ABSTRACT
The Amazonian ichthyofauna is one of the most diverse in the world, yet fishes from the adjacent coastal basins of Maranhão State in Northeastern Brazil remain poorly known. We use phylogeographic, community phylogenetic and phylogenetic beta diversity methods to study the biogeographic history of fishes from the coastal basins of Maranhão State. We report a total of 160 fish species from the basins of the Maranhão region, representing a 93% increase over results of previous studies. All the fish species assemblages from Maranhão are polyphyletic, with only a few putative sister species pairs inhabiting the region. The modern watershed divides among Maranhão basins do not form substantial barriers to dispersal for freshwater fish species, and are more effectively modelled as biogeographic islands than as biogeographic provinces. In combination these results suggest that the Maranhão ichthyofauna was assembled under the influence of several macroevolutionary (extinction, dispersal) and landscape evolution processes, during the Miocene and Pliocene, as well as by the modern ecological characteristics of the region. The results indicate that the distinctive geological and climatic conditions and history of Northeastern Brazil strongly constrained the formation of aquatic faunas in coastal basins of Maranhão State.
Keywords:
Biodiversity; Community phylogenetics; Paleogeography; Phylogenetic beta diversity; Phylogeography
RESUMO
A ictiofauna da Amazônia é uma das mais diversificadas do mundo, mas os peixes das bacias costeiras adjacentes do estado do Maranhão, no Nordeste do Brasil, ainda são pouco conhecidos. Utilizamos métodos filogeográficos, filogenia de comunidade e de diversidade beta filogenética para estudar a história biogeográfica de peixes das bacias costeiras do estado do Maranhão. Nós relatamos um total de 160 espécies de peixes das bacias da região do Maranhão, representando um aumento de 93% sobre os resultados de estudos anteriores. Todas as assembleias de espécies de peixes do Maranhão são polifiléticas, com apenas alguns supostos pares de espécies irmãs habitando a região. As divisões modernas das bacias hidrográficas do Maranhão não formam barreiras substanciais para a dispersão de espécies de peixes de água doce, e são mais efetivamente modeladas como ilhas biogeográficas do que como províncias biogeográficas. Em conjunto, esses resultados sugerem que a ictiofauna maranhense foi montada sob a influência de vários processos de evolução macroevolutiva (extinção, dispersão) e paisagística, durante o Mioceno e Plioceno, bem como pelas características ecológicas modernas da região. Os resultados indicam que as distintas condições geológicas e climáticas e a história do Nordeste do Brasil restringiram fortemente a formação de faunas aquáticas em bacias costeiras do estado do Maranhão.
Palavras-chave:
Beta diversidade filogenética; Biodiversidade; Comunidade filogenética; Filogeografia; Paleogeografia
Introduction
Relatively little is known about the fishes inhabiting coastal rivers of Maranhão State in Northeastern Brazil, mainly due to a lack of taxonomic and ecological studies (but see Piorski et al., 2007Piorski NM, Castro ACL, Sousa Neto AM. Peixes do cerrado da região sul maranhense. In: Barreto LN, editor. Cerrado Norte do Brasil. São Luís: USEB; 2007. p.177-212., 2017Piorski NM, Ferreira BRA, Guimarães EC, Ottoni FP, Nunes JLS, Brito PS. Peixes do Parque Nacional dos Lençóis Maranhenses. São Luis: Café & Lápis/Edufma; 2017.; Barros et al., 2011Barros MC, Fraga EC, Birindelli JLO. Fishes from the Itapecuru River basin, State of Maranhão, northeast Brazil. Braz J Biol. 2011; 71(2):375-80.; Ramos et al., 2014Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba River basin, northeastern Brazil. Biota Neotrop . 2014; 14(1):1-8.). Even less information has been published regarding the systematics, geographical variation and biogeography of the fishes of this region. To date no species lists have been published for the individual basins of the Maranhão region, and the region is frequently treated as a single geographic unit (e.g.Piorski et al., 1998Piorski NM, Castro ACL, Pereira LG, Muniz MEL. Ictiofauna do trecho inferior do rio Itapecuru, Nordeste do Brasil. B Lab Hidro. 1998; 11:15-24.; Rosa, 2003Rosa R. Diversidade e conservação dos peixes da Caatinga. In: Silva JMC, Tabarelli M, Fonseca MT, Lins LV, editors. Biodiversidade da caatinga: áreas e ações prioritárias para a conservação. Brasilia: Ministério do Meio Ambiente; 2003. p.149-162.; Soares, 2005Soares EC. Peixes do Mearim. São Luís: Instituto GEIA; 2005.; Barros et al., 2011Barros MC, Fraga EC, Birindelli JLO. Fishes from the Itapecuru River basin, State of Maranhão, northeast Brazil. Braz J Biol. 2011; 71(2):375-80.; Carvalho-Costa et al., 2011Carvalho-Costa LF, Piorski NM, Willis SC, Galetti PM, Ortí G. Molecular systematics of the neotropical shovelnose catfish genus Pseudoplatystoma Bleeker 1862 based on nuclear and mtDNA markers. Mol Phylogenet Evol. 2011; 59(1):177-94.). The river basins of Maranhão are sometimes combined with the Parnaíba basin (e.g.Rosa, 2003Rosa R. Diversidade e conservação dos peixes da Caatinga. In: Silva JMC, Tabarelli M, Fonseca MT, Lins LV, editors. Biodiversidade da caatinga: áreas e ações prioritárias para a conservação. Brasilia: Ministério do Meio Ambiente; 2003. p.149-162.; Ramos et al., 2014Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba River basin, northeastern Brazil. Biota Neotrop . 2014; 14(1):1-8.) that only partially occurs in Maranhão State. The fish diversity previously reported from Maranhão rivers includes 83 species in 65 genera, 31 families and 10 orders (Soares, 2005Soares EC. Peixes do Mearim. São Luís: Instituto GEIA; 2005.; Barros et al., 2011Barros MC, Fraga EC, Birindelli JLO. Fishes from the Itapecuru River basin, State of Maranhão, northeast Brazil. Braz J Biol. 2011; 71(2):375-80.).
This scarce knowledge of the Maranhão ichthyofauna is partially a consequence of the common misconception that this fauna is a subset of the lower Amazonas or Guianas rivers. Abell et al. (2008Abell R, Thieme ML, Revenga C, Bryer M, Kottelat M, Bogutskaya N, Coad B, Mandrak N, Contreras Balderas S, Bussing W, Stiassny MLJ, Skelton P, Allen GR, Unmack P, Naseka A, Ng R, Sindorf N, Robertson J, Armijo E, Higgins JV, Heibel TJ, Wikramanayake E, Olson D, López HL, Reis RE, Lundberg JG, Pérez MHS, Petry P. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. BioScience. 2008; 58(5):403-14.) contributed to this misperception by circumscribing the Maranhão region within the Amazonas Estuary and Coastal Drainages ecoregion (number 323). Depending on the approach used, the rivers of the Eastern Amazon are part of one or more distinct areas of endemism. Many studies have included the Eastern Amazon basins within the rest of Amazon (Géry, 1969Géry J. The freshwater fishes of South America. In: Fittkau EJ., Illies J, Klinge H, Schwabe GH, editors. Biogeography and ecology in South America. The Hague: Springer Netherlands; 1969. (Monographiae Biologicae, vol. 19). p.828-848.; Abell et al., 2008Abell R, Thieme ML, Revenga C, Bryer M, Kottelat M, Bogutskaya N, Coad B, Mandrak N, Contreras Balderas S, Bussing W, Stiassny MLJ, Skelton P, Allen GR, Unmack P, Naseka A, Ng R, Sindorf N, Robertson J, Armijo E, Higgins JV, Heibel TJ, Wikramanayake E, Olson D, López HL, Reis RE, Lundberg JG, Pérez MHS, Petry P. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. BioScience. 2008; 58(5):403-14.), or with Brazilian Northeastern basins (Vari, 1988Vari RP. The Curimatidae, a lowland neotropical fish family (Pisces: Characiformes): distribution, endemism and phylogenetic biogeography. In: Vanzolini PE, Heyer WR, editors. Proceedings of a workshop on Neotropical distribution patterns. Rio de Janeiro: Academia Brasileira de Ciências; 1988. p.343-377.). Some studies have considered these basins as independent areas (Lundberg et al., 1998Lundberg JG, Marshall LG, Guerrero J, Horton B, Malabarba MCSL, Wesselingh F. The stage for neotropical fish diversification: a history of tropical South American rivers. In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of Neotropical fishes. Porto Alegre: EDUPUCRS ; 1998. p.13-48.; Hubert, Renno, 2006Hubert N, Renno JF. Historical biogeography of South American freshwater fishes. J Biogeogr . 2006; 33(8):1414-36.; Dagosta, de Pinna, 2017Dagosta FCP, De Pinna M. Biogeography of Amazonian fishes: deconstructing river basins as biogeographic units. Neotrop Ichthyol .2017; 15(3): e170034, 2017. Available from: http://dx.doi.org/10.1590/1982-0224-20170034
http://dx.doi.org/10.1590/1982-0224-2017...
