and Petitions Subcommittee, 2019International Union for Conservation of Nature (IUCN). Standards and petitions subcommittee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Gland; 2019. Available from: https://www.iucnredlist.org/resources/redlistguidelines
https://www.iucnredlist.org/resources/re...
). Distribution information for all species was compiled and managed with Google Earth Pro and distribution maps were generated with the software QGis, following the tutorial presented by Calegari et al., (2016)Calegari BB, Delapieve MLS, Sousa LM. Tutorial para preparação de mapas de distribuição geográfica. Bol Soc Bras Ictiol. 2016; 118:15–30. Available from: https://www.sbi.bio.br/images/sbi/boletim-docs/2016/junho_118.pdf
https://www.sbi.bio.br/images/sbi/boleti...
.
RESULTS
Rhinotocinclus, new genus
urn:lsid:zoobank.org:act:6BB47659-B0D4-4153-B621-34E42CEE6227
Type-species.Parotocinclus longirostris Garavello, 1988.
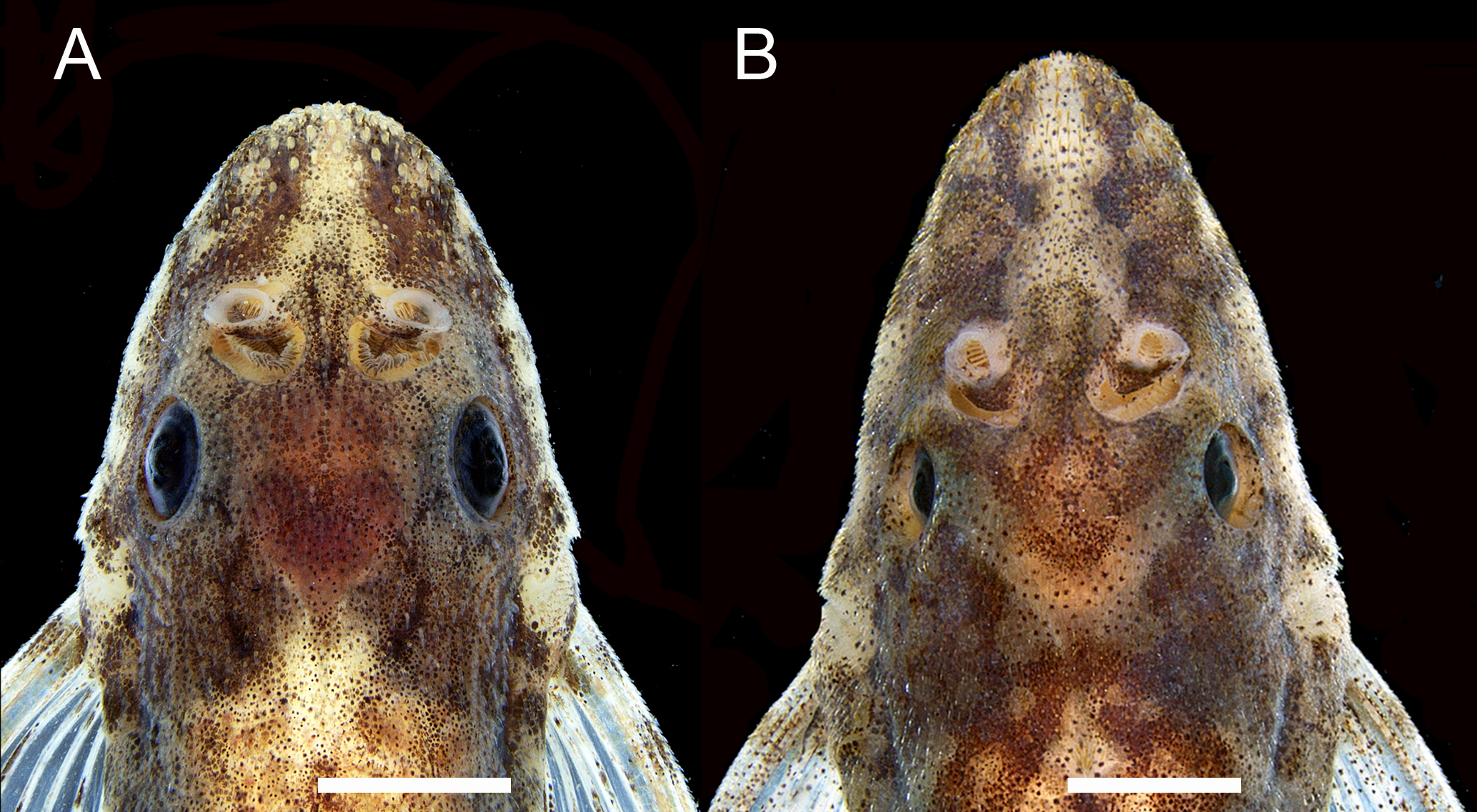
Diagnosis.Rhinotocinclus is diagnosed from all other genera in Hypoptopomatinae by having the canal cheek plate on the ventral surface of the head posteriorly elongated and contacting the cleithrum (Fig. 1; vs. canal cheek plate rounded or mesially elongated and not expanded backwards to contact the pectoral girdle). The new genus is also distinguished from other genera except Curculionichthys, Otocinclus, and Parotocinclus by having the dorsal-fin locking mechanism functional with the dorsal-fin spinelet V-shaped (vs. dorsal-fin locking mechanism non-functional and dorsal-fin spinelet roundish or absent). It is further distinguished from Curculionichthys by having a single rostral plate (vs. paired rostral plate) and less numerous lateral abdominal plates (2–5, but up to 8 in the R. collinsae Group; vs. 5–8). It is further distinguished from Parotocinclus by the shape of the coracoid, which is expanded anteriorly as a lamina partially covering the cleithrum ventrally (Fig. 1; vs. coracoid less expanded anteriorly), and from Otocinclus by having the preopercle exposed and bearing part of the mandibular branch of the laterosensory canal (vs. preopercle not exposed in the surface and not bearing laterosensory canal). Rhinotocinclus is further distinguished from the genera of Neoplecostomini (Euryochus Pereira & Reis, 2017, Hirtella Pereira, Zanata, Cetra & Reis, 2014, Isbrueckerichthys Derijst, 1996, Kronichthys Miranda Ribeiro, 1908, Neoplecostomus Eigenmann & Eigenmann, 1888, Pareiorhaphis Miranda Ribeiro, 1918, Pareiorhina Gosline, 1947) by having the pectoral girdle widely exposed and bearing odontodes on the ventral surface of both cleithrum and coracoid (vs. pectoral girdle covered by thick skin) and by the small body size (maximum standard length of its species 19.9–33.0 mm, vs. 49.2 mm in Hirtella, usually above 100 mm in remaining genera).
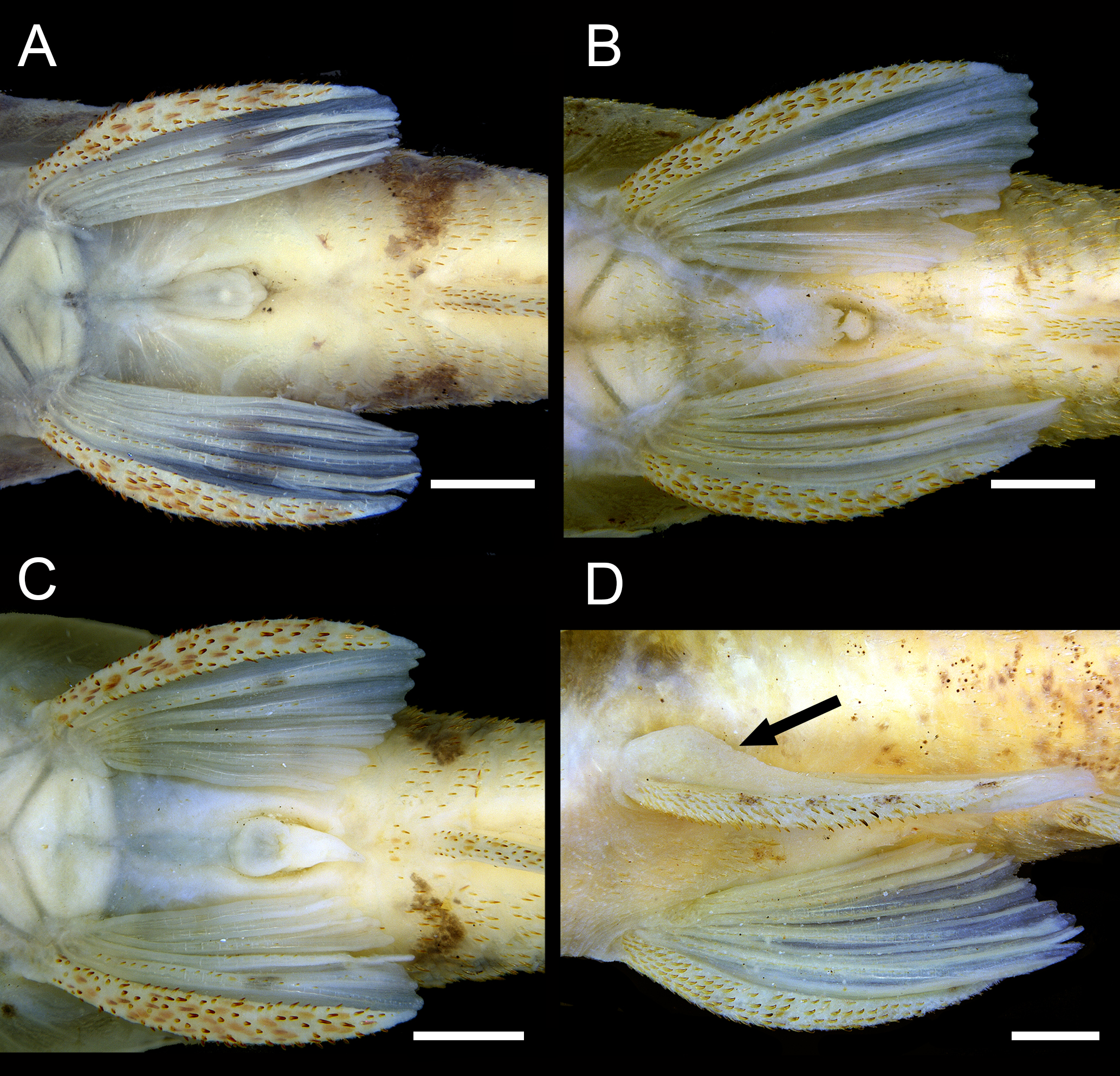
Canal cheek plate and pelvic girdle. A. Rhinotocinclus kwarup, MCP 32297; B. Rhinotocinclus yaka, MCP 53630; C. Parotocinclus maculicauda, MCP 17605; D. Curculionichthys scaius, MCP 53801. CCP = canal cheek plate; CL = cleithrum; CO = coracoid. Scale bars = 2 mm.
Sexual dimorphism. Species of Rhinotocinclus exhibit conspicuous secondary sexual dimorphism. As all hypoptopomatines, males of Rhinotocinclus possess a urogenital papilla immediately behind the anus (Figs. 2B,C), and a variably deep skin fold along the first, unbranched pelvic-fin ray (Fig. 2D), both characteristics being absent in females. In addition, males possess a much larger nostril than females (Fig. 3), causing the internarial distance to be smaller in males. The larger size of the olfactory organ of males also causes an elevation in the snout profile immediately in front of the eyes, which can be easily seen in lateral view (Fig. 3). In most species, males also possess longer pelvic fins, which reach or almost reach to the anal-fin origin (Figs. 2B,C), which does not happen in females. On the other hand, females usually attain larger size than males.
Pelvic fin and urogenital papilla of Rhinotocinclus. A. R. hardmani, AUM 62879, female; B. R. collinsae, MCP 54757, male; C. R. hardmani, AUM 62879, male; D. R. acuen, MCP 40543, male. Arrow points skin fold on first unbranched pelvic-fin ray. Scale bars = 1 mm.
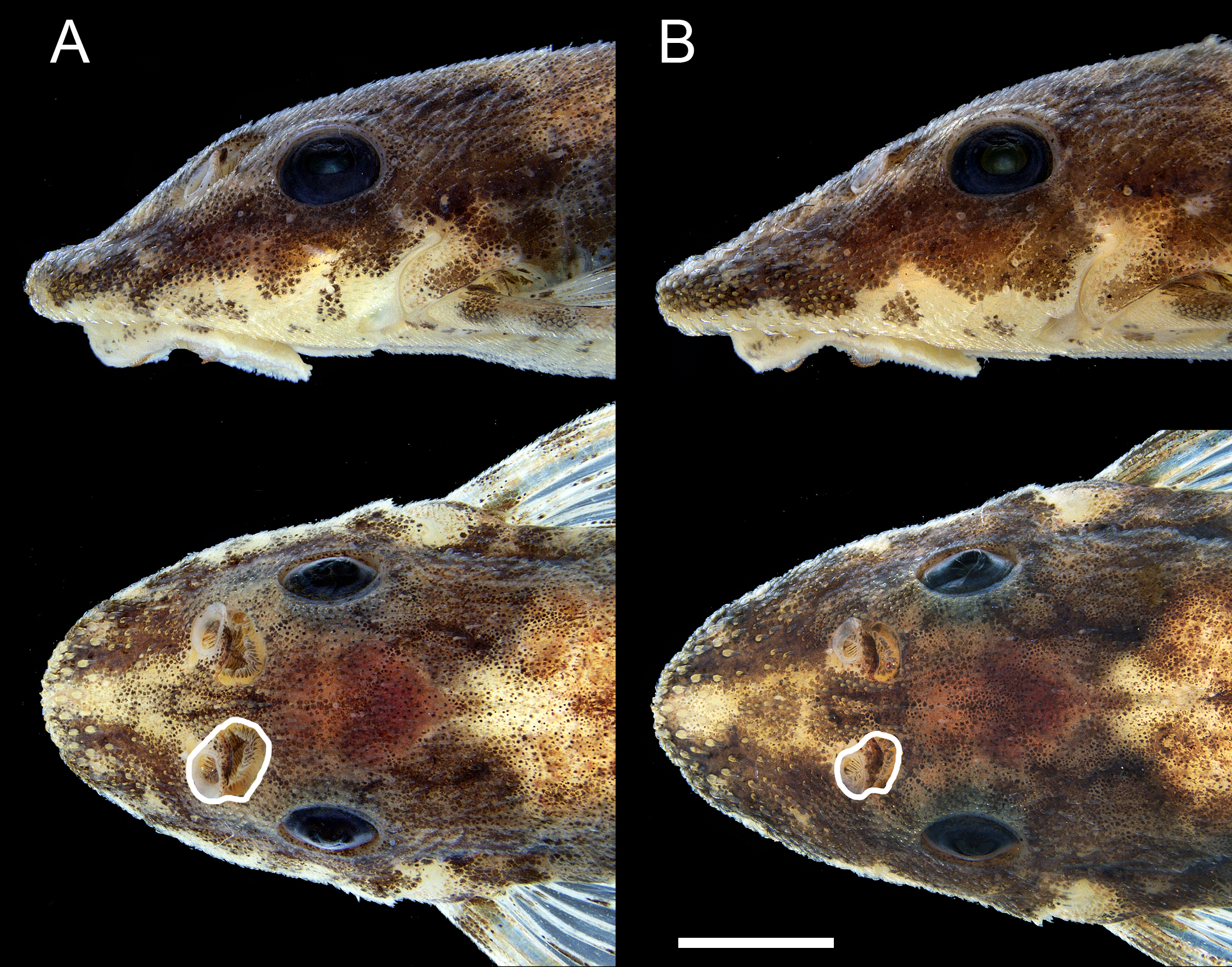
Snout shape and sexual dimorphism in Rhinotocinclus bristkii, ZUEC 16817. A. male: head wider at nostril level, nostril larger, internarial narrower, area between nostrils elevated; B. female: head narrower at nostril level, nostril smaller, internarial wider, area between nostrils not elevated. Scale bar = 2 mm.
Characters and phenotypic species groups. The genus Rhinotocinclus as herein rearranged includes 23 species previously attributed to Parotocinclus, Hisonotus, and Curculionichthys. These species can be grouped in four phenotypic species groups that share characters that may or may not have phylogenetic significance, but which are easily verified and useful to identify the species.
The Rhinotocinclus britskii Group. Species in this group were all described in Parotocinclus and are characterized by possessing (1) a normally developed adipose fin (Figs. 4A,B), (2) dark brown oral teeth (Fig. 5A), (3) a Y-shaped light mark from the snout tip to the nostrils (Figs. 6A,B), and (4) a dominant color pattern formed by five dark bars on body (first at the anterior portion of the dorsal fin, usually continuous with a triangular spot at the anterior portion of the dorsal fin and anteriorly inclined, second at posterior portion of dorsal fin, usually not reaching dorsal midline and posteriorly inclined, third from adipose to anal fin, fourth before end of the caudal peduncle, usually connected to the fifth, which is contiguous with a dark blotch at the base of the caudal fin (Figs. 7A,B). Species in this group include R. bristskii, R. eppleyi, R. kwarup, R. longirostris, R. polyochrus, R. variola, R. yaka, and three new species, which are widely distributed in the Amazon, Orinoco, and coastal rivers of the Guianas.
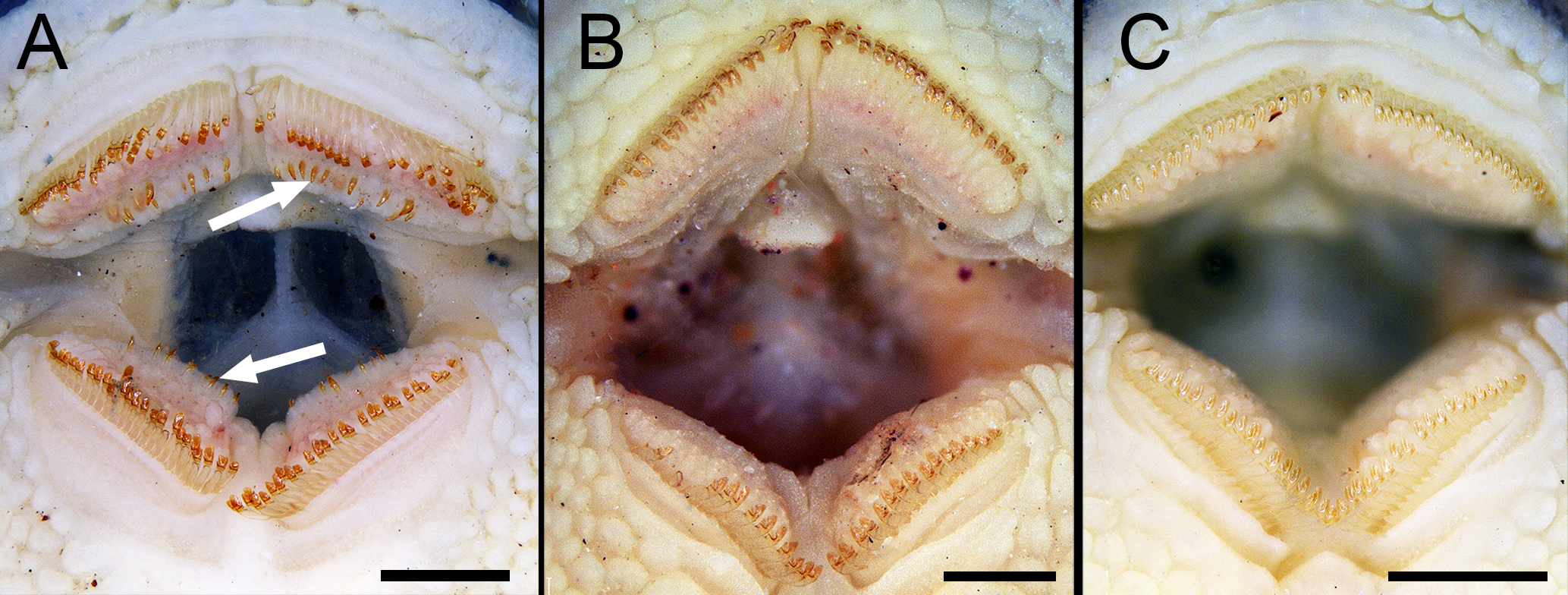
The Rhinotocinclus collinsae Group. Species in this group were also originally described in Parotocinclus and are characterized by possessing (1) an adipose fin, which may be coalesced to the dorsal plates and lack a membrane (Fig. 4C), (2) brown oral teeth (Fig. 5A), (3) lack of clearly defined light marks from the snout tip to the nostrils (Figs. 6G,H), (4) absence of a triangular dark spot in the dorsal fin (Fig. 8A) and dark bars 2 and 3 of body fused (Fig. 7C), (5) unicuspid accessory oral teeth on both the premaxilla and dentary (Fig. 5A, arrows), and (5) odontodes on the ventral surface of the pelvic-fin ray aligned with main ray axis and not bent mesially (Fig. 9B). Species in this group include R. collinsae, R. halbothi, and R. hardmani, which are distributed on the Guiana Shield in the coastal rivers of the Guianas and some northern tributaries of the lower Amazon in Brazil.
The Rhinotocinclus bockmanni Group. Species in this group were originally described either in Parotocinclus, Hisonotus, or Curculionichthys, and are characterized by having (1) small azygous platelets at the adipose-fin position (except for R. hera), (2) light yellow oral teeth (except R. hera, which has light ochre teeth; Figs. 5B,C), (3) two separate light lines from the snout tip diverging toward each nostril (Figs. 6E,F), (4) a dominant color pattern formed by four or five dark bars on body, usually connected by a lateral dark stripe of variable intensity (Fig. 7D). Species in this group include R. bockmanni, R. dani, R. hera, R. pentakelis, and two new species, which are distributed in Amazon tributaries draining the western portion of the Brazilian Shield.
Adipose fin of Rhinotocinclus. A. R. pilosus n. sp., UFRO-ICT 27700, normally developed; B. R. britskii, ZUEC 16817, normally developed; C. R. hardmani, AUM 62879, coalesced to dorsal plates. Scale bars = 2 mm.
Teeth color of Rhinotocinclus. A. R. collinsae, MCP 54757, brown; B. R. acuen, MCP 40543, ochre; C. R. dani, MCP 54756, yellow. Arrows point accessory teeth. Scale bars = 500 µ.
Snout color pattern of Rhinotocinclus. Y-shaped, A. R. longirostris, MZUSP 85786; B. R. eppleyi, MCP 54750; V-shaped, C. R. chromodontus, MCP 32660; D. R. jumaorum, MCP 54758; two separate lines, E. R. marginalis n. sp., MCP 54748; F. R. loxochelis n. sp., MPEG 38957; no light lines, G. R. collinsae, AUM 62851; H. R. hardmani, AUM 62850.
Lateral color pattern of Rhinotocinclus. A. R. britskii, MCP 54760; B. R. eppleyi, MCP 54750; C. R. collinsae, AUM 62851; D. R. dani, MCP 54756; E. R. acuen, MCP 40543. Numbers 1–5 are dark bars on body.
Dorsal-fin color pattern of Rhinotocinclus. A. R. collinsae, AUM 62851; B. R. yaka, MZUSP 123655; C. R. variola, MPEG 12431.
Odontodes on first pelvic-fin ray of Rhinotocinclus. A. R. acuen, MCP 40543, male; odontodes bent and turned mesially; B. R. hardmani, AUM 62879, male; odontodes aligned with main ray axis. Scale bars = 1 mm.
The Rhinotocinclus chromodontus Group. Species in this group were all described in Hisonotus and are characterized by (1) lacking an adipose fin and small azygous platelets at the adipose-fin position, (2) having brown or light ochre teeth (Figs. 5A,B), (3) a V-shaped light mark from the snout tip to the nostrils (Figs. 6C,D), and (4) a dominant color pattern formed by a dark stripe from the snout tip, passing through the eye and extending to the end of caudal peduncle, the transverse dark bars being inconspicuous laterally (Fig. 7E). Species in this group include R. acuen, R. chromodontus, R. dinizae, R. jumaorum, which are distributed in the rivers Araguaia, Xingu, Tapajós, and Madeira, all Amazon tributaries draining the Brazilian Shield.
Remarks. Among the Hypoptopomatini, some species of Hypoptopoma (H. brevirostratum Aquino & Schaefer, 2010, H. elongatum Aquino & Schaefer, 2010, H. guianense Boeseman, 1974, H. incognitum Aquino & Schaefer, 2010, H. inexspectatum (Holmberg 1893), and H. steindachneri Boulenger, 1895) may variably possess a small adipose fin, but their relationships were already demonstrated to be with their congeners and other hypoptopomatin genera (Delapieve et al., 2017Delapieve MLS, Lehmann A. P, Reis RE. An appraisal of the phylogenetic relationships of Hypoptopomatini cascudinhos with description of two new genera and three new species (Siluriformes: Loricariidae). Neotrop Ichthyol. 2017; 15(4):e170079. https://doi.org/10.1590/1982-0224-20170079
https://doi.org/10.1590/1982-0224-201700...
), and this character bears no phylogenetic signal among the two groups. The adipose fin is also present in most Neoplecostomini, but these are also distantly related to the new genus (Pereira, Reis, 2017Pereira EHL, Reis RE. Morphology-based phylogeny of the suckermouth armored catfishes, with emphasis on the Neoplecostominae (Teleostei: Siluriformes: Loricariidae). Zootaxa. 2017; 4264(1):1–104. https://doi.org/10.11646/zootaxa.4264.1.1
https://doi.org/10.11646/zootaxa.4264.1....
; Reis et al., 2017Reis RE, Calegari BB, Carvalho TP, Cramer CA, Delapieve MLS, Lehmann A. P, Pereira EHL. A phylogeny of the armored catfishes, with emphasis on the Neoplecostominae-Hypoptopomatinae clade (Siluriformes: Loricariidae): Integrating phenotypical and molecular data. Londrina: II International Symposium on Phylogeny and Classification of Neotropical Fishes; 2017.). The main diagnostic feature of Rhinotocinclus is the canal cheek plate posteriorly elongated and contacting the cleithrum. A very similar configuration of the cheek plate, however, occurs in a few species of Hisonotus from southern Brazil and Uruguay (H. megaloplax Carvalho & Reis, 2009, H. montanus Carvalho & Reis, 2009, H. ringueleti Aquino, Schaefer & Miquelarena, 2001, H. thayeri Martins & Langeani, 2016 and H. vireo Carvalho & Reis, 2011). Again, this similarity is better interpreted as a convergence as Hisonotus have been demonstrated not to be directly related to Rhinotocinclus (Cramer et al., 2011Cramer CA, Bonatto SL, Reis RE. Molecular phylogeny of the Neoplecostominae and Hypoptopomatinae (Siluriformes: Loricariidae) using multiple genes. Mol Phyl Evol. 2011; 59(1):43–52. https://doi.org/10.1016/j.ympev.2011.01.002
https://doi.org/10.1016/j.ympev.2011.01....
; Reis et al., 2017Reis RE, Calegari BB, Carvalho TP, Cramer CA, Delapieve MLS, Lehmann A. P, Pereira EHL. A phylogeny of the armored catfishes, with emphasis on the Neoplecostominae-Hypoptopomatinae clade (Siluriformes: Loricariidae): Integrating phenotypical and molecular data. Londrina: II International Symposium on Phylogeny and Classification of Neotropical Fishes; 2017.; Roxo et al., 2019Roxo FF, Messias FL, Silva GSC. A new species of Parotocinclus (Loricariidae: Hypoptopomatinae) from rio Tocantins basin, Brazil. Zootaxa. 2019; 4646(2):346–56. https://doi.org/10.11646/zootaxa.4646.2.9
https://doi.org/10.11646/zootaxa.4646.2....
).
Parotocinclus longirostris was chosen as type-species for the new genus following Recommendation 69A of the International Code of Zoological Nomenclature (ICZN, 1999International Commission on Zoological Nomenclature (ICZN). International Code of Zoological Nomenclature, Fourth Edition: adopted by the International Union of Biological Sciences. The International Trust for Zoological Nomenclature; 1999. ). It is adequately described and illustrated, type-material exists and is readily available at MZUSP, and it is a common and well distributed species in central Amazon.
Etymology.Rhinotocinclus masc., from the Greek Ρηιοσ (Rhinos), beak, snout and Otocinclus, a genus of Hypoptopomatinae, in allusion to the conspicuous and elegant snout of most of its species.
Key to the species of Rhinotocinclus
1a. Adipose fin absent, sometimes small platelets at adipose-fin position 14
1b. Adipose fin present, even if spine adnate to dorsal plates, without membrane 2
2a. Triangular dark spot on anterior portion of dorsal-fin membrane
(Figs. 8B,C); with Y-shaped light mark from snout tip to nostrils
(Figs. 6A,B); odontodes on ventral surface of first pelvic-fin ray
bent and pointing mesially (Fig. 9A);
eye large (25.5–40.5% snout length) 5 (R. britskii group)
2b. No triangular dark spot on anterior portion of dorsal-fin membrane (Fig. 8A);
no light mark on snout tip (Figs. 6G,H); odontodes on ventral surface
of pelvic-fin ray aligned with main ray axis (Fig. 9B);
eye small (18.9–24.6% snout length) 3 (R. collinsae group)
3a. Belly covered with middle abdominal plates between lateral abdominal plates
(Figs. 10A,B); males with dorsal skin fold on first pelvic-fin ray (Fig. 2D);
urogenital papilla normally developed
(approximately same size of anal tube; Fig. 2B) 4
3b. Belly naked or almost naked between lateral abdominal plates (Fig. 10C);
males without dorsal skin fold on first pelvic-fin ray;
urogenital papilla 3–4 times bigger than normal (2–3 times longer than
anal tube; Fig. 2C) R. hardmani (Essequibo River basin, Guyana)
4a. One irregular series of middle abdominal plates between the lateral
abdominal plates (Fig. 10B); adipose fin normally developed
(Figs. 4A,B) R. collinsae (Essequibo River basin, Guyana)
4b. Four to seven irregular series of middle abdominal plates between the
lateral abdominal plates (Fig. 10A); adipose-fin spine coalesced
to dorsal plates (Fig. 4C) R. halbothi
(Rio Trombetas basin, Brazil and upper Marowijne basin, Suriname)
5a. Snout more acutely pointed (Fig. 11B); dark bars on body wider and closer
(Fig. 7B); 2–4 plates between posterior border of rostral plate
and nostril (Fig. 12A) 7
5b. Snout more broadly rounded (Fig. 11A); dark bars on body narrower and
spaced (Fig. 7A); one plate between posterior border of rostral
plate and nostril (Fig. 12B) 6
6a. More numerous premaxillary (28–34, mode 32; Tab. 1) and dentary
(27–33, mode 29; Tab. 2) teeth; color pattern with more broken marks,
mottled R. kwarup (Upper rio Xingu basin, Brazil)
6b. Fewer premaxillary teeth (15–29, mode 23; Tab. 1) and dentary
(15–29, mode 20 and 25; Tab. 2) teeth; color pattern with less
broken marks R. britskii
(Coastal rivers of Guyana and Suriname; upper rio Branco basin in Brazil and Guyana; Cuyuni and Caroni rivers, Venezuela; lower and middle portions of tributaries to the eastern Amazon basin, Brazil)
7a. Zero to 2 (rarely 3) irregular series of middle abdominal plates
(Figs. 10B,C); four dark bars on body
(bars 1+2 or 2+3 fused) 9
7b. Four or 5 irregular series of middle abdominal plates (Fig. 10A);
five dark bars on body 8
8a. Fewer premaxillary (22–30, mode 26; Tab. 1 and dentary (21–27, mode 25;
Tab. 2) teeth; R. eppleyi (Upper Río Orinoco basin, Venezuela)
8b. More numerous premaxillary (28–36, mode 30;Tab. 1)
and dentary (27–31, mode 30; Tab. 2) teeth; R. longirostris
(Tributaries to central Amazon basin, Brazil)
9a. One or 2 lateral abdominal plates between pectoral-fin
axilla and pelvic-fin 10
9b. Three to 5 lateral abdominal plates between pectoral-fin
axilla and pelvic-fin 11
10a. Belly naked or almost naked between lateral abdominal plates (Fig. 10C);
caudal peduncle shallower
(6.3–7.5% SL) R. pilosus n. sp. (Rio Madeira basin near Humaitá, Brazil)
10b. Belly covered with 1–2 series of middle abdominal plates between lateral
abdominal plates (Fig. 10B); caudal peduncle deeper
(8.1–8.8% SL) R. isabelae n. sp. (Río Tigre and Río Nanay, Loreto, Peru)
11a. Fewer premaxillary (23–32) and dentary (19–31) teeth 13
11b. More numerous premaxillary (33–51) and dentary (33–43) teeth 12
12a. Dark dots smaller than pupil diameter broadly distributed dorsally and ventrally;
triangular dark spot on anterior portion of pectoral-fin membrane (Fig. 13B);
triangular dark spot occupying more than half of dorsal fin
(Fig. 8C) R. variola (Western Amazon basin, Colombia and Brazil)
12b. No dark dots smaller than pupil diameter (sometimes darkened sensory
pores on head); no triangular dark spot on pectoral-fin (Fig. 13A);
triangular dark spot occupying less than half of dorsal fin
(Fig. 8B) R. yaka (Rio Tiquié, Upper rio Negro basin, Brazil)
13a. Conspicuous light bar in front of dorsal fin, extended on head as Y-shaped
mark towards each eye; triangular dark spot of dorsal fin well developed;
body dark bars 1+2 fused; caudal peduncle shallower (6.8–7.1% SL);
pectoral-fin spine longer (29.2–32.3% SL); orbit smaller (38.9–40.6%
interorbital distance) R. polyochrus
(Río Mawarinuma, Río Orinoco basin at Neblina mountains, Venezuela)
13b. No light bar in front of dorsal fin and Y-shaped mark on head; triangular
dark spot of dorsal fin inconspicuous; body dark bars 2+3 fused;
caudal peduncle deeper (7.7–8.6% SL); pectoral-fin spine shorter
(24.8–27.3% SL); orbit larger (52.1–63.7% interorbital
distance) R. discolor n. sp. (Río Orinoco basin in southern Venezuela)
14a. Dominant color pattern formed by dark stripe from snout tip, through eye and extending to end of caudal peduncle (Fig. 7E);V-shaped light mark from snout tip diverging to each nostril
(Figs. 6C,D) 20 (R. chromodontus group)
14b. Dominant color patter formed by four or five dark bars on body (Fig. 7D);
two separate light lines from snout tip diverging to each nostril
(Figs. 6E,F); 15 (R. bockmanni group)
15a. No triangular dark spot on anterior portion of dorsal-fin membrane (Fig. 8A);
five dark bars on body, variably united by irregular dark stripe 16
15b. Triangular dark spot on anterior portion of dorsal-fin membrane (Fig. 8B);
dark bar 2 absent or inconspicuous, bars 1, 3–5 united by thin,
regular dark stripe R. bockmanni (Middle rio Tapajós basin, Brazil)
16a. One to 3 azygous platelets at adipose-fin position; tooth cusps light yellow 17
16b. Azygous platelets absent;
tooth cusps ochre R. hera (Rio Curuá-Una basin, Pará, Brazil)
17a. Dark bars on body regularly arranged, usually connected by irregular
midline dark stripe; nasal bone projected laterally
and contacting infraorbital 2 18
17b. Dark bars on body somewhat fragmented and inclined, such that they
connect to form a zig-zag pattern; nasal bone not contacting
infraorbital 2 R. loxochelis n. sp.