).
Géry (1969Géry J. The freshwater fishes of South America. In: Fittkau EJ., Illies J, Klinge H, Schwabe GH, editors. Biogeography and ecology in South America. The Hague: Springer Netherlands; 1969. (Monographiae Biologicae, vol. 19). p.828-848.) reported a stronger similarity between the Maranhão and Amazon ichthyofaunas, whereas Vari (1988Vari RP. The Curimatidae, a lowland neotropical fish family (Pisces: Characiformes): distribution, endemism and phylogenetic biogeography. In: Vanzolini PE, Heyer WR, editors. Proceedings of a workshop on Neotropical distribution patterns. Rio de Janeiro: Academia Brasileira de Ciências; 1988. p.343-377.) considered the Maranhão ichthyofauna a hybrid of the Northeastern and São Francisco rivers, grading to the Amazon river. Lundberg et al. (1998Lundberg JG, Marshall LG, Guerrero J, Horton B, Malabarba MCSL, Wesselingh F. The stage for neotropical fish diversification: a history of tropical South American rivers. In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of Neotropical fishes. Porto Alegre: EDUPUCRS ; 1998. p.13-48.) considered the Maranhão rivers a separate area, neither Amazon nor Northeastern, but a unique area, a conception followed by Hubert, Renno (2006Hubert N, Renno JF. Historical biogeography of South American freshwater fishes. J Biogeogr . 2006; 33(8):1414-36.) and Dagosta, de Pinna (2017Dagosta FCP, De Pinna M. Biogeography of Amazonian fishes: deconstructing river basins as biogeographic units. Neotrop Ichthyol .2017; 15(3): e170034, 2017. Available from: http://dx.doi.org/10.1590/1982-0224-20170034
http://dx.doi.org/10.1590/1982-0224-2017...
), with some differences in the area extents of these regions. It is however necessary to emphasize that none of these studies recognized a substantial number of species present in the Maranhão rivers, the largest number being 66 species in Dagosta, de Pinna (2017Dagosta FCP, De Pinna M. Biogeography of Amazonian fishes: deconstructing river basins as biogeographic units. Neotrop Ichthyol .2017; 15(3): e170034, 2017. Available from: http://dx.doi.org/10.1590/1982-0224-20170034
http://dx.doi.org/10.1590/1982-0224-2017...
).
The rivers of Maranhão State meander across a broad lowland coastal plain, a geography thought to have facilitated hydrological and faunal exchanges among the several basins through the capture of lateral tributaries and anastomosing river mouths (Huber, 1998Huber JH. Comparison of Old World and New World Tropical cyprinodonts. Paris: Societe francais de d’Ichthyologie; 1998.; Wilkinson et al., 2006Wilkinson MJ, Marshall LG, Lundberg JG. River behavior on megafans and potential influences on diversification and distribution of aquatic organisms.J South Am. Earth Sci. 2006; 21(1-2):151-72.). River or headwater capture changes the flow of water from one basin to another, expanding the receiver drainage and contracting the donor drainage (Christofoletti, 1975Christofoletti A. Enciclopédia Mirador Internacional. São Paulo: vol. 5; 1975. Capturas fluviais; p.2049-2051.; Oliveira, 2010Oliveira D. River capture as relief evolution evidences: a review. Rev Dep Geo. 2010; 20:37-50.). River capture has been hypothesized to help explain the observed fish species composition of the river basins of the Maranhão region (Piorski, 2010Piorski NM. Diversidade genética e filogeografia das espécies Hoplias malabaricus (Bloch, 1794) e Prochilodus lacustris Steindachner, 1907 no Nordeste do Brasil. [PhD Thesis]. São Carlos, SP: Universidade Federal de São Carlos; 2010.; Abreu, 2013Abreu JMS. Variação geográfica em Schizodon dissimilis (Garman, 1890) e diversidade genética e filogeográfica do grupo Schizodon fasciatus sensu lato (Characiformes: Anostomidae). [MSc Dissertation]. Belém, PA: Universidade Federal do Pará; 2013.). This continuous process, perennially connecting and separating portions of adjacent river basins, is thought to enhance biological diversity of obligate aquatic organisms, by increasing rates of dispersal and speciation, and reducing rates of extinction in some species (Albert, Crampton, 2010Albert JS, Crampton WGR. The geography and ecology of diversification in Neotropical freshwaters. Nature Education Knowledge. 2010; 3(10):13-19.; Albert, Reis, 2011Albert JS, Reis RE. Introduction to Neotropical freshwaters. In: Albert JS, Petry P, Reis RE, editors. Historical biogeography of Neotropical freshwater fishes. Berkeley, CA: University of California Press ; 2011. p.3-19.). Evidence for river capture in Maranhão rivers includes many elbows or abrupt changes in river courses (Piorski, 2010Piorski NM. Diversidade genética e filogeografia das espécies Hoplias malabaricus (Bloch, 1794) e Prochilodus lacustris Steindachner, 1907 no Nordeste do Brasil. [PhD Thesis]. São Carlos, SP: Universidade Federal de São Carlos; 2010.).
Quaternary eustatic sea-level changes are also thought to have affected the biogeography and biodiversity of river basins that drain across the Maranhão coastal plain. Successive marine transgressions (advances) and regressions (retreats) change coastlines and remodel coastal riverscapes (Dias et al., 2014Dias MS, Oberdorff T, Hugueny B, Leprieur F, Jézéquel C, Cornu JF, Brosse S, Grenouillet G, Tedesco PA. Global imprint of historical connectivity on freshwater fish biodiversity. Ecol Lett. 2014; 17(9):1130-40.; Hubert, Renno, 2006Hubert N, Renno JF. Historical biogeography of South American freshwater fishes. J Biogeogr . 2006; 33(8):1414-36.; Lovejoy et al., 2006Lovejoy NR, Albert JS, Crampton WGR. Miocene marine incursions and marine/freshwater transitions: Evidence from Neotropical fishes. J South Am Earth. 2006; 21(1-2):5-13.). Large marine transgressions into the Marajó and São Luís sedimentary basins occurred during late Miocene and Pliocene (11.6 - 2.6 Ma). These transient marine incursions resulted in shallow coastal seas known as ‘Mar de Pirabas’ (Soares Junior et al., 2011Soares Júnior AV, Hasui Y, Costa JBS, Machado FB. Evolução do rifteamento e paleogeografia da margem atlântica equatorial do Brasil: Triássico ao Holoceno. Geociências. 2011; 30(4):669-92.) and ‘Gulfo Maranhense’ (Ab’saber, 1960Ab’Saber AN. Contribuição à geomorfologia do Estado do Maranhão. Notícia Geomorfológica. 1960; 3(5):35-45.; Petri, Fúlfaro, 1983Petri AC, Fúlfaro VJ. Geologia do Brasil. São Paulo: Editora da Universidade Federal de São Paulo; 1983.), when sea-levels were up to 15 m above modern level, and the mouths of many coastal rivers were disconnected. At times of marine regression, as at the Last Glacial Maximum (LGM) c. 26.5 - 20.0 ka (Clark et al., 2009Clark PU, Dyke AS, Shakun JD, Carlson AE, Clark J, Wohlfarth B, Mitrovica JX, Hostetler SW, McCabe AM. The last glacial maximum. Science. 2009; 325(5941):710-14.), sea levels were as much as 122 m below modern level, and many rivers of the Maranhão coastal plain were connected at their mouths (Costa et al., 1997Costa JBS, Bemerguy RL, Hasui Y, Borges MDS, Júnior CRPF, Bezerra PL, Costa MLD, Fernandes JMG. Neotectônica da região amazônica: aspectos tectônicos, geomorfológicos e deposicionais. Geonomos. 1997; 4:23-44.; Piorski, 2010Piorski NM. Diversidade genética e filogeografia das espécies Hoplias malabaricus (Bloch, 1794) e Prochilodus lacustris Steindachner, 1907 no Nordeste do Brasil. [PhD Thesis]. São Carlos, SP: Universidade Federal de São Carlos; 2010.).
Here we provide a comprehensive study of fishes from the coastal basins of Maranhão State, with the goal to understand how landscape evolution and historical connections among rivers drives the formation of regional fish species assemblages. We provide the most complete list of fish species from the region to date, compare species richness and composition among basins, estimate the phylogenetic diversity of these faunas, and describe the faunal similarities of these rivers with those of the Amazon basin. We report a total of 160 fish species from the basins of the Maranhão region, representing 77 newly reported species, or a 93% increase over the greatest number previously reported. We conclude that past geological events and current ecological landscapes contribute to the modern patterns of fish species distributions in this region.
Material and Methods
Ichthyofauna composition. The survey of distributions of fish species inhabiting Maranhão State (Fig. 1) was conducted through examination of museum collections of Coleção de Peixes da Universidade Federal do Maranhão (CPUFMA), data accessed through species surveys published atlases, books and catalogs (Reis et al., 2003Reis RE, Kullander SO, Ferraris J, Carl J. Check List of the freshwater fishes of South and Central America. Porto Alegre: EDIPUCRS; 2003.; Soares, 2005Soares EC. Peixes do Mearim. São Luís: Instituto GEIA; 2005.; Buckup et al., 2007Buckup PA, Menezes NA, Ghazzi MS. Catálogo das espécies de peixes de água doce do Brasil. Rio de Janeiro: Museu Nacional, Universidade Federal do Rio de Janeiro; 2007. (Série Livros 23).; Lucinda et al., 2007Lucinda PHF, Freitas IS, Soares AB, Marques EE, Agostinho CS, Oliveira RJD. Fish, Lajeado reservoir, rio Tocantins drainage, state of Tocantins, Brazil. Check List . 2007; 3(2):70-83.; Soares et al., 2009Soares AB, Pelicice FM, Lucinda PHF, Akama A. Diversidade de peixes na área de influência da barragem de Peixe Angical, antes e após a formação do reservatório. In: Agostinho CS, Pelicice FM, Marques EE, editores. Reservatório de Peixe Angical: bases ecológicas para o manejo da ictiofauna. São Carlos: RiMa; 2009. p.15-27.; Mérona et al., 2010Mérona BJL, Juras AA, Santos GM, Cintra IHA. Os peixes e a pesca no baixo rio Tocantins: vinte anos depois da UHE Tucuruí. Belém: Eletronorte; 2010.; Barros et al., 2011Barros MC, Fraga EC, Birindelli JLO. Fishes from the Itapecuru River basin, State of Maranhão, northeast Brazil. Braz J Biol. 2011; 71(2):375-80.; Lima, Caires, 2011Lima FCT, Caires RA. Peixes da Estação Ecológica Serra Geral do Tocantins, bacias dos rios Tocantins e São Francisco, com observações sobre as implicações biogeográficas das “águas emendadas” dos rios Sapão e Galheiros. Biota Neotrop. 2011; 11(1):231-50.; Claro-García, Shibatta, 2013Claro-García A, Shibatta OA. The fish fauna of streams from the upper rio Tocantins basin, Goiás State, Brazil. Check List . 2013; 9(1):28-33.; Ramos et al., 2014Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba River basin, northeastern Brazil. Biota Neotrop . 2014; 14(1):1-8.; Melo et al., 2016Melo FAG, Buckup PA, Ramos TPA, Souza AKN, Silva CMA, Costa TC, Torres AR. Fish fauna of the lower course of the Parnaíba river, northeastern Brazil. Bol Mus Biol. Mello Leitão (N. Sér.). 2016; 38(4):363-400.; Bartolette et al., 2017Bartolette R, Vieira CS, Santos JFL, Santos CDC, Luduvice JSV, Passos TS, D’Avilla T, Nascimento BO, Ernesto D, Argolo FH, Aguiar AJM, Argolo F, Pereira MSA, Santos TF, Brito MFG. The ichthyofauna in the influence area of the Lajeado reservoir, Tocantins state, Brazil. Check List. 2017; 13(3):2156. Available from: http://dx.doi.org/10.15560/13.3.2156
http://dx.doi.org/10.15560/13.3.2156...
; Dagosta, de Pinna, 2017Dagosta FCP, De Pinna M. Biogeography of Amazonian fishes: deconstructing river basins as biogeographic units. Neotrop Ichthyol .2017; 15(3): e170034, 2017. Available from: http://dx.doi.org/10.1590/1982-0224-20170034
http://dx.doi.org/10.1590/1982-0224-2017...
; Piorski et al., 2017Piorski NM, Ferreira BRA, Guimarães EC, Ottoni FP, Nunes JLS, Brito PS. Peixes do Parque Nacional dos Lençóis Maranhenses. São Luis: Café & Lápis/Edufma; 2017.). We were unable to provide estimates of sampling completeness for these collection records, as the data necessary for rarefaction analysis are not available.
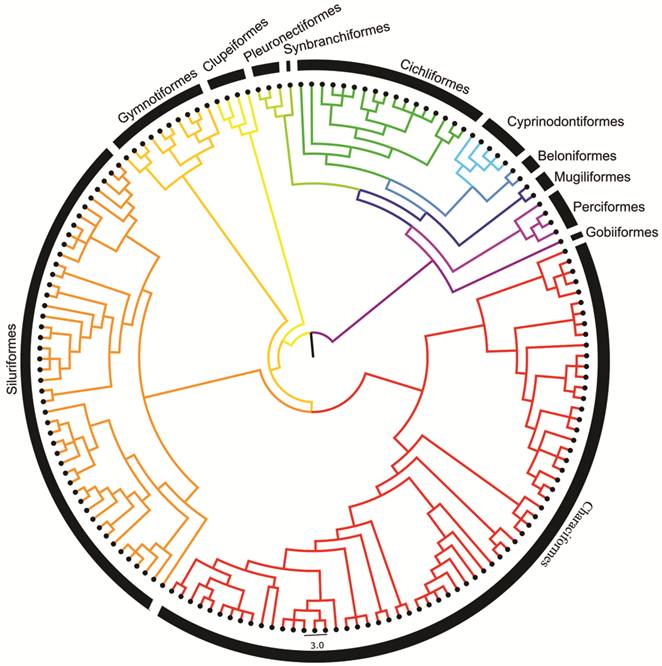
Community phylogenetic structure. Phylogenetic relationships among all 160 Maranhão fish species were estimated using a combination of published phylogenetic hypotheses following the methods of Aquino, Colli (2017Aquino PPU, Colli GR. Headwater captures and the phylogenetic structure of freshwater fish assemblages: a case study in central Brazil. J Biogeogr. 2017; 44(1):207-16.). Tree estimation was conducted in three steps. 1) The robust phylogeny of Betancur-R et al. (2013Betancur-R R, Richard E. Broughton, Wiley EO, Carpenter K, López JA, Li C, Holcroft NI, Arcila D, Sanciangco M, Cureton JC, Zhang F, Buser T, Campbell MA, Ballesteros JA, Roa-Varon A, Willis S, Borden WC, Rowley T, Reneau PC, Hough DJ, Lu G, Grande T, Arratia G, Orti G. The tree of life and a new classification of bony fishes. PLoS Curr Tree of Life. 2013:1-54.) generated from 20 nuclear genes and one (16S) mtDNA gene, was designated as a backbone. 2) Species found in the Maranhão region but absent from the backbone were manually added as polytomies with their closest present relatives. Published phylogenies were used to establish these relationships: Mirande (2010Mirande JM. Phylogeny of the family Characidae (Teleostei: Characiformes): From characters to taxonomy. Neotrop Ichthyol . 2010; 8(3):385-568.) for Characiformes; Bockmann (1998Bockmann FA. Análise filogenética da família Heptapteridae (Teleostei, Ostariophysi, Siluriformes) e redefinição de seus gêneros. [PhD Thesis]. São Paulo, SP: Universidade de São Paulo; 1998.), de Pinna (1998De Pinna MCC. Phylogenetic relationships of Neotropical Siluriformes (Teleostei: Ostariophysi): historical overview and synthesis of hypotheses. In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of Neotropical fishes. Porto Alegre: EDUPUCRS; 1998. p.279-330.), Albornoz (2006Albornoz PCL. Anatomia e relações filogenéticas da família Loricariidae (Ostariophysi: Siluriformes) com ênfase na subfamília Hypoptopomatinae. [PhD Thesis]. Porto Alegre, RS: Pontifícia Universidade Católica do Rio Grande do Sul; 2006.), Sullivan et al. (2006Sullivan JP, Lundberg JG, Hardman M. A phylogenetic analysis of the major groups of catfishes (Teleostei: Siluriformes) using rag1 and rag2 nuclear gene sequences. Mol Phylogenet Evol . 2006; 41(3):636-62.), Chiachio et al. (2008Chiachio MC, Oliveira C, Montoya-Burgos JI. Molecular systematic and historical biogeography of the armored Neotropical catfishes Hypoptopomatinae and Neoplecostominae (Siluriformes: Loricariidae). Mol Phylogenet Evol . 2008; 49(2):606-17.), Pereira (2008Pereira EHL. Relações filogenéticas de Neoplecostominae Regan, 1904 (Siluriformes: Loricariidae). [PhD Thesis]. Porto Alegre, RS: Pontifícia Universidade Católica do Rio Grande do Sul; 2008.), Cramer et al. (2011Cramer CA, Bonatto SL, Reis RE. Molecular phylogeny of the Neoplecostominae and Hypoptopomatinae (Siluriformes: Loricariidae) using multiple genes. Mol Phylogenet Evol . 2011; 59(1):43-52.) and Martins (2012Martins FO. Análise filogenética e revisão taxonômica de Pseudotothyris Britski & Garavello, 1984 (Loricariidae: Hypoptopomatinae). [MSc Dissertation]. São José do Rio Preto, SP: Universidade Estadual Paulista Júlio de Mesquita Filho; 2012.) for Siluriformes; Hrbek et al. (2007Hrbek T, Seekinger J, Meyer A. A phylogenetic and biogeographic perspective on the evolution of poeciliid fishes. Mol Phylogenet Evol . 2007; 43(3):986 98.) for Poecilidae; Albert (2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Ann Arbor: Museum of Zoology, University of Michigan; 2001. (Miscellaneous Publications; No. 190).), Lovejoy et al. (2010Lovejoy NR, Lester K, Crampton WGR, Marques FPL, Albert JS. Phylogeny, biogeography, and electric signal evolution of Neotropical knifefishes of the genus Gymnotus (Osteichthyes: Gymnotidae). Mol Phylogenet Evol . 2010; 54(1):278-90.) and Tagliacollo et al. (2016Tagliacollo VA, Bernt MJ, Craig JM, Oliviera C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol . 2016; 95:20-33.) for Gymnotiformes; Ilves et al. (2018Ilves KL, Torti D, López-Fernández H. Exon-based phylogenomics strengthens the phylogeny of Neotropical cichlids and identifies remaining conflicting clades (Cichliformes: Cichlidae: Cichlinae). Mol Phylogenet Evol . 2018; 118:232-43.) for Cichlidae, and Nakatani et al. (2011Nakatani M, Miya M, Mabuchi K, Saitoh K, Nishida M. Evolutionary history of Otophysi (Teleostei), a major clade of the modern freshwater fishes: Pangaea origin and Mesozoic radiation. BMC Evol Biol. 2011; 11:177. Available from: https://doi.org/10.1186/1471-2148-11-177
https://doi.org/10.1186/1471-2148-11-177...