(Jamanxim National Forest, rio Tapajós basin, Brazil)
18a. Caudal fin with one slanted dark band at base of rays and irregularly
dispersed dark dots; caudal peduncle shallower
(8.8–10.1% SL or 22.9–26.3% HL) 19
18b. Caudal fin with three slanted dark bands; caudal peduncle deeper
(10.1–11.7% SL or 26.6–29.7% HL) R. pentakelis
(Upper rio Tocantins basin, Brazil)
19a. Body dark bars 2 and usually 3 reaching to the ventral midline; more
numerous premaxillary teeth (19–28, mode 21; Tab. 1) and dentary (16–22,
mode 19; Tab. 2) R. dani (Rio Teles Pires and rio Jamanxim basins, Brazil)
19b. Body dark bars barely passing lateral dark stripe; fewer premaxillary teeth
(12–18, mode 16; Tab. 1) and dentary (11–16, mode 13;
Tab. 2) R. marginalis n. sp. (Lower rio Xingu and rio Iriri, Brazil)
20a. Tooth cusp chestnut brown or reddish brown (Fig. 5A);
20–40 premaxillary (Tab. 1) and 18–34 dentary (Tab. 2) teeth 21
20b. Tooth cusp light ochre (Fig. 5B); 17–22 premaxillary (Tab. 1)
and 14–19 dentary (Tab. 2) teeth 22
21a. Caudal fin mostly hyaline, with 2–3 irregular dark bands; dorsal- and
pectoral-fin spines with 2–3 dark dots; body narrower
(cleithral width 22.4–24.9% SL or 59.4–67.2% HL);
pectoral-fin spine shorter (23.3–26.7% SL or
60.8–68.9% HL) R. jumaorum (Lower rio Madeira basin, Brazil)
21b. Caudal fin mostly brown, with hyaline spot on upper and lower lobes;
dorsal- and pectoral-fin spines homogeneously dusky;
body wider (cleithral width 24.9–27.9% SL or 67.7–76.2% HL);
pectoral-fin spine longer (26.7–29.5% SL or
71.4–78.4% HL) R. chromodontus (Upper rio Tapajós basin, Brazil)
22a. Pectoral-fin spine short (23.1–26.9% SL); head short (37.0–40.0% SL),
4–6 lateral abdominal plates between pectoral-fin axilla and
pelvic–fin R. acuen (Upper rio Xingu basin, Brazil)
22b. Pectoral-fin spine long (28.1–30.0% SL); head long (40.0–41.8% SL);
3–4 lateral abdominal plates between pectoral-fin axilla and
pelvic-fin R. dinizae (Upper rio Araguaia basin, Brazil)
Frequency of premaxillary teeth of Rhinotocinclus species. Species listed by species groups (in bold).
Frequency of dentary teeth of Rhinotocinclus species. Species listed by species groups (in bold).
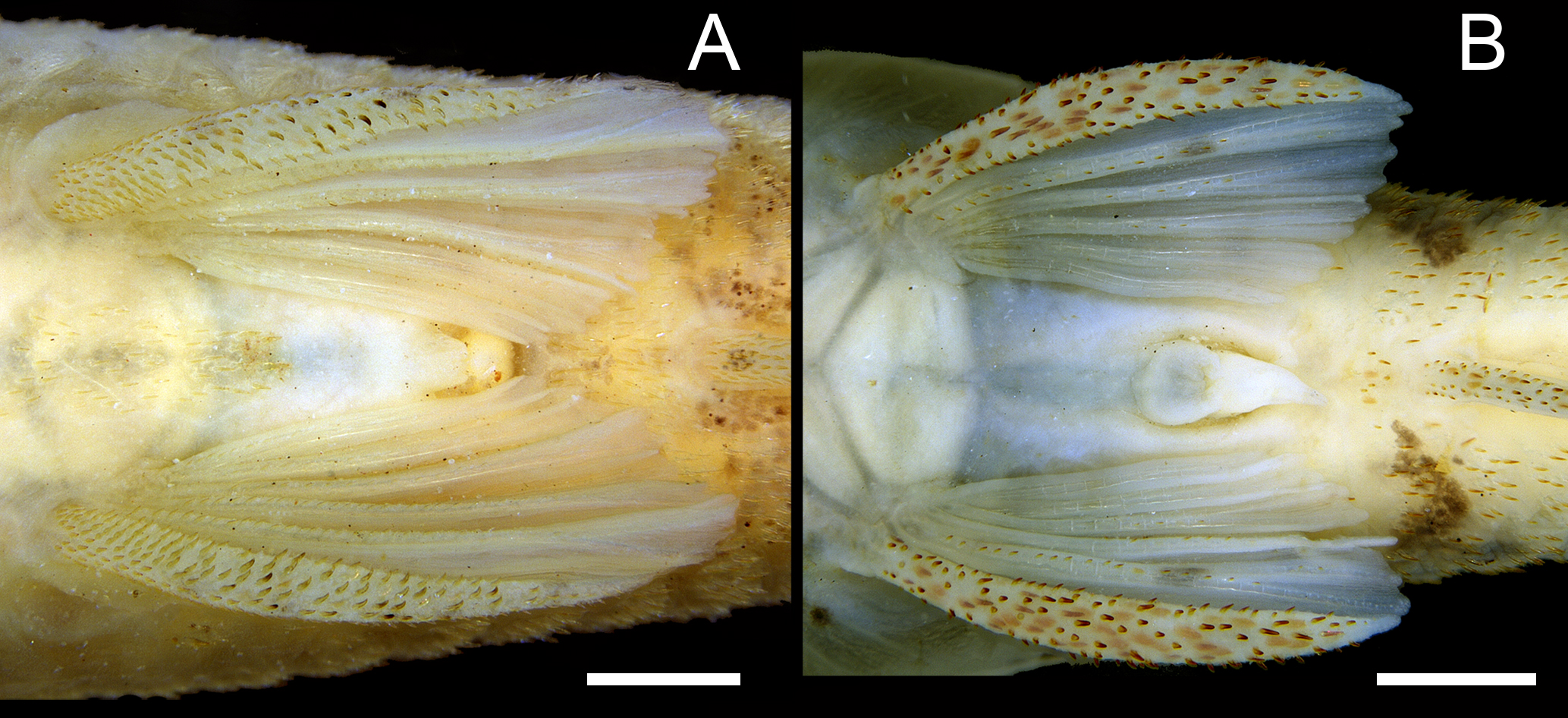
Middle abdominal plates series of Rhinotocinclus. A. R. bristskii, MCP 34709, 3–4 series; B. R. discolor n. sp., MHNLS 26183, 1–2 series; C. R. pilosus n. sp., UFRO-ICT 27700, abdomen naked.
Snout shape in Rhinotocinclus. A. R. britskii, ZUEC 16817, more broadly rounded; B. R. longirostris, INPA 25483, more acutely pointed. Scale bars = 2 mm.
Snout plates in Rhinotocinclus. A. R. yaka, MCP 53639; B. R. kwarup, MCP 32297. 1–3 = prenasal plates; N = nostril; NB = nasal bone; PRP = posrostral plates; RP = rostral plate. Scale bars = 2 mm.
Pectoral-fin color pattern in Rhinotocinclus. A. R. longirostris, INPA 25483, mostly hyaline; B. R. variola, MCP 48245, triangular dark spot at base of branched rays. Scale bar = 2 mm.
Species account. Species in this section are arranged by phenotypic species groups, as defined above, and by year of description inside each group.
Rhinotocinclus britskii Group
Rhinotocinclus britskii(Boeseman, 1974), new combination
Parotocinclus britskiiBoeseman 1974Boeseman M. On two Surinam species of Hypoptopomatinae, both new to science (Loricariidae, Siluriformes, Ostariophysi). Proc Konin Nederl Akad Wetensch. Ser. C, Biol Med Scienc. 1974; 77(3):251–71. :267 (Type-locality: Left tributary of Coppename River, 03°51’N 56°55’W, Surinam. Holotype: ZMA 106593).
Parotocinclus amazonensisGaravello, 1977Garavello JC. Systematics and geographical distribution of the genus Parotocinclus Eigenmann & Eigenmann, 1889 (Ostariophysii, Loricariidae). Arq Zool. 1977; 28(4):1–37. https://doi.org/10.11606/issn.2176-7793.v28i4p1-37
https://doi.org/10.11606/issn.2176-7793....
:7 (Type-locality: Ilha Sorubim, Rio Solimões, Amazonas, Brazil. Holotype: MZUSP 10145). NEW SYNONYM.
Parotocinclus aripuanensisGaravello, 1988Garavello JC. Three new species of Parotocinclus Eigenmann & Eigenmann, 1889 with comments on their geographical distribution (Pisces, Loricariidae). Naturalia. 1988; 13:117–28.:122 (Type-locality: Ingazeiro, 20 km upstream of Boca do Rio Canumã, Aripuanã, MT [Brazil]. Holotype: MZUSP 36899). NEW SYNONYM.
Diagnosis.Rhinotocinclus britskii is distinguished from R. acuen, R. bockmanni, R. chromodontus, R. dani, R. dinizae, R. hera, R. jumaorum, R. pentakelis, R. marginalis n. sp., and R. loxochelis n. sp. by possessing an adipose fin (vs. adipose fin absent), and by having a Y-shaped light mark from the snout tip to each nostril (Figs. 6A,B; vs. light mark V-shaped or present as two separate lines from snout tip diverging to each nostril; Figs. 6C,D and Figs. 6E,F, respectively). It is distinguished from R. collinsae, R. halbolthi, and R. hardmanni by lacking accessory teeth on both premaxilla and dentary (Figs. 5B,C; vs. accessory teeth present, Fig. 5A); the odontodes on the ventral surface of first pelvic-fin ray bent and pointing mesially (Fig. 9A; vs. odontodes aligned with main ray axis, Fig. 9B); a triangular dark spot on the anterior portion of the dorsal-fin membrane (Figs. 10B,C; vs. dorsal-fin spot absent); a Y-shaped light mark from snout tip to nostrils (vs. Y-shaped light mark absent); and a larger orbit, 27.8–39.0% snout length (vs. orbit 18.9–24.6% snout length). Rhinotocinclus britskii is distinguished from R. eppleyi, R. longirostris, R. polyochrus, R. variola, R. yaka, R. discolor n. sp., R. isabelae n. sp., and R. pilosus n. sp. by having the snout more broadly rounded (Fig. 12B; vs. snout more acutely pointed, Fig. 12A); dark bars on body narrower and more widely spaced (Fig. 7A; vs. dark bars on body wider and closer together, Fig. 7B); and one plate between the posterior border of the rostral plate and the nostril (Fig. 12B; vs. 2–4 plates, Fig. 12A). It is distinguished from R. kwarup by having fewer premaxillary teeth (15–29, mode 23, vs. 28–34, mode 32), and fewer dentary teeth (15–29, mode 20 and 25, vs. 27–33, mode 29; see Tabs. 1–2); and by having the color pattern with less broken marks (vs. color pattern with more broken marks, mottled).
Rhinotocinclus britskii, MCP 54760, 18.5 mm SL, female, Kwama creek, tributary to Coppename River, Sipaliwini, Suriname.
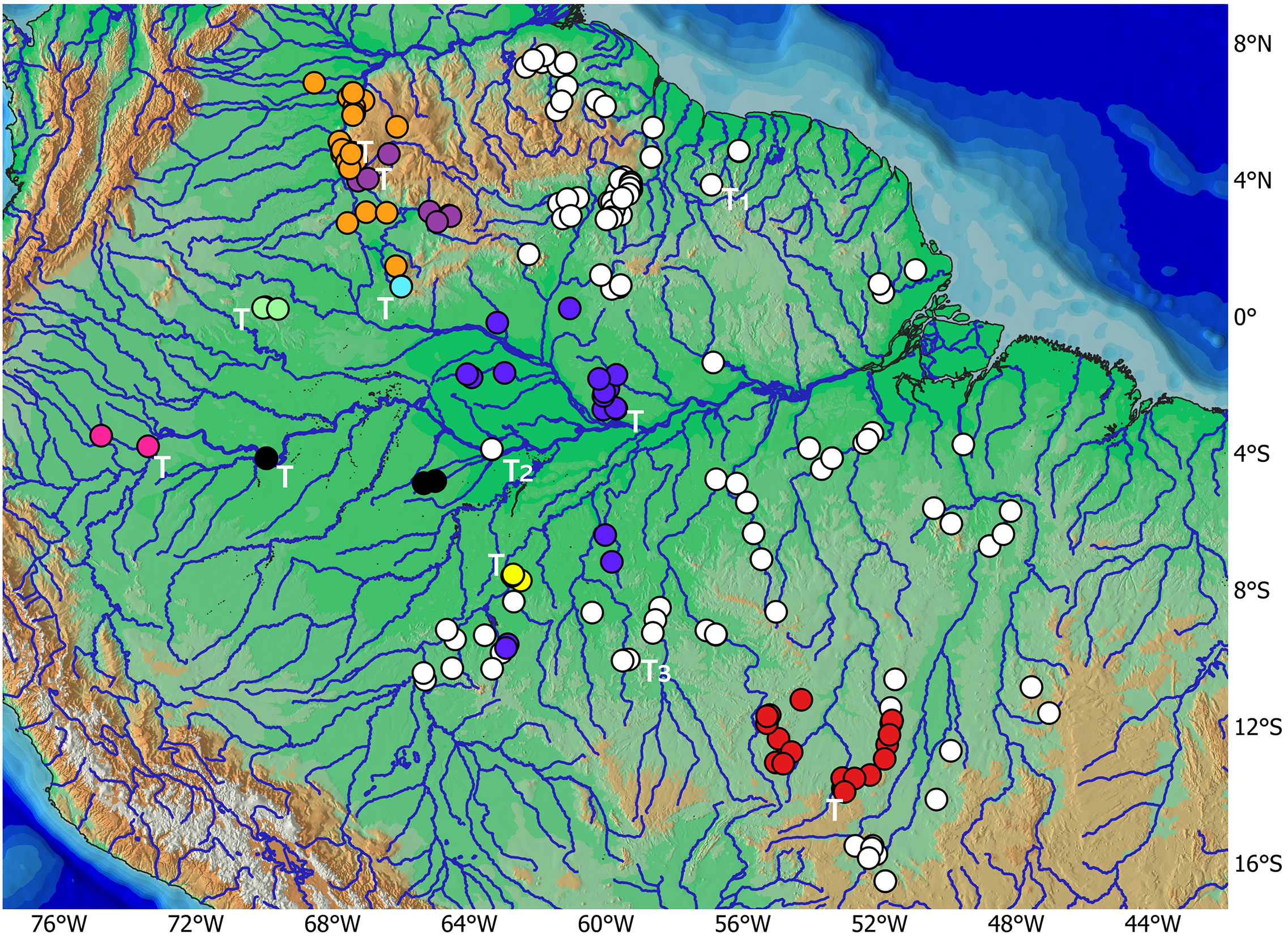
Geographical distribution.Rhinotocinclus britskii occurs in most of eastern Greater Amazon, in both Brazilian and Guianas Shields, including the coastal rivers of the Guianas from Amapá in Brazil to the Mazaruni River in Guyana, the Orinoco tributaries Caroni and Cuyuni, and the rio Branco in northern Amazon, as well as the middle and lower portions of the Madeira, Tapajós, Xingu, and Tocantins rivers, in the states of Amazonas, Goiás, Mato Grosso, Pará, Rondônia, and Tocantins in southern Amazon (Fig. 15).
Geographical distribution of Rhinotocinclus species of the R. britskii group in central and northwestern South America. Rhinotocinclus britskii (white dots); R. discolor n. sp. (purple dots); R. eppleyi (orange dots); R. isabelae n. sp. (pink dots); R. kwarup (red dots); R. longirostris (blue dots); R. pilosus n. sp. (yellow dots); R. polyochrus (cyan dot); R. variola (black dots); R. yaka (light green dots). T = Type-locality; T1 = type-locality of Parotocinclus britskii, T2 = type-locality of P. amazonensis, T3 = type-locality of P. aripuanensis.
Remarks.Rhinotocinclus britskii is the only species widely distributed among diverse river basins, occupying a large portion of the eastern Greater Amazon, including coastal rivers of the Guianas, the Cuyuni and Caroni rivers of the Orinoco basin, the rio Branco, and the tributaries to the eastern Amazon basin (Fig. 15). Three species names are available from this region, Parotocinclus britskii, P. amazonensis and P. aripuanensis, and populations from several river basins in this region were compared and tested for morphometric distinctness. Some of these population may possess small, subtle differences that overlap when compared basinwide or across basins, and we were unable to morphologically distinguish these populations (see Figs. 16–17 for a comparison). For this reason, both P. amazonensis and P. aripuanensis are herein synonymized with Rhinotocinclus britskii.
The geographic distribution of Rhinotocinclus britskii, widely distributed in rivers draining the Guianas and Brazilian Shields of eastern Greater Amazon, is not uncommon. Other exemples of such pattern are those of the characid Poptella compressa (Reis, 1989Reis RE. Systematic revision of the neotropical characid subfamily Stethaprioninae (Pisces, Characiformes). Comun Mus Ciênc PUCRS Sér Zool. 1989; 2(6):3–86.), Hoplias aimara (Mattox et al., 2006Mattox GMT, Toledo-Piza M, Oyakawa OT. Taxonomic study of Hoplias aimara (Valenciennes, 1846) and Hoplias macrophthalmus (Pellegrin, 1907) (Ostariophysii, Characiformes, Erythrinidae). Copeia. 2006; 2006(3):516–28. https://doi.org/10.1643/0045-8511(2006)2006[516:TSOHAV]2.0.CO;2
https://doi.org/10.1643/0045-8511(2006)2...
), Roeboexodon guianensis (Lima & Ribeiro, 2011), and Anostomus ternetzi (Lima & Ribeiro, 2011), which corroborate the hypothesis of evolution and differentiation on the highlands of the continental shields (Lima, Ribeiro, 2011Lima FCT, Ribeiro AC. Continental-scale tectonic controls of biogeography and ecology. In: Albert JS, Reis RE, editors. Historical biogeography of Neotropical freshwater fishes. California: University of California Press; 2011. p.145–64. https://doi.org/10.1525/california/9780520268685.003.0009
https://doi.org/10.1525/california/97805...
; Dagosta, de Pinna, 2019Dagosta FCP, de Pinna MCC. The fishes of the Amazon: distribution and biogeographical patterns, with a comprehensive list of species. Bull Am Mus Nat Hist. 2019; 431:1–163. https://doi.org/10.1206/0003-0090.431.1.1
https://doi.org/10.1206/0003-0090.431.1....
). However, the subtle morphometric variation of this species found among river basins might be indicative of undetected biodiversity. For this reason, a wide molecular study of R. britskii, comparing populations across the entire distribution area is in order. Rhinotocinclus britskii, listed as Parotocinclus amazonensis, is currently assessed as Least Concern (LC) in the Brazilian regional assessment by ICMBio, (2018)Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio). Livro vermelho da fauna brasileira ameaçada de extinção. Brasília: ICMBio; 2018..
Biplot of first and second Principal Components of morphometric proportions of standard length or head length in populations of Rhinotocinclus bristskii. Percent of variation included in PC1 is 23% and in PC2 is 17%. Head length and prepelvic distance loaded more strongly positively and head depth and interorbital distance loaded more strongly negatively on PC1. Head length and lower caudal-fin principal ray loaded more strongly positively and snout length loaded more strongly negatively on PC2.
Box-plots of morphometric variation in populations of Rhinotocinclus bristskii as percents of standard length (A–O) or head length (P–T). A. Body depth; B. Predosal distance; C. Prepelvic distance; D. Preanal distance; E. Preadipose distance; F. Dorsal-fin spine length; G. Anal-fin unbranched ray length; H. Adipose-fin spine length; I. Pectoral-fin spine length; J. Caudal peduncle depth; K. Dorsal-adipose fin distance: L. Dorsal-fin base length; M. Lower caudal-fin principal ray; N. Cleithral width; O. Head length; P. Head depth; Q. Interorbital distance; R. Orbital horizontal diameter; S. Snout length; T. Internarial distance. Number of individuals examined by geographic region: Tocantins (3), Araguaia (12), Xingu (10), Juruena (10), Tapajós (10), Madeira (10), Aripuanã (14), Branco (8), Uraricoera (10), Mazaruni (7), Essequibo (7), Coppename (12), and Amapá (8).
Material examined. ZMA 106593, holotype of Parotocinclus britskii (photos examined), left tributary of Coppename River, Suriname, approx. 03°51’N 56°55’W. MZUSP 10145, holotype of P. amazonensis, meander of rio Solimões at ilha Sorubim, upstream of Coari, Coari, Amazonas, Brazil, approx. 03°53’S 63°21’W. MZUSP 36899, holotype of P. aripuanensis (measured), MZUSP 36900-36906, 7 paratypes (3 measured), Ingazeiro, 20 km upstream of mouth of rio Canumã, Aripuanã, Mato Grosso, Brazil, approx. 10°10’S 59°27’W. Coppename River basin, Suriname: MHNG 2779.046, 124, MCP 54760, 10 (10 measured), Kwama creek, tributary to Coppename River, Sipaliwini, approx. 04°50’N 56°07’W. MHNG 2780.072, 5 of 12 (2 measured), Gran Tibiti River, tributary to Coppename River, Sipalawini, Suriname, approx. 04°51’N 56°07’W. Essequibo River basin, Guyana: ANSP 179132, 1, Rupununi River, road crossing 5.9 km WSW of village of Sand Creek, Rupununi (Region 9), 02°58’39.54”N 59°33’54.80”W. ANSP 179133, 3, Rupununi River, 3.7 km SSE village of Massara, Rupununi (Region 9), 03°52’3.54”N 59°17’03”W. ANSP 179135, 1, Essequibo River at Kurukupari, Upper Demerara-Berbice, 04°39’33.40”N 58°40’34.89”W. ANSP 179138, 16, MCP 34708, 5 (3 measured), Rupununi River, sand beach and inlet at Karanambo Ranch, Rupununi (Region 9), 03°45’02.47”N 59°18’08.96”W. ANSP 179210, 3, Rupununi River, 4.6 km NW of village of Massara, Rupununi (Region 9), 03°55’16.54”N 59°16’26.46”W. AUM 35721, 1, Essequibo River at Kurukupari, Upper Demerara-Berbice, 04°39’41.36”N 58°40’30.68”W. AUM 35724, 4, Simoni River, 4 sites from 6.6 km SE to 3.2 km W Karanambo, UpperTakutu-Upper Essequibo (03°43’09.01”N 59°15’40.36”W). AUM 35722, 4, Rupununi River 4.6 km NW Massara, Upper Takutu-Upper Essequibo, 03°55’33.71”N 59°16’49.33”W. AUM 35723, 2, Rupununi River 3.7 km SSE Massara, Upper Takutu-Upper Essequibo, 03°51’44.21”N 59°17’03.80”W. AUM 35725, 20, Rupununi River at Karanambo, Upper Takutu-Upper Essequibo, 03°45’00.14”N 59°18’30.06”W. INHS 49369, 1, Essequibo River at large sandbar and small cataract 31.9 mi SSW of Rockstone, Mazaruni-Potaro, 05°31’39.5”N 58°37’43.6”W. ROM 86161, 20, Rupununi River, in the vicinity of Yupukari-a stretch of river about 8 km in length, Upper Takutu-Upper Essequibo Region 9, 03°34’34.28”N 59°20’36.6”W. ROM 86412, 12, Rupununi River at Dadanawa, Upper Takutu-Upper Essequibo (Region 9), 02°49’54.02”W 59°31’41.17”W. Mazaruni River basin, Guyana: ROM 101591, 1, Mazaruni River, main channel, sandy beach with rocks, Cuyuni-Mazaruni, 06°08’46.03”N 60°02’05.75”W. ROM 102068, 5, Mazaruni River, Inaku Creek mouth, Cuyuni-Mazaruni, 06°08’49.81”N 60°01’54.84”W. ROM 102213, 1, Tamakay Creek, Cuyuni-Mazaruni, 06°20’09.96”N 60°17’25.01”W. Río Orinoco basin, Venezuela: ANSP 168171, 13, Río Miamo on Guasipati-El Miamo road ca. 20 km SW of El Miamo. Rio Yuruari/Cuyuni Drainage, Bolivar, 07°37’52.64”N 61°47’57.63”W. ANSP 168172, 14 + 2 cs, Río Botanamo, just beyond Vivero Florestal Intacmaca on road from Tumeremo to Bochinche, Río Cuyuni drainage, Bolivar, approx. 07°23’N 61°13’W. AUM 36607, 21, Río Macaruma, 134 km SE of Cuidad Guiana, 5 km SE of Guasipati, at old bridge just W of main road, Bolívar, 07°26’15.83”N 61°52’31.94”W. INHS 31733, 10 + 2 cs, Río Guanare at El Miamo, Bolivar, 07°38’29”N 61°46’39”W. MCNG 929, 1, rapids in Caño Negro, first tributary to Río Cuyuni upsteam Isla Anacoco, Bolivar, approx. 06°44’N 61°09’W. MCNG 16041, 1, Río Corumo, at bridge of Bochinche road, Bolivar, approx. 07°19’N 61°24’W. MCNG 16476, 13, Río Botanamo, at bridge of Bochinche road, Bolivar, approx. 07°25’N 61°11’W. MCNG 16656, 2, creek at bridge on km 91 of road to Santa Elena, Bolivar, approx. 06°02’N 61°27’W. MCNG 29427, 1, Río Yuruari E of El Manteco, Bolivar, approx. 07°18’N 62°21’W. MCNG 29463, 12 of 44, Río Guanare at balneario El Miamo, NW of Guasipati, Bolivar, 07°38’29”N 61°47’39”W. MCNG 29527, 1, Río Yuruari W of Guasipati near La Pastora, Bolivar, 07°30’14”N 62°07’56”W. MCNG 43374, 2, 50 m downstream bridge San Miguel de Betania, Las Claritas road, Bolivar, 06°17’42”N 61°18’00”W. Rio Branco basin, Guyana: ANSP 179139, 28, MCP 34709, 10 (5 measured), Sauriwau River, Takutu-Branco drainage, at crossing on road from Lethem to Sand Creek, 31.2 km NW Sand Creek Village, Upper Takutu-Upper Essequibo, Region 9, 03°06’41.19”N 59°46’36.43”W. ANSP 179130, 1, Pirara River, tributary to Ireng River, itself a tributary to Takutu River, 3.5 km NNW of Pirara, Upper Takutu-Upper Essequibo, Region 9, 03°38’43.36”N 59°41’21.67”W. ANSP 179131, 5, Yuora River, tributary to Ireng River, itself a tributary to Takutu River, 6.7 km NE of village of Karasabai on road to Tiger Creek village, Upper Takutu-Upper Essequibo, Region 9, 04°04’21.19”N 59°28’59.83”W. ANSP 179134, 1, Moco-Moco River, tributary to Takutu River, below dam at Moco-Moco Hydro Power Station, 18.8 km SE of Lethem, Upper Takutu-Upper Essequibo, Region 9, 03°19’30.31”N 59°41’26.36”W. ANSP 179137, 1, Ireng River, tributary to Takutu River, 6.9 km WSW village of Karasabai, Upper Takutu-Upper Essequibo, Region 9, 04°00’33.77”N 59°35’30.40”W. ANSP 179498, 6, Takutu River, ca. 2.75 km W of Saint Ignatius, Upper Takutu-Upper Essequibo, Region 9, 03°20’26.84”N 59°49’47.24”W. AUM 35730, 37, Sauriwau River, tributaqry to Takutu River, 31.2 km NW village of Sand Creek, Upper Takutu-Upper Essequibo, 03°06’51.55”N 59°46’31.58”W. ROM 95997, 1, Katorwau River, downstream crossing, Upper Takutu-Upper Essequibo, Region 9, 02°53’43.14”N 59°51’20.34”W. ROM 96082, 44, Katorwau River, Kodowidpao, Upper Takutu-Upper Essequibo, Region 9, 02°52’29.64”N 59°49’50.22”W. ROM 96223, 30, Takutu River, rapids at shelf of rock, Upper Takutu-Upper Essequibo, Region 9, 02°50’09.49”N 59°59’25.55”W. Rio Branco basin, Roraima, Brazil: MCP 46094, 1, rio Anauzinho on vicinal road to highway BR-210, São Luiz, 01°12’32”N 60°09’15”W. MCP 46147, 1, rio Jauaperi on vicinal road to highway BR-210 between Caroebe and São João da Bahia, 00°48’14”N 59°49’52”W. MCP 46175, 10, rio Jauaperi on vicinal road 5, ca. 10 km from highway BR-210, Caroebe, 00°54’47”N 59°34’23”W. INPA 1641, 26, rio Uraricoera, Boa Vista, approx. 03°28’N 60°49’W. INPA 1649, 18, furo Santa Rosa, rio Uraricoera at cachoeira Tiporena, Boa Vista, approx. 03°18’N 61°23’W. INPA 1863, 8, rio Uraricoera at Beiradão, Boa Vista, approx. 03°26’N 61°08’W. INPA 6064, 18 (2 measured) + 2 cs, rio Mucajaí at cachoeira Paredão II, Mucajaí, approx. 02°52’N 61°16’W. INPA 8147, 13 + 2 cs, igarapé do Arraia, km 114 on road from Boa Vista to Bonfim, 03°21’21.2”N 59°54’12.7”W. INPA 8164, 8, rio Takutu on road from Boa Vista to Bonfim, near market in Bonfim, 03°21’19.9”N 59°49’51.5”W. INPA 12962, 6, igarapé Surrão near mouth of rio Takutu, on road from Boa Vista to Bonfim, 03°18’23.9”N 59°50’53.9”W. INPA 16419, 35 (3 measured) + 2 cs, rio Catrimani at cachoeira Arapari, upstream mouth of rio Arapari, Boa Vista, approx. 01°49’N 62°16’W. LBP 22255, 2, rio Takutu, Bonfim, 03°21’17.3”N 59°54’16.6”W. MCP 46161, 6 (2 measured), igarapé Cocó on vicinal road 3 between Caroebe and Entre Rios, Caroebe, 00°51’22”N 59°37’02”W. MZUSP 117036, 28 of 58 (3 measured), rio Cauame near mouth of igarapé Au-au, rio Branco basin, Boa Vista, 02º56’27”N 61º01’55”W. Amapá coastal rivers, Brazil: ZUEC 16446, 5 of 11, rio Cachorrinho, tributary to rio Amapari, near Cupixi, Porto Grande, Amapá, 00º41’52”N 51º53’25”W. ZUEC 16582, 16 of 35, rio Ariramba, tributary to rio Tartarugalzinho, Tartarugalzinho, Amapá, 01º21’24”N 50º57’04”W. ZUEC 16817, 9 of 19, rio Amapari, Colônia Água Branca, Água Branca do Amapari, Amapá, 00º56’59”N 52º00’23”W. Rio Trombetas basin, Brazil: MZUSP 15839, 1, beach on rio Trombetas upstrem mouth of lago Jacaré, Trombetas Biological Reserve, Oriximiná, Pará, approx. 01º21’S 56º52’W. Rio Tapajós basin, Brazil: CPUFMT 1451, 30 (3 measured), rio Teles Pires upstream from Sete Quedas rapids, Paranaíta, Mato Grosso, 09°19’00.1”S 56°46’56.7”W. LBP 22254, 3, igarapé Urubutu near Itaituba, Pará, 04°46’10”S 56°46’46”W. LIRP 7442, 1 (measured), rio Jamanxim at cachoeira da Fazenda, Morais de Almeida district, Itaituba, Pará, 06°21’07”S 55°41’06”W. LIRP 7443, 3 + 1 cs, MCP 47078, 2, rio Jamanxim at cachoeira da Fazenda, Morais de Almeida district, Itaituba, Pará, 06°21’07”S 55°41’06”W. LIRP 7444, 5 + 1 cs, rio Jamanxin at first rapids, Novo Progresso, Pará, 07°06’43”S 55°27’17”W. MCP 47077 (formerly LIRP 7444), 5, rio Jamanxim at first rapids, Novo Progresso, Pará, 07°06’43”S 55°27’17”W. INPA 6674, 4, rio Jamanxim at ilha Terra Preta, Trairão, Pará, approx. 05°27’S 55°53’W. INPA 35522, 10 (3 measured), creek on left margin of Sete Quedas rapids on rio Teles Pires, Paranaíta, Mato Grosso, 09°18’58”S 56°47’38”W. INPA 29103, 328 (4 measured), rio Juruena near Salto Augusto, Cotriguaçu, Mato Grosso, approx. 09°16’S 58°38’W. MCP 47684, 30 (5 measured), rio Juruena near Salto Augusto, Mato Grosso, 08°53’20”S 58°33’30”W. MCP 47977, 1 (measured), igarapé Santa Úrsula, rio Juruena basin, Apuí, Amazonas, 08°31’39”S 58°25’27”W. MCP 51603, 1, igarapé tributary to rio Taburari on road BR-163 between Trairão and Caracol, Trairão, Pará, 04°53’38.6”S 56°10’43.8”W. MCP 53608, 1, igarapé tributary to rio Taburari on road BR-163 between Trairão and Caracol, Trairão, Pará, 04°53’38.6”S 56°10’43.8”W. MZUSP 96096, 40 of 68, creek on left bank of rio Teles Pires, Jacareacanga, Pará, 09°18’26”S 56°47’25”W. MZUSP 99254, 11 (3 measured), rio Apiacás near mouth, Apiacás, Mato Grosso, 09°11’36”S 57°04’02”W. Rio Curuá-Una basin, Brazil: MCP 54749, 1, rio Tutuí on Transamazon road ca. 20 km E of Placas, Uruará, Pará, 03°51’33.8”S 54°03’40.5”W. Rio Madeira basin, Brazil: INPA 837, 3, rio Preto do Candeias, Costa Marques, Rondônia, approx. 09°20’S 63°33’W. INPA 1134, 2, igarapé Vertente, upper rio Formoso, serra Pacaás Novos, Ariquêmes, Rondônia, approx. 10°18’S 64°30’W. INPA 1143, 7, igarapé Boa Vista at km 54 of road BR-421 from Ariquêmes to Campo Novo, Ariquêmes, Rondônia, approx. 10°19’S 63°20’W. INPA 4590, 10, rio Machado, Cururu, Costa Marques, Rondônia, approx. 08°21’S 62°42’W. INPA 32983, 73 (6 measured), igarapé Bom Jesus, right margin tributary to rio Guariba at RESEX Guariba, Apuí, Amazonas, 08°40’16”S 60°24’30”W. LBP 10981, 36, rio Lajeado, Guajará-Mirim, Rondônia, 10°26’23.5”S 65°20’34.1”W. MCP 35879, 54 (4 measured) + 4 cs, igarapé Bananeiras on road BR-425 N of Guajará-Mirim and ca. 110 km S of road BR-364, Guajará-Mirim, Rondônia, 10°38’28”S 65°17’34”W. MCP 35883, 1, rio Branco, ca. 10 km N of Ariquemes on road BR-364, Ariquemes, Rondônia, 09°50’40”S 63°03’35”W. MCP 35884, 1, creek tributary to rio Preto do Crespo ca. 27 km E of road BR-364, Rio Crespo, Rondônia, 09°42’21”S 62°55’38”W. MCP 47128, 10 (4 measured), igarapé Karipuna downstream from rapids, Jaci-Paraná, Rondônia, 09°10’26”S 64°39’39”W. MCP 47130, 3 (2 measured), lago Três Praias on rio Jaci-Paraná, Jaci-Paraná, Rondônia, 09°27’17”S 64°25’03”W. NUP 6763, 1 (measured), creek tributary to igarapé Guaribal, rio Madeira basin, Aripuanã, Mato Grosso, 10°04’47”S 59°31’04”W. NUP 7654, 1 (measured), creek tributary to rio Praia Grande, rio Madeira basin, Aripuanã, Mato Grosso, 10°02’43”S 59°20’19”W. NUP 8550, 2 (measured), creek tributary to igarapé Guaribal, rio Madeira basin, Aripuanã, Mato Grosso, 10°04’14”S 59°30’11”W. Rio Xingu basin, Altamira, Pará State, Brazil: MCP 48608 (formerly ANSP 199559), 5 (1 measured), rio Xingu, ca. 44 km SW of Altamira, 03°36’29.3”S 52°20’57.2”W. NUP 19609, 3 (3 measured), rio Três de Maio, tributary to rio Curuá, rio Xingu basin, 08°38’53.7”S 55°01’42.55”W. INPA 30943, 1, ROM 112206, 2 tissue samples, rio Iriri at Barrinha, 04°09’05”S 53°23’28”W. INPA 30944, 4, rio Iriri, Barrinha, rapids in middle of canal, 04°09’05”S 53°23’28”W. INPA 31120, 4, rio Iriri, above mouth of rio Novo, 04°28’11”S 53°41’38”W. MZUSP 111561, 13 (4 measured), black water creek tributary to rio Xingu near cachoeira do Espelho, 03°42’32”S 52°27’11”W. MZUSP 117156, 3 (2 measured), igarapé Babaquara, tributary to rio Xingu near ilha Babaquara, 03°24’42”S 52°12’32”W. Rio Araguaia basin, Brazil: LBP 12274, 47 (7 measured), ribeirão Insula, rio das Mortes basin, Barra do Garças, Mato Grosso, 15°29’57.3”S 52°12’10”W. MCP 40477, 3, córrego Pium, on road BR-158 ca. 22 km S of Posto da Mata, Posto da Mata, Mato Grosso (11°53’58”S 51°39’26”W). MCP 40576, 1, rio Preto on road BR-158 ca. 72 km S of Porto Alegre do Norte, Mato Grosso, 11°27’47”S 51°40’42”W. MCP 40788, 21 (1 measured), rio Paciguara on road BR-158, 2 km N of Confresa, Confresa, Mato Grosso, 10°37’34”S 51°32’51”W. MCP 41427, 2 (measured), rio Piranhas near mouth of córrego das Pedras, Piranhas, Goiás, 16°32’03”S 51°49’58”W. MCP 43740, 4 (1 measured), rio Piranhas near mouth of córrego das Pedras, Piranhas, Goiás, 16°32’03”S 51°49’58”W. MHNG 2550.60, 1, rio Araguaia at waterfall between São João [do Araguaia] and São Bento [do Tocantins], Pará, approx. 5°42’S 48°10’W. MZUEL 7698, 2, rio Taquaral, Barra do Garças, Mato Grosso, 15°40’41.4”S 52°17’52.3”W. MZUEL 7699, 14, rio Corrente, Barra do Garças, Mato Grosso, 15°29’56.9”S 52°12’10.5”W. MZUEL 7700, 11, rio Corrente, Barra do Garças, Mato Grosso, 15°29’56.9”S 52°12’10.5”W. MZUEL 7701, 5, creek tributary to rio Insula, Barra do Garças, Mato Grosso, 15°34’19.7”S 52°13’25.6”W. MZUEL 7702, 5, rio Corrente, Barra do Garças, Mato Grosso, 15°29’56.9”S 52°12’10.5”W. MZUEL 7703, 5, córrego Fundo, Barra do Garças, Mato Grosso (15°51’32.2”S 52°19’01.0”W). MZUEL 7704, 5 rio Corrente, Barra do Garças, Mato Grosso (15°29’56.9”S 52°12’10.5”W). MZUSP 105578, 5, creek tributary to rio Tapirapé, upstream from Bacaba Base of Tapirapé Biological Reserve, Marabá, Pará, 05°36’54”S 50°24’46”W. MZUSP 52162, 43 of 93 (1 measured), rio Água Fria at fazenda Praia Alta 2, on road TO-181 from Araguaçú to Barreira do Pequi, 27 km N of Araguaçú, Tocantins, approx. 12°43’S 49°55’W. MZUSP 89332, 24, córrego Pitomba, tributary to rio Crixás-Mirim at road GO-336, ca. 5 km S of Nova Crixás, Goiás, 14°08’35”S 50°20’13”W. NUP 9733, 1, rio Araguaia between Aragominas, Tocantins and Piçarras, Pará, 06°43’22.62”S 48°46’55.46”W. NUP 19114, 4, córrego Grande, tributary to rio Araguaia, Barra do Garças, Mato Grosso, 15°44’31.04”S 52°05’37.75”W. ZUEC 7422, 14, rio Itacaiúnas, Serra dos Carajás, Igarapé Azul, Parauapebas, Pará, Brasil, 06º04’03”S 49º54’08”W. Rio Tocantins basin, Brazil: INPA 6066, 2, igarapé Canoal, tributary to rio Tocantins, Breu Branco, Pará, approx. 03°45’S 49°33’W. MZUSP 103111, 1 (measured), rio Manuel Alves da Natividade at Porto Alegre do Tocantins, Tocantins, 11°36’41”S 47°02’39”W. MZUSP 106562, 4 (2 measured), rio Pacu near Racha Placa, Mozantinópolis, Canaã dos Carajás, Pará, 06°26’44”S 50°12’50”W.