), Betancur-R et al. (2013Betancur-R R, Richard E. Broughton, Wiley EO, Carpenter K, López JA, Li C, Holcroft NI, Arcila D, Sanciangco M, Cureton JC, Zhang F, Buser T, Campbell MA, Ballesteros JA, Roa-Varon A, Willis S, Borden WC, Rowley T, Reneau PC, Hough DJ, Lu G, Grande T, Arratia G, Orti G. The tree of life and a new classification of bony fishes. PLoS Curr Tree of Life. 2013:1-54.) and Chen et al. (2013Chen WJ, Lavoué S, Mayden RL. Evolutionary origin and early biogeography of otophysan fishes (Ostariophysi: Teleostei). Evolution. 2013; 67(8):2218-39.) for all other groups. 3) Species not present in the Maranhão region were pruned from the tree using Phylomatic version 3, available online (http://phylodiversity.net/phylomatic/).
The resulting tree includes the 160 species from the Maranhão region with all branches of equal length. Tree dating was estimated using the Branch Length Adjustment (BLADJ) algorithm in Phylocom 4.2 (Webb et al., 2008Webb CO, Ackerly DD, Kembel SW. Phylocom: Software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics. 2008; 24(18):2098-100.). The pruned, dated working phylogeny used in all community phylogenetic analyses is represented in Fig. 2 and the Nexus file (S1).
To complement this working phylogeny, we next constructed a presence-absence matrix for all 160 species and each river basin studied to assess phylogenetic diversity using the R package picante 1.7 (Kembel et al., 2010Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO. Picante: R tools for integrating phylogenies and ecology. Bioinformatics. 2010; 26(11):1463-64.). We used Mean Pairwise Distance (MPD) and Mean Nearest Taxon Distance (MNTD) indices to measure the phylogenetic structure of the local assemblages in each river basin (Webb et al., 2002Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. Phylogenies and community ecology. Annu Rev Ecol Syst. 2002; 33(1):475-505.). MPD calculates the phylogenetic distances among all possible species pairs as an estimate of the phylogenetic structure of species assemblages. MNTD calculates distances between each species and its nearest neighbor in the same assemblage, capturing variations at the tip of the phylogeny (Webb, 2000Webb CO. Exploring the phylogenetic structure of ecological communities: an example for rain forest trees. Am Nat . 2000; 156(2):145 -55.). The MPD and MNTD values observed in each assemblage are then compared by z-test to a sample of 999 randomly-generated phylogenies to assess whether they diverge significantly from chance. Scores greater than 2.0 with z-test p-value <0.05 indicate phylogenetic over-dispersion, with a greater phylogenetic distance between species than expected. Values lower than -2.0 with p> 0.95 indicate that assemblages are phylogenetically clustered; i.e. the phylogenetic distance between them is shorter than expected on a randomly generated tree. Values between -2.0 and 2.0 indicate phylogenetic randomness, that is, the distance between species occurs at random (Cavender-Bares et al., 2004Cavender-Bares J, Ackerly DD, Baum DA, Bazzaz FA. Phylogenetic overdispersion in Floridian oak communities. Am Nat. 2004; 163(6):823 -43.). To reduce the impact of sampling effect on the results, all analyses were performed using the Standardized Effect Size (SES) algorithm available in picante (Kembel et al., 2010Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO. Picante: R tools for integrating phylogenies and ecology. Bioinformatics. 2010; 26(11):1463-64.), through 999 randomizations comparing the communities with a null model then applying a Z test.
We also used picante to calculate species richness (SR) and Faith’s phylogenetic diversity (PD) (Faith, 1992Faith DP. Conservation evaluation and phylogenetic diversity. Biol Conserv. 1992; 61(1):1-10.). The SR index is calculated as the sum of the number of species in the studied area, without taking other indices into account, while the PD index is calculated as the sum of the lengths of tree branches for each species present in the area and thus supplied in millions of years (Ma) (Faith, 1992Faith DP. Conservation evaluation and phylogenetic diversity. Biol Conserv. 1992; 61(1):1-10.).
We used the PhyloSor index, which quantifies the fraction of branch lengths shared by sites (Bryant et al., 2008Bryant JA, Lamanna C, Morlon H, Kerkhoff AJ, Enquist BJ, Green JL. Microbes on mountainsides: Contrasting elevational patterns of bacterial and plant diversity. Proc Natl Acad Sci USA. 2008(Suppl 1):11505-11.), to assess phylogenetic beta diversity between assemblages. Phylogenetic beta diversity was calculated using the picante package to measure the degree of shared species among basins. This index was compared with two measures of geographical distance: Euclidian and Floodplains distances (e.g.Albert et al., 2011aAlbert JS, Carvalho TP, Petry P, Holder MA, Maxime EL, Espino J, Corahua I, Quispe R, Rengifo B, Ortega H, Reis RE. Aquatic biodiversity in the Amazon: Habitat specialization and geographic isolation promote species richness. Animals. 2011a; 1(4):205-41.). Phylogenetic beta diversity between each pair of river basins was then compared to Euclidian (straight-line) and thalweg (valley-line) measures of geographical distance between those basins using a Mantel test with 99,999 permutations in ape 5.1 package (Paradis et al., 2004Paradis E, Claude J, Strimmer K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics. 2004; 20(2):289-90.).
Reconstruction of paleodrainages. To understand the influence of sea-level changes in the distribution of the freshwater fishes species in this area, we estimated paleodrainage boundaries for the maximum and minimum sea level over the past 10 My based in the result from community phylogenetic structure analyze. For this we used topographical and bathymetric information extracted from a digital elevation model (DEM) GEBCO_2014 at 30 arc-second resolution (c. 1 km; http://www.gebco.net/) in QGIS3.2.3 using the method described by Thomaz et al. (2015Thomaz AT, Malabarba LR, Bonatto SL, Knowles LL. Testing the effect of palaeodrainages versus habitat stability on genetic divergence in riverine systems: study of a Neotropical fish of the Brazilian coastal Atlantic Forest. J Biogeogr . 2015; 42(12):2389-401.).
These paleodrainage reconstruction shows that all nine hydrographic basins of modern Maranhão drained into just three paleodrainages at the LGM when the sea-level was 122 m below the modern level and the most areas of these basins are totally or partially submersed when the sea-level was 35 m above the modern level at 5.5 Ma (Hansen et al., 2013Hansen J, Sato M, Russell G, Kharecha P. Climate sensitivity, sea level and atmospheric carbon dioxide. Phil Trans R Soc A. 2013; 371(2001): 20120294. Available from: https://doi.org/10.1098/rsta.2012.0294
https://doi.org/10.1098/rsta.2012.0294...
) (Fig. 3), these results show that the sea-level changes can influence the distribution of the species in this area as well as the limits of the basins. The three paleodrainages identified for LGM are structured as follow. One of these paleodrainages included the areas of the modern Gurupi, Maracaçumé, and Turiaçu basins, and part of Litoral Ocidental basin. A second paleodrainage was formed by the modern Mearim, Itapecuru, Munim basins and part of Litoral Ocidental and Periá basins. A third paleodrainage was formed by the modern Preguiças basin and part of Periá basin.
Reconstruction of paleodrainages area of Maranhão. a. 35 m above the modern level. b. 122 m below the modern level. Note that all modern rivers flowed through three paleodrainages at the LGM (-122 m). Note that similarities of the fish faunas can be explained in part by past connection among the basins. These paleo-connections also justify how basins with low species richness were combined for the analyses of historical biogeography.
To avoid biased results due to low species numbers we combined the basins with low species number with other basins in the community phylogenetic structure analysis. For this we used two criteria: 1) mouth connection at the LGM reconstruction and, 2) high faunal similarity (number of species shared) when the basins are connected with more than one basin at the LGM reconstruction. So, based on these criteria, the area called the “Turiaçu” corresponds here to the combined Turiaçu, Gurupi, Maracaçumé and Litoral Ocidental basins in the analyses developed in this paper.
Results
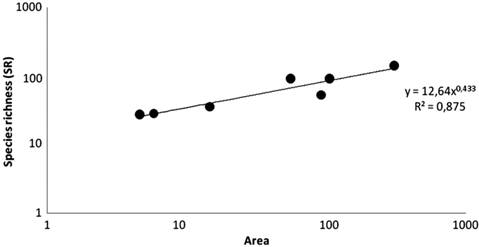
Community phylogenetic structure. We report a species richness of 160 freshwater fish species distributed among the hydrographic basins of Maranhão state representing 12 orders and 39 families (S2). This increases the known diversity of this region by 77 species (~93%), eight families (~26%) and two orders (20%) over all previous studies. Of these 160 species, 16 are considered endemic for this region, sometimes shared with the adjacent Parnaíba basin, and one introduced species in Brazil, Oreochromis niloticus (Linnaeus, 1758) originally imported for commercial purposes (Vicente et al., 2014Vicente IST, Elias F, Fonseca-Alves CE. Perspectivas da produção de tilápia do Nilo (Oreochromis niloticus) no Brasil. Revista de Ciências Agrárias. 2014; 37:392-98.) and probably released after captive breeding. The orders with the highest number of species are Characiformes and Siluriformes, with 65 and 46 records, respectively. The families with greatest representation are Characidae, Cichlidae and Loricariidae with 24, 17 and 15 species respectively (Tab. 1). Fish species richness of Maranhão hydrographic basins is significantly correlated with basin area (r2 = 0.875, p = 0.007919), and the species-area exponent (z =0.43) value is in the range classified as “archipelagic” by Rosenzweig (2004Rosenzweig ML. Applying species-area relationships to the conservation of species diversity. In Lomolino MV, Heaney LR, editors. Frontiers of biogeography. New directions in the geography of nature. Sunderland, MA: Sinauer Associates Inc.; 2004. p.325-343.) (Fig. 4).
Species-area relationships (SAR) for freshwater fishes of Maranhão basins. Data for 160 species.
Summary of data used in analyses of Maranhão basins. Number of fish species, genera, families, areal extent, and geographic category for each basin. Numbers of taxonomic units to not sum to Total due to partially overlapping taxonomic composition of basins. 1 = ANA, 2015ANA - Agência Nacional de Águas (Brasil). Conjuntura dos recursos hídricos no Brasil: regiões hidrográficas brasileiras - Edição Especial. Brasília: ANA; 2015.; 2 = NUGEO, 2016NUGEO - Núcleo Geoambiental/Universidade Estadual do Maranhão. Bacias hidrográficas e climatologia no Maranhão. São Luís: UEMA; 2016..
The results of the MPD and MNTD analyses for each basin were not significantly different from those drawn from a random phylogeny (Tab. 2), indicating that the phylogenetic distance between species is not significantly different from what would be expected at random. This provides no evidence for either phylogenetic clustering or phylogenetic overdispersion either at the tips of the phylogeny or over its entire area.
Summary of indices comparing species richness and phylogenetic diversity among Maranhão basins. Note these results are consistent with recent colonization events and short diversification times. PD values and species richness from Itapecuru and Mearim basins indicate older events. All basins exhibit a phylogenetically random species composition, meaning that the phylogenetic distance between clades (MPD) or sister species-pairs (MNTD) is not significantly different from what would be expected at random.
In the analysis of PD, we recover recent colonization events and short diversification times. Taken together with the recovered SR index, this suggests that the Itapecuru and Mearim basins underwent events that caused species to diverge for a longer time than among other basins, 9.8 and 9.7 My, respectively (Tab. 2).
The results of the phylobeta diversity analysis (Tab. 3) and the other measures of geographical distance among basins (Tab. 4), showed that the basin boundaries do not constitute substantial barriers to dispersal and gene flow, and that geographic distance does not strongly hinder dispersal in freshwater fishes of this region.
Distance among Maranhão basins using three methods: phylobeta diversity (Phylosor), Euclidian distances and floodplain (thalweg) distances. Numbers below the diagonal are z-stat values, and above the diagonal are p-values.
Discussion
Ichthyofauna composition. The numbers of fish species in Maranhão are substantially higher than those reported in previously published studies (Piorski et al., 1998Piorski NM, Castro ACL, Pereira LG, Muniz MEL. Ictiofauna do trecho inferior do rio Itapecuru, Nordeste do Brasil. B Lab Hidro. 1998; 11:15-24., 2003Piorski NM, Castro ACL, Pinheiro CUB. A prática da pesca entre os grupos indígenas das bacias dos rios Pindaré e Turiaçu, no Estado do Maranhão, Nordeste do Brasil. B Lab Hidro . 2003; 16:67-74., 2007Piorski NM, Castro ACL, Sousa Neto AM. Peixes do cerrado da região sul maranhense. In: Barreto LN, editor. Cerrado Norte do Brasil. São Luís: USEB; 2007. p.177-212., 2017Piorski NM, Ferreira BRA, Guimarães EC, Ottoni FP, Nunes JLS, Brito PS. Peixes do Parque Nacional dos Lençóis Maranhenses. São Luis: Café & Lápis/Edufma; 2017.; Reis et al., 2003Reis RE, Kullander SO, Ferraris J, Carl J. Check List of the freshwater fishes of South and Central America. Porto Alegre: EDIPUCRS; 2003.; Soares, 2005Soares EC. Peixes do Mearim. São Luís: Instituto GEIA; 2005.; Barros et al., 2011Barros MC, Fraga EC, Birindelli JLO. Fishes from the Itapecuru River basin, State of Maranhão, northeast Brazil. Braz J Biol. 2011; 71(2):375-80.). This increase in the number of species is principally due to the incorporation of the available data from CPUFMA. Among basins exclusive to the state of Maranhão, the Mearim and Itapecuru basins are the most intensively studied to date, primarily due to their being the largest basins, and therefore having a greater economic importance (Soares, 2005Soares EC. Peixes do Mearim. São Luís: Instituto GEIA; 2005.; Barros et al., 2011Barros MC, Fraga EC, Birindelli JLO. Fishes from the Itapecuru River basin, State of Maranhão, northeast Brazil. Braz J Biol. 2011; 71(2):375-80.). Piorski et al. (1998Piorski NM, Castro ACL, Pereira LG, Muniz MEL. Ictiofauna do trecho inferior do rio Itapecuru, Nordeste do Brasil. B Lab Hidro. 1998; 11:15-24.) found, for the lower region of the Itapecuru River basin, 41 species belonging to 36 genera and 13 families, while Barros et al. (2011Barros MC, Fraga EC, Birindelli JLO. Fishes from the Itapecuru River basin, State of Maranhão, northeast Brazil. Braz J Biol. 2011; 71(2):375-80.) recorded 69 species, representing 65 genera, 29 families and 10 orders, in the three sectors of the basin. The larger number of species of orders Characiformes, Siluriformes and Cichliformes corroborates the existing literature, in which these groups are the most diverse for the region and for nearby basins, as well as families Characidae, Cichlidae and Loricariidae (Piorski et al., 1998Piorski NM, Castro ACL, Pereira LG, Muniz MEL. Ictiofauna do trecho inferior do rio Itapecuru, Nordeste do Brasil. B Lab Hidro. 1998; 11:15-24.; Reis et al., 2003Reis RE, Kullander SO, Ferraris J, Carl J. Check List of the freshwater fishes of South and Central America. Porto Alegre: EDIPUCRS; 2003.; Ramos et al., 2005Ramos RTC, Ramos TPA, Rosa RS, Beltrão GBM, Groth F. Diversidade de peixes (Ictiofauna) da bacia do rio Curimatau, Paraíba. In: Araujo FS, Rodal MJN, Barbosa MRV, editores. Análise das variações da biodiversidade do bioma caatinga: suporte das estratégias regionais de conservação. Brasília: Ministério do Meio Ambiente; 2005. p.291-318., 2014Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba River basin, northeastern Brazil. Biota Neotrop . 2014; 14(1):1-8.; Buckup et al., 2007Buckup PA, Menezes NA, Ghazzi MS. Catálogo das espécies de peixes de água doce do Brasil. Rio de Janeiro: Museu Nacional, Universidade Federal do Rio de Janeiro; 2007. (Série Livros 23).; Barros et al., 2011Barros MC, Fraga EC, Birindelli JLO. Fishes from the Itapecuru River basin, State of Maranhão, northeast Brazil. Braz J Biol. 2011; 71(2):375-80.).