Descriptive morphometrics of Rhinotocinclus species. Values given as percent of standard length or head length. Range includes the holotype (Hol), Hol1 = holotype of Parotocinclus amazonensis, Hol2 = holotype of P. aripuanensis, Para = paratype, SD = standard deviation.
Rhinotocincluslongirostris(Garavello, 1988), new combination
Parotocincluslongirostris Garavello, 1988Garavello JC. Three new species of Parotocinclus Eigenmann & Eigenmann, 1889 with comments on their geographical distribution (Pisces, Loricariidae). Naturalia. 1988; 13:117–28.:120 (Type-locality: Rio Preto da Eva, Manaus-Itacoatiara highway, km 80, Manaus, [Amazonas, Brazil]. Holotype: MZUSP 36891).
Diagnosis.Rhinotocinclus longirostris is distinguished from R. acuen, R. bockmanni, R. chromodontus, R. dani, R. dinizae, R. hera, R. jumaorum, R. pentakelis, R. marginalis n. sp., and R. loxochelis n. sp. by possessing an adipose fin (vs. adipose fin absent), and by having a Y-shaped light mark from the snout tip to each nostril (Figs. 6A,B; vs. light mark V-shaped or present as two separate lines from snout tip diverging to each nostril). It is distinguished from R. collinsae, R. halbolthi, and R. hardmanni by lacking accessory teeth on both premaxilla and dentary (Figs. 5B,C; vs. accessory teeth present); the odontodes on the ventral surface of first pelvic-fin ray bent and pointing mesially (Fig. 9A; vs. odontodes aligned with main ray axis, Fig. 9B); a triangular dark spot on the anterior portion of the dorsal-fin membrane (Fig. 8B; vs. dorsal-fin spot absent); a Y-shaped light mark from snout tip to nostrils (vs. light mark absent); and a larger orbit, 26.7–31.4% snout length (vs. orbit 18.9–24.6% snout length). Rhinotocinclus longirostris is distinguished from R. britskii and R. kwarup, by having the snout more acutely pointed (Fig. 11B; vs. snout more broadly rounded, Fig. 11A); dark bars on body wider and closer together (Fig. 7B; vs. dark bars on body narrower and more widely spaced, Fig. 7A); and 2–3 plates between the posterior border of the rostral plate and the nostril (Fig. 12A; vs. one plate). Rhinotocinclus longirostris is distinguished from R. variola, R. yaka, R. discolor n. sp., R. isabelae n. sp., and R. pilosus n. sp. by having 4–5 irregular series of middle abdominal plates (vs. 0–2, rarely 3 irregular series); and five dark bars on body (Fig. 7B; vs. four dark bars on body [bars 1+2 or 2+3 fused], Fig. 7C). It is distinguished from R. eppleyi by having more numerous premaxillary, 28–36 (mode 30) and dentary, 27–31 (mode 30) teeth (see Tabs. 1–2, vs. fewer premaxillary (22–30, mode 26) and dentary (21–27, mode 25) teeth).
Geographical distribution. Rhinotocinclus longirostris occurs in tributaries to the central Amazon, including the Madeira and Negro basins, in the states of Amazonas, Rondônia, and Roraima, Brazil (Fig. 15).
Remarks.Rhinotocinclus longirostris, from the central Amazon basin, is most similar and barely distinguishable phenotypically from R. eppleyii, which inhabits the middle Orinoco. The only morphological differences found between these species are the number of oral teeth, as R. eppleyi has 22–30 (mode 26) premaxillary and 21–27 (mode 25) dentary teeth, while R. longirostris has 28–36 (mode 30) premaxillary and 27–31 (mode 30) dentary teeth. These counts are not fully discrete and partially overlap (see Tabs. 1–2). In addition, body proportions also overlap (compare measurements in Tabs. 3–4). Rhinotocinclus longirostris, listed as Parotocinclus longirostris, is currently assessed as Least Concern (LC) in the Brazilian regional assessment by ICMBio, (2018)Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio). Livro vermelho da fauna brasileira ameaçada de extinção. Brasília: ICMBio; 2018. and in the global assessment by IUCN.
Material examined: Rio Amazonas basin, Brazil: Amazonas State: DEPRJ 8484, 4, igarapé Solimõezinho, bacia do rio Unini, Barcelos, 01º39’18”S 62º58’15”W. MZUSP 36891, holotype (measured), MZUSP 36892–36894, 4 paratypes, rio Preto da Eva, km 80 on road from Manaus to Itacoatiara, Manaus, 02°41’55” 59°42’15”W. MZUSP 36895–36898, 4 paratypes, igarape Tarumãzinho, on road from Manaus to Caracaraí, Manaus, 02°40’28”S 60°02’46”W. INPA 15479, 10, igarapé Caititu, rio Uatumã, 01°42’46.1”S 59°41’58”W. INPA 25483, 25 (5 measured), creek on Fazenda Dimona (PDBFF), approx. 80 km N of Manaus, 02º19’45”S 60º04’39”W. INPA 15885, 1 + 1 cs, igarapé Tarumazinho, tributary of rio Tarumã-Açu, on km 28 of road BR-174, 02º43’31”S 60º04’62”W. INPA 30063, 11, rio Cuieiras, Fazenda DIMONA (PDBFF), Manaus, 02º12’44”S 60º03’20”W. INPA 30278, 21 (5 measured), igarapé Ajuricaba, Presidente Figueiredo, 02º07’04”S 59º56’06”W. INPA 33983, 10, igarapé Solimõezinho, tributary to rio Unini, Barcelos, 01º39’18”S 62º58’15”W. INPA 33984, 30, upper rio Unini, ca. 40 km upstream of confluence with rio Preto, Barcelos, 01º41’32”S 64º04’00”W. INPA 33986, 40, rio Preto, ca. 30 km upstream from confluence with rio Unini, Barcelos, approx. 01º47’S 63º56’W. LBP 13234, 1, igarape da Canoa near Presidente Figueiredo, 01º49’41.35”S 60º12’21.02”W. MHNG 2691.052, 1, rio Araca, tributary to rio Negro, approx. 00°11’S 63°11’W. MZUSP 85786, 18 (5 measured) + 3 cs, rio Preto da Eva, ca. 4 km upstream of Rio Preto da Eva town, 02º40’49.2”S 59º43’15.2”W. MZUSP 117500, 1, creek tributary to rio Aripuanã at Apuí, 07°11’10.10”S 59°50’01.39”W. MZUSP 117630, 1, creek tributary to rio Juma between Novo Aripuanã and km 100, Novo Aripuanã, 06°23’57.7”S 60°01’36.25”W. Rondônia State: MCP 35876, 2, rio Preto do Crespo, ca. 13 km N of Rio Crespo, 09º35’56”S 62º52’43”W. MCP 35882, 2 + 1 cs, creek tributary to rio Crespo, ca. 20 km E of highway BR-364 towards Rio Crespo, 09º42’21”S 62º55’38”W. Roraima State: MCP 46076, 2, rio Jauaperi on vicinal road near highway BR-174, ca. 47 km from Jundiá, Rorainópolis, 00º13’55”S 61º03’52”W.
Rhinotocinclus longirostris, MZUSP 85786, 22.8 mm SL, male, rio Preto da Eva, ca. 4 km upstream of Rio Preto da Eva town, Amazonas, Brazil.
Rhinotocincluspolyochrus(Schaefer, 1988), new combination
ParotocincluspolyochrusSchaefer, 1988Schaefer SA. A new species of the loricariid genus Parotocinclus from southern Venezuela (Pisces: Siluroidei). Copeia. 1988; 1988(1):182–88. https://doi.org/10.2307/1445936
https://doi.org/10.2307/1445936...
:184 (Type-locality: Venezuela, Território Federal Amazonas, Depto. Río Negro, Río Mawarinuma tributary at Neblina base camp, on right bank of riffle; 00°55’N 66°10’W, elevation 120 m. Holotype: AMNH 74482).
Diagnosis.Rhinotocinclus polyochrus is distinguished from R. acuen, R. bockmanni, R. chromodontus, R. dani, R. dinizae, R. hera, R. jumaorum, R. pentakelis, R. marginalis n. sp., and R. loxochelis n. sp. by possessing an adipose fin (vs. adipose fin absent), and by having a Y-shaped light mark from the snout tip to each nostril (vs. light mark V-shaped or present as two separate lines from snout tip diverging to each nostril). It is distinguished from R. collinsae, R. halbolthi, and R. hardmanni by lacking accessory teeth on both premaxilla and dentary (Figs. 5B,C; vs. accessory teeth present); the odontodes on the ventral surface of first pelvic-fin ray bent and pointing mesially (Fig. 9A; vs. odontodes aligned with main ray axis, Fig. 9B); a triangular dark spot on the anterior portion of the dorsal-fin membrane (Fig. 8B; vs. dorsal-fin spot absent); a Y-shaped light mark from snout tip to nostrils (vs. Y-shaped light mark absent); and a larger orbit, 26.3–28.7% snout length (vs. orbit 18.9–24.6% snout length). Rhinotocinclus polyochrus is distinguished from R. britskii and R. kwarup, by having the snout more acutely pointed (Fig. 11B; vs. snout more broadly rounded, Fig. 11A); dark bars on body wider and closer together (Fig. 7B; vs. dark bars on body narrower and more widely spaced, Fig. 7A); and 2 plates between the posterior border of the rostral plate and the nostril (Fig. 12A; vs. one plate). Rhinotocinclus polyochrus is distinguished from R. eppleyi and R. longirostris by having 3–4 irregular series of middle abdominal plates (vs. 4–5 irregular series); and dark bars on body 1+2 fused (vs. dark bars on body separated). It is distinguished from R. variola, R. yaka, R. discolor n. sp., R. isabelae n. sp., and R. pilosus n. sp. by having a conspicuous light bar in front of dorsal fin, extended on head as a Y-shaped mark towards each nostril (vs. bar and Y-shaped mark on head absent). It is further distinguished from P. variola and R. yaka by having fewer premaxillary teeth (23–32 vs. 33–51 and 34–39, respectively); from R. discolor n. sp. and R. pilosus n. sp. by having smaller eye (38.9–40.6% interorbital distance vs. 52.1–63.7% and 45.7–55.2%, respectively); and from R. isabelae n. sp. by having a shallower caudal peduncle (6.8–7.1% SL vs. 8.1–8.8% SL) and 3–4 lateral abdominal plates (vs. 1–2 lateral abdominal plates).
Geographical distribution.Rhinotocinclus polyochrus is only known from the type-locality in the Río Mawarinuma, at the Neblina Ridge in the state of Amazonas, Venezuela (Fig. 15). The Río Mawarinuma is a tributary to the Río Baria which empties in the Casiquiare Canal, which drains to the upper Negro.
Remarks.Rhinotocinclus polyochrus is most similar to R. longirostris and R. eppleyi, from which it is distinguished by having 3–4 irregular series of middle abdominal plates, while R. longirostris and R. eppleyi have 4–5 series, and by having dark bars 1+2 fused on body, versus all bars separated. Coincidently, R. polyochrus and R. longirostris were described in the same year (Schaefer, 1988Schaefer SA. A new species of the loricariid genus Parotocinclus from southern Venezuela (Pisces: Siluroidei). Copeia. 1988; 1988(1):182–88. https://doi.org/10.2307/1445936
https://doi.org/10.2307/1445936...
and Garavello, 1988Garavello JC. Three new species of Parotocinclus Eigenmann & Eigenmann, 1889 with comments on their geographical distribution (Pisces, Loricariidae). Naturalia. 1988; 13:117–28., respectively) and both authors were apparently unaware of the description of the other species, as they were not compared in either description. Unfortunately, R. polyochrus is only known from the type-material, the holotype and one paratype, both being females. Rhinotocinclus polyochrus, listed as Parotocinclus polyochrus, is currently assessed as Least Concern (LC) by the IUCN.
Material examined. AMNH 74482, holotype (measured), AMNH 77520, 1 paratype (measured), creek tributary to Río Mawarinuma at Neblina base camp, Amazonas, Venezuela, approx. 00°55’N 66°10’W.
Rhinotocinclus polyochrus, holotype, AMNH 74482, 27.6 mm SL, female, creek tributary to Río Mawarinuma at Neblina base camp, Amazonas, Venezuela.
Rhinotocincluseppleyi(Schaefer & Provenzano, 1993), new combination
ParotocincluseppleyiSchaefer & Provenzano, 1993Schaefer SA, Provenzano F. The Guyana Shield Parotocinclus: systematics, biogeography, and description of a new Venezuelan species (Siluroidei: Loricariidae). Ichthyol Explor Freshw. 1993; 4(1):39–56.:46 (Type-locality: Venezuela, Amazonas, caño Curicurito, approx. 1 km above its mouth into the Río Autana, Río Sipapo drainage, 04°47’N 67°25’W. Holotype: MBUCV-V-22530).
Diagnosis.Rhinotocinclus eppleyi is distinguished from R. acuen, R. bockmanni, R. chromodontus, R. dani, R. dinizae, R. hera, R. jumaorum, R. pentakelis, R. marginalis n. sp., and R. loxochelis n. sp. by possessing an adipose fin (vs. adipose fin absent), and by having a Y-shaped light mark from the snout tip to each nostril (Figs. 7A,B; vs. light mark V-shaped or present as two separate lines from snout tip diverging to each nostril). It is distinguished from R. collinsae, R. halbolthi, and R. hardmanni by lacking accessory teeth on both premaxilla and dentary (Figs. 5B, C; vs. accessory teeth present, Fig. 5A); the odontodes on the ventral surface of first pelvic-fin ray bent and pointing mesially (Fig. 9A; vs. odontodes aligned with main ray axis, Fig. 9B); a triangular dark spot on the anterior portion of the dorsal-fin membrane (Fig. 8B; vs. dorsal-fin spot absent); a Y-shaped light mark from snout tip to nostrils (vs. Y-shaped light mark absent); and a larger orbit, 26.7–31.4% snout length (vs. orbit 18.9–24.6% snout length). Rhinotocinclus eppleyi is distinguished from R. britskii and R. kwarup, by having the snout more acutely pointed (Fig. 11B; vs. snout more broadly rounded, Fig. 11A); dark bars on body wider and closer together (Fig. 7B; vs. dark bars on body narrower and more widely spaced, Fig. 7A); and 3–4 plates between the posterior border of the rostral plate and the nostril (Fig. 12A; vs. one plate). Rhinotocinclus eppleyi is distinguished from R. polyochrus, R. variola, R. yaka, R. discolor n. sp., R. isabelae n. sp., and R. pilosus n. sp. by having 4–5 irregular series of middle abdominal plates (vs. 0–2, rarely 3 irregular series); and five dark bars on body; vs. four dark bars with bars 1+2 or 2+3 fused). It is distinguished from R. longirostris by having fewer premaxillary (22–30, mode 26) and dentary (21–27, mode 25) teeth (Tabs. 1–2, vs. more numerous premaxillary, 28–36, mode 30 and dentary, 27–31, mode 30 teeth).
Geographical distribution.Rhinotocinclus eppleyi occurs in the upper Río Orinoco basin in the states of Apure, Bolivar, and Amazonas of Venezuela (Fig. 15). Despite no specimens were available, R. eppleyi also occurs in the states of Vichada and Guainía of Colombia, just across the Río Orinoco (Maldonado-Ocampo et al., 2008Maldonado-Ocampo JA, Vari RP, Usma JS. Checklist of the freshwater fishes of Colombia. Biota Colombiana. 2008; 9(2):143–237. Available from: http://www.redalyc.org/articulo.oa?id=49120960001
http://www.redalyc.org/articulo.oa?id=49...
; DoNascimiento et al., 2017DoNascimiento C, Herrera-Collazos EE, Herrera-R GA, Ortega-Lara A, Villa-Navarro FA, Oviedo JSU, Maldonado-Ocampo JA. Checklist of the freshwater fishes of Colombia: a Darwin Core alternative to the updating problem. ZooKeys. 2017; 708:25–138. https://doi.org/10.3897/zookeys.708.13897
https://doi.org/10.3897/zookeys.708.1389...
).
Remarks.Rhinotocinclus eppleyi is most similar and scarcely distinguished from R. longirostris, the only phenotypical differences being the numbers of premaxillary and dentary teeth – see Remarks for R. longirostris for details. Rhinotocinclus eppleyi is currently not assessed by IUCN.
Material examined. Río Orinoco basin, Venezuela: ANSP 165845, 11 + 2 cs paratypes, Caño Potrerito, tributary to Río Cinaruco, 24 km S of Rio Cinaruco on road from San Fernando de Apure to Puerto Paez, Apure, approx. 06°25’N 67°32’W. ANSP 165846, 1 paratype, Caño Horeda, at border of Bolivar-Amazonas states, ca. 68 km NE of Puerto Ayacucho, on road from Puerto Ayacucho to Puerto Paez, Amazonas, 06°07’39”N 67°22’01”W. ANSP 169470, 8 paratypes, Caño Curicurito, ca. 1 km above its mouth into Río Autana, Amazonas, 04°47’16”N 67°24’30”W. MCP 33313, 8 (8 measured) + 2 cs paratypes (formerly MBUCV-V 22524), Caño Curicurito, ca. 1 km from mouth of Río Autana, Río Sipapo basin, Amazonas, approx. 04°47’N 67°25’W. ANSP 160700, 5 + 1 cs, Río Sipapo, ca. 6 km upstream from Pendare, Amazonas, 04°52’29”N 67°43’16”W. AUM 22338, 2, Caño Ore, ca. 50 km SW of Los Pijiguaos, on road from Caicara to Puerto Ayacucho, Bolívar (06°18’57”N 67°05’46”W). AUM 22635, 6, Caño Potrerito, ca.15 km N of Puerto Paez on road to San Fernando, Apure, 06°24’43”N 67°31’55”W. FMNH 85863, 1, Río Orinoco, 13 km S of Puerto Nuevo toward Puerto Ayacucho, Rio Orera basin, Amazonas, 05°06’52.73”N 67°48’10.46”W. FMNH 103541, 40 of 162, Caño Curcurito, ca. 1 km above mouth in Río Autana, Amazonas, 04°43’48”N 67°37’12”W. FMNH 108763, 1, Río Sipapo, ca. 1.5 h above Pendare, Amazonas, 04°45’24”N 67°43’17”W. MCNG 3010, 3 of 4, Río Manapiare near Yutaje, Amazonas, 05°32’50”N 66°06’57”W. MCNG 6957, 15 of 111, Río Naure near Hato Lagunitas, S of Apure, Apure, 06°50’10”N 68°32’10”W. MCNG 21655, 1, Río Guayapo ca. 100 km from mouth with Río Sipapo, Amazonas, approx. 04°37’N 67°19’W. MCNG 21698, 3, Río Sipapo ca. 150 m from Salto Remo, Amazonas, 04°34’28”N 67°18’31”W. MCNG 22184, 1, flooded forest on left bank of Río Guayapo, 9 km from mouth into Río Sipapo, Amazonas (approx. 04°31’N 67°35’W). MCNG 22306, 1, Río Guayapo at Aguacate rapids, upstrem from confluence with Río Sipapo, Amazonas, approx. 04°20’N 67°30’W. MCNG 23323, 3, beach of Río Guainia at Maroa, Amazonas, approx. 02°44’N 67°34’W. MCNG 23591, 2, Caño Chimita, tributary to Río Atacavi-Atabapo, 10 km from confluence with Río Atacavi, Amazonas, approx. 03°03’N 67°01’W. MCNG 25886, 1, Río Orinoco downstream from Village of Cariche, Amazonas, approx. 03°02’N 66°25’W. MCNG 26630, 20 of 30, Caño Topocho at road from Caicara to Puerto Ayacucho, Amazonas, approx. 05°57’N 67°22’W. MCNG 33131, 4 + 1 cs, Río Autana at Raudal Pereza, Amazonas, 04°46’4.22”N 67°27’28.34”W. MCNG 34147, 2, Río Cinaruco, Apure, approx. 06°32’N 67°24’W. MCNG 39512, 13, beach at Río Cinaruco, Apure, 06°32’45”N 67°25’00”W. MCNG 41450, 3, Río Cinaruco, Apure, 06°32’31”N 67°24’31”W. MCNG 41460, 3, Río Cinaruco, Apure, 06°32’30”N 67°24’05”W. MCNG 44274, 3, Río Yatua, Amazonas, 01°28’01”N 66°08’55”W. ROM 88301, 12 (2 measured), MCP 54750 10, (3 measured), Caño Parhueña, upstream route 12, ca. 35 km NE of Puerto Ayacucho, Amazonas, 05°53’30.66”N 67°24’13.54”W.
Descriptive morphometrics of Rhinotocinclus species. Values given as percent of standard length or head length. Range includes the holotype (Hol), SD = standard deviation.
Rhinotocinclus eppley, MCP 54750, 21.7 mm SL, female, Caño Parhueña, Puerto Ayacucho, upstream of Route 12 bridge, ca. 35 km NE of Puerto Ayacucho, Amazonas, Venezuela.
Live specimen of Rhinotocinclus eppleyi from lot ROM 88301, Caño Parhueña, Puerto Ayacucho, upstream of Route 12 bridge, ca. 35 km NE of Puerto Ayacucho, Amazonas, Venezuela. Photo by Nathan Lujan.
Rhinotocinclusvariola(Lehmann, Schvambach & Reis, 2015), new combination
Parotocinclusvariola Lehmann, Schvambach & Reis, 2015:47 (Type-locality: Colombia, Departamento Amazonas, Leticia, Quebrada Tacana, tributary to rio Amazonas at km 6.5 of road from Leticia to Tarapacá, 04°09’15”S 69°56’09”W. Holotype: ICNMHN 18685).
Diagnosis.Rhinotocinclus variola is distinguished from R. acuen, R. bockmanni, R. chromodontus, R. dani, R. dinizae, R. hera, R. jumaorum, R. pentakelis, R. marginalis n. sp., and R. loxochelis n. sp. by possessing an adipose fin (vs. adipose fin absent), and by having a Y-shaped light mark from the snout tip to each nostril (Figs. 7A,B; vs. light mark V-shaped or present as two separate lines from snout tip diverging to each nostril). It is distinguished from R. collinsae, R. halbolthi, and R. hardmanni by lacking accessory teeth on both premaxilla and dentary (Figs. 5B,C; vs. accessory teeth present, Fig. 5A); the odontodes on the ventral surface of first pelvic-fin ray bent and pointing mesially (Fig. 9A; vs. odontodes aligned with main ray axis, Fig. 9B); a triangular dark spot on the anterior portion of the dorsal-fin membrane (Fig. 8B; vs. dorsal-fin spot absent); a Y-shaped light mark from snout tip to nostrils (vs. Y-shaped light mark absent); and a larger orbit, 27.1–31.0% snout length (vs. orbit 18.9–24.6% snout length). Rhinotocinclus variola is distinguished from R. britskii, R. discolor n. sp., R. eppleyi, R. isabelae n. sp., R. kwarup, R. longirostris, R. pilosus n. sp., R. variola, and R. yaka by having dark dots smaller than a pupil diameter broadly distributed dorsally and ventrally on body (vs. dark dots absent, but sometimes darkened sensory pores may be present on head); a triangular dark spot on anterior portion of the pectoral-fin membrane (Fig. 13B; vs. dark spot absent); and, except for R. isabelae n. sp., the triangular dark spot of the dorsal fin occupying more than half of the fin (Fig. 8C; vs. dark spot occupying less than half of dorsal fin).
Geographical distribution.Rhinotocinclus variola is known from the type-locality in the Quebrada Tacana, a creek tributary to the Amazon in Leticia, Departamento Amazonas, Colombia and the rio Urucu, an Amazon tributary in the state of Amazonas, Brazil (Fig. 15). Rhinotocinclus variola is probably more widespread in creeks tributaries to the rio Amazonas in the western Amazon, in Brazil, Colombia, and Peru.
Remarks. This species was so far known from its type-locality in the Quebrada Tacana near Leticia, Colombia. We herein report the presence of R. variola for the first time in Brazil, in creeks tributary to the rio Urucu, a right margin tributary to the Amazon between the rio Juruá and the rio Purus. Rhinotocinclus variola was tentatively categorized as Least Concern (LC) by Lehmann et al., (2015)Lehmann A P, Schvambach LJ, Reis RE. A new species of the armored catfish Parotocinclus (Loricariidae: Hypoptopomatinae), from the Amazon basin in Colombia. Neotrop Ichthyol. 2015; 13(1):47–52. https://doi.org/10.1590/1982-0224-20140113
https://doi.org/10.1590/1982-0224-201401...
in the original description.
Material examined. Rio Amazonas basin: Colombia: ICNMHN 18685, holotype, ICNMHN 10114, 5 paratypes + 1 juv, ICNMHN 10326, 3 paratypes, ICNMHN 10349, 10 paratypes, MCP 48244, 5 paratypes (5 measured), MCP 48245, 4 (4 measured) + 2 cs +1 juv paratypes, Quebrada Tacana, tributary to Río Amazonas at km 6.5 of road from Leticia to Tarapacá, Leticia, Departamento Amazonas, 04°09’15”S 69°56’09”W. Brazil, Amazonas State: MPEG 12260, 1, igarapé Tartaruga, rio Urucu basin, Coari, Amazonas, 04º53’55.32”S 65º19’18.40”W. MPEG 12341, 6 (2 measured), igarapé Tartaruga, rio Urucu basin, Coari, 04º53’55.32”S 65º19’18.40”W. MPEG 12420, 6 (3 measured), igarapé IMT, rio Urucu basin, Coari, approx. 04º49’28”S 65º01’50”W. MPEG 12563, 6 of 11 (4 measured), igarapé IMT, rio Urucu basin, Coari, approx. 04º49’28”S 65º01’50”W.
Rhinotocinclus variola, paratype, MCP 48245, 24.3 mm SL, male, Quebrada Tacana, tributary to Río Amazonas at km 6.5 of road from Leticia to Tarapacá, Leticia, Departamento Amazonas, Colombia.
Rhinotocinclus variola, MPEG 12341, 20.2 mm SL, female, rio Coari, Coari, Amazonas, Brazil.
Rhinotocinclus yaka(Lehmann, Lima & Reis, 2018), new combination
Parotocinclus yakaLehmann, Lima & Reis, 2018:586 (Type-locality: Brazil, Amazonas, Rio Tiquié drainage, Igarapé Açaí near São Pedro Village, approx. 00°16’N 69°58’W. Holotype: MZUSP 123655).
Diagnosis.Rhinotocinclus yaka is distinguished from R. acuen, R. bockmanni, R. chromodontus, R. dani, R. dinizae, R. hera, R. jumaorum, R. pentakelis, R. marginalis n. sp., and R. loxochelis n. sp. by possessing an adipose fin (vs. adipose fin absent), and by having a Y-shaped light mark from the snout tip to each nostril (Figs. 6A,B; vs. light mark V-shaped or present as two separate lines from snout tip diverging to each nostril). It is distinguished from R. collinsae, R. halbolthi, and R. hardmanni by lacking accessory teeth on both premaxilla and dentary (Figs. 5B,C; vs. accessory teeth present, Fig. 5A); the odontodes on the ventral surface of first pelvic-fin ray bent and pointing mesially (Fig. 9A; vs. odontodes aligned with main ray axis, Fig. 9B); a triangular dark spot on the anterior portion of the dorsal-fin membrane (Fig. 8B; vs. dorsal-fin spot absent); a Y-shaped light mark from snout tip to nostrils (vs. Y-shaped light mark absent); and a larger orbit, 26.9–32.5% snout length (vs. orbit 18.9–24.6% snout length). Rhinotocinclus yaka is distinguished from R. britskii and R. kwarup, by having the snout more acutely pointed (Fig. 11B; vs. snout more broadly rounded, Fig. 11A); dark bars on body wider and closer together (Fig. 7B; vs. dark bars on body narrower and more widely spaced, Fig. 7A); and 2–3 plates between the posterior border of the rostral plate and the nostril (Fig. 12A; vs. one plate). Rhinotocinclus yaka is distinguished from R. eppleyi and R. longirostris by having 1–2 irregular series of middle abdominal plates (vs. 4–5 irregular series); and dark bars on body 1+2 fused (vs. five dark bars on body). It is distinguished from R. polyochrus, R. discolor n. sp., and R. isabelae n. sp. by having more numerous premaxillary teeth (34–39; vs. 23–32, 23–26 and 21–29 respectively). It is further distinguished from R. polyochrus and and R. isabelae n. sp. by its shallower body (body depth 14.0–17.2% SL; vs. 17.9–20.0% and 17.8–20.9% SL, respectively); and from R. discolor n. sp. by having the triangular dorsal-fin dark spot occupying nearly one third of the fin (vs. small dorsal-fin spot). Rhinotocinclus yaka is distinguished from P. variola by having the triangular dorsal-fin dark spot occupying nearly one third of the fin (Fig. 8B; vs. dorsal-fin spot occupying more than half of the dorsal fin, Fig. 8C), and by lacking dark dots smaller than a pupil diameter broadly distributed dorsally and ventrally on body (vs. dots present). Rhinotocinclus yaka is finally distinguished from R. pilosus n. sp. by having 1–2 irregular series of large middle abdominal plates between the lateral abdominal plates (Fig. 10B; vs. belly naked or with one series of granular plates in the middle, Fig. 10C), and by having 3–4 lateral abdominal plates (vs. 1–2 such plates).
Geographical distribution.Rhinotocinclus yaka occurs in the upper rio Tiquié, a tributary to the upper rio Negro, state of Amazonas, Brazil (Fig. 15). Rhinotocinclus yaka is probably more widespread in the rio Tiquié and perhaps in the rio Uaupés basin, both in Brazil and Colombia.
Remarks.Rhinotocinclus yaka was tentatively categorized as Least Concern (LC) by Lehmann et al., (2018)Lehmann A P, Lima FCT, Reis RE. Parotocinclus yaka, a new species of armored catfish (Loricariidae: Hypoptopomatinae), from the Amazon basin in Brazil. Zootaxa. 2018; 4521(4):584–92. https://doi.org/10.11646/zootaxa.4521.4.7
https://doi.org/10.11646/zootaxa.4521.4....
in the original description.
Material examined. Rio Negro basin, Amazonas State, Brazil: MZUSP 123655, holotype (measured), ANSP 206002, 2 paratypes (1 measured), MCP 53639, 5 (4 measured) + 2 cs paratypes, MZUSP 81408, 20 paratypes (5 measured), ZUEC 17032, 2 paratypes (1 measured), igarapé Açaí near São Pedro Village, rio Tiquié basin, approx. 00°16’N 69°58’W. MZUSP 85053, 2 paratypes (2 measured), black water creek tributary to rio Tiquié near Fronteira Village, approx. 00°15’N 70°02’W. MZUSP 93308, 2 paratypes, igarapé Cunuri (or Macucu), on opposite shore of São José II Village, rio Tiquié basin, approx. 00°13’N 69°36’W.