However, the total number of species identified for the region is greater than recent literature reports. Hubert, Renno (2006Hubert N, Renno JF. Historical biogeography of South American freshwater fishes. J Biogeogr . 2006; 33(8):1414-36.) included 33 characiforms species for the entire Maranhão endemism region, which encompasses the rivers exclusive to Maranhão and some basins in the state of Pará. In our list we recorded the occurrence of 65 characiforms, almost twice number previously reported. In a more recent work about the biogeography of neotropical freshwater fish, in which some Maranhão basins were considered, Dagosta, de Pinna (2017Dagosta FCP, De Pinna M. Biogeography of Amazonian fishes: deconstructing river basins as biogeographic units. Neotrop Ichthyol .2017; 15(3): e170034, 2017. Available from: http://dx.doi.org/10.1590/1982-0224-20170034
http://dx.doi.org/10.1590/1982-0224-2017...
) built a larger database on species distribution for the Amazonian region, with approximately 5,000 species. However, and despite the efforts undertaken by the authors, they could not obtain much information on the distribution of fish in the basins of Maranhão, thus grouping the Gurupi basin with the Capim basin due to a gap in information and considering only the basins of Mearim and Itapecuru for their analysis, reaching a total of 66 species for the region.
The results described above demonstrate that the Maranhão basins has been under-sampled or even disregarded in previous studies of the fish fauna. In view of that, the present study comprises the largest database already registered for freshwater fish species from the coastal drainages of Maranhão, with a total of 160 species.
Community phylogenetic structure. The phylogenetic diversity analysis shows that the Itapecuru and Mearim basins have the highest SR and PD values, what is compatible with areas that have experienced events related to species diversification (extinction and speciation) for the longest time and present the most phylogenetically distinct species composition (Webb et al., 2002Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. Phylogenies and community ecology. Annu Rev Ecol Syst. 2002; 33(1):475-505.). On the other hand, the other drainages have a composition of species that are closer phylogenetically, which is characteristic of areas that are newly colonized or that have been through more recent events, having less time for speciation (Wiens, Donoghue, 2004Wiens JJ, Donoghue MJ. Historical biogeography, ecology and species richness. Trends Ecol Evol. 2004; 19(12):639- 44.; Proches et al., 2006Proches S, Wilson JRU, Cowling RM. How much evolutionary history in a 10x10m plot? Proc R Soc B. 2006; 273(1590):1143-48.; Davies et al., 2007Davies RG, Orme CDL, Webster AJ, Jones KE, Blackburn TM, Gaston KJ. Environmental predictors of global parrot (Aves: Psittaciformes) species richness and phylogenetic diversity. Global Ecol Biogeogr. 2007; 16(2):220-33.; Pavoine, Bonsall, 2011Pavoine S, Bonsall MB. Measuring biodiversity to explain community assembly: a unified approach. Biol Rev. 2011; 86(4):792-812.; Aquino, Colli, 2017Aquino PPU, Colli GR. Headwater captures and the phylogenetic structure of freshwater fish assemblages: a case study in central Brazil. J Biogeogr. 2017; 44(1):207-16.).
This is also evidence that historical events, such as marine incursions in the late Miocene and Pliocene, may have left a signature on the fish species composition of Maranhão (Lundberg et al., 1998Lundberg JG, Marshall LG, Guerrero J, Horton B, Malabarba MCSL, Wesselingh F. The stage for neotropical fish diversification: a history of tropical South American rivers. In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of Neotropical fishes. Porto Alegre: EDUPUCRS ; 1998. p.13-48.; Soares Junior et al., 2011Soares Júnior AV, Hasui Y, Costa JBS, Machado FB. Evolução do rifteamento e paleogeografia da margem atlântica equatorial do Brasil: Triássico ao Holoceno. Geociências. 2011; 30(4):669-92.; Albert et al., 2018Albert JS, Val P, Hoorn C. The changing course of the Amazon River in the Neogene: Center stage for Neotropical diversification. Neotrop Ichthyol. 2018; 16(3):e180033. Available from: http://dx.doi.org/10.1590/1982-0224-20180033
http://dx.doi.org/10.1590/1982-0224-2018...
). Formation of the fauna present in northern South America was greatly affected by marine incursion events (López- Fernández, Albert, 2011López-Fernández H, Albert JS. Paleogene radiations. In: Albert JS, Petry P, Reis RE, editors. Historical biogeography of Neotropical freshwater fishes. Berkeley, CA: University of California Press ; 2011. p.105-117.). The Maranhão State was strongly affected by marine incursions, as the majority of its area is below 300 m elevation (Albert, Reis, 2011Albert JS, Reis RE. Introduction to Neotropical freshwaters. In: Albert JS, Petry P, Reis RE, editors. Historical biogeography of Neotropical freshwater fishes. Berkeley, CA: University of California Press ; 2011. p.3-19.). During these periods, and due to their location and size, the Mearim and Itapecuru basins were the least affected drainages in the region, being more buffered by geographic distance and topography from the paleogeographic changes that affected the other basins.
According to Hansen et al. (2013Hansen J, Sato M, Russell G, Kharecha P. Climate sensitivity, sea level and atmospheric carbon dioxide. Phil Trans R Soc A. 2013; 371(2001): 20120294. Available from: https://doi.org/10.1098/rsta.2012.0294
https://doi.org/10.1098/rsta.2012.0294...
), sea level oscillations caused by the glacial periods promoted a rise of up to 30 m above our current level during the Miocene, about 8 Ma, and influenced the region until the middle Pleistocene (~1 Ma), the Maranhão basins being directly affected by these variations. As all these basins empty to the sea on the modern landscape, and are strongly influenced by tidal variation, they experienced considerable impacts during various periods of sea level rise, some lasting up to 800,000 years (Lundberg et al., 1998Lundberg JG, Marshall LG, Guerrero J, Horton B, Malabarba MCSL, Wesselingh F. The stage for neotropical fish diversification: a history of tropical South American rivers. In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of Neotropical fishes. Porto Alegre: EDUPUCRS ; 1998. p.13-48.; Nores, 2004Nores M. The implications of Tertiary and Quaternary sea level rise events for avian distribution patterns in the lowlands of northern South America. Global Ecol Biogeogr . 2004; 13(2):149-61.). In addition to marine incursions, geological data (Petri, Fúlfaro, 1983Petri AC, Fúlfaro VJ. Geologia do Brasil. São Paulo: Editora da Universidade Federal de São Paulo; 1983.; Costa et al., 1997Costa JBS, Bemerguy RL, Hasui Y, Borges MDS, Júnior CRPF, Bezerra PL, Costa MLD, Fernandes JMG. Neotectônica da região amazônica: aspectos tectônicos, geomorfológicos e deposicionais. Geonomos. 1997; 4:23-44.) show that the reactivation of tectonic faults during the Miocene and Pliocene may have affected watershed boundaries among Maranhão basins. In combination, these climatological and geological events may have resulted in biotic exchanges among the several drainages by dispersal along the coastal plain and by headwater tributary exchanges, thereby increasing the faunal similarities between some basins.
According to Hubert, Renno (2006Hubert N, Renno JF. Historical biogeography of South American freshwater fishes. J Biogeogr . 2006; 33(8):1414-36.) some freshwater fish species may have persisted in upstream portions of the Parnaíba and Tocantins basins during marine incursions about 5 Ma. Combining this information with the PD values found for the coastal drainages of Maranhão, we propose that these upstream areas may have served as a phylogenetic refugium, as has been proposed for other geographically circumscribed regions of northern South America (e.g. Maracaibo basin; Albert et al., 2006Albert JS, Lovejoy NR, Crampton WGR. Miocene tectonism and the separation of cis-and trans-Andean river basins: Evidence from Neotropical fishes. J South Am Earth Sci. 2006; 21(1-2): 14-27.). These upstream areas may then have served as a source area for the subsequent formation of the lowland Itapecuru and Mearim fish species assemblages. Species dispersal among these areas after marine incursion events was possibly aided by the river captures and geodispersal. These events may have occurred between the Parnaíba and Itapecuru basins, in the region of Mirador State Park, where the two basins share the longest extent of their common watershed.
Another possible area of trans-basin headwater river capture is the area between Tocantins, Mearim and the springs of Gurupi and Turiaçu, in a paleodelta (~ 2.5 Ma) located near the modern city of Tucuruí. Such a link between these basins may have provided enough connection time to promote a flow of species, even after the initial emergence of the Tiracambu mountain range at about 5.3 Ma, the main divisor of these drainages on the modern landscape (Costa et al., 1997Costa JBS, Bemerguy RL, Hasui Y, Borges MDS, Júnior CRPF, Bezerra PL, Costa MLD, Fernandes JMG. Neotectônica da região amazônica: aspectos tectônicos, geomorfológicos e deposicionais. Geonomos. 1997; 4:23-44.; Soares Junior et al., 2011Soares Júnior AV, Hasui Y, Costa JBS, Machado FB. Evolução do rifteamento e paleogeografia da margem atlântica equatorial do Brasil: Triássico ao Holoceno. Geociências. 2011; 30(4):669-92.).
Historical biogeography. In combination, the phylogeographic, community phylogenetic and phylogenetic beta diversity analyses of this study indicate the importance of geological events and sea level fluctuations during the late Miocene and Pliocene in the formation of Maranhão fish species assemblages. By contrast, the modern watershed borders of Maranhão basins do not appear to constitute substantial barriers to dispersal and gene flow for the freshwater fishes of this region. The species-area exponent (z = 0.43) of the Maranhão region is consistent with the interpretation of these basins as a biogeographic archipelago, where species richness is controlled by dispersal and extinction, and where speciation does not contribute substantially to the formation of species richness. As a result, the Maranhão basins are more effectively modelled as biogeographic islands than as biogeographic provinces (Rosenzweig, 2003Rosenzweig ML. Reconciliation ecology and the future of species diversity. Oryx. 2003; 37(2):194-205.; Albert et al., 2011aAlbert JS, Petry P, Reis RE. Major biogeographic and phylogenetic patterns. In: Albert JS, Petry P, Reis RE, editors. Historical biogeography of Neotropical freshwater fishes. Berkeley, CA: University of California Press; 2011a. p.21-56.).
These results also shed light on intra-specific genetic variation found in certain fish species in this area (Piorski, 2010Piorski NM. Diversidade genética e filogeografia das espécies Hoplias malabaricus (Bloch, 1794) e Prochilodus lacustris Steindachner, 1907 no Nordeste do Brasil. [PhD Thesis]. São Carlos, SP: Universidade Federal de São Carlos; 2010.; Carvalho-Costa et al., 2011Carvalho-Costa LF, Piorski NM, Willis SC, Galetti PM, Ortí G. Molecular systematics of the neotropical shovelnose catfish genus Pseudoplatystoma Bleeker 1862 based on nuclear and mtDNA markers. Mol Phylogenet Evol. 2011; 59(1):177-94.; Abreu, 2013Abreu JMS. Variação geográfica em Schizodon dissimilis (Garman, 1890) e diversidade genética e filogeográfica do grupo Schizodon fasciatus sensu lato (Characiformes: Anostomidae). [MSc Dissertation]. Belém, PA: Universidade Federal do Pará; 2013.; Luz et al., 2015Luz LA, Reis LL, Sampaio I, Barros MC, Fraga E. Genetic differentiation in the populations of red piranha, Pygocentrus nattereri Kner (1860) (Characiformes: Serrasalminae), from the river basins of northeastern Brazil. Braz J Biol . 2015; 75(4):838-45.). In these studies, different species share haplotypes among the Mearim, Itapecuru and Parnaíba basins, indicating dispersal and perhaps geodispersal events (Hubert et al., 2007Hubert N, Duponchelle F, Nuñez J, Garcia-Davila C, Paugy D, Renno JF. Phylogeography of the piranha genera Serrasalmus and Pygocentrus: implications for the diversification of the Neotropical ichthyofauna. Mol Ecol. 2007; 16(10):2115-36.). In the studies that includes sequences from Turiaçu basin, these samples formed a separate clade normally closer to Amazonian fish than to Maranhão fish (Abreu JMS, personal observation), an indicative of vicariance events (Hubert et al., 2007Hubert N, Duponchelle F, Nuñez J, Garcia-Davila C, Paugy D, Renno JF. Phylogeography of the piranha genera Serrasalmus and Pygocentrus: implications for the diversification of the Neotropical ichthyofauna. Mol Ecol. 2007; 16(10):2115-36.). In addition, more specific analyses of taxonomic groups of the Turiaçu river indicate the occurrence of new taxa that are apparently unique to this drainage (Saraiva et al., in prep; Garavello et al., in prep.). The paleodrainage reconstruction plus the emergence of Tiracambu mountain (Costa et al., 1997Costa JBS, Bemerguy RL, Hasui Y, Borges MDS, Júnior CRPF, Bezerra PL, Costa MLD, Fernandes JMG. Neotectônica da região amazônica: aspectos tectônicos, geomorfológicos e deposicionais. Geonomos. 1997; 4:23-44.; Soares Junior et al., 2011Soares Júnior AV, Hasui Y, Costa JBS, Machado FB. Evolução do rifteamento e paleogeografia da margem atlântica equatorial do Brasil: Triássico ao Holoceno. Geociências. 2011; 30(4):669-92.) are a reasonable explanation for the separation of the Turiaçu basin from the other basins, promoting the isolation of this area and the differentiation of the associated ichthyofauna.
Available geological data suggest that events during the Miocene and Pliocene were important time periods for the separation of Maranhão, Tocantins and Amazon basins. An important event was the emergence of the Tiracambu range, the main divider between the Maranhão and Tocantins-Amazon drainages. At the same time, the Parnaíba basin was exposed to the same events that the Maranhão basins. These events suggest the regional fauna is relatively young, and that species may yet be colonizing the area. Speciation among populations of the several basins may be influenced by the ecological configuration of the region. The Maranhão area exhibits a singular ecological configuration, with a unique and distinctive geological and biotic history, and is more than just a transitional area between the more well-studied areas of the humid Amazon and arid Cerrado and Caatinga ecozones.
Acknowledgments
This work was undertaken with support from FAPEMA 011644/2016 to J.M.S.A., and FAPEMA 01490/16 to N.M.P.
References
- Abell R, Thieme ML, Revenga C, Bryer M, Kottelat M, Bogutskaya N, Coad B, Mandrak N, Contreras Balderas S, Bussing W, Stiassny MLJ, Skelton P, Allen GR, Unmack P, Naseka A, Ng R, Sindorf N, Robertson J, Armijo E, Higgins JV, Heibel TJ, Wikramanayake E, Olson D, López HL, Reis RE, Lundberg JG, Pérez MHS, Petry P. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. BioScience. 2008; 58(5):403-14.
- Abreu JMS. Variação geográfica em Schizodon dissimilis (Garman, 1890) e diversidade genética e filogeográfica do grupo Schizodon fasciatus sensu lato (Characiformes: Anostomidae). [MSc Dissertation]. Belém, PA: Universidade Federal do Pará; 2013.
- Ab’Saber AN. Contribuição à geomorfologia do Estado do Maranhão. Notícia Geomorfológica. 1960; 3(5):35-45.
- Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Ann Arbor: Museum of Zoology, University of Michigan; 2001. (Miscellaneous Publications; No. 190).
- Albert JS, Carvalho TP, Petry P, Holder MA, Maxime EL, Espino J, Corahua I, Quispe R, Rengifo B, Ortega H, Reis RE. Aquatic biodiversity in the Amazon: Habitat specialization and geographic isolation promote species richness. Animals. 2011a; 1(4):205-41.
- Albert JS, Crampton WGR. The geography and ecology of diversification in Neotropical freshwaters. Nature Education Knowledge. 2010; 3(10):13-19.
- Albert JS, Lovejoy NR, Crampton WGR. Miocene tectonism and the separation of cis-and trans-Andean river basins: Evidence from Neotropical fishes. J South Am Earth Sci. 2006; 21(1-2): 14-27.
- Albert JS, Petry P, Reis RE. Major biogeographic and phylogenetic patterns. In: Albert JS, Petry P, Reis RE, editors. Historical biogeography of Neotropical freshwater fishes. Berkeley, CA: University of California Press; 2011a. p.21-56.
- Albert JS, Reis RE. Introduction to Neotropical freshwaters. In: Albert JS, Petry P, Reis RE, editors. Historical biogeography of Neotropical freshwater fishes. Berkeley, CA: University of California Press ; 2011. p.3-19.
- Albert JS, Val P, Hoorn C. The changing course of the Amazon River in the Neogene: Center stage for Neotropical diversification. Neotrop Ichthyol. 2018; 16(3):e180033. Available from: http://dx.doi.org/10.1590/1982-0224-20180033
» http://dx.doi.org/10.1590/1982-0224-20180033 - Albornoz PCL. Anatomia e relações filogenéticas da família Loricariidae (Ostariophysi: Siluriformes) com ênfase na subfamília Hypoptopomatinae. [PhD Thesis]. Porto Alegre, RS: Pontifícia Universidade Católica do Rio Grande do Sul; 2006.
- ANA - Agência Nacional de Águas (Brasil). Conjuntura dos recursos hídricos no Brasil: regiões hidrográficas brasileiras - Edição Especial. Brasília: ANA; 2015.
- Aquino PPU, Colli GR. Headwater captures and the phylogenetic structure of freshwater fish assemblages: a case study in central Brazil. J Biogeogr. 2017; 44(1):207-16.
- Bartolette R, Vieira CS, Santos JFL, Santos CDC, Luduvice JSV, Passos TS, D’Avilla T, Nascimento BO, Ernesto D, Argolo FH, Aguiar AJM, Argolo F, Pereira MSA, Santos TF, Brito MFG. The ichthyofauna in the influence area of the Lajeado reservoir, Tocantins state, Brazil. Check List. 2017; 13(3):2156. Available from: http://dx.doi.org/10.15560/13.3.2156
» http://dx.doi.org/10.15560/13.3.2156 - Barros MC, Fraga EC, Birindelli JLO. Fishes from the Itapecuru River basin, State of Maranhão, northeast Brazil. Braz J Biol. 2011; 71(2):375-80.