Rhinotocinclus yaka, holotype, MZUSP 123655, 29.4 mm SL, female, igarapé Açaí near São Pedro Village, rio Tiquié basin, Amazonas, Brazil.
Rhinotocincluskwarup(Lehmann & Reis, 2021), new combination
Parotocincluskwarup Lehmann & Reis, 2021:449 (Type-locality: Couto de Magalhães River near Vila São José do Couto, Campinápolis, MT, Brazil [13°50’17”S 53°03’53”W]. Holotype: MZUSP 125830).
Diagnosis.Rhinotocinclus kwarup is distinguished from R. acuen, R. bockmanni, R. chromodontus, R. dani, R. dinizae, R. hera, R. jumaorum, R. pentakelis, R. marginalis n. sp., and R. loxochelis n. sp. by possessing an adipose fin (vs. adipose fin absent), and by having a Y-shaped light mark from the snout tip to each nostril (Figs. 6A,B; vs. light mark V-shaped or present as two separate lines from snout tip diverging to each nostril). It is distinguished from R. collinsae, R. halbolthi, and R. hardmanni by lacking accessory teeth on both premaxilla and dentary (Figs. 5B,C; vs. accessory teeth present, Fig. 5A); the odontodes on the ventral surface of first pelvic-fin ray bent and pointing mesially (Fig. 9A; vs. odontodes aligned with main ray axis, Fig. 9B); a triangular dark spot on the anterior portion of the dorsal-fin membrane (Fig. 8B; vs. dorsal-fin spot absent); a Y-shaped light mark from snout tip to nostrils (vs. Y-shaped light mark absent); and a larger orbit, 31.2–40.5% snout length (vs. orbit 18.9–24.6% snout length). Rhinotocinclus kwarup is distinguished from R. discolor n. sp., R. eppleyi, R. isabelae n. sp., R. longirostris, R. pilosus n. sp., R. polyochrus, R. variola, and R. yaka by having the snout more broadly rounded (Fig. 11A; vs. snout more acutely pointed, Fig. 11B); dark bars on body narrower and more widely spaced (Fig. 7A; vs. dark bars on body wider and closer together, Fig. 7B); and one plate between the posterior border of the rostral plate and the nostril (Fig. 12B; vs. 2–4 plates). It is distinguished from R. britskii by having more numerous premaxillary, 28–34 (mode 32), and dentary, 27–33 (mode 29) teeth (vs. fewer premaxillary and dentary teeth, 15–29 (modes 23 and 20/25 respectively), see Tabs. 1–2; and by having the color pattern with more broken marks, mottled (vs. color pattern with less broken marks).
Geographical distribution.Rhinotocinclus kwarup occurs in the tributaries of the upper rio Xingu in the state of Mato Grosso, Brazil (Fig. 15).
Remarks.Rhinotocinclus kwarup, from the upper rio Xingu basin, is most similar to R. bristkii. The morphological differences found between these two species are the color pattern, which has more broken marks, creating a mottled appearance in the former, while R. britskii has the body bars and head marks more unbroken, causing a clearer pattern, and the number of oral teeth, as R. kwarup has 28–34 (mode 32) premaxillary and 27–33 (mode 29) dentary teeth, while all analyzed populations of R. britskii have 15–29 (mode 23) premaxillary and 15–29 (mode 20 and 25) dentary teeth, see Tabs. 1 and 2 for tooth count distribution. These counts are not fully discrete and partially overlap, and this species should probably be included in a wide molecular assessment of P. britskii populations. Rhinotocinclus kwarup was tentatively categorized as Least Concern (LC) by Lehmann, Reis, (2021)Lehmann A P, Reis RE. A new species of the armored catfish Parotocinclus (Loricariidae: Hypoptopomatinae) from the upper Xingu River basin, Brazil. Ichthyol Herpetol. 2021; 109(2):449–55. https://doi.org/10.1643/i2021046
https://doi.org/10.1643/i2021046...
in the original description.
Material examined. Rio Xingu basin, Mato Grosso State, Brazil: MZUSP 125830, holotype (measured) and MZUSP 95576, 61 paratypes (8 measured), rio Couto de Magalhães near Vila São José do Couto, Campinápolis, 13°50’17”S 53°03’53”W. LBP 15894, 6 paratypes, creek tributary to rio Coluene, Canarana, 13°25’30.9”S 52°16’47.0”W. MCP 32146, 3 paratypes, rio Von der Stainer (= rio Atelchu) on road from Santa Terezinha to Iberê, ca. 28 km W of Iberê, Nova Ubiratã, 12°47’05”S 54°40’49”W. MCP 32296, 12 + 3 cs paratypes, rio Arraias on road from Vera to Feliz Natal, ca. 5 km NW of Feliz Natal, Mato Grosso, 12°21’46”S 54°57’30”W. MCP 32297, 32 (5 measured) + 3 cs paratypes, rio Azul on road MT-140, ca. 7 km NNW of Santa Carmen, 11°54’42”S 55°17’48”W. MCP 32298, 2 paratypes, creek on road from Santa Terezinha to Iberê, ca. 10 km W of Iberê, Nova Ubiratã, 12°45’19”S 54°34’25”W. MCP 32299, 2 paratypes, creek tributary to rio Azul on road MT-423, ca. 51 km SW of Cláudia, Sinop, 11°40’17”S 55°12’54”W. MCP 32300, 1 paratype, córrego Etnéia on road MT-423, ca. 60 km SW of Cláudia, Sinop, 11°42’21”S 55°17’03”W. MCP 32301, 1 paratype, creek tributary to rio da Saudade on road MT-423 ca. 38 km SE of Marcelândia, Analândia do Norte, 11°13’23”S 54°17’24”W. MCP 32302, 1 paratype, rio Ferro on road from Novo Mato Grosso to Nova Ubiratã, ca. 25 km SW of Novo Mato Grosso, Nova Ubiratã, 13°03’32”S 55°02’12”W. MCP 39805, 1 cs paratype, córrego da Caporã, tributary to córrego Três Marias, rio Suiazinho, on road BR-158, Ribeirão Cascalheira, 12°32’10”S 51°46’45”W. MNRJ 24970, 64 paratypes, ribeirão das Traíras, tributary to rio Comandante Fontoura on road BR-158, S of Posto da Mata, Alto Boa Vista, 11°49’43”S 51°38’09”W. MNRJ 25073, 1 paratype, córrego Trinta, Alô Brasil, 12°14’54”S 51°42’45”W. MNRJ 25138, 2 paratypes, ribeirão Bonito, tributary to rio Suiazinho, Ribeirão Cascalheira, 12°57’09”S 51°51’07”W. MZUSP 95679, 9 paratypes, ribeirão da Anta and marginal pool in its mouth into rio Culuene, Gaúcha do Norte, 13°30’53”S 53°05’34”W. MZUSP 95709, 21 paratypes (6 measured), rio Coronel Vandick, ca. 20 km of Vila do rio Culuene, Gaúcha do Norte, 13°31’34”S 52°43’52”W. MZUSP 97040, 89 paratypes, rio Couto de Magalhães at mouth of córrego Água Clara, Meu Ranchinho Farm, Campinápolis, 13°48’02”S 53°03’43”W. MZUSP 97068, 51 paratypes, córrego Água Fria, tributary to rio Couto de Magalhães, ca. 2.5 km S of Vila São José do Couto, Campinápolis, 13°49’25”S 53°04’30”W. MZUSP 99007, 3 paratypes, rio Von den Steinen (= rio Atelchu) at Fazenda A.R.S., Nova Ubiratã, 13°05’35”S 54°49’08”W. MZUSP 99060, 4, rio Couto de Magalhães at Airton Espirito Santo Farm, Campinápolis, 13°55’16”S 53°01’27”W.
Descriptive morphometrics of Rhinotocinclus species. Values given as percent of standard length or head length. Range includes the holotype (Hol), SD = standard deviation.
Rhinotocinclus kwarup, holotype, MZUSP 125830, 21.6 mm SL, female, rio Couto de Magalhães near Vila São José do Couto, Campinápolis, Mato Grosso, Brazil.
Rhinotocinclus discolor, new species
urn:lsid:zoobank.org:act:BDD5D811-D797-4190-97D6-B3479866A445
Holotype. MHNLS 26183, 24.4 mm SL, Caño Pasa, tributary of Río Sipapo, 98 km from Puerto Ayacucho airport, Amazonas, Venezuela, 05°03’40.28”N 67°46’46.45”W, 20 Apr 2010, J. Birindelli, N. Lujan, V. Meza & O. Leon.
Paratypes. Amazonas, Venezuela: AUM 53425, 12, 22.1–24.1 mm SL (3 measured, 23.6–24.1 mm SL); MCP 54752, 3, 21.8–24.6 mm SL (3 measured), MHNLS 26184, 2, 21.6–21.8 mm SL (2 measured), MZUSP 125868, 2, 21.8–23.3 mm SL (2 measured), ROM 94295, 4, 22.0–25.1 mm SL (2 measured, 23.3–25.1 mm SL), 2 tissue vouchers, collected with holotype. ANSP 160731, 1, 25.2 mm SL (measured), creek entering Río Sipapo at raudal del Caldero, ca. 3 km upstream of confluence with Río Orinoco, Departamento Apure, approx. 05°04’N 67°46’W, 14 Nov 1985, B. Chernoff, W. Saul, R. Royero & O. Brull. AUM 40978, 2, 24.0–24.2 mm SL, Río Ventuari, beach ca. 116 km NE of Macuruco, 169 km NE of San Fernando de Atabapo, 04°45’09.4”N 66°21’17.5”W, 7 Apr 2004, D. C. Werneke, N. K. Lujan, M. H. Sabaj, & L. S. Sousa. AUM 42102, 1, 23.6 mm SL, Río Orinoco at beach and bedrock outcrop ca. 50 km E of San Fernando de Atabapo, 03°58’13”N 67°15’18”W, 2 Mar 2005, N. K. Lujan, D.C. Werneke, M. H. Sabaj, M. Arce & R. Betancur. AUM 46313, 13, 17.8–22.9 mm SL, Caño Maraya, beach near mouth, 04°01’50”N 66°57’56”W, 12 Nov 2003, C. Montaña & S. Willis. MCNG 22280, 6, 21.8–24.6 mm SL (6 measured) + 1 cs, creek entering Río Sipapo at raudal Caldero, Departamento Apure, approx. 05°01’20”N 67°46’W, 16 May 1989, L. Nico and others.
Rhinotocinclus discolor, holotype, MHNLS 26183, 24.4 mm SL, male, Caño Pasa, tributary of Río Sipapo, 98 km from Puerto Ayacucho airport, Amazonas, Venezuela.
Non-paratypes. Amazonas, Venezuela: MCNG 23757, 1, Río Matacuni, Departamento Atabapo, approx. 03°04’N 65°10’W, 22 Jan 1990, B. Stergios et al., MCNG 24047, 2, Río Putaco at confluence with Río Ocamo, Departamento Atabapo, approx. 2°57’N 64°36’W, 3 Feb 1990, L. Nico et al., MCNG 24270, 9 + 1 cs, Río Putaco near raudal Chicrita-Porã, Departamento Atabapo, approx. 02°56’N 64°33’W, 4 Feb 1990, L. Nico et al., MCNG 24302, 1, Río Ocamo, immediately above mouth of caño Jenita, Departamento Atabapo, 02°45’49.6”N 64°56’34.3”W, 7 Feb 1990, L. Nico et al.
Diagnosis.Rhinotocinclus discolor is distinguished from R. acuen, R. bockmanni, R. chromodontus, R. dani, R. dinizae, R. hera, R. jumaorum, R. loxochelis n. sp., R. pentakelis, and R. marginalis n. sp. and by possessing an adipose fin (vs. adipose fin absent), and by having a Y-shaped light mark from the snout tip to each nostril (Figs. 6A,B; vs. light mark V-shaped or present as two separate lines from snout tip diverging to each nostril). It is distinguished from R. collinsae, R. halbolthi, and R. hardmani by lacking accessory teeth on both premaxilla and dentary (Figs. 5B,C; vs. accessory teeth present, Fig. 5A); the odontodes on the ventral surface of first pelvic-fin ray bent and pointing mesially (Fig. 9A; vs. odontodes aligned with main ray axis, Fig. 9B); a Y-shaped light mark from snout tip to nostrils (vs. Y-shaped light mark absent); and a larger orbit, 29.8–36.2% snout length (vs. orbit 18.9–24.6% snout length). Rhinotocinclus discolor is distinguished from R. britskii, R. eppleyi, R. kwarup, R. isabelae n. sp., R. longirostris, R. pilosus n. sp., R. polyochrus, R. variola, and R. yaka by having a small, inconspicuous triangular spot at the dorsal-fin base (Fig. 8A; vs. spot large and conspicuous). It is further distinguished by having the dark transverse bar 2+3 fused together (vs. bars not fused in R. britskii, R. eppleyi, R. kwarup, and R. longirostris, or bars 1+2 fused in R. isabelae n. sp., R. pilosus n. sp., R. polyochrus, R. variola, and R. yaka).
Description. Proportional measurements in Tab. 5. Dorsal profile of head concave from snout tip to area between nares, convex from that point to parieto-supraoccipital tip and straight to slightly concave from that point to origin of dorsal fin. Dorsal profile of body mostly straight and descending from dorsal-fin origin to insertion of caudal fin. Trunk horizontally ovoid to roundly triangular and caudal peduncle vertically ovoid in cross section, vaguely flattened ventrally and compressed caudally. Body progressively narrowing posteriorly from cleithrum, more so behind dorsal fin.
Head flat to slightly convex between orbits; dorsal margin of orbit slightly elevated. Snout elongated, depressed, its anterior margin rounded in dorsal view, with small depression anterior to naris. Eye comparatively large, positioned dorsolaterally, with small to medium dorsal iris operculum. Posterior tip of parieto-supraoccipital without patch of enlarged odontodes. Slightly enlarged odontodes on snout border, especially on rostral and postrostral plates and on lower surface of pectoral and pelvic spines; enlarged odontodes curved and posteriorly oriented. Odontodes on head and trunk otherwise of uniform size and distribution. Canal cheek plate bent and elongated posteroventrally, contacting cleithum. Lips rounded, narrow, covered with minute papillae; papillae slightly decreasing in size towards lip margin. Lip margin with uniformly distributed papillae forming delicate fringe. Maxillary barbel completely adnate to lower lip without free distal portion. Teeth moderately slender, bifid. Larger, medial cusp blade-like and slightly rounded, not elongated. Smaller, lateral cusp minute and pointed. Premaxillary teeth 23–26 (24); dentary teeth 19–24 (24); accessory teeth absent.
Body entirely covered by dermal plates except for ventral surface of head around lips, area around pelvic-fin insertion, and area around anus. Lateral plates arranged in five longitudinal series on trunk. Dorsal plate series complete, beginning at origin of dorsal fin, with 19 plates; mid-dorsal series incomplete, with 6–7 plates; middle series complete, with two ossified tubes and 24 plates. Lateral line on middle plate series with two ossified tubes, six pored plates followed by one unperforated plate, then 14–15 plates bearing canal, and 2–3 terminal plates without canal. Mid-ventral series incomplete with 15 plates; series terminating below adipose fin. Ventral series complete and continuous from pelvic-fin origin to caudal-fin base, with 18 plates. Predorsal plates forming two transverse rows anterior to nuchal plate. Single preadipose azygous plate. Coracoid completely exposed ventrally, twice longer than cleithrum; cleithrum exposed laterally with medial area and arrector fossa covered by skin. Lateral abdominal plates 3–5 (4/3); plates transversely elongate, clearly arranged in line between coracoid and pelvic-fin origin. Middle abdominal plates 1–2 series, between the lateral abdominal plates. Preanal plate large, single or double, bordered anteriorly by two or three plates. First anal-fin pterygiophore exposed in front of anal-fin as small, plate-like bone supporting odontodes. Total vertebrae 27, in one dissected specimen.
Dorsal-fin rays I,7; spine slightly arched. Dorsal-fin origin slightly posterior to vertical through pelvic-fin origin. Dorsal-fin spinelet present, plate-like, roundly triangular dorsally and V-shaped anteriorly. Spinelet articulated to first dorsal-fin pterygiophore and dorsal-fin spine locking mechanism functional. Adipose fin present and well developed. Pectoral-fin rays I,6. Large spine slightly arched; tip of adpressed spine reaching between middle and distal third of pelvic fin. Pectoral-fin axillary slit present, with large slanted opening ventral to tip of posterior process of cleithrum. Pelvic-fin rays i,5, fin short, with tip of adpressed fin almost reaching or reaching to anal-fin origin in males, falling short of that point in females. Adult males with conspicuous fleshy flap along posterodorsal margin of thickened first pelvic-fin ray. Odontodes on ventral surface of thickened first pelvic-fin ray bent and oriented mesially. Anal-fin rays i,5. Caudal-fin rays i,14,i, with lower unbranched ray slightly longer than upper.
Color in alcohol. Dorsal portions of head and trunk light brown, lighter laterally, and cream to pale yellow ventrally. Dorsal and lateral surface of snout with patches of dark chromatophores forming darker lines. Light Y-shaped mark from snout tip diverging towards nostrils. Compound pterotic and most of parieto-supraoccipial behind eyes dark brown. Posterior portion of parieto-supraoccipital and predorsal region lighter than surrounding areas, but not forming inverted Y-shaped mark. Trunk with four conspicuous dark brown bars; bars 2 and 3 coalesced in wider bar. Bars extending transversely from dorsal midline and narrowing ventrally, barely reaching to ventral midline. Ventral surface mostly unpigmented, but small dark spots sometimes on cheeks, lateral abdominal plates, and caudal peduncle. Tooth cusps reddish brown. Fins with transverse, conspicuous brown bands formed by concentration of chromatophores on rays; bands more numerous on leading rays; membranes mostly hyaline. Dorsal fin with small dark triangular spot at anterior portion of membrane, spine with 3–4 dark brown spots, branched rays with 1–2 dark bands, especially in distal half. Pectoral-fin spine with 4–6 dark spots, branched rays with 2–3 irregular dark bands. Pelvic fin with 2 irregular dark bands. Anal fin with 1–2 dark bands. Adipose fin with 1–2 dark spot at anterior portion of spine. Caudal fin with dark transverse blotch at base and 2–3 irregular dark brown bands.
Sexual dimorphism. Males have a conspicuous urogenital papilla immediately behind the anus and a deep skin fold along the first, unbranched pelvic-fin ray, both characteristics being absent in females. Males also possess a larger nostril than females, and the snout profile somewhat elevated immediately in front of the eyes. Males also have slightly longer pelvic fins, which reach or almost reach to the anal-fin origin and fall short of that point in females.
Geographical distribution.Rhinotocinclus discolor is known from creeks of the Río Orinoco basin in the State of Amazonas, Venezuela (Fig. 15).
Etymology.Rhinotocinclus discolor, from the Latin color, color, and the prefix dis-, meaning not of the same color, in allusion to the remarkable color pattern with the second and third dark bars coalesced. A noun in apposition.
Conservation status. The extinction risk of Rhinotocinclus discolor is assessed as low despite the limited knowledge of its geographic distribution. The species is so far known from ten localities in southern Venezuela, with an Extension of Occurrence (EOO) estimated by the convex polygon of those localities of approximately 33,000 square kilometers. The region is relatively well preserved but logging, gold mining and cattle ranching are common throughout the area. For this reason, R. discolor is preliminarily categorized as Least Concern (LC) according to the International Union for Conservation of Nature (IUCN) categories and criteria (IUCN Standards and Petitions Subcommittee, 2019International Union for Conservation of Nature (IUCN). Standards and petitions subcommittee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Gland; 2019. Available from: https://www.iucnredlist.org/resources/redlistguidelines
https://www.iucnredlist.org/resources/re...
).
Remarks. Four lots collected in 1990 from the Departamento Atabapo (MCNG 23757, MCNG 24047, MCNG 24270, MCNG 24302) are not well preserved, having the entire body softened and heavily darkened, not appropriate for a careful morphometric comparison, and for this reason were regarded as non-paratypes. Rhinotocinclus discolor is sympatric and probably syntopic with R. eppleyi, from which it is easily distinghished. The new species has one or two irregular series of middle abdominal plates (vs. four or five), a small, inconspicuous triangular spot at the dorsal-fin base (vs. spot large and conspicuous), and the dark transverse bars 2+3 fused together (vs. dark bars no fused in R. eppleyi).
Rhinotocinclus pilosus, new species
urn:lsid:zoobank.org:act:5D604B71-5C91-4452-BB9F-5CA4CF804ED2
Holotype. UFRO-ICT 27700, 22.6 mm SL, igarapé Traíra, ca. 35 km E of rio Madeira on Transamazon road, Humaitá, Amazonas, Brazil, 07°35’30.9”S 62°44’45.4”W, 8 Aug 2012, W. M. Ohara.
Paratypes. Amazonas State, Brazil: INPA 59644, 3, 15.7–20.6 mm SL (1 measured, 20.6 mm SL), MCP 54753, 10, 15.0–21.8 mm SL (7 measured, 19.1–21.8 mm SL), ROM 111485, 2, 17.9–20.1 mm SL (1 measured, 20.1 mm SL), UFRO-ICT 23448, 16, 13.2–20.9 mm SL (5 measured, 19.2-20.9 mm SL), collected with holotype. MCP 35874, 1, 19.9 mm SL (measured), creek crossing Transamazon road ca. 68 km E of rio Madeira, Humaitá, 07°43’58”S 62°29’40”W, 27 Jul 2004, R. Reis, F. Langeani, E. Pereira & A. Cardoso. MCP 35875, 3, 14.1–14.6 mm SL + 2 cs, igarapé Traíra, ca. 35 km E of rio Madeira on Transamazon road, Humaitá, 07°35’33”S 62°44’45”W, 27 Jul 2004, R. Reis, F. Langeani, E. Pereira & A. Cardoso. MCP 35878, 3, 15.2–19.1 mm SL (1 measured, 19.1 mm SL) + 1 cs, rio Maicimirim, ca. 45 km E of rio Madeira on Transamazon road, Humaitá, 07°37’56”S 62°39’44”W, 27 Jul 2004, R. Reis, F. Langeani, E. Pereira & A. Cardoso. UFRO-ICT 17593, 1, 15.1 mm SL, flooded area in right bank of rio Marmelos, Humaitá, 07°33’22.4”S 62°42’59.9”W, 10 Aug 2012, W. Ohara. UFRO-ICT 18382, 11, 15.0–21.0 mm SL (3 measured, 18.1–21.0 mm SL), rio Marmelos, Humaitá, 07°33’22.4”S 62°42’59.9”W, 10 Aug 2012, LIP team col.
Rhinotocinclus pilosus, holotype, UFRO 27700, 22.6 mm SL, female, igarapé Traíra, ca. 35 km E of rio Madeira on Transamazon road, Humaitá, Amazonas, Brazil.
Live specimen of Rhinotocinclus pilosus from lot MCP 35878, rio Maicimirim, ca. 45 km E of rio Madeira on Transamazon road, Humaitá, Amazonas, Brazil.
Diagnosis.Rhinotocinclus pilosus is distinguished from R. acuen, R. bockmanni, R. chromodontus, R. dani, R. dinizae, R. hera, R. jumaorum, R. loxochelis n. sp., R. marginalis n. sp., and R. pentakelis by possessing an adipose fin (vs. adipose fin absent), and by having a Y-shaped light mark from the snout tip to each nostril (Figs. 6A,B; vs. light mark V-shaped or present as two separate lines from snout tip diverging to each nostril). It is distinguished from R. collinsae, R. halbolthi, and R. hardmanni by lacking accessory teeth on both premaxilla and dentary (Figs. 5B, C; vs. accessory teeth present, Fig. 5A); the odontodes on the ventral surface of first pelvic-fin ray bent and pointing mesially (Fig. 9A; vs. odontodes aligned with main ray axis, Fig. 9B); a triangular dark spot on the anterior portion of the dorsal-fin membrane (Fig. 8B; vs. dorsal-fin spot absent); a Y-shaped light mark from snout tip to nostrils (vs. Y-shaped light mark absent); and a larger orbit, 27.1–34.9% snout length (vs. orbit 18.9–24.6% snout length). Rhinotocinclus pilosus is distinguished from R. britskii, R. discolor, R. eppleyi, R. isabelae n. sp., R. kwarup, R. longirostris, R. polyochrus, R. variola, and R. yaka by having the belly naked or almost naked between lateral abdominal plates, with none or one row of granular platelets in the middle (Fig. 10C; vs. belly fully plated, Figs. 10A,B). It is further distinguished from the species above except R. isabelae n. sp., by having 1–2 lateral abdominal plates (vs. 3–6 plates); and from R. isabelae n. sp. by the shallower caudal peduncle (6.3–7.5% vs. 8.1–8.8% SL), and by having 31–37 premaxillary teeth (vs. 21–29 teeth).
Description. Proportional measurements in Tab. 5. Dorsal profile of head straight to slightly concave from snout tip to area between nares, straight between orbits and convex from that point to parieto-supraoccipital tip; straight to slightly convex from that point to origin of dorsal fin. Dorsal profile of body mostly straight and descending from dorsal-fin origin to insertion of caudal fin. Trunk horizontally ovoid to roundly triangular and caudal peduncle vertically ovoid in cross section, vaguely flattened ventrally and compressed caudally. Body progressively narrowing posteriorly from cleithrum, more so behind dorsal fin.
Head widely flat between orbits; dorsal margin of orbit slightly elevated. Snout elongated, depressed, anterior margin rounded in dorsal view, with small depression anterior to naris. Snout quickly becoming wide, sides of head becoming more parallel at level of second postrostral plate. Eye comparatively small, positioned dorsolaterally, with small to medium dorsal iris operculum. Posterior tip of parieto-supraoccipital with two keels of enlarged odontodes, disappearing in larger specimens. Slightly enlarged odontodes on snout border, especially on rostral and postrostral plates and on lower surface of pectoral and pelvic spines; enlarged odontodes curved and posteriorly oriented. Odontodes on trunk and fins, especially posterior to dorsal fin, elongate and acicular, giving hairy appearance. Canal cheek plate bent and elongated posteroventrally, contacting cleithum. Lips rounded, narrow, covered with minute papillae; papillae slightly decreasing in size towards lip margin. Lip margin with uniformly distributed papillae forming delicate fringe. Maxillary barbel mostly adnate to lower lip with small free distal portion. Teeth moderately slender, bifid. Larger, medial cusp blade-like and slightly rounded, not elongated. Smaller, lateral cusp minute and pointed. Premaxillary teeth 31–37 (35); dentary teeth 29–35 (34); accessory teeth absent.
Body entirely covered by dermal plates except for ventral surface of head around lips, abdomen between lateral abdominal plates, and area around anus. Few plateles sometimes present in single, irregular series in middle abdomen or in preanal area. Lateral plates arranged in five longitudinal series on trunk. Dorsal plate series complete, beginning at origin of dorsal fin, with 20 plates; mid-dorsal series incomplete, with 5–6 plates; middle series complete, with two ossified tubes and 23 plates. Lateral line on middle plate series with two ossified tubes, 20–22 plates bearing canal, and 1–3 terminal plates without canal. Mid-ventral series incomplete with 16 plates; series terminating below adipose fin. Ventral series complete and continuous from pelvic-fin origin to caudal-fin base, with 17 plates. Predorsal plates forming two transverse rows anterior to nuchal plate. Preadipose azygous plates 1–2. Coracoid almost completely exposed ventrally, with medial portion covered with skin, somewhat longer than cleithrum; cleithrum exposed laterally with medial area and arrector fossa covered by skin. Lateral abdominal plates 1–2 (2/1); plates small, transversely elongate to rounded, close to coracoid. Middle abdominal plates zero to one series of small platelets embedded in skin in middle of abdomen. Preanal plate absent, none to five platelets embedded in skin in preanal area. First anal-fin pterygiophore exposed in front of anal-fin as small, plate-like bone supporting odontodes. Total vertebrae 27, in two dissected specimens.
Dorsal-fin rays I,7; spine slightly arched. Dorsal-fin origin slightly posterior to vertical through pelvic-fin origin. Dorsal-fin spinelet present, plate-like, roundly triangular dorsally and V-shaped anteriorly. Spinelet articulated to first dorsal-fin pterygiophore and dorsal-fin spine locking mechanism functional. Adipose fin present and well developed. Pectoral-fin rays I,6. Large spine straight to slightly arched; tip of adpressed spine reaching between distal third and distal fourth of pelvic fin. Pectoral-fin axillary slit present, with large slanted opening ventral to tip of posterior process of cleithrum. Pelvic-fin rays i,5, unbranbched ray shorter than branched rays, especially in females; fin short, with tip of adpressed fin falling short of anal-fin origin in both males and females. Adult males with low fleshy flap along posterodorsal margin of thickened first pelvic-fin ray. Odontodes on ventral surface of thickened first pelvic-fin ray strongly bent and oriented mesially. Anal-fin rays i,5. Caudal-fin rays i,14,i, with lower unbranched ray slightly longer than, or equal to upper.
Color in alcohol. Dorsal portions of head and trunk light brown, lighter laterally, and cream to pale yellow ventrally. Dorsal and lateral surface of snout with patches of dark chromatophores forming dark brown lines. Light Y-shaped mark from snout tip diverging towards nostrils. Compound pterotic and most of parieto-supraoccipial behind eyes dark brown. Posterior tip of parieto-supraoccipital and predorsal region lighter than surrounding areas, but not forming inverted Y-shaped mark. Trunk with four conspicuous dark brown bars; bars 1 and 2 coalesced in wider bar. Bars extending transversely from dorsal midline and narrowing ventrally, ventral tip of bar 2 reaching to ventral midline. Ventral surface mostly unpigmented, but small dark chromatophores scattered on cheeks, abdomen, and caudal peduncle. Tooth cusps reddish brown. Fins with transverse, conspicuous brown bands formed by concentration of chromatophores on rays; bands more numerous on leading rays; membranes mostly hyaline. Dorsal fin with dark triangular spot on anterior portion, spine with 4–5 dark brown spots, branched rays with one inconspicuous dark band on distal half. Pectoral-fin spine homogeneously dusky or with 4–5 incospicuous dark spots, branched rays with 2 irregular dark bands. Pelvic fin with 2 irregular dark bands. Anal fin with 1–2 dark bands. Adipose fin with 1 dark spot on anterior portion of spine. Caudal fin with dark transverse blotch at base and melanophores scattered on branched rays, forming incospicuous dark brown band on posterior third.
Sexual dimorphism. Males have a small urogenital papilla immediately behind the anus and a low skin fold along the first, unbranched pelvic-fin ray, both characteristics being absent in females. Males also have slightly longer pelvic-fin unbranched ray which is almost as long as first branched ray; in females unbranched ray in clearly shorter than first unbranched ray. Males also possess a slightly larger nostril than females.
Geographical distribution.Rhinotocinclus pilosus is known from few localities in creeks tributary to the rio Madeira east of Humaitá, Amazonas, Brazil (Fig. 15).
Etymology.Rhinotocinclus pilosus from the Latin pilosus, meaning hairy, in allusion to the dense cover of hyperthrophied odontodes typical of the species. An adjective.
Conservation status. The extinction risk of Rhinotocinclus pilosus is assessed as low despite its restricted range and the limited knowledge of its geographic distribution. The species is so far known from few localities near Humaitá, Amazonas State of Brazil, with an Extension of Occurrence (EOO) estimated by the convex polygon of those localities of approximately 82 km2. Despite being crossed by the Trans-Amazon road, where most specimens were collected, the region is relatively well preserved and surrounded by protected areas (Humaitá National Forest south of the Trans-Amazon road and the Indigenous Lands Nove de Janeiro, Pirahã, and Ipixuna north of the road). Despite logging, gold mining, and cattle ranching are common throughout the Trans-Amazon road, these threats are not believed to impact the species. For this reason, R. pilosus is preliminarily categorized as Least Concern (LC) according to the International Union for Conservation of Nature (IUCN) categories and criteria (IUCN Standards and Petitions Subcommittee, 2019International Union for Conservation of Nature (IUCN). Standards and petitions subcommittee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Gland; 2019. Available from: https://www.iucnredlist.org/resources/redlistguidelines
https://www.iucnredlist.org/resources/re...
).
Rhinotocinclus isabelae, new species
urn:lsid:zoobank.org:act:6A076A5D-9972-41D4-9BEE-036C45B7102F
Holotype. MUSM 69737, 17.6 mm SL, Río Nanay, between Puerto Almendra and Tarapoto, Iquitos, Loreto, Peru, 03°48’15”S 73°23’53”W, 5 Aug 2019, E. Mejia & Ornamental fishers.
Paratypes. Loreto, Peru: MUSM 69738, 4, 14.6–17.2 mm SL (4 measured), MCP 54222, 5, 14.1–17.3 mm SL (4 measured, 14.8–17.3 mm SL), collected with holotype. MCP 50154, 4, 14.6–17.9 mm SL (3 measured, 17.0–17.9 mm SL), aquarium shop, imported from Iquitos, obtained 12 Mar 2016, R. E. Reis. MCP 54755, 2, 16.8–16.9 mm SL (2 measured) + 1 cs, MZUSP 125878, 3, 15.4–17.6 mm SL, ROM 104102, 16, 14.8–19.0 mm SL (1 measured, 17.9 mm SL) + 5 tissue vouchers + 1 cs, Río Tigre near community of Intuto, estimated 03°29’34.3”S 74°46’13.9”W, Jan 2018, Ornamental fishers for Oliver Lucanus (most fixed in ethanol).
Rhinotocinclus isabelae, holotype, MUSM 69737, 17.6 mm SL, female, Río Nanay, between Puerto Almendra and Tarapoto, Iquitos, Loreto, Peru.
Live specimen of Rhinotocinclus isabelae photographed in aquarium. Photo by Daniel Konn-Vetterlein.
Diagnosis.Rhinotocinclus isabelae is distinguished from R. acuen, R. bockmanni, R. chromodontus, R. dani, R. dinizae, R. hera, R. jumaorum, R. pentakelis, R. loxochelis n. sp., and R. marginalis n. sp. by possessing an adipose fin (vs. adipose fin absent), and by having a Y-shaped light mark from the snout tip to each nostril (Figs. 6A,B; vs. light mark V-shaped or present as two separate lines from snout tip diverging to each nostril). It is distinguished from R. collinsae, R. halbolthi, and R. hardmanni by lacking accessory teeth on both premaxilla and dentary (Figs. 5B,C; vs. accessory teeth present, Fig. 5A); the odontodes on the ventral surface of first pelvic-fin ray bent and pointing mesially (Fig. 9A; vs. odontodes aligned with main ray axis, Fig. 9B); a triangular dark spot on the anterior portion of the dorsal-fin membrane (Fig. 8C; vs. dorsal-fin spot absent); a Y-shaped light mark from snout tip to nostrils (vs. Y-shaped light mark absent); and a larger orbit, 29.4–34.3% snout length (vs. orbit 18.9–24.6% snout length). Rhinotocinclus isabelae is distinguished from the remaining congeners with an adipose fin, except R. variola, by having the dorsal-fin dark spot occupying more than half of the fin (Fig. 8C); and except for R. pilosus, by having 1–2 lateral abdominal plates (vs. 3–6 plates). It is further distinguished from R. variola by lacking dark dots smaller than a pupil diameter broadly distributed dorsally and ventrally on body (vs. dots present), and by having 21–29 premaxillary teeth (vs. 33–51 teeth); and from R. pilosus by having the abdomen fully plated (vs. abdomen mostly naked), and by the deeper caudal peduncle (8.1–8.8% vs. 6.3–7.5% SL). Rhinotocinclus isabelae is further distinguished from all congeners by its small body size (maximum standard length 17.9 mm).
Description. Proportional measurements in Tab. 5. Dorsal profile of head straight to slightly concave from snout tip to area between nares, convex from that point to parieto-supraoccipital tip; straight to slightly convex from that point to origin of dorsal fin. Supraoccipital tip wih enlarged odontodes and slightly elevated. Dorsal profile of body mostly straight and descending from dorsal-fin origin to insertion of caudal fin. Trunk horizontally ovoid to roundly triangular and caudal peduncle vertically ovoid in cross section, vaguely flattened ventrally and compressed caudally. Body progressively narrowing posteriorly from cleithrum, more so behind dorsal fin.
Head widely flat between orbits; dorsal margin of orbit slightly elevated. Snout elongated, depressed, its anterior margin rounded in dorsal view, with small depression anterior to naris. Eye comparatively large, positioned dorsolaterally, with small to medium dorsal iris operculum. Posterior tip of parieto-supraoccipital with two keels of enlarged odontodes, fading in larger specimens. Slightly enlarged odontodes on snout border, especially on rostral and postrostral plates and on lower surface of pectoral and pelvic spines; enlarged odontodes curved and posteriorly oriented. Odontodes on trunk, especially posterior to dorsal fin, somewhat aligned. Canal cheek plate bent and elongated posteroventrally, contacting cleithum. Lips rounded, narrow, covered with minute papillae; papillae slightly decreasing in size towards lip margin. Lip margin with uniformly distributed papillae forming delicate fringe. Maxillary barbel mostly adnate to lower lip with small free distal portion. Teeth slender and delicate, bifid. Larger, medial cusp blade-like and slightly rounded, not elongated. Smaller, lateral cusp minute and pointed. Premaxillary teeth 21–29 (22); dentary teeth 18–24 (20); accessory teeth absent.
Body entirely covered by dermal plates except for ventral surface of head around lips, area around pelvic-fin insertion, and area around anus. Lateral plates arranged in five longitudinal series on trunk. Dorsal plate series complete, beginning at origin of dorsal fin, with 19 plates; mid-dorsal series incomplete, with 6–7 plates; middle series complete, with two ossified tubes and 23–24 plates. Lateral line on middle plate series with two ossified tubes, 7–9 plates bearing canal, 1–3 unperforated plates, 9–11 plates with canal, and 1–3 terminal plates without canal. Mid-ventral series incomplete with 12–16 plates; series terminating below or slightly anterior to adipose fin. Ventral series complete and continuous from pelvic-fin origin to caudal-fin base, with 18–20 plates. Predorsal plates forming two transverse rows anterior to nuchal plate. Preadipose azygous plates 1. Coracoid completely exposed ventrally, much longer than cleithrum; cleithrum exposed laterally with medial area and arrector fossa covered by skin. Lateral abdominal plates 1–3 (2/2). Middle abdominal plates arranged in 1–2 series, usually leaving unplated spaces between them and lateral abdominal plates. One or two preanal plates, usually anteriorly bordered by two plates. First anal-fin pterygiophore exposed in front of anal-fin as small, plate-like bone supporting odontodes. Total vertebrae 27, in two dissected specimens.
Dorsal-fin rays I,7; spine arched. Dorsal-fin origin slightly posterior to vertical through pelvic-fin origin. Dorsal-fin spinelet present, plate-like, roundly triangular dorsally and V-shaped anteriorly. Spinelet articulated to first dorsal-fin pterygiophore and dorsal-fin spine locking mechanism functional. Adipose fin present and well developed. Pectoral-fin rays I,6. Large spine slightly arched; tip of adpressed spine reaching distal third of pelvic fin. Pectoral-fin axillary slit present, with large slanted opening ventral to tip of posterior process of cleithrum. Pelvic-fin rays i,5, unbranbched ray shorter than branched rays, especially in females; fin short, with tip of adpressed fin almost reaching or reaching to anal-fin origin. Adult males with low fleshy flap along posterodorsal margin of thickened first pelvic-fin ray. Odontodes on ventral surface of thickened first pelvic-fin ray bent and oriented mesially. Anal-fin rays i,5. Caudal-fin rays i,14,i, with lower unbranched ray slightly longer than, or equal to upper.
Color in alcohol. Dorsal portions of head and trunk yellowish cream, cream to pale yellow ventrally. Dorsal and lateral surface of snout and cheeks mostly dark brown, sometimes with clear bar from nostril to ventral margin of snot. Light Y-shaped mark from snout tip diverging towards nostrils. Compound pterotic and most of parieto-supraoccipial behind eyes dark brown. Posterior tip of parieto-supraoccipital and predorsal region yellowish cream and not forming inverted Y-shaped mark. Trunk with four conspicuous dark brown bars; bars 1 and 2 coalesced in wider bar; bars 4 and 5 very close or coalesced. Bars extending transversely from dorsal midline and narrowing ventrally, ventral tip of bar 2–4 reaching to ventral midline. Ventral surface mostly pigmented. Patches of dark chromatophores scattered on ventral head, especially on cheeks, dorsal surface of upper and lower lips, and gular region behind lower lip; branchiostegal membrane reddish brown. Tooth cusps reddish brown. Abdomen with heavy sparkling of dark chromatophores, caudal peduncle with dark brown pigmentation from lateral bars. Fins with transverse, conspicuous brown bands formed by concentration of chromatophores on rays and membrane. Dorsal fin with large dark triangular spot on anterior portion; spine with anterior third dark or reddish brown, soft rays with anterior 2/3 dark brown. Pectoral-fin spine with 4–5 dark brown spots, branched rays with dark brown and reddish brown pigmentation forming 2 irregular dark bands. Pelvic fin with one irregular dark band as continuation of dark bar 1. Anal fin with anterior half to 2/3 dark brown as continuation of dark bars 2 and 3. Adipose fin with one dark spot on preadipose aziguous plate and on middle portion of spine; these marks sometimes united. Caudal fin with reddish brown transverse blotch at base – bar 5, and cospicuous reddish brown band on posterior third; heavier on lobe tips.
Sexual dimorphism. Males have a small urogenital papilla immediately behind the anus and a low skin fold along the first, unbranched pelvic-fin ray, both characteristics being absent in females. Males also have slightly longer pelvic-fin unbranched ray which is almost as long as first branched ray than females. Males also possess larger nostrils and a more elevated lateral proifile of snout than females.
Geographical distribution.Rhinotocinclus isabelae is known from two localities near Iquitos, Loreto, Peru, one in the nearby Río Nanay and other in the Río Tigre (Fig. 15).
Etymology.Rhinotocinclus isabelae is named in honor to Isabela Alho dos Reis, younger daughter of the senior author. Isabela is an enthusiast of biodiversity conservation and interested in aquarium fishes since she was very young. A patronym.
Conservation status.Rhinotocinclus isabelae is endemic to Peru and is currently known from two localities, the Río Nanay near Iquitos and the Río Tigre, a tributary to the Río Marañon. As only two localities are known the Extension of Occurrence (EOO) can not be estimated. Despite the species is apparently common, based on the high number of specimens exported by the aquarium trade, the impact of this activity is not understood and R. isabelae is preliminarily categorized as Data Deficient (DD) according to the International Union for Conservation of Nature (IUCN) categories and criteria (IUCN Standards and Petitions Subcommittee, 2019International Union for Conservation of Nature (IUCN). Standards and petitions subcommittee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Gland; 2019. Available from: https://www.iucnredlist.org/resources/redlistguidelines
https://www.iucnredlist.org/resources/re...
).
Remarks. This species is heavily harvested for the aquarium trade in Loreto, Peru, having been exported for many years. It is common in aquarium shops in Europe and easily found in aquarium webshops, where it is commercialized as Parotocinclus sp. “Peru” or Bumble Bee Otocinclus.
Rhinotocinclus collinsae Group
Rhinotocinclus collinsae(Schmidt & Ferraris, 1985), new combination
Parotocinclus collinsaeSchmidt & Ferraris, 1985Schmidt RE, Ferraris Jr. CJ. A new species of Parotocinclus (Pisces: Loricariidae) from Guyana. Proc Biol Soc Wash. 1985; 98(2):341–46. Available from: https://www.biodiversitylibrary.org/page/34648620#page/365/mode/1up
https://www.biodiversitylibrary.org/page...
:341 (Type-locality: Guyana, Essequibo Province, tributary to [Little] Takutu River about 2 mi from Mazarahally Takutu lumber camp in Takutu Mountains, approx. 06°15’N 59°5’W. Holotype: AMNH 55433).
Diagnosis.Rhinotocinclus collinsae is distinguished from all congeners, except R. halbolthi and R. hardmanni, by having accessory teeth on both premaxilla and dentary (Fig. 5A; accessory teeth absent); the odontodes on the ventral surface of first pelvic-fin ray aligned with main ray axis (Fig. 9B; vs. odontodes bent and pointing mesially, Fig. 9A); and lacking a light mark from the snout tip to nostrils (Figs. 6G,H; vs. light mark present and Y-, V-shaped or as two separate lines). Rhinotocinclus collinsae is further distinguished from R. britskii, R. discolor, R. eppleyi, R. isabelae, R. kwarup, R. longirostris, R. pilosus, R. polyochrus, R. variola, and R. yaka by lacking a triangular dark spot on the anterior portion of the dorsal-fin membrane (vs. dorsal-fin spot present); and from R. acuen, R. bockmanni, R. chromodontus, R. dani, R. dinizae, R. hera, R. jumaorum, R. loxochelis n. sp., R. marginalis n. sp., and R. pentakelis by possessing an adipose fin (vs. adipose fin absent). Rhinotocinclus collinsae is distinguished from R. halbolthi and R. hardmanni by having a normally developed adipose fin (Figs. A,B; vs. adipose-fin spine coalesced to the dorsum, Fig. 4C). It is further distinguished from R. halbolthi by having one series of middle abdominal plates (vs. 4–7 series), and a longer pectoral fin (58.2–69.1% vs. 46.3–55.8% HL); and from R. hardmanii by having many and large accessory teeth (vs. few and minute teeth), a normally developed urogenital papilla in males (vs. urogenital papilla 3–4 times bigger than normal), skin flap on first pelvic-fin ray of males (vs. skin flap absent), and belly fully plated between the lateral abdominal plates (vs. belly naked or almost naked between lateral abdominal plates).
Geographical distribution.Rhinotocinclus collinsae occurs in the Essequibo River basin, including the Potaro and Mazaruni rivers, in the Regions of Cuyuni-Mazarui and Potaro-Siparuni of Guyana (Fig. 32).
Remarks. The type-locality of R. collinsae was originally described as “Guyana, Essequibo Province, tributary to Takutu River about 2 mi from Mazarahally Takutu lumber camp in Takutu Mountains, approx. 06o15’N 59o05’W, the type specimens being collected by Robert E. Schmidt and Antonios Pappantoniou on 17 August 1983. Despite the approximate coordinates provided suggest the locality is in the lower Mazaruni River, the Takutu River is a well know tributary to the upper Branco of the Amazon basin. Trying to solve this apparent inconsistency, we found a collecting locality of Apistogramma ortmanni at the AMNH collected by the same collector in 1983 (AMNH 72979; field station RES-83-16) that reads: “Little Takutu River and tributaries, Mazarahally Takutu lumber camp, west shore Mazaruni River”. Despite we could not locate this site precisely on a map, it names the river as “Little Takutu”, which differentiates from the Branco tributary Takutu River, and undoubtedly sets the locality in the lower Mazaruni basin. For this reason, the original type-locality of Rhinotocinclus collinsae is herein corrected to Little Takutu River. Rhinotocinclus collinsae is currently not assessed by IUCN or other regional initiative.
Material examined. Essequibo River basin, Guyana: AMNH 55433, holotype (measured), creek tributary to [Little] Takutu River, Cuyuni-Mazaruni, approx. 06°15’N 59°05’W. AMNH 55434, 2 of 4 (2 measured) paratypes, same data as holotype. ANSP 175927 1, creek crossing Kurupukari-Surama River road ca. 3 miles from Kurupukari field station, Siparuni VIII-2, Potaro-Siparuni, 04°22’29”N 58°50’30”W. ANSP 175923, 2 cs, blackwater creek 5 minutes downstream from Burro Burro campsite, Potaro-Siparuni, 04°43’58”N 58°51’18”W. ANSP 179140, 7, MCP 34710, 3, Whitewater Creek, small blackwater creek tributary to Mazaruni River, 6.8 km SW of Bartica, Cuyuni-Mazaruni, 06°22’41”N 58°40’25”W. AUM 28118, 7 + 2 cs, Potaro River and Amatuk cataract and beach just below cataract, Potaro-Siparuni, 05°18’14”S 59°18’40”W. AUM 28143, 2, Potaro River at Waratuk cataract, Potaro-Siparuni, 05°15’32”S 59°24’01”W. AUM 35577, 10 (8 measured) + 1 cs, Whitewater Creek, small blackwater creek tributary to Mazaruni River, 6.8 km SW of Bartica, Cuyuni-Mazaruni, 06°22’41”N 58°40’25”W. AUM 62851, 13 of 24, MCP 54757, 10, Kuribrong River at Grass Shoals Rapids, Potaro-Siparuni, 05°28’44.7”S 59°31’54.4”W. AUM 62880, 1, Kuribrong River at Ram Sheep Rapids, Potaro-Siparuni, 05°26’32.5”S 59°30’07.2”W. AUM 62896, 3, Grass Falls Creek (Kiwikparu Creek), just upstream from mouth of Kuribrong River, Potaro-Siparuni, 05°24’23.4”S 59°32’01”W. MHNG 2607.046, 2 of 3, river tributary to Essequibo River, 21.6 km from Linden on road from Linden to Rochstone, Mazaruni-Potaro, 05°59’02”N 58°31’48”W. ROM 83660, 2, Apanachi Creek, 26 mi from Issano River, along Issano-Barbica Road, Potaro-Siparuni, 05°37’14.22”W 59°13’36.46”W. ROM 101797, 2, Kurupung River, Cuyuni-Mazaruni, 06°12’40.39”N 60°09’53.35”W. ROM 102182, 3, upper Eping Creek, Cuyuni-Mazaruni, 06°06’22.75”N 60°06’24.12”W.
Descriptive morphometrics of Rhinotocinclus species. Values given as percent of standard length or head length. Range includes the holotype (Hol), SD = standard deviation.
Rhinotocinclus collinsae, holotype, AMNH 55433, 23.9 mm SL, female, creek tributary to Little Takutu River, Cuyuni-Mazaruni, Guyana.
Geographical distribution of Rhinotocinclus species of the R. collinsae group in southeastern Guiana Shield. Rhinotocinclus collinsae (yellow dots); R. halbothi (red dots); R. hardmani (blue dots). T = Type-locality
Rhinotocinclushalbothi(Lehmann, Lazzarotto & Reis, 2014), new combination
Parotocinclushalbothi Lehmann, Lazzarotto & Reis, 2014Lehmann A P, Lazzarotto H, Reis RE. Parotocinclus halbothi, a new species of small armored catfish (Loricariidae: Hypoptopomatinae), from the Trombetas and Marowijne River basins, in Brazil and Suriname. Neotrop Ichthyol. 2014; 12(1):27–33. https://doi.org/10.1590/S1679-62252014000100002
https://doi.org/10.1590/S1679-6225201400...
:28 (Type-locality: Brazil, Pará, Oriximiná, creek tributary to igarapé do Moura at Platô Monte Branco, rio Trombetas drainage, Amazon basin, 01°35’58.09”S 56°31’21.83”W. Holotype: MCP 48029).
Diagnosis.Rhinotocinclus halbothi is distinguished from all congeners, except R. collinsae and R. hardmanni, by having accessory teeth on both premaxilla and dentary (Fig. 5A; accessory teeth absent, Figs. 5B,C); the odontodes on the ventral surface of first pelvic-fin ray aligned with main ray axis (Fig. 9B; vs. odontodes bent and pointing mesially, Fig. 9A); and lacking a light mark from the snout tip to nostrils (vs. light mark present and Y-, V-shaped or as two separate lines). Rhinotocinclus halbothi is further distinguished from R. britskii, R. discolor, R. eppleyi, R. isabelae, R. kwarup, R. longirostris, R. pilosus, R. polyochrus, R. variola, and R. yaka by lacking a triangular dark spot on the anterior portion of the dorsal-fin membrane (vs. dorsal-fin spot present); and from R. acuen, R. bockmanni, R. chromodontus, R. dani, R. dinizae, R. hera, R. jumaorum, R. pentakelis, R. loxochelis n. sp., and R. marginalis n. sp. by possessing an adipose fin (vs. adipose fin absent). Rhinotocinclus halbothi is distinguished from R. collinsae and R. hardmanni by having 4–7 series of middle abdominal plates (vs. 0–1 series). It is further distinguished from R. collinsae by having the adipose-fin spine coalesced to the dorsum (vs. adipose fin normally developed), and by a shorter pectoral fin (46.3–55.8% vs. 58.2–69.1% HL); and from R. hardmanii by having many and large accessory teeth (vs. few and minute teeth), a normally developed urogenital papilla in males (Fig. 2B; vs. urogenital papilla 3–4 times bigger than normal, Fig. 2C), skin flap on first pelvic-fin ray of males (vs. skin flap absent), and belly fully plated between the lateral abdominal plates (vs. belly naked or almost naked between lateral abdominal plates).
Geographical distribution.Rhinotocinclus halbothi occurs in the rio Trombetas basin, a tributary to the Amazon in the states of Amazonas and Pará, Brazil, and in the upper Marowijne River, in southern Suriname (Fig. 32).
Remarks.Rhinotocinclus halbothi, listed as Parotocinclus halbothi, is currently assessed as Least Concern (LC) in the Brazilian regional assessment by ICMBio, (2018)Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio). Livro vermelho da fauna brasileira ameaçada de extinção. Brasília: ICMBio; 2018..
Material examined. Brazil: MCP 48029, holotype (measured), MCP 48030, 6 paratypes (6 measured), MCP 48098, 1 cs paratype (measured), INPA 39890, 4 + 1 immature paratypes (4 measured), creek tributary to igarapé do Moura at Platô Monte Branco, rio Trombetas drainage, Oriximiná, Pará, 01°35’58.09”S 56°31’21.83”W. MPEG 17299, 2 paratypes, igarapé 1500 on track 4, Estação Ecológica Grão-Pará, upper rio Mapuera, rio Trombetas basin, Pará, 01º16’20.8”N 58º41’09.2”W. Suriname: USNM 408454, 1 paratype, left tributary to upper Paloemeu River, 1 km downstream of basecamp, Marowijne River basin, Sipaliwini, 02º28’38”N 55º38’17”W. USNM 409918, 2 paratypes, downstream waterfall in right tributary of upper Paloemeu River, Marowijne River basin, Sipaliwini, 02º27’21”N 55º37’35”W.
Rhinotocinclus halbothi, holotype, MCP 48029, 19.4 mm SL, female, creek tributary to igarapé do Moura at Platô Monte Branco, rio Trombetas drainage, Oriximiná, Pará, Brazil.
Rhinotocinclushardmani(Lehmann, Lujan & Reis, 2022), new combination
Parotocinclushardmani Lehmann, Lujan & Reis, 2022:2 (Type-locality: Kuribrong River at Grass Shoals Rapids, Potaro-Siparuni, Guyana, 05°28’44.7”N 59°31’54.4”W. Holotype: CSBD F3618).
Diagnosis.Rhinotocinclus hardmanni is distinguished from all congeners, except R. collinsae and R. halbolthi, by having accessory teeth on both premaxilla and dentary (Fig. 5A; accessory teeth absent, Figs. 5B,C); the odontodes on the ventral surface of first pelvic-fin ray aligned with main ray axis (Fig. 9B; vs. odontodes bent and pointing mesially, Fig. 9A); and lacking a light mark from the snout tip to nostrils (vs. light mark present and Y-, V-shaped or as two separate lines). Rhinotocinclus hardmanni is further distinguished from R. britskii, R. discolor, R. eppleyi, R. isabelae, R. kwarup, R. longirostris, R. pilosus, R. polyochrus, R. variola, and R. yaka by lacking a triangular dark spot on the anterior portion of the dorsal-fin membrane (vs. dorsal-fin spot present); and from R. acuen, R. bockmanni, R. chromodontus, R. dani, R. dinizae, R. hera, R. jumaorum, R. loxochelis n. sp., R. marginalis n. sp., and R. pentakelis by possessing an adipose fin (vs. adipose fin absent). Rhinotocinclus hardmanni is distinguished from R. collinsae and R. halbothi by having few and minute teeth accessory teeth (vs. many and large), a large urogenital papilla in males, 3–4 times bigger than normal (Fig. 2C; vs. normally developed urogenital papilla, Fig. 2B), by lacking a skin flap on first pelvic-fin ray of males (vs. skin flap present), and having the belly naked or almost naked between lateral abdominal plates (vs. fully plated belly). It is further distinguished from R. collinsae by having the adipose-fin spine coalesced to the dorsum (Fig. 4C; vs. adipose fin normally developed, Figs. 4A,B), and from R. halbothi by having fewer premaxillary teeth (18–22 vs. 28–32 teeth).
Geographical distribution.Rhinotocinclus hardmani occurs in the Potaro River, Essequibo River basin in the Region of Potaro-Siparuni, Guyana (Fig. 32).
Remarks.Rhinotocinclus hardmani is sympatric and syntopic with R. collinsae in the Potaro River, having been collected together at the same spot. Despite both species are superficially similar, they have many distinguishing characters. Rhinotocinclus hardmani is easily diagnosed from R. collinsae by having few and minute teeth accessory teeth, while they are numerous and large in R. collinsae, by the large urogenital papilla of males, which is 3–4 times bigger than that of R. collinsae, by males lacking a skin flap on first pelvic-fin ray, which is present in R. collinsae, by having the belly naked or almost naked between lateral abdominal plates, while it is fully plated in R. collinsae, and by having the adipose-fin spine coalesced to the dorsum, while it is free from the dorsum on R. collinsae. Rhinotocinclus hardmani was tentatively categorized as Least Concern (LC) by Lehmann et al., (2022)Lehmann A P, Lujan NK, Reis RE. A new species of armored catfish (Loricariidae: Hypoptopomatinae) syntopic and superficially similar to Parotocinclus collinsae, from the Potaro River basin, Guyana. Ichthyol Herpetol. Forthcoming 2022. in the original description.
Material examined. Essequibo River basin, Guyana: CSBD F3618, holotype (measured), AUM 62850, 78 paratypes (9 measured), CSBD F3619, 10 paratypes, MCP 54588, 17 (9 measured) + 3 cs paratypes, ROM 110801, 10 paratypes, UMMZ 252792, 10 paratypes, Kuribrong River at Grass Shoals Rapids, Potaro-Siparuni, 05°28’44.7”N 59°31’54.4”W. AUM 62879, 17 of 33 paratypes, Kuribrong River at Ram Sheep Rapids, Potaro-Siparuni, 05°26’32.5”S 59°30’07.2”W. AUM 62895, 14 of 26 paratypes, Grass Falls Creek (Kiwikparu Creek), just upstream from mouth of Kuribrong River, Potaro-Siparuni, 05°24’23.4”S 59°32’01”W. INHS 49522, 5 + 1 cs paratypes, Potaro River at Amatuk Cataract, Potaro-Siparuni, 05°18’13.5”N 59°18’40.2”W. INHS 49555, 2 paratypes, 19.1–22.8 mm SL + 1 cs, INHS 49556, 1, 23.5 mm SL, Potaro River at Waratuk Cataract, Potaro-Siparuni, Guyana, 05°15’31.9”N 59°24’ 01.0”W. ROM 91423, 17 paratypes, Mikobe Creek, approx. 0.5 km upstream from mouth, at rapids beyond first rapid blocking upstream boat entry, Potaro-Siparuni, 05°24’50.25”N 59°28’12.91”W. ROM 111040, 49 paratypes, creek entering Kuribrong River at upper Grass Falls, at lowermost series of rapids near mouth, Potaro-Siparuni, 05°24’26.55”N 59°31’57.05”W.
Rhinotocinclus hardmani, holotype, CSBD F3618, 22.7 mm SL, female, Kuribrong River at Grass Shoals Rapids, Potaro-Siparuni, Guyana.
Rhinotocinclus bockmanni Group
Rhinotocinclus bockmanni (Carvalho & Datovo, 2012), new combination
Hisonotus bockmanniCarvalho & Datovo, 2012Carvalho M, Datovo A. A new species of cascudinho of the genus Hisonotus (Siluriformes: Loricariidae: Hypoptopomatinae) from the upper Rio Tapajós basin, Brazil. Copeia. 2012; 2012(2):266–75. https://doi.org/10.1643/CI-11-016
https://doi.org/10.1643/CI-11-016...
:266 (Type-locality: Brazil, Pará State, Jacareacanga Municipality, sandbank at Rio Cururu, tributary to Rio Teles Pires, tributary to Rio Tapajós, 08°53’42.2”S 57°14’27.8”W, 140 m asl. Holotype: LIRP 8139).
Diagnosis.Rhinotocinclus bockmanni is distinguished from all congeners, except for R. dani, R. hera, R. pentakelis, R. marginalis n. sp., and R. loxochelis n. sp. by having the dominant color pattern formed by dark bars on body well separated and distinct (Fig. 7D; vs. dominant color pattern formed by wide dark bars partially coalesced or closed together, or formed by a dark stripe from the snout tip, through the eye and extending to end of caudal peduncle); having two separate light lines from snout tip diverging to each nostril (Fig. 6E, F; vs. light lines on snout absent, Y- or V-shaped); and by having teeth with yellow cusps (Fig. 5C; vs. cusps brown or light ochre). Rhinotocinclus bockmanni is further distinguished from R. britskii, R. kwarup, R. eppleyi, R. longirostris, R. polyochrus, R. variola, R. yaka, R. discolor, R. isabelae, and R. pilosus by lacking an adipose fin (vs. adipose fin present). Rhinotocinclus bockmanni is further distinguished from congeners, except R. dani, R. pentakelis, R. marginalis n. sp., and R. loxochelis n. sp., by having small platelets at adipose-fin position (vs. small platelets absent). Rhinotocinclus bockmanni is distinguished from R. dani, R. hera, R. pentakelis, R. marginalis n. sp., and R. loxochelis n. sp. by having a triangular dark spot at the dorsal-fin membrane (vs. dorsal-fin dark spot absent), and by lacking or having a very inconspicuous dark bar 2 on body (vs. bar 2 present and conspicuous).
Geographical distribution.Rhinotocinclus bockmanni occurs in the lower and middle rio Tapajós basin, including the rio Teles Pires in the state of Pará, Brazil (Fig. 36).
Remarks.Rhinotocinclus bockmanni was originally described and so far known from the rio Cururu, tributary to rio Teles Pires near Jacareacanga, Pará. We herein record this species to the lower Tapajós downstream from the mouth of rio Jamanxim, extending the distribution o the north by ca. 500 km in straight line. Rhinotocinclus bockmanni, listed as Hisonotus bockmanni, is currently assessed as Data Deficient (DD) in the Brazilian regional assessment by ICMBio, (2018)Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio). Livro vermelho da fauna brasileira ameaçada de extinção. Brasília: ICMBio; 2018.. Based on the new record this species should be reassessed.
Material examined. Rio Tapajós basin, Pará State, Brazil: LIRP 8139, holotype, LIRP 8140, 2 of 3 + 1 cs paratypes (2 measured), MCP 46046, 3 paratypes (3 measured), sand bank of rio Cururu, tributary to rio Teles Pires, rio Tapajós basin, Jacareacanga, 08°53’42.2”S 57°14’27.8”W. INPA 6924, 3 (3 measured), rio Tapajós at Pimental, below mouth of rio Jamanxin, Itaituba, 04°33’41”S 56°15’50”W.
Descriptive morphometrics of Rhinotocinclus species. Values given as percent of standard length or head length. Range includes the holotype (Hol), SD = standard deviation.
Rhinotocinclus bockmanni, paratype, MCP 46046, 23.0 m SL, female, sand bank of rio Cururu, tributary to rio Teles Pires, rio Tapajós basin, Jacareacanga, Pará, Brazil.
Geographic distribution of Rhinotocinclus species of the R. bockmanni group in southeastern Amazon basin. Rhinotocinclus bockmanni (black dots); R. dani (red dots); R. hera (pink dots); R. loxochelis n. sp. (blue dots); R. marginalis n. sp. (white dots); R. pentakelis (yellow dots). T = Type-locality.
Rhinotocinclusdani (Roxo, Silva & Oliveira, 2016), new combination
Parotocinclus daniRoxo, Silva & Oliveira, 2016Roxo FF, Silva GSC, Oliveira C. Description of a new species of Parotocinclus (Siluriformes, Hypoptopomatinae) from the rio Tapajós basin. ZooKeys. 2016; 634:125–36. https://doi.org/10.3897/zookeys.634.9917
https://doi.org/10.3897/zookeys.634.9917...
:127 (Type-locality: municipality of Peixoto de Azevedo, Mato Grosso State, small tributary of rio Peixoto de Azevedo, drainage of rio Teles Pires, rio Tapajós basin, 10°23’10”S 54°18’22”W. Holotype: MZUSP 120737).
Diagnosis.Rhinotocinclus dani is distinguished from all congeners, except for R. bockmanni, R. hera, R. pentakelis, R. marginalis n. sp., and R. loxochelis n. sp. by having the dominant color pattern formed by five dark bars on body well separated and distinct (Fig. 7D; vs. dominant color pattern formed by four or five wide dark bars partially coalesced or closed together, or formed by a dark stripe from the snout tip, through the eye and extending to end of caudal peduncle); having two separate light lines from snout tip diverging to each nostril (Figs. 6E,F; vs. light lines on snout absent, Y- or V-shaped); and by having teeth with yellow (Fig, 5C; vs. cusps brown or light ochre). Rhinotocinclus dani is further distinguished from R. britskii, R. discolor, R. eppleyi, R. kwarup, R. isabelae, R. longirostris, R. pilosus, R. polyochrus, R. variola, and R. yaka by lacking an adipose fin (vs. adipose fin present). Rhinotocinclus dani is further distinguished from congeners, except R. bockmanni, R. loxochelis n. sp., R. marginalis n. sp., and R. pentakelis by having small platelets at adipose-fin position (vs. small platelets absent). Rhinotocinclus dani is distinguished from R. bockmanni by lacking a triangular dark spot at the dorsal-fin membrane (vs. dorsal-fin dark spot present), and by having a conspicuous dark bar 2 on body (vs. bar 2 absent or inconspicuous). It is distinguished from R. hera by having small platelets at adipose-fin position (vs. small platelets absent) and yellow teeth cusps (vs. light ochre cusps); and from R. pentakelis by the shallower body (body depth 16.3–18.3% vs. 18.4–20.8% SL; caudal peduncle depth 8.8–9.9% vs. 10.1–11.7% SL; head depth 38.0–43.5% vs. 43.6–49.5% HL) and shorter dorsal-fin spine (24.0–27.0% vs. 27.0–30.8% SL). Rhinotocinclus dani is further distinguished from R. loxochelis n. sp. by the regularly arranged dark bars on body (vs. dark bars on body somewhat fragmented and inclined, such that they connect to form a zig-zag pattern), the shallower caudal peduncle (8.8–9.9% vs. 10.3–11.2% SL) and smaller orbital diameter (13.6–16.7% vs. 16.9–18.1% HL); and from R. marginalis n. sp. by the body dark bars 2 and usually 3 reaching to the ventral midline (vs. dark bars barely passing lateral dark stripe), and more numerous premaxillary (19–28, mode 21; Tab. 1) and dentary (16–22, mode 19; Tab. 2) teeth (vs. fewer premaxillary, 12–18, mode 16, and dentary, 11–16, mode 13, teeth).
Geographical distribution.Rhinotocinclus dani occur in the rio Teles Pires and rio Jamanxim basins of the rio Tapajós drainage, in the states of Mato Grosso and Pará, Brazil (Fig. 36).
Remarks. Extintion risk of Rhinotocinclus dani is currently not assessed.
Material examined. Rio Tapajós basin, Mato Grosso State, Brazil: MZUSP 120737, holotype (measured), cachoeira da Neblina in tributary to rio Peixoto de Azevedo, rio Teles Pires basin, Peixoto de Azevedo, 10°23’10”S 54°18’22”W. MZUSP 96194, 20 paratypes (3 measured), island on rio Teles Pires, Paranaíta, 09°27’31”S 56°29’19”W. MZUSP 96225, 5 paratypes (2 measured), marginal lagoon from gold mining activities in rio Tapajós, Paranaíta, 09°25’44”S 56°32’36”W. INPA 35523, 37 (7 measured), MCP 54756, 13 (7 measured) + 2 cs, rio Paranaíta, Paranaíta, Mato Grosso, 09°30’57”S 56°42’36”W. MCP 32676, 3 (1 measured) + 2 cs, rio Kaiapá on road MT-320, ca. 5 km N of Nova Canaã do Norte, 10°36’16”S 55°42’26”W.
Rhinotocinclus dani, MCP 54756, 26.1 mm SL, female, rio Paranaíta, Paranaíta, Mato Grosso, Brazil.
Rhinotocinclus hera (Gamarra, Calegari & Reis, 2019), new combination
Curculionichthys hera Gamarra, Calegari & Reis, 2019:2 (Type-locality: igarapé do Onça, tributary to rio Curuá-Una on road BR-163 between Belterra and Rurópolis, Belterra, Pará, Brazil, 03º33’35.3”S 54°52’09.2”W, elevation 80 m asl. Holotype: MCP 52500).
Diagnosis.Rhinotocinclus hera is distinguished from all congeners, except for R. bockmanni, R. dani, R. pentakelis, R. marginalis n. sp., and R. loxochelis n. sp. by having the dominant color pattern formed by dark bars on body well separated and distinct (Fig. 7E; vs. dominant color pattern formed by wide dark bars partially coalesced or closed together, or formed by a dark stripe from the snout tip, through the eye and extending to end of caudal peduncle); having two separate light lines from snout tip diverging to each nostril (Fig. 6E, F; vs. light lines on snout absent, Y- or V-shaped); and, also except from R. acuen and R. dinizae, by having teeth with light ochre cusps (Fig. 5B; vs. cusps brown). Rhinotocinclus hera is further distinguished from R. britskii, R. kwarup, R. eppleyi, R. longirostris, R. polyochrus, R. variola, R. yaka, R. discolor, R. isabelae, and R. pilosus by lacking an adipose fin (vs. adipose fin present). Rhinotocinclus hera is further distinguished from congeners, except R. acuen, R. bockmanni, R. dinizae, and R. jumaorum by lacking both an adipose fin and small platelets at adipose-fin position (vs. adipose fin or small platelets present). Rhinotocinclus hera is further distinguished from R. bockmanni by lacking a triangular dark spot at the dorsal-fin membrane (vs. dorsal-fin dark spot present), and by having a conspicuous dark bar 2 on body (vs. bar 2 absent or inconspicuous); and from R. dani by having small platelets at adipose-fin position (vs. small platelets absent); and proportionally larger orbit and narrower interorbital distance (orbital diameter 44.0–50.4%; vs. 36.2–44.1% interorbital distance). Rhinotocinclus hera is further distinguished from R. pentakelis by its shallower body (body depth 14.6–17.5% vs. 18.4–20.8% SL; and caudal peduncle depth 7.8–9.3% vs. 10.1–11.7% SL); and shorter dorsal-fin spine length (21.5–25.1% vs. 27.0–30.8% SL); and from R. loxochelis n. sp. by the regularly arranged dark bars on body (vs. dark bars on body somewhat fragmented and inclined, such that they connect to form a zig-zag pattern), the shallower caudal peduncle (7.8–9.3% vs. 10.3–11.2% SL) and narrower interorbital distance (33.7–37.7% vs. 38.8–43.9% HL). Rhinotocinclus hera is further distinguished from R. marginalis n. sp. by the comparatively shallower caudal peduncle (21.3–23.5% vs. 23.3–26.3% HL).
Geographical distribution.Rhinotocinclus hera occurs in creeks tributary to the rio Curuá-Una, a small river draining to the Amazon immediately east of the town of Santarém, Pará State, Brazil (Fig. 36).
Remarks.Rhinotocinclus hera was originally described as Curculionichthys hera, and accordingly was compared to other species of Curculionichthtys. Remarkably, the authors distinguished the new species from congeners, except C. jumaorum (originally described as Hisonotus) and C. karipuna Silva, Roxo, Melo & Oliveira, 2016, by possessing a single rostral plate (vs. paired rostral plates); and except C. jumaorum and C. sabaji Roxo, Silva, Ochoa & Oliveira, 2015, by having darkened tooth-crowns (vs. hyaline to light yellow tipped teeth). As demonstrated in the presente study, both C. hera and H. jumaorum, belong to Rhinotocinclus and the single rostral plate and brown tooth crown are typical of its species. Rhinotocinclus hera was tentatively categorized as Least Concern (LC) by Gamarra et al., (2019)Gamarra SP, Calegari BB, Reis RE. A new species of Curculionichthys (Siluriformes: Loricariidae) from the north edge of the Brazilian Shield, lower Amazon basin. Neotrop Ichthyol. 2019; 17(2):e190001. https://doi.org/10.1590/1982-0224-20190001
https://doi.org/10.1590/1982-0224-201900...
in the original description.
Material examined. Rio Curuá-Una basin, Pará State, Brazil: MCP 52500, holotype (measured), AMNH 267150, 7 paratypes, MCP 51571, 70 (6 measured) + 2 cs paratypes, NUP 20539, 7 paratypes, MZUSP 123948, 7 paratypes, igarapé do Onça, tributary to rio Curuá-Una, on road BR-163 between Belterra and Rurópolis, Belterra, 03º33’35.3”S 54°52’09.2”W. MCP 51600, 15 paratypes (3 measured), igarapé Moju, tributary to rio Curuá-Una, on road BR-163 between Belterra and Rurópolis, Belterra, 03°25’05.8”S 54°54’46.7”W. MCP 51604, 10, rio Curuá-Una at mouth or rio Curuá-Tinga, ca. 11 km N of Transmazon road, Placas, 03°47’56.1”S 54°21’19.3”W. MCP 53424, 2, rio Curuá-Una at mouth or rio Curuá-Tinga, ca. 11 km N of Transamazon road, Placas, 03°47’56.1”S 54°21’19.3”W. MCP 51599, 5 (4 measured) + 1 tissue sample, rio Tutuí on Transamazon road ca. 20 km E of Placas, Uruará, 03°51’33.8”S 54°03’40.5”W.
Rhinotocinclus hera, holotype, MCP 52500, 25.2 mm SL, female, igarapé do Onça, tributary to rio Curuá-Una, on road BR-163 between Belterra and Rurópolis, Belterra, Pará, Brazil.
Rhinotocinclus pentakelis (Roxo, Messias & Silva, 2019), new combination
Parotocinclus pentakelisRoxo, Messias & Silva, 2019Roxo FF, Messias FL, Silva GSC. A new species of Parotocinclus (Loricariidae: Hypoptopomatinae) from rio Tocantins basin, Brazil. Zootaxa. 2019; 4646(2):346–56. https://doi.org/10.11646/zootaxa.4646.2.9
https://doi.org/10.11646/zootaxa.4646.2....
:347 (Type-locality: Brazil, Tocantins State, municipality Lavandeira, Rio Palmas, Rio Tocantins basin; 12°48’05”S 46°28’38”W. Holotype: MZUSP 124900).
Diagnosis.Rhinotocinclus pentakelis is distinguished from all congeners, except for R. bockmanni, R. dani, R. hera, R. marginalis n. sp., and R. loxochelis n. sp. by having the dominant color pattern formed by dark bars on body well separated and distinct (Fig. 7D; vs. dominant color pattern formed by wide dark bars partially coalesced or closed together, or formed by a dark stripe from the snout tip, through the eye and extending to end of caudal peduncle); having two separate light lines from snout tip diverging to each nostril (Figs. 6E,F; vs. light lines on snout absent, Y- or V-shaped); and by having teeth with yellow cusps (Fig. 5C; vs. cusps light ochre or brown). Rhinotocinclus pentakelis is further distinguished from R. britskii, R. discolor, R. eppleyi, R. isabelae, R. kwarup, R. longirostris, R. pilosus, R. polyochrus, R. variola, and R. yaka by lacking an adipose fin (vs. adipose fin present). Rhinotocinclus pentakelis is further distinguished from congeners, except R. bockmanni, R. dani, R. loxochelis n. sp., and R. marginalis n. sp., by having small platelets at adipose-fin position (vs. small platelets absent). Rhinotocinclus pentakelis is distinguished from R. bockmanni by lacking a triangular dark spot at the dorsal-fin membrane (vs. dorsal-fin dark spot present), and by having a conspicuous dark bar 2 on body (vs. bar 2 absent or inconspicuous). It is distinguished from R. hera by having small platelets at adipose-fin position (vs. small platelets absent) and yellow teeth cusps (vs. light ochre cusps); and from R. dani by the deeper body (body depth 18.4–20.8% vs. 16.3–18.3% SL; caudal peduncle depth 10.1–11.7% vs. 8.8–9.9% SL; head depth 43.6–49.5% vs. 38.0–43.5% HL), and longer dorsal-fin spine (27.0–30.8% vs. 24.0–27.0% SL). Rhinotocinclus pentakelis is further distinguished from R. loxochelis n. sp. by the regularly arranged dark bars on body (vs. dark bars on body somewhat fragmented and inclined, such that they connect to form a zig-zag pattern), and the comparatively shorter head (head length 37.5–39.7% vs. 39.3–41.3% HL); and from R. marginalis n. sp. by the comparatively deeper caudal peduncle (10.1–11.7% vs. 9.1–10.1% SL and 26.3–29.7%; vs. 23.3–26.3% HL).
Geographical distribution.Rhinotocinclus pentakelis is only known from two localities in tributaries to the rio Tocantins in the state of Tocantins, Brazil (Fig. 36).
Remarks. In the original description, Parotocinclus pentakelis was named and diagnosed from remaining Parotocinclus species, except P. dani, by possessing five conspicuous dark bars on body, compared to three or four dark bars of other Parotocinclus species. In fact, however, remaining Amazon basin Parotocinclus, now all transferred to Rhinotocinclus, possess five dark bars (Fig. 7). Extintion risk of Rhinotocinclus pentakelis is currently not assessed.
Material examined. MCP 54394, 36 (16 measured) + 2 cs, rio Palma at Lavandeira, rio Tocantins basin, Tocantins, Brazil, 12°47’41.03”S 46°30’46.20”W.
Descriptive morphometrics of Rhinotocinclus species. Values given as percent of standard length or head length. Range includes the holotype (Hol), SD = standard deviation.
Rhinotocinclus pentakelis, MCP 54394, 23.4 mm SL, female, rio Palma at Lavandeira, rio Tocantins basin, Tocantins, Brazil.
Rhinotocinclus marginalis, new species
urn:lsid:zoobank.org:act:89E47C73-8B40-4676-86A4-C97EFBB8034C
Holotype. MCP 54748, 19.6 mm SL, rio Iriri at Cachoeira Grande, ca. 15 km upstream from confluence with rio Xingu, Piranhaquara, Pará, 03°50’35.5”S 52°44’08.3”W, 10 Oct 2012, M. H. Sabaj, M. A. Arce & L. M. Sousa.
Paratypes. Rio Xingu basin, Pará, Brazil: ANSP 193022, 55 (not measured), MCP 48609, 8, 17.8–19.9 mm SL (1 measured, 19.9 mm SL) + 1 cs, same data as holotype. INPA 31155, 29, 15.0–20.3 mm SL (4 measured, 18.2–20.3 mm SL) + 2 cs, rio Iriri, ca. 4 h below mouth of rio Novo, Altamira, 04°14’14”S 53°24’34”W, 22 Aug 2008, H. López-Fernandez, N. Meliciano, G. Ortí & C. Röepke. INPA 33916, 10, 15.3–20.5 mm SL (4 measured, 18.5–20.5 mm SL), Altamira National Forest, Itaitúba, approx. 05°50’S 54°55’W, 19 Jun 2009, R. Leitão & F. Ribeiro. INPA 59657, 3, 18.8–20.8 mm SL (3 measured), rio Iriri, above mouth of rio Novo, ltamira, 04°28’11”S 53°41’38”W, 21 Aug 2008, H. López-Fernandez, N. Meliciano, G. Ortí & C. Röepke. INPA 59658, 9, 16.1–19.1 mm SL, ROM 112205, 2 tissue samples, rio Iriri at Barrinha, Altamira, 04°09’05”S 53°23’28”W, 19 Aug 2008, H. López-Fernandez, N. Meliciano, G. Ortí & C. Röepke. LBP 16685, 6, 20.6–25.1 mm SL, unnamed creek in Altamira, 03°24’19.8”S 52°05’48”W, 30 Sep 2012, C. Oliveira, R. Britzke, L. M. Sousa & D. A. Bastos (preserved in ethanol).
Rhinotocinclus marginalis, holotype, MCP 54748, 19.6 mm SL, rio Iriri at Cachoeira Grande, ca. 15 km upstream from confluence with rio Xingu, Piranhaquara, Pará, Brazil.
Diagnosis.Rhinotocinclus marginalis is distinguished from all congeners, except for R. bockmanni, R. dani, R. hera, R. pentakelis, and R. loxochelis n. sp. by having the dominant color pattern formed by dark bars on body well separated and distinct (Fig. 5D; vs. dominant color pattern formed by wide dark bars partially coalesced or closed together, or formed by a dark stripe from the snout tip, through the eye and extending to end of caudal peduncle); having two separate light lines from snout tip diverging to each nostril (Figs. 6E,F; vs. light lines on snout absent, Y- or V-shaped); and by having teeth with yellow cusps (Fig. 5C; vs. cusps light ochre or brown). Rhinotocinclus marginalis is further distinguished from R. britskii, R. discolor, R. eppleyi, R. isabelae, R. kwarup, R. longirostris, R. pilosus, R. polyochrus, R. variola, and R. yaka by lacking an adipose fin (vs. adipose fin present). Rhinotocinclus marginalis is further distinguished from congeners, except R. bockmanni, R. dani, R. loxochelis n. sp., and R. pentakelis, by having small platelets at adipose-fin position (vs. small platelets absent). Rhinotocinclus marginalis is distinguished from R. bockmanni by lacking a triangular dark spot at the dorsal-fin membrane (vs. dorsal-fin dark spot present), and by having a conspicuous dark bar 2 on body (vs. bar 2 absent or inconspicuous). It is distinguished from R. hera by having small platelets at adipose-fin position (vs. small platelets absent) and yellow teeth cusps (vs. light ochre cusps); and from R. pentakelis by the shallower caudal peduncle (9.1–10.1% vs. 10.1–11.7% SL and 23.3–26.3% vs. 26.6–29.7% HL). Rhinotocinclus marginalis is further distinguished from R. loxochelis n. sp. by the regularly arranged dark bars on body (vs. dark bars on body somewhat fragmented and inclined, such that they connect to form a zig-zag pattern), the shallower caudal peduncle 9.1–10.1% vs. 10.3–11.2% SL) and shorter pectoral-fin spine (26.7–30.3% vs. 30.4–32.1% SL); and from R. dani by the dark bars on body barely passing lateral dark stripe (vs. dark bars 2 and usually 3 reaching to the ventral midline), and fewer premaxillary (12–18, mode 16; Tab. 1) and dentary (11–16, mode 13; Tab. 2) teeth (vs. more numerous premaxillary, 19–28, mode 21, and dentary, 16–22, mode 19, teeth).
Description. Proportional measurements in Tab. 8. Dorsal profile of head concave from snout tip to area between nares, convex from that point to parieto-supraoccipital tip and straight from that point to origin of dorsal fin. Dorsal profile of body mostly straight and descending from dorsal-fin origin to insertion of caudal fin. Trunk horizontally ovoid to roundly triangular and caudal peduncle vertically ovoid in cross section, vaguely flattened ventrally and compressed caudally. Body progressively narrowing posteriorly from cleithrum, more so behind dorsal fin.
Head slightly convex between orbits; dorsal margin of orbit slightly elevated. Snout elongated, depressed, its anterior margin rounded in dorsal view, with small depression anterior to naris. Eye comparatively large, positioned dorsolaterally, with small dorsal iris operculum. Posterior tip of parieto-supraoccipital with patch of enlarged odontodes. Slightly enlarged odontodes on snout border, especially on rostral and postrostral plates and on lower surface of pectoral and pelvic spines; enlarged odontodes curved and posteriorly oriented. Odontodes on head and trunk otherwise of uniform size and distribution. Canal cheek plate bent and elongated posteroventrally, almost contacting cleithum. Lips rounded, narrow, covered with minute papillae; papillae slightly decreasing in size towards lip margin. Lip margin with uniformly distributed papillae forming delicate fringe. Maxillary barbel with large free distal portion. Teeth moderately robust, bifid. Larger, medial cusp blade-like and slightly rounded, not elongated. Smaller, lateral cusp minute and pointed. Premaxillary teeth 12–18 (14); dentary teeth 11–16 (13); accessory teeth absent.
Body entirely covered by dermal plates except for ventral surface of head around lips, area around pelvic-fin insertion, and area around anus. Lateral plates arranged in five longitudinal series on trunk. Dorsal plate series complete, beginning at origin of dorsal fin, with 19–20 plates; mid-dorsal series incomplete, with 7–8 plates; middle series complete, with two ossified tubes and 22–24 plates. Lateral line on middle plate series with two ossified tubes, 20–22 pored plates followed by 2–3 terminal plates without canal. Mid-ventral series incomplete with 19–20 plates. Ventral series complete and continuous from pelvic-fin origin to caudal-fin base, with 20-–21 plates. Predorsal plates forming two transverse rows anterior to nuchal plate. Coracoid completely exposed ventrally, twice longer than cleithrum; cleithrum exposed laterally with medial area and arrector fossa covered by skin. Lateral abdominal plates 3–4 (3/3); plates transversely elongate, clearly arranged in line between coracoid and pelvic-fin origin. Middle abdominal plates 1–2 series, between the lateral abdominal plates. Preanal plate large, single or double, bordered anteriorly by two or three plates. First anal-fin pterygiophore exposed in front of anal-fin as small, plate-like bone supporting odontodes. Total vertebrae 28, in one dissected specimen.
Dorsal-fin rays I,7; spine slightly arched. Dorsal-fin origin slightly posterior to vertical through pelvic-fin origin. Dorsal-fin spinelet present, plate-like, roundly triangular dorsally and V-shaped anteriorly. Spinelet articulated to first dorsal-fin pterygiophore and dorsal-fin spine locking mechanism functional. Adipose fin absent; single azygous plate at adipose-fin position in 1 out of 3 cs specimens. Pectoral-fin rays I,6. Large spine slightly arched; tip of adpressed spine reaching between distal fourth and tip of pelvic fin. Pectoral-fin axillary slit present, with large slanted opening ventral to tip of posterior process of cleithrum. Pelvic-fin rays i,5, fin short, with tip of adpressed fin almost reaching or reaching to anal-fin origin in males, falling short of that point in females. Adult males with small, delicate fleshy flap along posterodorsal margin of thickened first pelvic-fin ray. Odontodes on ventral surface of thickened first pelvic-fin ray bent and oriented mesially. Anal-fin rays i,5. Caudal-fin rays i,14,i, with upper and lower unbranched rays subequal.
Color in alcohol. Dorsal portions of head and trunk light brown, and cream to pale yellow ventrally. Two separate light mark from snout tip diverging towards nostrils. Compound pterotic and most of parieto-supraoccipial behind eyes dark brown. Posterior portion of parieto-supraoccipital lighter than surrounding areas, but not forming inverted Y-shaped mark. Trunk with five conspicuous dark brown bars; bars 1 merged with darkened predorsal area. Bars extending transversely from dorsal midline to middle lateral series of plates. Dark bars connected by lateral dark stripe from compound pterotic to caudal-fin base; dark bars barely passing ventrally to such stripe. Ventral surface mostly unpigmented, but small concentrations of chromatophores on cheeks, lateral abdominal plates, and caudal peduncle. Tooth cusps light yellow. Fins with transverse, brown bands formed by concentration of chromatophores on rays; bands more numerous on leading rays; membranes mostly hyaline. Dorsal fin without dark triangular spot at anterior portion of membrane, spine with 2–3 dark brown spots, branched rays with 2–3 dark bands, especially in distal half. Pectoral-fin spine with 4–5 dark spots, branched rays with 1–2 irregular dark bands. Pelvic fin hyaline or with one indistinct dark band. Anal fin with 1–2 dark bands. Caudal fin with dark transverse blotch at base and 1–2 irregular, indistinct brown bands.
Sexual dimorphism. Males have a conspicuous urogenital papilla immediately behind the anus and a low skin fold along the first, unbranched pelvic-fin ray, both characteristics being absent in females. Males also possess a larger nostril than females, and the snout profile greatly elevated immediately in front of the eyes. Males also have slightly longer pelvic fins, which reach or almost reach to the anal-fin origin and fall short of that point in females.
Geographical distribution.Rhinotocinclus marginalis is known from tributaries to the lower rio Xingu and rio Iriri, in the state of Pará, Brazil (Fig. 36).
Etymology.Rhinotocinclus marginalis, from the Latin margo, meaning margin, and -alis, in allusion to the distribution of the species in the northern margin of the Brazilian Shield. An adjective.
Conservation status. The extinction risk of Rhinotocinclus marginalis is assessed as low despite the limited knowledge of its geographic distribution. The species is known from six localities in the lower Xingu and the Iriri rivers, with an Extension of Occurrence (EOO) estimated by the convex polygon of those localities of approximately 6,570 km2. Despite logging and gold mining are common throughout the area, these threats are not believed to put the population in risk. In addition, the entire area of distribution of the species is located inside preservations areas (Altamira National Forest, Terra do Meio Ecological Station, Riozonho do Afrísio and Iriri Extractive Reserves, and at least three Indigenous Lands). For this reason, R. marginalis is preliminarily categorized as Least Concern (LC) according to the International Union for Conservation of Nature (IUCN) categories and criteria (IUCN Standards and Petitions Subcommittee, 2019International Union for Conservation of Nature (IUCN). Standards and petitions subcommittee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Gland; 2019. Available from: https://www.iucnredlist.org/resources/redlistguidelines
https://www.iucnredlist.org/resources/re...
).
Rhinotocinclus loxochelis, new species
urn:lsid:zoobank.org:act:1B9ABD2F-5756-49C4-AB59-3C15B820D98D
Holotype. MPEG 38957, 22.6 mm SL, creek in Jamanxim National Forest, Itaituba, Pará, 08°23’45.7”S 55°39’27.6”W, 28 May 2008, F. B. N. Ribeiro.
Paratypes. Rio Jamanxim basin, Pará State, Brazil: MPEG 15033, 3, 17.4–23.1 mm SL (2 measured, 20.5–23.1 m SL) + 1 cs (measured, 22.4 mm SL), MCP 54747, 2, 21.0–22.6 mm SL (2 measured), collected with holotype. MPEG 17644, 3, 20.3–21.4 mm SL (3 measured), rio Novo basin, tributary to rio Jamanxim, Jamanxim National Forest, Novo Progresso, 07°18’45.64”S 56°10’10.83”W, 24 Sep 2008, F. R. Silva. MPEG 17681, 4, 20.2–21.6 mm SL, rio Claro, tributary to rio Jamanxim, Jamanxim National Forest, Novo Progresso, 07°02’50.6”S 55°47’12.5”W, 27 Aug 2008, F. R. Silva.
Rhinotocinclus loxochelis, holotype, MPEG 38957, 22.6 mm SL, female, creek in Jamanxim National Forest, Itaituba, Pará, Brazil.
Diagnosis.Rhinotocinclus loxochelis is distinguished from all congeners by having the dominant color pattern formed by four dark bars on body somewhat fragmented and inclined, such that they connect to form a zig-zag pattern (Fig. 41; vs. dominant color pattern formed by dark bars separated and distinct, or dark bars wide and partially coalesced, or formed by a dark stripe from the snout tip, through the eye and extending to end of caudal peduncle). Rhinotocinclus loxochelis is further distinguished from R. britskii, R. discolor, R. eppleyi, R. isabelae, R. kwarup, R. longirostris, R. pilosus, R. polyochrus, R. variola, and R. yaka by lacking an adipose fin (vs. adipose fin present). Rhinotocinclus loxochelis is further distinguished from congeners, except R. marginalis, R. bockmanni, R. dani, and R. pentakelis, by having small platelets at adipose-fin position (vs. small platelets absent). Rhinotocinclus loxochelis is distinguished from R. bockmanni by lacking a triangular dark spot at the dorsal-fin membrane (vs. dorsal-fin dark spot present), and from R. hera by having yellow teeth cusps (Fig. 5C; vs. light ochre cusps). Rhinotocinclus loxochelis is further distinguished from R. marginalis by the dark bar 2 on body reaching to the ventral midline (vs. dark bars on body barely passing lateral dark stripe), and by the deeper caudal peduncle 10.3–11.2% vs. 9.1–10.1% SL) and longer pectoral-fin spine (30.4–32.1% vs. 26.7–30.3% SL); and from R. dani by the deeper body (body depth 18.3–20.4% vs. 16.3–18.3% SL, caudal peduncle depth 10.3–11.2% vs. 8.8–9.9% SL, and head depth 44.9–49.4% vs. 38.0–43.5% HL).
Description. Proportional measurements in Tab. 8. Dorsal profile of head slightly concave from snout tip to area between nares, convex from that point to parieto-supraoccipital tip and straight to slightly convex from that point to origin of dorsal fin. Dorsal profile of body mostly straight and descending from dorsal-fin origin to insertion of caudal fin. Trunk horizontally ovoid to roundly triangular and caudal peduncle vertically ovoid in cross section, vaguely flattened ventrally and compressed caudally. Body progressively narrowing posteriorly from cleithrum, more so behind dorsal fin.
Head convex between orbits; dorsal margin of orbit not elevated. Snout elongated, depressed, its anterior margin rounded in dorsal view, with small depression anterior to naris. Eye comparatively large, positioned more laterally than dorsolaterally, with small dorsal iris operculum. Posterior tip of parieto-supraoccipital with patch of slightly enlarged odontodes. Slightly enlarged odontodes on snout border, especially on rostral and postrostral plates and on lower surface of pectoral and pelvic spines; enlarged odontodes curved and posteriorly oriented. Odontodes on head and trunk otherwise of uniform size and distribution. Canal cheek plate bent and elongated posteroventrally, almost contacting cleithum. Lips rounded, narrow, covered with minute papillae; papillae slightly decreasing in size towards lip margin. Lip margin with uniformly distributed papillae forming delicate fringe. Maxillary barbel with large free distal portion. Fleshy keel on lower lip behind each dentary. Teeth moderately robust, bifid. Larger, medial cusp blade-like and slightly rounded, not elongated. Smaller, lateral cusp minute and pointed. Premaxillary teeth 16–22 (20); dentary teeth 15–19 (17); accessory teeth absent.
Body entirely covered by dermal plates except for ventral surface of head around lips, area around pelvic-fin insertion, and area around anus. Lateral plates arranged in five longitudinal series on trunk. Dorsal plate series complete, beginning at origin of dorsal fin, with 18 plates; mid-dorsal series incomplete, with 5–7 plates; middle series complete, with two ossified tubes and 22–23 plates. Lateral line on middle plate series with two ossified tubes, 21–22 pored plates followed by 1 terminal plate without canal. Mid-ventral series incomplete with 18 plates. Ventral series complete and continuous from pelvic-fin origin to caudal-fin base, with 19 plates. Predorsal plates forming two transverse rows anterior to nuchal plate. Coracoid completely exposed ventrally, twice longer than cleithrum; cleithrum exposed laterally with medial area and arrector fossa covered by skin. Lateral abdominal plates 3–5 (3/4); plates transversely elongate, clearly arranged in line between coracoid and pelvic-fin origin. Middle abdominal plates 1–2 series, between the lateral abdominal plates. Preanal plate large, single or double, bordered anteriorly by one or two plates. First anal-fin pterygiophore exposed in front of anal-fin as small, plate-like bone supporting odontodes. Total vertebrae 27, in one dissected specimen.
Dorsal-fin rays I,7; spine slightly arched. Dorsal-fin origin slightly posterior to vertical through pelvic-fin origin. Dorsal-fin spinelet present, plate-like, roundly triangular dorsally and V-shaped anteriorly. Spinelet articulated to first dorsal-fin pterygiophore and dorsal-fin spine locking mechanism functional. Adipose fin absent; single azygous plate at adipose-fin position in one cs specimen. Pectoral-fin rays I,6. Large spine slightly arched; tip of adpressed spine almost reaching or reaching to tip of pelvic fin. Pectoral-fin axillary slit present, with large slanted opening ventral to tip of posterior process of cleithrum. Pelvic-fin rays i,5, fin short, with tip of adpressed fin falling short of anal-fin origin in females, males so far unknown. Odontodes on ventral surface of thickened first pelvic-fin ray bent and oriented mesially. Anal-fin rays i,5. Caudal-fin rays i,14,i, with upper and lower unbranched rays subequal.
Color in alcohol. Dorsal portions of head and trunk light brown o pale yellow, cream to pale yellow ventrally. Two separate light marks from snout tip diverging towards nostrils. Compound pterotic and most of parieto-supraoccipial behind eyes brown. Posterior portion of parieto-supraoccipital lighter than surrounding areas, but not forming inverted Y-shaped mark. Trunk with five conspicuous dark brown bars; bar 1 merged with darkened predorsal area; bar 4 sometimes duplicated. Bars extending transversely from dorsal midline to ventral surface. Dark bars connected by zig-zag lateral dark stripe from compound pterotic to caudal-fin base. Ventral surface mostly unpigmented, but small concentrations of chromatophores on cheeks, lateral abdominal plates, and caudal peduncle. Bar 2 elongated ventrally to anal-fin origin; bars 3–4 reaching or almost reaching to ventral midline. Tooth cusps light yellow. Fins with transverse, brown bands formed by concentration of chromatophores on rays; bands more numerous on leading rays; membranes mostly hyaline. Dorsal fin without dark triangular spot at anterior portion of membrane, spine with 3–4 dark brown spots, branched rays with 2–3 dark bands. Pectoral-fin spine with 4–5 dark spots, branched rays with 2–3 irregular dark bands. Pelvic fin with 1–2 dark band. Anal fin with 1–2 indistinct dark bands. Caudal fin with dark transverse blotch at base and 2–3 irregular brown bands.
Sexual dimorphism. Males unknown for this species; all 14 specimens examined are females.
Geographical distribution.Rhinotocinclus loxochelis is known from creeks tributary to the rio Jamanxim, rio Tapajós basin, in the Jamanxim National Forest, Pará, Brazil (Fig. 36).
Etymology.Rhinotocinclus loxochelis, from the Greek λοξοσ (loxos), slanting, crosswise, and κελισ (kelis), stain, spot, in allusion to the broken and oblique dark bars of the species. A noun in apposition.
Conservation status. The extinction risk of Rhinotocinclus loxochelis is assessed as low despite its restricted range. The species is known from three localities in the rio Jamanxim basin, with an Extension of Occurrence (EOO) estimated by the convex polygon of those localities of approximately 3,400 km2. Both logging and gold mining are common in the region, but these threats are not believed to put the population in risk. Also, all three known localities are located inside the Jamanxim National Forest. For this reason, R. loxochelis is preliminarily categorized as Least Concern (LC) according to the International Union for Conservation of Nature (IUCN) categories and criteria (IUCN Standards and Petitions Subcommittee, 2019International Union for Conservation of Nature (IUCN). Standards and petitions subcommittee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Gland; 2019. Available from: https://www.iucnredlist.org/resources/redlistguidelines
https://www.iucnredlist.org/resources/re...
).
Rhinotocinclus chromodontus Group
Rhinotocinclus chromodontus (Britski & Garavello, 2007), new combination
Hisonotus chromodontus Bristski & Garavello, 2007:414 (Type-locality: Brazil: Mato Grosso State: Diamantino, creek number 1, tributary of Rio Preto, on road to São Francisco, Rio Arinos drainage, ca. 14°18’S 56°20’W. Holotype: MZUSP 45355).
Diagnosis.Rhinotocinclus chromodontus is distinguished from congeners, except R. acuen, R. jumaorum, and R. dinizae, by having the dominant color pattern formed by a dark stripe from the snout tip, through the eye and extending to end of caudal peduncle (Fig. 7E; vs. dominant color pattern formed by dark bars separated and distinct, or dark bars wide and partially coalesced or closed together), and by having a V-shaped light mark from the snout tip to each nostril (Figs. 6C,D; vs. light mark absent, Y-shaped or present as two separate lines from snout tip diverging to each nostril). It is also distinguished from congeners, except R. acuen, R. hera, R. jumaorum, and R. dinizae, by lacking an adipose fin or platelets at the adipose-fin position (vs. adipose fin or platelets present). Rhinotocinclus chromodontus is distinguished from R. acuen, R. jumaorum, and R. dinizae by having the caudal fin mostly brown, with two hyaline spots on upper and lower lobes (Fig. 42; vs. caudal fin with a dark blotch at base and two irregular dark bands), and from R. acuen, R. dinizae, R. hera by having the oral teeth reddish brown (vs. teeth light ochre). It is further distinguished from R. acuen, R. dinizae, R. hera, and R. jumaorum by the dorsal- and pectoral-fin spines homogeneously dusky (vs. spines with 2–3 dark dots).
Geographical distribution.Rhinotocinclus chromodontus occurs in the upper rio Tapajós basin, in the state of Mato Grosso (Fig. 43).
Remarks. Two species of Hisonotus were described by Britski, Garavello, (2007)Britski HA, Garavello JC. Description of two new sympatric species of the genus Hisonotus Eigenmann and Eigenmann, 1889, from upper rio Tapajós, Mato Grosso State, Brazil (Pisces: Ostariophysi: Loricariidae). Braz J Biol. 2007; 67(3):631–37. https://doi.org/10.1590/S1519-69842007000300005
https://doi.org/10.1590/S1519-6984200700...
, H. chromodontus and H. luteofrenatus, from the upper rio Tapajós, being thus compared to other Hisonotus species. Hisonotus luteofrenatus has already been transferred to Curculionichthys and H. chromodontus is herein being transferred to Rhinotocinclus. We take this opportunity to mention that figures 3a and b are interchanged in Britski, Garavello, (2007)Britski HA, Garavello JC. Description of two new sympatric species of the genus Hisonotus Eigenmann and Eigenmann, 1889, from upper rio Tapajós, Mato Grosso State, Brazil (Pisces: Ostariophysi: Loricariidae). Braz J Biol. 2007; 67(3):631–37. https://doi.org/10.1590/S1519-69842007000300005
https://doi.org/10.1590/S1519-6984200700...
, as can be easily noticed by the single rostral plate of Rhinotocinclus (compared to the paired rostral plate of Curculionichthys) and the posteriorly projected canal cheek plate (compared to the mesially projected plate of Curculionichthys). Rhinotocinclus chromodontus, listed as Hisonotus chromodontus, is currently assessed as Least Concern (LC) in the Brazilian regional assessment by ICMBio, (2018)Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio). Livro vermelho da fauna brasileira ameaçada de extinção. Brasília: ICMBio; 2018..
Material examined. Brazil, Mato Grosso State, Rio Tapajós basin: MZUSP 45255, holotype (measured), riacho Três, tributary to rio Preto on road to São Francisco, Diamantino, approx. 14°16’S 56°19’W. MCP 37636, 5 paratypes (4 measured), rio Sumidouro Grande, tributary to rio Arinos on farm Arrossensal, Nortelândia, approx. 14°05’S 56°45’W. AUM 73895, 10, INPA 59666, 10, MCP 35873, 150+ 10 cs, MHNG 2787.079, 10, ROM 111487, 10, rio Sauê-Uiná below dam on road BR-364 towards Sapezal, Sapezal, 13°32’22”S 58°50’48”W. MCP 32648, 3, creek on road MT-338, ca. 158 km N of Tapurah, Simione, 11°42’13”S 56°56’17”W. MCP 32649, 28, creek on road MT-338, ca. 33 km N of Tapurah, Tapurah, 12°29’46”S 56°40’43”W. MCP 32650, 9, creek ca. 20 km NW of São José do Rio Claro, on road to Nova Maringá, São José do Rio Claro, 13°20’29”S 56°51’07”W. MCP 32651, 6 (3 measured), creek on road MT-010, from Diamantino to São José do Rio Claro, 96 km NW of Diamantino, São José do Rio Claro, 13°37’05”S 56°34’27”W. MCP 32652, 6, ribeirão Macuco on road BR-163, ca. 74 km N of Sinop, Sinop, 11°17’35”S 55°19’10”W. MCP 32653, 1, rio Roquete on road BR-163 ca. 45 km N of Sinop, Sinop, 11°32’20”S 55°23’35”W. MCP 32654, 16, creek on road MT-338, ca. 89 km N of Tapurah, Tapurah, 12°01’49”S 56°33’59”W. MCP 32655, 2, creek on road from road MT-010 to Nova Mutum, ca. 11 km W from ferry crossing rio Arinos, São José do Rio Claro, 13°37’23”S 56°29’18”W. MCP 32656, 2, creek ca. 22 km NW of São José do Rio Claro, on road to Nova Maringá, São José do Rio Claro, 13°19’24”S 56°51’22”W. MCP 32657, 1, creek on road MT-338, ca. 46 km N of Tapurah, Tapurah, 12°23’14”S 56°41’54”W. MCP 32658, 1, creek on road MT-338, ca. 131 km N of Tapurah, Simione, 11°47’40”S 56°44’09”W. MCP 32659, 4, creek on farm Esplanada Arinos, on road from road MT-010 and ferry to Nova Mutum, São José do Rio Claro, 13°36’31”S 56°32’10”W. MCP 32660, 134 (3 measured), igarapé Ribeirão Preto, on road MT-338, ca. 26 km ESE of Porto dos Gaúchos, Porto dos Gaúchos, 11°39’27”S 57°12’07”W. MCP 32674, 1, creek tributary to rio Caibi on road MT-140 from Santa Carmen to Vera, Vera, 12°10’40”S 55°20’23”W. MCP 32675, 62 + 5 cs, rio Celeste ca. 9 km W of Nova Ubiratã, on road to Sorriso, Nova Ubiratã, 13°03’08”S 55°21’13”W. MCP 32677, 5 + 1 cs, creek tributary to rio Celeste ca. 47 km NW of Nova Ubiratã on road to Sorriso, Sorriso, 12°45’47”S 55°31’05”W. MCP 32678, 19 + 2 cs, córrego Maria (or Quinze), on road BR-163 ca. 23 km N of Sinop, Sinop, 11°43’16”S 55°27’33”W. MCP 35871, 1, córrego Água Quente, on road from Sapezal to rio Papagaio, Sapezal, 13°32’25”S 58°43’32”W. MCP 44413, 16, creek on road MT-235 from Nova Mutum to Santa Rita do Trivelato, Nova Mutum, 13°48’55”S 56°02’39”W. MCP 44419, 2, córrego Conguinha on road MT-235, from Nova Mutum to Santa Rita do Trivelato, Nova Mutum, 13°47’44”S 55°53’25”W. MCP 44447, 10, rio Criquiri, ca. 8 km from Nova Mutum, Nova Mutum, 13°48’11”S 56°09’23”W. MCP 44473, 2, rio dos Patos on road MT-235 from Nova Mutum to Santa Rita do Trivelato, Nova Mutum, 13°48’00”S 56°01’36”W. MZUEL 7882, 18, córrego Água Quente, tributary to rio Papagaio, rio Juruena basin, Sapezal, 13°32’25.5”S 58°43’34.8”W. MZUEL 9067, 7, córrego Santa Cruz, tributary to rio Papagaio, rio Juruena basin, Brasnorte, 12°48’21.7”S 58°09’21.5”W. MZUEL 9068, 10, rio do Calor, tributary to rio Papagaio, rio Juruena basin, Sapezal, 13°02’57.1”S 58°39’15.7”W. MZUEL 9070, 3, tributary to rio Norato (or Honorato), itself a tributary to rio do Sangue, rio Juruena basin, ca. 7 km S of Brasnorte, 12°11’12.9”S 57°59’33.3”W. MZUEL 9073, 3, tributary to rio Juruena at km 363 of road MT-170, Juína, 11°31’35.0”S 58°24’29.3”W. MZUSP 61115, 5, rio Criquiri, tributary to rio dos Patos, Nova Mutum, approx. 13°51’S 56°11’W.
Descriptive morphometrics of Rhinotocinclus species. Values given as percent of standard length or head length. Range includes the holotype (Hol), SD = standard deviation.
Rhinotocinclus chromodontus, MCP 32660, 25.2 mm SL, male, igarapé Ribeirão Preto, on road MT-338, ca. 26 km ESE of Porto dos Gaúchos, Porto dos Gaúchos, Mato Grosso, Brazil.
Geographic distribution of Rhinotocinclus species of the R. chromodontus group in southern Amazon. Rhinotocinclus acuen (red dots); R. chromodontus (blue dots); R. dinizae (cyan dots); R. jumaorum (yellow dots). T = Type-locality.
Rhinotocinclus acuen (Silva, Roxo & Oliveira, 2014), new combination
Hisonotus acuenSilva, Roxo & Oliveira, 2014Silva GSC, Roxo FF, Oliveira FF. Hisonotus acuen, a new and phenotypically variable cascudinho (Siluriformes, Loricariidae, Hypoptopomatinae) from the upper rio Xingu basin, Brazil. ZooKeys. 2014; 442:105–25. https://doi.org/10.3897/zookeys.442.7870
https://doi.org/10.3897/zookeys.442.7870...
:107 (Type-locality: Brazil, Mato Grosso State, municipality of Querência, affluent of rio Toguro, rio Xingu basin, 13°00’26”S 52°11’27”W. Holotype: MZUSP 115350).
Diagnosis.Rhinotocinclus acuen is distinguished from congeners, except R. chromodontus, R. jumaorum, and R. dinizae, by having the dominant color pattern formed by a dark stripe from the snout tip, through the eye and extending to end of caudal peduncle (Fig. 7E; vs. dominant color pattern formed by dark bars separated and distinct, or dark bars wide and partially coalesced or closed together), and by having a V-shaped light mark from the snout tip to each nostril (Figs. 6C,D; vs. light mark absent, Y-shaped or present as two separate lines from snout tip diverging to each nostril). It is also distinguished from congeners, except R. chromodontus, R. dinizae, R. hera, and R. jumaorum, by lacking an adipose fin or platelets at the adipose-fin position (vs. adipose fin or platelets present). Rhinotocinclus acuen is distinguished from R. chromodontus by having oral teeth light ochre (Fig. 5B; vs. teeth reddish brown), caudal fin with a dark blotch at the base and two irregular dark bands (vs. caudal fin mostly brown, with two hyaline spot on upper and lower lobes), the dorsal- and pectoral-fin spines with 2–3 dark dots (vs. spines homogeneously dusky), narrower body (cleithral width 21.7–24.1% vs. 24.9–27.9% SL and 57.6–62.7% vs. 67.7–76.2% HL), and fewer teeth (17–22 premaxillary, 14–19 dentary; vs. 26–40 and 21–34, respectively). It is distinguished from R. jumaorum by having teeth light ochre (vs. teeth brown), and by the relative proportion of caudal peduncle depth and body width (caudal peduncle depth 39.2–45.0% vs. 35.4–39.0% cleithral width). Rhinotocinclus acuen is distinguished from R. dinizae by having shorter fins (dorsal-fin spine 20.0–22.8% vs. 22.8–25.5% SL, anal-fin unbranched ray 15.0–17.4% vs. 18.5–19.5% SL, and pectoral-fin spine 23.1–26.9% vs. 28.1–30.0% SL), and a shorter head (head length 37.0–40.0% vs. 40.0–41.8% SL).
Geographical distribution.Rhinotocinclus acuen occurs in the tributaries of the upper rio Xingu in the state of Mato Grosso, Brazil (Fig. 43).
Remarks.Rhinotocinclus acuen was also described as a member of Hisonotus, and accordingly compared to other species of the later genus (Silva et al., 2014Silva GSC, Roxo FF, Oliveira FF. Hisonotus acuen, a new and phenotypically variable cascudinho (Siluriformes, Loricariidae, Hypoptopomatinae) from the upper rio Xingu basin, Brazil. ZooKeys. 2014; 442:105–25. https://doi.org/10.3897/zookeys.442.7870
https://doi.org/10.3897/zookeys.442.7870...
). Interestingly, in the diagnosis of the new species the authors begin saying “Hisonotus acuen differs from all congeners except H. bockmanni, H. chromodontus, H. insperatus, H. luteofrenatus, H. oliveirai, and H. paresi by having a functional V-shaped spinelet”. Both H. bockmanni and H. chromodontus are herein being tranferred to Rhinotocinclus and the remaining species have already been transferred to Curculionichthys, the functional V-shaped spinelet indeed diagnosing R. acuen and, in fact, all Rhinotocinclus and Curculionichthys species, from Hisonotus. Extintion risk of Rhinotocinclus acuen is currently not assessed.
Material examined. Rio Xingu basin, Mato Grosso State, Brazil: MZUSP 115350, holotype (measured), creek tributary to rio Toguro, Querência, 13°00’26”S 52°11’27”W. MCP 40543, 22 (5 measured), AUM 73894, 10, INPA 59665, 10, ROM 111486, 10, córrego Trinta, tributary to rio Suaizinho on road BR-153 between Alô Brasil and Ribeirão Cascalheira, Ribeirão Cascalheira, 12°14’54”S 51°42’45”W. MCP 32671, 2, creek tributary to rio Azul on road MT-140, Santa Carmen, 11°59’55”S 55°16’39”W. MCP 32672, 5, rio Ferro on road from Novo Mato Grosso to Nova Ubiratã, ca. 25 km SW of Novo Mato Grosso, Nova Ubiratã, 13°03’32”S 55°02’12”W. MCP 32673, 10 (3 measured) + 2 cs, creek tributary to rio da Saudade on road MT-423, ca. 38 km SE of Marcelândia, Analândia do Norte, 11°13’23”S 54°17’24”W. MCP 32679, 2, córrego Tatu on road MT-423, 14 km W of Cláudia, Cláudia, 11°28’36”S 54°58’47”W. MCP 32680, 1, córrego Saudade on road from Santa Helena to Marcelândia, 66 km SE of road BR-163, Marcelândia, 11°07’07”S 54°40’15”W. MCP 32681, 3, ribeirão Camparina on road MT-320 from Santa Helena to Marcelândia, 79 km SE of road BR-163, Marcelândia, 11°06’27”S 54°33’03”W. MCP 32682, 9, rio Azul on road MT-140, ca. 7 km NNW of Santa Carmen, Santa Carmen, 11°54’42”S 55°17’48”W. MCP 32683, 3, creek on road from Santa Terezinha to Iberê, ca. 10 km W of Iberê, Nova Ubiratã, 12°45’19”S 54°34’25”W. MCP 32684, 7 + 1 cs, creek tributary to rio Tartaruga on road from Vera to Feliz, Vera, 12°15’55”S 55°15’37”W. MCP 32685, 3, rio Azul on road MT-423, 16 km w of Cláudia, Cláudia, 11°28’20”S 54°59’40”W. MCP 32686, 6, rio Tartaruga on road MT-423 ca. 20 km E of Vera, Cláudia, 11°30’36”S 54°42’43”W. MCP 32687, 16, creek on road from Novo Mato Grosso to Nova Ubiratã, 7 km SW of Novo Mato Grosso, Nova Ubiratã, 12°57’10”S 54°54’46”W. MCP 32688, 3, rio Tartaruga at Nova Ubiratã, Nova Ubiratã, 13°02’03”S 55°16’07”W. MCP 32689, 1, creek on road MT-320 from Santa Helena to Marcelândia, 73 km SE of road BR-163, Marcelândia, 11°07’39”S 54°36’52”W. MCP 32690, 6, rio da Saudade on road MT-423, 31 km SE of Marcelândia, Marcelândia, 11°11’11”S 54°19’27”W. MCP 32691, 2, creek on road MT-423, 22 km SE of Marcelândia, Marcelândia, 11°12’21”S 54°23’19”W. MCP 32692, 4, creek tributary to rio Tartaruga on road from Novo Mato Grosso to Nova Ubiratã, ca. 44 km WSW of Novo Mato Grosso, Nova Ubiratã, 13°02’43”S 55°12’21”W. MCP 32693, 2, rio Von der Steinen (or Atelchu) on road from Santa Terezinha to Iberê, ca. 28 km W of Iberê, Nova Ubiratã, 12°47’05”S 54°40’49”W. MCP 32694, 6, creek tributary to rio Manissamá-Miçu on road MT-423 ca. 11.6 km SE of Marcelândia, Marcelândia, 11°09’46”S 54°27’01”W. MCP 32695, 2, rio Tartaruga on road from Vera to Feliz Natal, 20 km NE of Vera, Vera, 12°14’21”S 55°07’11”W. MCP 32696, 4, creek ca. 2 km N of Novo Mato Grosso, Nova Ubiratã, 12°53’49”S 54°50’54”W. MCP 40283, 45, MHNG 2787.080, 10, rio Traíras ca. 3 km W of Posto da Mata on road BR 242, Posto da Mata, 11°42’40”S 51°39’18”W. MCP 40299, 12, córrego da Caaporã, tributary to córrego Três Marias, rio Suiazinho on road BR-158, Ribeirão Cascalheira, 12°32’10”S 51°46’45”W. MCP 40365, 16 (2 measured), creek N of Ribeirão Cascalheira, Ribeirão Cascalheira, 12°35’50”S 51°46’55”W. MCP 40402, 23, creek tribuatry to rio Suiazinho on road BR-158, ca. 1 km N of Ribeirão Cascalheira, Ribeirão Cascalheira, 12°29’21”S 51°46’06”W. MCP 40720, 10, creek tributary to rio Suiazinho on road BR 158, ca. 1 km N of Ribeirão Cascalheira, Ribeirão Cascalheira, 12°29’21”S 51°46’06”W. MCP 40732, 18, córrego Trinta, tributary to rio Suaizinho on road BR-153 between Alô Brasil and Ribeirão Cascalheira, Ribeirão Cascalheira, 12°14’54”S 51°42’45”W. MCP 40790, 5, creek N of Ribeirão Cascalheira, Ribeirão Cascalheira, 12°35’50”S 51°46’55”W. MCP 40814, 5, rio Traíras ca. 3 km W of Posto da Mata on road BR-242, Posto da Mata, 11°42’40”S 51°39’18”W.
Rhinotocinclus acuen, MCP 40543, 27.4 mm SL, female, córrego Trinta, tributary to rio Suaizinho on road BR-153 between Alô Brasil and Ribeirão Cascalheira, Ribeirão Cascalheira, Mato Grosso, Brazil.
Rhinotocinclus jumaorum (Dias, Silva, Oliveira & Roxo, 2018), new combination
Hisonotus jumaorum Dias, Silva, Oliveira & Roxo, 2018:578 (Type-locality: Brazil, Amazonas state, municipality of Apuí, rio Juma, tributary of rio Aripuanã, rio Madeira basin, 07°12’43.7”S 59°55’19.6”W. Holotype: MZUSP 123835).
Diagnosis.Rhinotocinclus jumaorum is distinguished from congeners, except R. acuen, R. chromodontus, and R. dinizae, by having the dominant color pattern formed by a dark stripe from the snout tip, through the eye and extending to end of caudal peduncle (Fig. 7E; vs. dominant color pattern formed by dark bars separated and distinct, or dark bars wide and partially coalesced or closed together), and by having a V-shaped light mark from the snout tip to each nostril (Figs. 6C,D; vs. light mark absent, Y-shaped or present as two separate lines from snout tip diverging to each nostril). It is also distinguished from congeners, except R. acuen, R. chromodontus, R. dinizae, and R. hera, by lacking an adipose fin or platelets at the adipose-fin position (vs. adipose fin or platelets present). Rhinotocinclus jumaorum is distinguished from R. acuen by having oral teeth brown (vs. light ochre), and by the relative proportion of caudal peduncle depth and body width (caudal peduncle depth 35.4–39.0% vs. 39.2–45.0% cleithral width). It is distinguished from R. chromodontus by having the caudal fin with a dark blotch at the base and two irregular dark bands (vs. caudal fin mostly brown, with two hyaline spot on upper and lower lobes), the dorsal- and pectoral-fin spines with 2–3 dark dots (vs. spines homogeneously dusky), a shorter pectoral-fin spine (23.3–26.7% vs. 26.7–29.5% SL and 60.8–68.9% vs. 71.4–78.4% HL), and narrower body (cleithral width 22.4–24.9% vs. 24.9–27.9% SL and 59.4–67.2% vs. 67.7–76.2% HL). Rhinotocinclus jumaorum is distinguished from R. dinizae by having teeth with brown cusps (vs. cusps light ochre); a shorter pectoral-fin spine (23.3–26.7% vs. 28.1–30.0% SL); a shorter head (36.4–39.7% vs. 40.0–41.8% SL); and more numerous teeth (20–33 premaxillary vs. 16–19, and 18–28 dentary vs. 12–15).
Geographical distribution.Rhinotocinclus jumaorum occurs in the rio Juma, a small tributary to the rio Aripuanã and the rio Acari, a tributary to the rio Canumã, rio Madeira basin, in state of Amazonas, Brazil (Fig. 43).
Remarks.Rhinotocinclus jumaorum was also described as a member of Hisonotus by Dias et al., (2018)Dias AC, Silva GSC, Oliveira C, Roxo FF. A new species of Hisonotus (Siluriformes: Loricariidae) from Aripuanã river, Amazon basin, Brazil. Zootaxa. 2018; 4504(4):577–85. https://doi.org/10.11646/zootaxa.4504.4.8
https://doi.org/10.11646/zootaxa.4504.4....
, disregarding the study of Calegari et al., (2017)Calegari BB, Morlis WG, Reis RE. A new species of Otothyropsis (Siluriformes: Loricariidae) from the upper Río Paraná basin, Paraguay, with a discussion of the limits between Otothyropsis and Hisonotus. Zootaxa. 2017; 4244(2):231–45. https://doi.org/10.11646/zootaxa.4244.2.5
https://doi.org/10.11646/zootaxa.4244.2....
which redefined Hisonotus and restricted its distribution to the La Plata basin and coastal rivers from Uruguay to Espirito Santo State, Brazil. For this reason, and based on the fact that Hisonotus jumaorum shares some diagnostic features of Curculionichthys and is distributed in Amazon tributaries running on the Brazilian Shield, Calegari et al., (2018)Calegari BB, Gamarra SP, Reis RE. A new species of Curculionichthys (Siluriformes: Hypoptopomatinae) from the western border of the Brazilian Shield, Madeira River basin, Brazil. Copeia. 2018; 106(4):663–70. https://doi.org/10.1643/CI-18-133
https://doi.org/10.1643/CI-18-133...
reallocated the species as Curculionichthys jumaorum. Roxo et al., (2019)Roxo FF, Messias FL, Silva GSC. A new species of Parotocinclus (Loricariidae: Hypoptopomatinae) from rio Tocantins basin, Brazil. Zootaxa. 2019; 4646(2):346–56. https://doi.org/10.11646/zootaxa.4646.2.9
https://doi.org/10.11646/zootaxa.4646.2....
, however, transfered the species back to Hisonotus. Extintion risk of Rhinotocinclus jumaorum is currently not assessed.
Material examined. Rio Madeira basin, Apuí, Amazonas State, Brazil: MZUSP 123835, holotype (measured), rio Juma, tributary to rio Aripuanã on Transamazon road (BR-230) between vila do 180 and Apuí, 07°12’43”S 59°55’18”W. MZUSP 122325, 26 (4 measured), rio dos Pombos on vicinal road dos Pombos, rio Juma basin, 07°10’59.45”S 60°01’50.88”W. MZUSP 122389, 9, MCP 54758, 8 (3 measured) + 2 cs, rio Juma on vicinal road ca. 6 km from Transamazon road (BR-230), 07°16’45”S 59°57’03”W. MZUSP 122482, 12 (3 measured), creek 2, on vicinal road Dom Pedro, rio Acari basin, 06°50’22.34”S 59°42’26.89”W. MZUSP 122505, 1 (measured), creek 1, tributary to rio Coruja on vicinal road Coruja, rio Juma basin, 07°16’39.61”S 59°51’28.76”W. MZUSP 122639, 1 (measured), igarapé Andorinha, tributary to rio Acari on vicinal road Três Estados, 07°06’31.87”S 59°35’52.62”W. MZUSP 122687, 1 (measured), creek on South branch of Transamazon road (BR-230), 35 km S of Apuí, rio Acari basin, 07°26’21.59”S 59°50’53.81”W. MZUSP 122369, 1, igarapé do Mutum on vicinal road Brasília at Fazenda Nova Esperança, ca. 5 km from Transamazon road (BR-230), 07°14’57”S 59°58’41”W. MZUSP 122975, 4, rio Juma on Transamazon road (BR-230), 07°12’43.49”S 59°55’18.78”W.
Rhinotocinclus jumaorum, MCP 54758, 27.0 mm SL, male, rio Juma on vicinal road ca. 6 km from Transamazon road (BR-230), Apuí, Amazonas, Brazil.
Rhinotocinclus dinizae (Ribeiro-Silva, Silva, Venere, Silva & Roxo, 2020), new combination
Hisonotus dinizae Ribeiro-Silva, Silva, Venere, Silva & Roxo, 2020Ribeiro-Silva LR, Silva GSC, Venere PC, Silva HP, Roxo FF. Description of a new species of Hisonotus (Loricariidae: Siluriformes) from rio Araguaia basin. Zootaxa. 2020; 4860(4):553–62. https://doi.org/10.11646/zootaxa.4860.4.5
https://doi.org/10.11646/zootaxa.4860.4....
:554 (Type-locality: Brazil, Mato Grosso state, municipality of Barra do Garças, córrego Grande, drainage of rio Pindaíba, rio Araguaia basin, -15.7417 -52.0936 [15°44’30.12”S 52°05’36.96”W]. Holotype: MZUSP 125790).
Diagnosis.Rhinotocinclus dinizae is distinguished from congeners, except R. acuen, R. chromodontus, and R. jumaorum, by having the dominant color pattern formed by a dark stripe from the snout tip, through the eye and extending to end of caudal peduncle (Fig. 7E; vs. dominant color pattern formed by dark bars separated and distinct, or dark bars wide and partially coalesced or closed together), and by having a V-shaped light mark from the snout tip to each nostril (Figs. 6C,D; vs. light mark absent, Y-shaped or present as two separate lines from snout tip diverging to each nostril). It is also distinguished from congeners, except R. acuen, R. chromodontus, R. hera, and R. jumaorum, by lacking an adipose fin or platelets at the adipose-fin position (vs. adipose fin or platelets present). Rhinotocinclus dinizae is further distinguished from R. hera by the smaller orbit (12.9–14.8% vs. 15.1–17.3% HL, 38.1–43.0% vs. 44.0–50.4% interorbital distance, and 23.8–28.0% vs. 29.2–32.5% snout length), and from R. acuen, R. chromodontus, and R. jumaorum by the longer head (40.0–41.8% vs. 37.0–40.0%, 36.2–38.5%, and 36.4–39.8% SL, respectively). Rhinotocinclus dinizae is further distinguished from R. acuen by having longer fins (dorsal-fin spine 22.8–25.5% vs. 20.0–22.8% SL, anal-fin unbranched ray 18.5–19.5% vs. 15.0–17.4% SL, and pectoral-fin spine 28.1–30.0% vs. 23.1–26.9% SL). It is further distinguished from R. chromodontus and R. jumaorum by the fewer teeth (16–19 premaxillary, 12–15 dentary vs. 26–40, 21–34, and 20–33, 18–28, respectively).
Geographical distribution.Rhinotocinclus dinizae is known from two localities in the upper rio Araguaia near Barra do Garças, Mato Grosso State, Brazil (Fig. 43).
Remarks. Another species originally described as Hisonotus, it was compared to congeners and diagosed as “Hisonotus dinizae differs from all congeners, except H. acuen, H. bockmanni, H. chromodontus, H. jumaorum and H. vespuccii by having a V-shaped spinelet...”. Except for H. vespuccii, which is probably a member of Otothyropsis, all remaining species are now transferred to Rhinotocinclus and the functional V-shaped spinelet indeed diagnoses R. dinizae, as well as all Rhinotocinclus and Curculionichthys species, from Hisonotus. Extintion risk of Rhinotocinclus dinizae is currently not assessed.
The holotype and MZUSP paratypes of Hisonous dinizae have never been sent to MZUSP after the species original description and were unavailable for the present study, possibly having been lost. Five paratypes (LBP 4932), however, were examined and compared to other Rhinotocinclus species.
Material examined. LBP 4932, 4 + 1 cs paraypes (4 measured), córrego Correntes, rio Araguaia basin, Barra do Garças, Mato Grosso, Brazil, 15°29’59”S 52°12’12”W, 22 Mar 2007, P. C. Vênere & V. Garutti.
Rhinotocinclus dinizae, LBP 4932, 19.5 mm SL, paratype, córrego Correntes, rio Araguaia basin, Barra do Garças, Mato Grosso, Brazil.
DISCUSSION
This study includes the description of a new genus and a taxonomic revision of its 23 species. Despite few phylogenetic analyses have included representatives of Rhinotocinclus, parts of this genus have already been recognized as a possible clade by previous authors (Reis, Lehmann, 2011Reis RE, Lehmann A P. A new genus and five new species of cascudinhos of the subfamily Hypoptopomatinae (Siluriformes: Loricariidae) from northern South America. Minneapolis, USA: Abstracts of the 2011 meetings of the American Society of Ichthyologists and Herpetologists; 2011.; Lehmann, Reis, 2012Lehmann A P, Reis RE. A new species of Parotocinclus (Siluriformes: Loricariidae) from the upper Rio São Francisco, Brazil. Zootaxa. 2012; 3390(1):56–64. https://doi.org/10.11646/zootaxa.3390.1.5
https://doi.org/10.11646/zootaxa.3390.1....
; Reis et al., 2017Reis RE, Calegari BB, Carvalho TP, Cramer CA, Delapieve MLS, Lehmann A. P, Pereira EHL. A phylogeny of the armored catfishes, with emphasis on the Neoplecostominae-Hypoptopomatinae clade (Siluriformes: Loricariidae): Integrating phenotypical and molecular data. Londrina: II International Symposium on Phylogeny and Classification of Neotropical Fishes; 2017.; Dias et al., 2018Dias AC, Silva GSC, Oliveira C, Roxo FF. A new species of Hisonotus (Siluriformes: Loricariidae) from Aripuanã river, Amazon basin, Brazil. Zootaxa. 2018; 4504(4):577–85. https://doi.org/10.11646/zootaxa.4504.4.8
https://doi.org/10.11646/zootaxa.4504.4....
; Gamarra et al., 2019Gamarra SP, Calegari BB, Reis RE. A new species of Curculionichthys (Siluriformes: Loricariidae) from the north edge of the Brazilian Shield, lower Amazon basin. Neotrop Ichthyol. 2019; 17(2):e190001. https://doi.org/10.1590/1982-0224-20190001
https://doi.org/10.1590/1982-0224-201900...
; Roxo et al., 2019Roxo FF, Messias FL, Silva GSC. A new species of Parotocinclus (Loricariidae: Hypoptopomatinae) from rio Tocantins basin, Brazil. Zootaxa. 2019; 4646(2):346–56. https://doi.org/10.11646/zootaxa.4646.2.9
https://doi.org/10.11646/zootaxa.4646.2....
). Two phylogenetic studies containing species of this clade have so far included a limited number of its members. Notwithstanding, a clade containing representatives of all four species groups defined in the present study, including Curculionichthys hera (treated as Curculionichthys sp. n.), Hisonotus acuen, H. bockmanni, H. chromodontus, and four species of Amazonian Parotocinclus, P. amazonensis, P. britskii, P. collinsae, and P. eppleyi has been recovered in a combined molecular and morphological phylogenetic analysis of the Hypoptopomatinae (Reis et al., 2017Reis RE, Calegari BB, Carvalho TP, Cramer CA, Delapieve MLS, Lehmann A. P, Pereira EHL. A phylogeny of the armored catfishes, with emphasis on the Neoplecostominae-Hypoptopomatinae clade (Siluriformes: Loricariidae): Integrating phenotypical and molecular data. Londrina: II International Symposium on Phylogeny and Classification of Neotropical Fishes; 2017.). Similarly, Roxo et al. (2019)Roxo FF, Ochoa LE, Sabaj MH, Lujan NK, Covain R, Silva GSC, Melo BF, Albert JS, Chang J, Foresti F, Alfaro ME, Oliveira C. Phylogenomic reappraisal of the Neotropical catfish family Loricariidae (Teleostei: Siluriformes) using ultraconserved elements. Mol Phyl Evol. 2019; 135:148–65. https://doi.org/10.1016/j.ympev.2019.02.017
https://doi.org/10.1016/j.ympev.2019.02....
, in a genomic phylogenetic analysis of the loricariids using ultraconserved elements, found H. acuen, H. chromodontus, Parotocinclus aripuanensis (= P. britskii), and an undescribed Parotocinclus from the Amazon to cluster in a clade, which they termed “New Genus 2”. Despite few species having been included in both studies, members of the four species-groups were analyzed and clustered as a monophyletic group separated from Parotocinclus, supporting the need for a new genus name. There are currently different initiatives underway to resolve more in depth the phylogenetic relationships of the Hypoptopomatinae and the Loricariidae as a whole, and they include all or most species of Rhinotocinclus.
The Greater Amazon, or the Amazon, Orinoco and Guianan coastal basins, is home to few other groups of Hypoptopomatinae. The most conspicuous and typical of the region is the tribe Hypoptopomatini, a well supported clade composed of Acestridium, Hypoptopoma, Leptotocinclus, Nannoptopoma, Nannoxyropsis, Niobichthys, Otocinclus, and Oxyropsis (Delapieve et al., 2017Delapieve MLS, Lehmann A. P, Reis RE. An appraisal of the phylogenetic relationships of Hypoptopomatini cascudinhos with description of two new genera and three new species (Siluriformes: Loricariidae). Neotrop Ichthyol. 2017; 15(4):e170079. https://doi.org/10.1590/1982-0224-20170079
https://doi.org/10.1590/1982-0224-201700...
). In addition to the Hypoptopomatini, members of a clade composed of Corumbataia and Curculionichthys are also found in Amazon tributaries draining the north and northwestern versants of the Brazilian Shield, in addition to the upper Paraná and São Francisco rivers. Rhinotocinclus is diagnosed from all these genera by having the canal cheek plate on the ventral surface of the head posteriorly elongated and contacting the cleithrum, compared to the canal cheek plate rounded or mesially elongated and not expanded backwards to contact the pectoral girdle. Rhinotocinclus is also distinguished from the Hypoptopmatini by having the preopercle exposed and bearing part of the mandibular branch of the laterosensory canal, as opposed to the preopercle not exposed at the surface and not bearing laterosensory canal. From Corumbataia, it is distinguished by the V-shaped dorsal-fin spinelet, which is rounded or absent in Corumbataia, and the much slender and delicate aspect of Rhinotocinclus species compared to the robust aspect of Corumbataia species. Finally, Rhinotocinclus is easily separated from Curculionichthys by the single rostral plate, opposed to the paired rostral plate of the later and less numerous lateral abdominal plates (2–5), compared to 5–8 in Curculionichthys. One exception to this last character are the members of the R. collinsae group, which may have up to eight lateral abdominal plates.
With the removal of Rhinotocinclus acuen, R. bockmanni, R. chromodonus, R. dinizae, and R. jumaorum from Hisonotus, this later genus becomes absent from the Amazon basin. Remaining species currently assigned to Hisonotus from outside the lower La Plata basin and coastal drainages between Uruguay and Espírito Santo State of Brazil are most probably members of Otothyropsis. The only exception is H. bocaiuva from the rio Jequitaí, rio São Francisco basin in Minas Gerais, which possibly represents a lineage of Parotocinclus wihout an adipose fin. This hypothesis, as well as the validity of Otothyropsis as a separate clade from Hisonotus, will have to be tested by future phylogenetic analyses.
Comparative material examined. Argentina.Hisonotus maculipinnis: MCP 48067, 2 cs, Laguna El Rey, Río Salado basin, Santa Fé. Bolivia. Otocinclus vestitus: CBF 3945, 4 + 1 cs, small creek on right margin of Río Nareuda, ca. 5 km of Río Tahuamano mouth, Río Beni basin, Nicolás Suarez, Pando. Brazil. Plesioptopoma curvidens: MCP 36861, 8 + 2 cs paratypes, MNRJ 21426, 3 +1 cs paratypes, rio Paraopeba at old bridge, ca. 100 m downstream of road BR-040, Cristiano Otoni, Minas Gerais. Pseudotocinclus juquiae: MCP 45129, 2 + 2 cs, creek on farm Estio, on road from Santa Rita to Juquitiba, ca 8 km S of Santa Rita, Juquitiba, São Paulo. LBP 1160, 5 + 1 cs [voucher 9664], rio Ribeira de Iguape at Fazenda Estio, Juquitiba, São Paulo. Pseudotocinclus parahybae: MCP 45094, 2 + 1 cs, creek tributary to rio Ribeirão Grande on farm São Sebastião do Ribeirão Grande, Pindamonhangaba, São Paulo. MZUSP 47581, 1 cs paratype, ribeirão Grande, near farm São Sebastião do Ribeirão Grande, Pindamonhangaba, São Paulo. Pseudotocinclus tietensis: MCP 20090, 2 + 1 cs, riacho Paraitinguinha on road from Salesópolis to Jacareí, ca. 3 km N of Salesópolis, Salesópolis, São Paulo. MCP 20111, 9 + 2 cs, rio Taiaçupeba near to power station Tijuco Preto at Taiaçupeba, Mogi das Cruzes, São Paulo. Corumbataia canoeiro: MCP 54366, 12 + 2 cs, creek tributary to rio das Almas, tributary to rio Paranã, Cavalcante, Goiás. Corumbataia cuestae: LBP 1309, 47 + 3 cs, córrego da Jacutinga, tributary to Rio Tietê, Bofete, São Paulo. Corumbataia tocantinensis: MCN 13462, 21 + 3 cs, córrego Moquém on road between Colinas do Sul and Cavalcante at Chapada dos Veadeiros National Park, Cavalcante, Goiás. Corumbataia veadeiros: MCP 41477, 5 + 3 cs paratypes, creek on km 2 of road GO-241, tributary to rio Ribeirão dos Bois, Teresina de Goiás, Goiás. MCP 54376, 8 + 2 cs, córrego do Elói on road GO-241 ca. 2 km W of Teresina de Goiás, Teresina de Goiás, Goiás. Gymnotocinclus anosteos: MCP 41726, 5 + 3 cs paratypes, ribeirão das Cobras, near road GO-327, Alto Paraiso de Goiás, Goiás. Curculionichthys coxipone: MCP 49998, 8 + 1 cs, córrego Piraputanga on farm Piraputanga, Diamantino, Mato Grosso. Curculionichthys insperatus: MCP 47070, 5 + 3 cs, ribeirão Tamanduá, tributary to rio Verde near São Domingos Hydroelectric Power Plant, Água Clara, Mato Grosso do Sul. Curculionichthys itaim: MCP 51569, 18 + 1 cs, cachoeira of rio Itapacurú between Miritituba and Pimental, Itaituba, Pará. Curculionichthys luteofrenatus: MCP 32665, 3 + 1 cs, creek tributary to rio Celeste ca. 47 km NW of Nova Ubiratã, on road to Sorriso, Sorriso, Mato Grosso. MCP 32670, 9 + 1 cs, igarapé Ribeirão Preto, on road MT-338, ca. 26 km ESE of Porto dos Gaúchos, Porto dos Gaúchos, Mato Grosso. Curculionichthys sagarana: MCP 47133, 13 + 1 cs, ribeirão Arrenegado on road BR-364 between Guarda-Mor and Bunti Grande, Guarda-Mor, Minas Gerais. Curculionichthys scaius: MCP 53801, 16 + 3 cs paratypes, creek tributary to rio Aripuanã, Aripuanã, Mato Grosso. Otothyris lophophanes: DZSJRP 13069, 48 + 1 cs, creek tributary to rio Macaé, Macaé, Rio de Janeiro. Otothyris travassosi: MCP 18105, 28 + 2 cs paratypes, rio Braço Norte, tributary to rio São Mateus, on road ES-130 near Boa Esperança, Boa Esperança, Espírito Santo. Pseudotothyris ignota: MCP 20030, 5 + 1 cs paratypes, artificial ditches in Guaraguaçú by the road PR-407, 7 km SE of rio da Vila, Paranaguá, Paraná. Pseudotothyris obtusa: MCP 31728, 9 + 2 cs, creek tributary to rio Preto [or Branco] ca. 2 km of State Airport, Itanhaém, São Paulo. Schizolecis guentheri: MCP 31558, 100 + 3 cs, rio São Roque on road BR-101 near to Tarituba, Parati, Rio de Janeiro. Parotocinclus adamateus: MCP 54151, 4 + 3 cs paratypes, ribeirão de Baixo near its mouth in rio São José, Lençóis, Bahia. MCP 54160, 1 cs paratype, rio Garapa, tributary to rio São José‚ ca. 600m upstreat from road from Lençóis to Andaraí, Andaraí, Bahia. Parotocinclus cabessadecuia: MCP 54409, 40 +2 cs, rio Gurguéia, near to Macaco Village, São Gonçalo do Gurguéia, Bahia. Parotocinclus cesarpintoi: MCP 30562, 9 +2 cs, rio Ipojuca near mouth of rio do Brejo, at Vila dos Pilões, Primavera, Pernambuco. Parotocinclus cristatus: MCP 36813, 10 + 2 cs, creek tributary to rio da Fazenda, itself a tributary to rio Peruípe, Caravelas, Bahia. MCP 18116, 31 + 2 cs, creek tributary to rio Almada in Duas Barras, on road from Coaraci to Almadina, Coaraci, Bahia. Parotocinclus doceanus: MCP 18084, 14 + 4 cs, rio Mucuri in Mayrink, ca 16 km W of Nanuque, 0,5 km upstram from bridge of road BR-418, Mayrink, Minas Gerais. Parotocinclus fluminense: MCP 20077, 4 cs, rio do Salto, ca. 14 km NW of Rio Dourado, tributary to rio Macaé, Macaé, Rio de Janeiro. Parotocinclus haraldoi: MNRJ 11383, 15 + 3 cs, córrego do Otaviano, Poço do Sanharé, Piauí. Parotocinclus jequi: MCP 44933, 11 + 1 cs, ribeirão Correntes in Mandaçaia, Leme do Prado, Minas Gerais. MCP 49171, 5 + 2 cs, creek at Novo Peixe Cru, Minas Gerais. Parotocinclus jimi: MCP 33329, 3 + 1 cs, rio Preto do Criciuma, tributary to rio de Contas between Jequi and Jitauna, Jequi, Bahia. Parotocinclus jumbo: MCP 31107, 84 + 7 cs, rio Ipanema in Batalha, tributary to rio São Francisco, Batalha, Alagoas. Parotocinclus maculicauda: MCP 17605, 3 + 2 cs, rio Cubatão near São Bonifácio, on road SC-431, vila Queçaba, Águas Mornas, Santa Catarina. Parotocinclus minutus: MCP 40034, 8 + 2 cs, rio Vaza Barris, at APA Serra Branca, Raso da Catarina, Jeremoabo, Bahia. Parotocinclus planicauda: MCP 31317, 8 + 2 cs, córrego Limoeiro in Praça Oito, tributary to rio Doce, Itarana, Espírito Santo. Parotocinclus prata: MCP 28322, 18 + 1 cs, córrego Altinas, ca. 10 km from Presidente Olegário on road form Presidente Olegário to Galena, Presidente Olegário, Minas Gerais. Parotocinclus robustus: MCP 16746, 7 + 1 cs paratypes, creek 45 km S of Montes Claros on road BR-135 towards Bocaiúva, Bocaiúva, Minas Gerais. MCP 36847, 9 + 2 cs paratypes, córrego Diamante on road between Buriti Grande and road BR-135, Francisco Dumont, Minas Gerais. Parotocinclus seridoensis: MCP 31463, 18 + 2 cs, rio Piranhas on road BR-230, between Pombal and Sousa, ca. 5 km from Pombal, Pombal, Paraíba. Parotocinclus spilosoma: MCP 39165, 425 + 4 cs, rio Salgado, tributary to rio Jaguaribe, Jardim, Ceará. AUM 20581, 26 + 2 cs, rio Curu on road BR-222, São Luiz do Curu, Ceará. Chauliocheilos saxatilis: MCP 49308, 11 + 1 cs, creek tributary to rio Itamarandiba in Retiro da Serra, Itamarandiba, Minas Gerais. Rhinolekos britskii: DZSJRP 6884, 2 paratypes, rio Corumbá in Fazenda Arapuca, ca. 25 km from road GO-020, Bela Vista de Goiás, Goiás. Rhinolekos capetinga: UFRGS 9957, 12 + 2 cs, creek on road GO-118 between São João da Aliança and Planaltina de Goiás, Planaltina de Goiás, Goiás. Rhinolekos garavelloi: MCP 47112, 19 + 1 cs, creek on Farm Lageado, near road GO-213, Caldas Novas, Goiás. Rhinolekos schaeferi: MCP 44056, 10 + 2 cs paratypes, creek on farm Fernanda, Alexânia, Goiás. Microlepidogaster arachas: MCP 28319, 16 + 3 cs paratypes, córrego Grande on road from Davinópolis to the rio Paranaíba ferry, ca. 1 km of Davinópolis, Davinópolis, Goiás. MCP 28333, 4 + 1 cs paratypes, creek on road from Ibi to Argenita, rio Quebra Anzol basin, Ibi, Minas Gerais. Microlepidogaster dimorpha: DZSJRP 10543, 17 + 1 cs paratype, riacho Grotão on road BR-262, farn Nossa Senhora da Abadia, Uberaba, Minas Gerais. Microlepidogaster discontenta: UFRGS 9877, 3 + 1 cs paratype, córrego Arrependido on road BR-251, Unaí, Minas Gerais. Microlepidogaster discus: DZSJRP 19493, 16 + 2 cs, creek on dust road from Itacambira to Botumirim, rio Jequitinhonha basin, Botumirim, Minas Gerais. Microlepidogaster longicolla: MCP 23323, 13 + 5 cs paratypes, ribeirão Santana, tributary to rio São João on road from Cidade Ocidental to Brasília, Brasília, Distrito Federal. Microlepidogaster negomata: DZSJRP 21003, 1 cs paratype, ribeirão Bebedouro near road to Chaves, upstream of Arapuá, Arapuá, Minas Gerais. Microlepidogaster perforatus: MCP 17717, 5 + 1 cs, rio Carandaí, tributary to rio Grande, upstream Carandaí, Carandaí, Minas Gerais. ANSP 17471, 1 + 1 cs, rio Carandaí upstream Carandaí, Minas Gerais. Otothyropsis alicula: MCP 23500, 10 + 2 cs paratypes, rio Santo Antonio, tributary to rio Sapucaí, itself a tributary to rio Grande, Delfim Moreira, Minas Gerais. MNRJ 23957, 59 + 4 cs paratype, rio Santo Antônio at Água Limpa, near mouth of ribeirão Salto, Delfim Moreira, Minas Gerais. Otothyropsis biamnicus: MCP 37164, 5 + 1 cs paratypes, rio dos Patos on road PR-427 between Lapa and Campo do Tenente, Lapa, Paraná. Otothyropsis marapoama: MCP 42119, 1 cs, rio Boa Esperança, tributary to rio Jacaré Guaçu, Gavião Peixoto, São Paulo. MCP 38303, 9 +1 cs paratypes, córrego Cubatão at Sítio Cubatão, Catanduva, São Paulo. Otothyropsis polyodon: MCP 45756, 4 +1 cs paratypes, rio Verde at mouth of Ribeirão Tamanduá, Ribas do Rio Pardo, Mato Grosso do Sul. MCP 47038, 8 + 1 cs paratypes, creek tributary to rio Verde on road from Mutum to São Domingos Hydropower Plant, Água Clara, Mato Grosso do Sul. Hisonotus aky: MCP 41474, 37 + 3 cs, rio Forquilha at Balneário do Espraiado, on secondary road from Maximiliano de Almeida to Paim Filho, Paim Filho, Rio Grande do Sul. Hisonotus alberti: MCP 17019, 13 + 2 cs, rio Poções in São Sebastião dos Poções, ca. 11 km S of Montalvânia, Montalvânia, Minas Gerais. Hisonotus armatus: MCP 25458, 7 + 3 cs, arroio Corup on road between Agudo and Dona Francisca Hydropower Plant, Agudo, Rio Grande do Sul. Hisonotus bocaiuva: MCP 16740, 17 + 1 cs, creek 45 km S of Montes Claros on road BR-135 towards Bocaiúva, Bocaiúva, Minas Gerais. Hisonotus brunneus: MCP 22701, 27 + 3 cs paratypes, rio Passo Novo at exit from Cruz Alta tu Ibirubá, Cruz Alta, Rio Grande do Sul. Hisonotus carreiro: MCP 40945, 3 + 2 cs paratypes, rio Carreiro downstream of Balneário Carreiro, Serafina Corrêa, Rio Grande do Sul. Hisonotus charrua: MCP 27539, 4 + 2 cs, arroio do Tigre on road from/Ijucapirama to road BR-453, ca. 2.5 km NE of road BR-453, Jaguari, Rio Grande do Sul. Hisonotus depressicauda: MZUSP 103693, 4 + 1 cs, rio Grande on dust road between Araçauva and Campo Grande, Santo André, São Paulo. Hisonotus francirochai: MCP 34630, 1 + 1 cs, córrego José Mendes, tributary to rio São João, Fortaleza de Minas, Minas Gerais. Hisonotus heterogaster: MCP 41073, 5 + 2 cs paratypes, arroio Felício on road from Nova Palma to Júlio de Castilhos, Júlio de Castilhos, Rio Grande do Sul. Hisonotus iota: MCP 40029, 18 + 3 cs paratypes, rio Chapecó near vila São Miguel, Coronel Freitas, Santa Catarina. Hisonotus laevior: MCP 37684, 58 + 4 cs, arroio Arambaré, ca. 5 km S of Vila Basílio, on road to Pedro Osório, Pedro Osório, Rio Grande do Sul. MCP 11521, 2 cs, rio Jacuí, Rio Grande do Sul. Hisonotus leucofrenatus: MCP 11540, 60 + 3 cs, rio Cubatão, near road BR-101, Joinville, Santa Catarina. Hisonotus leucophrys: MCP 41354, 3 + 2 cs paratypes, rio Ariranhas on road SC-466, Xavantina, Santa Catarina. Hisonotus megaloplax: MCP 31779, 9 + 3 cs paratypes, rio Passo Fundo downstream form Corsan Dam, Passo Fundo, Rio Grande do Sul. Hisonotus montanus: MCP 41459, 16 + 3 cs paratypes, rio Rufino on road SC-427 at town Ro Rufino, Rio Rufino, Santa Catarina. Hisonotus nigricauda: MCP 40761, 11 + 3 cs, arroio Banhado Grande on road BR-153 from Bagé to Caçapava do Sul, Bagé, Rio Grande do Sul. MCP 26865, 89 + 3 cs, arroio do Salso on road BR 158, tributary to rio Ibicuí da Armada, Rosário do Sul, Rio Grande do Sul. Hisonotus notatus: MNRJ 38147, 40 + 1 cs, rio Maratuá, São Gonçalo, Rio de Janeiro. MNRJ 13552, 24 + 3cs, rio São João at bridge downstream from mouth of rio Panelas, Silva Jardim, Rio de Janeiro. Hisonotus notopagos: MCP 25924, 1 + 2 cs paratypes, arroio da Mantiqueira, Lavras do Sul, Rio Grande do Sul. Hisonotus pachysarkos: NUP 5569, 26 + 4 cs paratypes, rio Barra Grande, Prudentópolis, Paraná. Hisonotus prata: MCP 22204, 9 + 3 cs paratypes, rio da Prata at Passo do Despraiado, between Guabiju and André da Rocha, Guabiju, Rio Grande do Sul. Hisonotus ringueleti: MCP 11215, 128 + 4 cs, arroio Quaraí-Mirim on road from Quaraí to Alegrete, Quaraí, Rio Grande do Sul. Hisonotus taimensis: MCP 17417, 29 + 3 cs, new channel of arroio Taim, Taim Ecological Station, Rio Grande, Rio Grande do Sul. Hisonotus thayeri: MCP 44806, 65 + 2 cs, arroio Santo Antonio towards Menino Jesus, tributary to rio Itapemirim, Muniz Freire, Espírito Santo. Hisonotus vespuccii: MCP 17153, 113 + 2 cs, creek in São João das Missões, on road between Itacarambi and Manga, Manga, Minas Gerais. Hisonotus vireo: MCP 14619, 4 + 3 cs, rio dos Sinos, ca. 5 km upstream Caraá, Caraá, Rio Grande do Sul. Hisonotus yasi: NUP 790, 2 cs, Caxias hydroelectric reservoir, Capitão Leônidas Marques, Paraná. Lampiella gibbosa: MCP 31588, 1 + 1 cs, rio Bonito, tributary to rio Pardo, Rio Bonito, Barra do Turvo, São Paulo. Eurycheilichthys apocremnus: MCP 25678, 10 + 2 cs paratypes, creek ca. 7 km N of Barros Cassal, tributary to rio Fão, Barros Cassal, Rio Grande do Sul. Eurycheilichthys castaneus: MCP 22134, 13 + 2 cs paratypes, arroio Burro Preto on road RS-324, between Passo Fundo and Marau, Marau, Rio Grande do Sul. Eurycheilichthys coryphaenus: MCP 40667, 1 cs paratype, creek tributary to rio Tainhas, ca. 4 km N of Tainhas, Tainhas, Rio Grande do Sul. Eurycheilichthys limulus: MCP 13086, 46 + 2 cs paratypes, rio Jacuí on road RS-324, between Marau and Passo Fundo, Passo Fundo, Rio Grande do Sul. Eurycheilichthys luisae: MCP 25566, 17 + 2 cs paratypes, arroio Jabuticaba near mouth at Jaboticaba, tributary to rio das Antas, Veranópolis, Rio Grande do Sul. Eurycheilichthys pantherinus: MCP 22373, 29 + 2 cs, rio Silveira in Silveira, at exit to Bom Jardim da Serra, São José dos Ausentes, Rio Grande do Sul. Eurycheilichthys paucidens: MCP 22800, 6 + 2 cs paratypes, arroio Tavoquá, near Farm Cambará, Muitos Capões, Rio Grande do Sul. Eurycheilichthys planus: MCP 22261, 41 + 2 cs paratypes, arroio Água Branca in Água Branca, ca. 20 km N of Nova Prata, Guabijú, Rio Grande do Sul. Eurycheilichthys vacariensis: MCP 22782, 17 + 2 cs paratypes, arroio Espeto [or rio dos Soares], on road from Muitos Capões to Vacaria, Muitos Capões, Rio Grande do Sul. Epactionotus bilineatus: MCP 29116, 25 + 3 cs, arroio Forqueta, tributary to rio Maquiné, Barra do Ouro, Maquiné, Rio Grande do Sul. UFRGS 4491, 18 + 2 cs, rio Maquiné and arroio do Ouro, between Maquiné and Barra do Ouro, Maquiné, Rio Grande do Sul. Epactionotus gracilis: MCP 11615, 15 + 2 cs paratypes, rio Jordão, Jordão Alto, rio Ararangua basin, Nova Veneza, Santa Catarina. Epactionotus itaimbezinho: MCP 14708, 12 + 3 cs paratypes, rio Canoas ca. 8 km of Praia Grande towards Mãe dos Homens, rio Mampituba basin, Praia Grande, Santa Catarina. Epactionotus advenus: MCP 54449, 4 + 2 cs paratypes, rio Rachadel and Small tributary creeks, rio Biguaçu basin, Antonio Carlos, Santa Catarina. Otocinclus flexilis: MCP 17414, 11 + 2 cs, arroio Itaeté, Passo das Pedras, Capão do Leão, Rio Grande do Sul. Otocinclus mura: MCP 22550, 17 + 2 cs, igarapé Urucure on road from Tomé Açu to Moju, ca. 49 km W of Tomé Açu, Tomé Açu, Pará. Otocinclus xakriaba: MCP 16879, 21 + 4 cs, rio Peru-Açu at Fabião, Januária, Minas Gerais. MCP 23506, 3 + 1 cs, rio Paraopeba dowstream Igarape Power Plant, Juatuba, Minas Gerais. Acestridium discus: MZUSP 85321, 2 cs, igarapé do Manu, Rio Preto da Eva, Amazonas. MZUSP 74275, 2 cs, igarapé Jaradá, tributary to rio Cuieiras, ca. 40 km from mouth, Manaus, Amazonas. Acestridium scutatum: MCP 37785, 10 + 2 cs paratypes, rio Traíra ca. 35 km E of rio Madeira on the Transamazon Road, Humaitá, Amazonas. Acestridium triplax: MPEG 12614, 7 + 1 cs, Juruti, Pará. Nannoxyropsis acicula: MCP 51462, 7 + 3 cs paratypes, rio Tapajós at Mamãe Anã Village, Jacareacanga, Pará. Leptotocinclus ctenistus: MCP 51457, 2 + 2 cs paratypes, igarapé São Sebastião, tributary to igarapé do Baré, lago Amanã basin, Maraã, Amazonas. Leptotocinclus madeirae: MCP 35888, 3 + 2 cs paratypes, igarapé do Vinte e Dois, Recanto do Sanari, ca. 20 km W of Humaitá, Humaitá, Amazonas. UFRO-I 15681, 11 + 1 cs paratypes, rio Realidade on road BR-319, at Realidade Village, ca. 100 km N of Humaitá, Amazonas. Oxyropsis carinata: MCP 30632, 1 cs, rio Solimões, lago Capivara at Costa das Capivaras, Tefé, Amazonas. Nannoptopoma sternoptychum: MCP 46940, 5 + 1 cs, rio Solimões, south margin of ilha Panamim, Tefé, Amazonas. Hypoptopoma elongatum: MPEG 27055, 3 + 1 cs, Vila Penedo, Jacareacanga, Pará. Hypoptopoma gulare: MCP 25489, 4 + 1 cs, rio Peritoró at Peritoró, Peritoró, Maranhão. Hypoptopoma inexspectatum: MCP 15744, 22 + 2 cs, rio Paraguai at Cáceres and neighborhoods, Cáceres, Mato Grosso. Hypoptopoma thoracatum: MCP 35872, 122 + 3 cs, igarapé Mapinguari on road BR-364, Bujari, Acre. Colombia. Leptotocinclus ctenistus: MCP 51461, 2 + 2 cs paratypes, Quebrada Tacana, tributary to Río Amazonas, km 6.5 of road from Letícia to Tarapacá, Leticia, Amazonas. Guyana. Oxyropsis ephippia: MCP 49017, 1 + 1 cs paratypes, Essequibo River, approx. 3h upstream of Kurupukari basecamp, Potaro-Siparuni. Paraguay. Otothyropsis dialeukos: MCP 49901, 5 + 1 cs paratypes, Arroyo Itá, ca. 30 m upstream bridge at Paso Itá, Hernandarias, Alto Parana. Otothyropsis piribebuy: MCP 44394, 22 + 3 cs paratypes, Arroyo Piribebuy, Eusebio Ayala, Cordillera. Peru. Hypoptopoma incognitum: MCP 46393, 2 + 1 cs, Cocha Soledad at lodge Soledad in Río Las Piedras, Río Madre de Dios drainage, Madre de Dios. Nannoptopoma spectabile: MCP 41467, 2 + 1 cs, upper Río Tapiche, Sierra del Divisor Reserve, Requena, Loreto. Otocinclus cocama: MCP 34842, 9 + 2 cs paratypes, Quebrada Yanayacu, tributary to Cocha Supay, Jenaro Herrera, Loreto. Oxyropsis carinata: MCP 26208, 12 + 1 cs, Caño Yarina at second security station (INRENA), tributary to Río Pacaya, Pacaya-Samiria National Reserve, Maynas, Loreto. Oxyropsis wrightiana: MCP 34503, 24 + 3 cs, Río Pacaya, Lago Tamara, Loreto. Uruguay. Otothyris rostrata: MCP 36786, 3 + 1 cs, creek tributary to Río Cebollati, Maldonado. Venezuela. Acestridium martini: MCP 35015, 4 + 1 cs, Río Sipapo, Pendare, Amazonas. Niobichthys ferrarisi: MCP 34810, 3 + 1 cs, Río Baria, ca. 200 m upstream basecamp La Neblina, Amazonas.
ACKNOWLEDGEMENTS
The studies to write this paper were developed during the last two decades and we are grateful to many colleagues and institutions. We are thankful to Carlos and Margarete Lucena for their continued help and support at MCP, and to Edson Pereira and Barbara Calegari for many years of fieldwork collecting cascudinhos and discussing their systematics. For donating or sending specimens on loan, or providing information on specimens or localities, we are much indebted to following colleagues and institution (in alphabetical order of names and institutions): Barbara Brown and Scott Schaefer (AMNH), Mariangeles Arce, John Lundberg and Mark Sabaj (ANSP), Jon Armbruster and Dave Werneke (AUM), James Maclaine (BMNH), Soraya Barrera (CBF), Francisco Langeani (DZSJRP), Barry Chernoff and Mary Ann Rogers (FMNH), Maria Elina Bichuette (IBUSP), John Lynch and Ivan Mojica (ICNMHN), Larry Page and Christopher Taylor (INHS), Lúcia Rapp Py-Daniel and Renildo Ribeiro (INPA), Michael Hardman (LACM), Cláudio Oliveira (LBP), Karsten Hartel (MCZ), Donald Taphorn (MHNG), Oscar Lasso (MHNLS), Santiago Ayerbe (MHNUC), Marcelo Britto and Paulo Buckup (MNRJ), Ângelo Dourado and Wolmar Wosiacki (MPEG), Fernando Jerep (MZUEL), Stefano Vanni (MZUF), Aléssio Datovo, Michel Gianeti, Osvaldo Oyakawa, Mário de Pinna, and Vinicius Reis (MZUSP), Helmut Wellendorf (NMW), Sven Kullander (NRM), Esther Dondorp and Martien van Oijen (RMNH), Mary Burridge, Mariano Galati, Erling Holm, Nathan Lujan, and Don Stacey (ROM), Carla Pavanelli (UEM), Robert H. Robins (UF), Hugmar Pains da Silva (UFMT), Luiz R. Malabarba and Juliana Wingert (UFRGS), Aline Andriolo, Carolina Doria, and William Ohara (UFRO), Hernan López-Fernández and Randy Singer (UMMZ), Cristiano Moreira (UNIFESP), Alessandra Bono (UNISINOS), Isaäc Isbrücker (formerly ZMA), Karina Gomes and Flavio Lima (ZUEC). We thank Juliano Romanzini for help with microphotography and lab assistance, and Nathan Lujan and Daniel Konn-Vetterlein for the photos of live specimens of Rhinotocinclus eppleyi and R. isabelae, respectively. We thank Neil Woodward of Pier Aquatics, Wigan, UK, for calling our attention to and donating the first specimens of R. isabelae. Funding for fieldwork yielding the holotype and 23 paratypes of Rhinotocinclus discolor provided by grants to Nathan K. Lujan from the Coypu Foundation and National Geographic Committee for Research and Exploration (grant 8721–09). RER and PLA are partially funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq grants #306455/2014–5 and #400166/2016-0 to RER and # 483060/2013–5 and 312112/2021–1 to PLA).
REFERENCES
- Albert JS, Petry P, Reis RE. Major biogeographic and phylogenetic patterns. In: Albert JS, Reis RE, editors. Historical biogeography of Neotropical freshwater fishes. California: University of California Press; 2011. p.21–57. https://doi.org/10.1525/california/9780520268685.001.0001
» https://doi.org/10.1525/california/9780520268685.001.0001 - Boeseman M. The genus Hypostomus Lacépède, 1803, and its Surinam representatives (Siluriformes, Loricariidae). Zool Verhandel. 1968; 99:1–89.
- Boeseman M. On two Surinam species of Hypoptopomatinae, both new to science (Loricariidae, Siluriformes, Ostariophysi). Proc Konin Nederl Akad Wetensch. Ser. C, Biol Med Scienc. 1974; 77(3):251–71.
- Britski HA, Garavello JC. Description of two new sympatric species of the genus Hisonotus Eigenmann and Eigenmann, 1889, from upper rio Tapajós, Mato Grosso State, Brazil (Pisces: Ostariophysi: Loricariidae). Braz J Biol. 2007; 67(3):631–37. https://doi.org/10.1590/S1519-69842007000300005
» https://doi.org/10.1590/S1519-69842007000300005 - Calegari BB, Delapieve MLS, Sousa LM. Tutorial para preparação de mapas de distribuição geográfica. Bol Soc Bras Ictiol. 2016; 118:15–30. Available from: https://www.sbi.bio.br/images/sbi/boletim-docs/2016/junho_118.pdf
» https://www.sbi.bio.br/images/sbi/boletim-docs/2016/junho_118.pdf - Calegari BB, Gamarra SP, Reis RE. A new species of Curculionichthys (Siluriformes: Hypoptopomatinae) from the western border of the Brazilian Shield, Madeira River basin, Brazil. Copeia. 2018; 106(4):663–70. https://doi.org/10.1643/CI-18-133
» https://doi.org/10.1643/CI-18-133 - Calegari BB, Morlis WG, Reis RE. A new species of Otothyropsis (Siluriformes: Loricariidae) from the upper Río Paraná basin, Paraguay, with a discussion of the limits between Otothyropsis and Hisonotus. Zootaxa. 2017; 4244(2):231–45. https://doi.org/10.11646/zootaxa.4244.2.5
» https://doi.org/10.11646/zootaxa.4244.2.5 - Carvalho M, Datovo A. A new species of cascudinho of the genus Hisonotus (Siluriformes: Loricariidae: Hypoptopomatinae) from the upper Rio Tapajós basin, Brazil. Copeia. 2012; 2012(2):266–75. https://doi.org/10.1643/CI-11-016
» https://doi.org/10.1643/CI-11-016 - Cramer CA, Bonatto SL, Reis RE. Molecular phylogeny of the Neoplecostominae and Hypoptopomatinae (Siluriformes: Loricariidae) using multiple genes. Mol Phyl Evol. 2011; 59(1):43–52. https://doi.org/10.1016/j.ympev.2011.01.002
» https://doi.org/10.1016/j.ympev.2011.01.002 - Dagosta FCP, de Pinna MCC. The fishes of the Amazon: distribution and biogeographical patterns, with a comprehensive list of species. Bull Am Mus Nat Hist. 2019; 431:1–163. https://doi.org/10.1206/0003-0090.431.1.1
» https://doi.org/10.1206/0003-0090.431.1.1 - Delapieve MLS, Lehmann A. P, Reis RE. An appraisal of the phylogenetic relationships of Hypoptopomatini cascudinhos with description of two new genera and three new species (Siluriformes: Loricariidae). Neotrop Ichthyol. 2017; 15(4):e170079. https://doi.org/10.1590/1982-0224-20170079
» https://doi.org/10.1590/1982-0224-20170079 - Dias AC, Silva GSC, Oliveira C, Roxo FF. A new species of Hisonotus (Siluriformes: Loricariidae) from Aripuanã river, Amazon basin, Brazil. Zootaxa. 2018; 4504(4):577–85. https://doi.org/10.11646/zootaxa.4504.4.8
» https://doi.org/10.11646/zootaxa.4504.4.8 - Gamarra SP, Calegari BB, Reis RE. A new species of Curculionichthys (Siluriformes: Loricariidae) from the north edge of the Brazilian Shield, lower Amazon basin. Neotrop Ichthyol. 2019; 17(2):e190001. https://doi.org/10.1590/1982-0224-20190001
» https://doi.org/10.1590/1982-0224-20190001 - Garavello JC. Systematics and geographical distribution of the genus Parotocinclus Eigenmann & Eigenmann, 1889 (Ostariophysii, Loricariidae). Arq Zool. 1977; 28(4):1–37. https://doi.org/10.11606/issn.2176-7793.v28i4p1-37
» https://doi.org/10.11606/issn.2176-7793.v28i4p1-37 - Garavello JC. Three new species of Parotocinclus Eigenmann & Eigenmann, 1889 with comments on their geographical distribution (Pisces, Loricariidae). Naturalia. 1988; 13:117–28.
- Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol Electron. 2001; 4(1):1–9. Available from: https://palaeo-electronica.org/2001_1/past/past.pdf
» https://palaeo-electronica.org/2001_1/past/past.pdf - Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio). Livro vermelho da fauna brasileira ameaçada de extinção. Brasília: ICMBio; 2018.
- International Commission on Zoological Nomenclature (ICZN). International Code of Zoological Nomenclature, Fourth Edition: adopted by the International Union of Biological Sciences. The International Trust for Zoological Nomenclature; 1999.
- International Union for Conservation of Nature (IUCN). Standards and petitions subcommittee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Gland; 2019. Available from: https://www.iucnredlist.org/resources/redlistguidelines
» https://www.iucnredlist.org/resources/redlistguidelines - Lehmann A P, Lazzarotto H, Reis RE Parotocinclus halbothi, a new species of small armored catfish (Loricariidae: Hypoptopomatinae), from the Trombetas and Marowijne River basins, in Brazil and Suriname. Neotrop Ichthyol. 2014; 12(1):27–33. https://doi.org/10.1590/S1679-62252014000100002
» https://doi.org/10.1590/S1679-62252014000100002 - Lehmann A P, Lima FCT, Reis RE Parotocinclus yaka, a new species of armored catfish (Loricariidae: Hypoptopomatinae), from the Amazon basin in Brazil. Zootaxa. 2018; 4521(4):584–92. https://doi.org/10.11646/zootaxa.4521.4.7
» https://doi.org/10.11646/zootaxa.4521.4.7 - Lehmann A P, Lujan NK, Reis RE. A new species of armored catfish (Loricariidae: Hypoptopomatinae) syntopic and superficially similar to Parotocinclus collinsae, from the Potaro River basin, Guyana. Ichthyol Herpetol. Forthcoming 2022.
- Lehmann A P, Reis RE. A new species of Parotocinclus (Siluriformes: Loricariidae) from the upper Rio São Francisco, Brazil. Zootaxa. 2012; 3390(1):56–64. https://doi.org/10.11646/zootaxa.3390.1.5
» https://doi.org/10.11646/zootaxa.3390.1.5 - Lehmann A P, Reis RE. A new species of the armored catfish Parotocinclus (Loricariidae: Hypoptopomatinae) from the upper Xingu River basin, Brazil. Ichthyol Herpetol. 2021; 109(2):449–55. https://doi.org/10.1643/i2021046
» https://doi.org/10.1643/i2021046 - Lehmann A P, Schvambach LJ, Reis RE. A new species of the armored catfish Parotocinclus (Loricariidae: Hypoptopomatinae), from the Amazon basin in Colombia. Neotrop Ichthyol. 2015; 13(1):47–52. https://doi.org/10.1590/1982-0224-20140113
» https://doi.org/10.1590/1982-0224-20140113 - Lima FCT, Ribeiro AC. Continental-scale tectonic controls of biogeography and ecology. In: Albert JS, Reis RE, editors. Historical biogeography of Neotropical freshwater fishes. California: University of California Press; 2011. p.145–64. https://doi.org/10.1525/california/9780520268685.003.0009
» https://doi.org/10.1525/california/9780520268685.003.0009 - Maldonado-Ocampo JA, Vari RP, Usma JS. Checklist of the freshwater fishes of Colombia. Biota Colombiana. 2008; 9(2):143–237. Available from: http://www.redalyc.org/articulo.oa?id=49120960001
» http://www.redalyc.org/articulo.oa?id=49120960001 - Mattox GMT, Toledo-Piza M, Oyakawa OT. Taxonomic study of Hoplias aimara (Valenciennes, 1846) and Hoplias macrophthalmus (Pellegrin, 1907) (Ostariophysii, Characiformes, Erythrinidae). Copeia. 2006; 2006(3):516–28. https://doi.org/10.1643/0045-8511(2006)2006[516:TSOHAV]2.0.CO;2
» https://doi.org/10.1643/0045-8511(2006)2006[516:TSOHAV]2.0.CO;2 - Miranda Ribeiro P. Um Paraotocinclus [sic] do Nordeste Brasileiro (Peixes-Larocaridae [sic]-Hypoptopomatinae). Bol Biol. 1939; 4(3):364–66.
- DoNascimiento C, Herrera-Collazos EE, Herrera-R GA, Ortega-Lara A, Villa-Navarro FA, Oviedo JSU, Maldonado-Ocampo JA. Checklist of the freshwater fishes of Colombia: a Darwin Core alternative to the updating problem. ZooKeys. 2017; 708:25–138. https://doi.org/10.3897/zookeys.708.13897
» https://doi.org/10.3897/zookeys.708.13897 - Pereira EHL, Reis RE. Morphology-based phylogeny of the suckermouth armored catfishes, with emphasis on the Neoplecostominae (Teleostei: Siluriformes: Loricariidae). Zootaxa. 2017; 4264(1):1–104. https://doi.org/10.11646/zootaxa.4264.1.1
» https://doi.org/10.11646/zootaxa.4264.1.1 - Reis RE. Systematic revision of the neotropical characid subfamily Stethaprioninae (Pisces, Characiformes). Comun Mus Ciênc PUCRS Sér Zool. 1989; 2(6):3–86.
- Reis RE, Calegari BB, Carvalho TP, Cramer CA, Delapieve MLS, Lehmann A. P, Pereira EHL. A phylogeny of the armored catfishes, with emphasis on the Neoplecostominae-Hypoptopomatinae clade (Siluriformes: Loricariidae): Integrating phenotypical and molecular data. Londrina: II International Symposium on Phylogeny and Classification of Neotropical Fishes; 2017.
- Reis RE, Lehmann A P. A new genus and five new species of cascudinhos of the subfamily Hypoptopomatinae (Siluriformes: Loricariidae) from northern South America. Minneapolis, USA: Abstracts of the 2011 meetings of the American Society of Ichthyologists and Herpetologists; 2011.
- Ribeiro-Silva LR, Silva GSC, Venere PC, Silva HP, Roxo FF. Description of a new species of Hisonotus (Loricariidae: Siluriformes) from rio Araguaia basin. Zootaxa. 2020; 4860(4):553–62. https://doi.org/10.11646/zootaxa.4860.4.5
» https://doi.org/10.11646/zootaxa.4860.4.5 - Roxo FF, Messias FL, Silva GSC. A new species of Parotocinclus (Loricariidae: Hypoptopomatinae) from rio Tocantins basin, Brazil. Zootaxa. 2019; 4646(2):346–56. https://doi.org/10.11646/zootaxa.4646.2.9
» https://doi.org/10.11646/zootaxa.4646.2.9 - Roxo FF, Ochoa LE, Sabaj MH, Lujan NK, Covain R, Silva GSC, Melo BF, Albert JS, Chang J, Foresti F, Alfaro ME, Oliveira C. Phylogenomic reappraisal of the Neotropical catfish family Loricariidae (Teleostei: Siluriformes) using ultraconserved elements. Mol Phyl Evol. 2019; 135:148–65. https://doi.org/10.1016/j.ympev.2019.02.017
» https://doi.org/10.1016/j.ympev.2019.02.017 - Roxo FF, Silva GSC, Oliveira C. Description of a new species of Parotocinclus (Siluriformes, Hypoptopomatinae) from the rio Tapajós basin. ZooKeys. 2016; 634:125–36. https://doi.org/10.3897/zookeys.634.9917
» https://doi.org/10.3897/zookeys.634.9917 - Sabaj MH. Codes for natural history collections in ichthyology and herpetology. Copeia. 2020; 108(3):593–669. https://doi.org/10.1643/ASIHCODONS2020
» https://doi.org/10.1643/ASIHCODONS2020 - Schaefer SA. A new species of the loricariid genus Parotocinclus from southern Venezuela (Pisces: Siluroidei). Copeia. 1988; 1988(1):182–88. https://doi.org/10.2307/1445936
» https://doi.org/10.2307/1445936 - Schaefer SA. The Neotropical cascudinhos: systematics and biogeography of the Otocinclus catfishes (Siluriformes: Loricariidae). Proc Acad Nat Sci Phila. 1997; 148:1–120. https://www.jstor.org/stable/4065046
» https://www.jstor.org/stable/4065046 - Schaefer SA, Provenzano F. The Guyana Shield Parotocinclus: systematics, biogeography, and description of a new Venezuelan species (Siluroidei: Loricariidae). Ichthyol Explor Freshw. 1993; 4(1):39–56.
- Schmidt RE, Ferraris Jr. CJ. A new species of Parotocinclus (Pisces: Loricariidae) from Guyana. Proc Biol Soc Wash. 1985; 98(2):341–46. Available from: https://www.biodiversitylibrary.org/page/34648620#page/365/mode/1up
» https://www.biodiversitylibrary.org/page/34648620#page/365/mode/1up - Silva GSC, Roxo FF, Oliveira FF Hisonotus acuen, a new and phenotypically variable cascudinho (Siluriformes, Loricariidae, Hypoptopomatinae) from the upper rio Xingu basin, Brazil. ZooKeys. 2014; 442:105–25. https://doi.org/10.3897/zookeys.442.7870
» https://doi.org/10.3897/zookeys.442.7870 - Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985; 9(2):107–19.
- Van der Sleen P, Albert JS. Field guide to the fishes of the Amazon, Orinoco, and Guianas. New Jersey: Princeton University Press; 2017.
ADDITIONAL NOTES
-
HOW TO CITE THIS ARTICLE
Reis RE, Lehmann A. P. A new genus of armored catfish (Siluriformes: Loricariidae) from the Greater Amazon, with a review of the species and description of five new species. Neotrop Ichthyol. 2022; 20(2):e220002. https://doi.org/10.1590/1982-0224-2022-0002
Edited-by
Publication Dates
-
Publication in this collection
08 July 2022 -
Date of issue
2022
History
-
Received
13 Jan 2022 -
Accepted
20 May 2022