- Betancur-R R, Richard E. Broughton, Wiley EO, Carpenter K, López JA, Li C, Holcroft NI, Arcila D, Sanciangco M, Cureton JC, Zhang F, Buser T, Campbell MA, Ballesteros JA, Roa-Varon A, Willis S, Borden WC, Rowley T, Reneau PC, Hough DJ, Lu G, Grande T, Arratia G, Orti G. The tree of life and a new classification of bony fishes. PLoS Curr Tree of Life. 2013:1-54.
- Bockmann FA. Análise filogenética da família Heptapteridae (Teleostei, Ostariophysi, Siluriformes) e redefinição de seus gêneros. [PhD Thesis]. São Paulo, SP: Universidade de São Paulo; 1998.
- Bryant JA, Lamanna C, Morlon H, Kerkhoff AJ, Enquist BJ, Green JL. Microbes on mountainsides: Contrasting elevational patterns of bacterial and plant diversity. Proc Natl Acad Sci USA. 2008(Suppl 1):11505-11.
- Buckup PA, Menezes NA, Ghazzi MS. Catálogo das espécies de peixes de água doce do Brasil. Rio de Janeiro: Museu Nacional, Universidade Federal do Rio de Janeiro; 2007. (Série Livros 23).
- Carvalho-Costa LF, Piorski NM, Willis SC, Galetti PM, Ortí G. Molecular systematics of the neotropical shovelnose catfish genus Pseudoplatystoma Bleeker 1862 based on nuclear and mtDNA markers. Mol Phylogenet Evol. 2011; 59(1):177-94.
- Cavender-Bares J, Ackerly DD, Baum DA, Bazzaz FA. Phylogenetic overdispersion in Floridian oak communities. Am Nat. 2004; 163(6):823 -43.
- Chen WJ, Lavoué S, Mayden RL. Evolutionary origin and early biogeography of otophysan fishes (Ostariophysi: Teleostei). Evolution. 2013; 67(8):2218-39.
- Chiachio MC, Oliveira C, Montoya-Burgos JI. Molecular systematic and historical biogeography of the armored Neotropical catfishes Hypoptopomatinae and Neoplecostominae (Siluriformes: Loricariidae). Mol Phylogenet Evol . 2008; 49(2):606-17.
- Clark PU, Dyke AS, Shakun JD, Carlson AE, Clark J, Wohlfarth B, Mitrovica JX, Hostetler SW, McCabe AM. The last glacial maximum. Science. 2009; 325(5941):710-14.
- Claro-García A, Shibatta OA. The fish fauna of streams from the upper rio Tocantins basin, Goiás State, Brazil. Check List . 2013; 9(1):28-33.
- Costa JBS, Bemerguy RL, Hasui Y, Borges MDS, Júnior CRPF, Bezerra PL, Costa MLD, Fernandes JMG. Neotectônica da região amazônica: aspectos tectônicos, geomorfológicos e deposicionais. Geonomos. 1997; 4:23-44.
- Cramer CA, Bonatto SL, Reis RE. Molecular phylogeny of the Neoplecostominae and Hypoptopomatinae (Siluriformes: Loricariidae) using multiple genes. Mol Phylogenet Evol . 2011; 59(1):43-52.
- Christofoletti A. Enciclopédia Mirador Internacional. São Paulo: vol. 5; 1975. Capturas fluviais; p.2049-2051.
- Dagosta FCP, De Pinna M. Biogeography of Amazonian fishes: deconstructing river basins as biogeographic units. Neotrop Ichthyol .2017; 15(3): e170034, 2017. Available from: http://dx.doi.org/10.1590/1982-0224-20170034
» http://dx.doi.org/10.1590/1982-0224-20170034 - Davies RG, Orme CDL, Webster AJ, Jones KE, Blackburn TM, Gaston KJ. Environmental predictors of global parrot (Aves: Psittaciformes) species richness and phylogenetic diversity. Global Ecol Biogeogr. 2007; 16(2):220-33.
- De Pinna MCC. Phylogenetic relationships of Neotropical Siluriformes (Teleostei: Ostariophysi): historical overview and synthesis of hypotheses. In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of Neotropical fishes. Porto Alegre: EDUPUCRS; 1998. p.279-330.
- Dias MS, Oberdorff T, Hugueny B, Leprieur F, Jézéquel C, Cornu JF, Brosse S, Grenouillet G, Tedesco PA. Global imprint of historical connectivity on freshwater fish biodiversity. Ecol Lett. 2014; 17(9):1130-40.
- Faith DP. Conservation evaluation and phylogenetic diversity. Biol Conserv. 1992; 61(1):1-10.
- Géry J. The freshwater fishes of South America. In: Fittkau EJ., Illies J, Klinge H, Schwabe GH, editors. Biogeography and ecology in South America. The Hague: Springer Netherlands; 1969. (Monographiae Biologicae, vol. 19). p.828-848.
- Hansen J, Sato M, Russell G, Kharecha P. Climate sensitivity, sea level and atmospheric carbon dioxide. Phil Trans R Soc A. 2013; 371(2001): 20120294. Available from: https://doi.org/10.1098/rsta.2012.0294
» https://doi.org/10.1098/rsta.2012.0294 - Hrbek T, Seekinger J, Meyer A. A phylogenetic and biogeographic perspective on the evolution of poeciliid fishes. Mol Phylogenet Evol . 2007; 43(3):986 98.
- Huber JH. Comparison of Old World and New World Tropical cyprinodonts. Paris: Societe francais de d’Ichthyologie; 1998.
- Hubert N, Duponchelle F, Nuñez J, Garcia-Davila C, Paugy D, Renno JF. Phylogeography of the piranha genera Serrasalmus and Pygocentrus: implications for the diversification of the Neotropical ichthyofauna. Mol Ecol. 2007; 16(10):2115-36.
- Hubert N, Renno JF. Historical biogeography of South American freshwater fishes. J Biogeogr . 2006; 33(8):1414-36.
- Ilves KL, Torti D, López-Fernández H. Exon-based phylogenomics strengthens the phylogeny of Neotropical cichlids and identifies remaining conflicting clades (Cichliformes: Cichlidae: Cichlinae). Mol Phylogenet Evol . 2018; 118:232-43.
- Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO. Picante: R tools for integrating phylogenies and ecology. Bioinformatics. 2010; 26(11):1463-64.
- Lima FCT, Caires RA. Peixes da Estação Ecológica Serra Geral do Tocantins, bacias dos rios Tocantins e São Francisco, com observações sobre as implicações biogeográficas das “águas emendadas” dos rios Sapão e Galheiros. Biota Neotrop. 2011; 11(1):231-50.
- López-Fernández H, Albert JS. Paleogene radiations. In: Albert JS, Petry P, Reis RE, editors. Historical biogeography of Neotropical freshwater fishes. Berkeley, CA: University of California Press ; 2011. p.105-117.
- Lovejoy NR, Albert JS, Crampton WGR. Miocene marine incursions and marine/freshwater transitions: Evidence from Neotropical fishes. J South Am Earth. 2006; 21(1-2):5-13.
- Lovejoy NR, Lester K, Crampton WGR, Marques FPL, Albert JS. Phylogeny, biogeography, and electric signal evolution of Neotropical knifefishes of the genus Gymnotus (Osteichthyes: Gymnotidae). Mol Phylogenet Evol . 2010; 54(1):278-90.
- Lucinda PHF, Freitas IS, Soares AB, Marques EE, Agostinho CS, Oliveira RJD. Fish, Lajeado reservoir, rio Tocantins drainage, state of Tocantins, Brazil. Check List . 2007; 3(2):70-83.
- Lundberg JG, Marshall LG, Guerrero J, Horton B, Malabarba MCSL, Wesselingh F. The stage for neotropical fish diversification: a history of tropical South American rivers. In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of Neotropical fishes. Porto Alegre: EDUPUCRS ; 1998. p.13-48.
- Luz LA, Reis LL, Sampaio I, Barros MC, Fraga E. Genetic differentiation in the populations of red piranha, Pygocentrus nattereri Kner (1860) (Characiformes: Serrasalminae), from the river basins of northeastern Brazil. Braz J Biol . 2015; 75(4):838-45.
- Martins FO. Análise filogenética e revisão taxonômica de Pseudotothyris Britski & Garavello, 1984 (Loricariidae: Hypoptopomatinae). [MSc Dissertation]. São José do Rio Preto, SP: Universidade Estadual Paulista Júlio de Mesquita Filho; 2012.
- Melo FAG, Buckup PA, Ramos TPA, Souza AKN, Silva CMA, Costa TC, Torres AR. Fish fauna of the lower course of the Parnaíba river, northeastern Brazil. Bol Mus Biol. Mello Leitão (N. Sér.). 2016; 38(4):363-400.
- Mérona BJL, Juras AA, Santos GM, Cintra IHA. Os peixes e a pesca no baixo rio Tocantins: vinte anos depois da UHE Tucuruí. Belém: Eletronorte; 2010.
- Mirande JM. Phylogeny of the family Characidae (Teleostei: Characiformes): From characters to taxonomy. Neotrop Ichthyol . 2010; 8(3):385-568.
- Nakatani M, Miya M, Mabuchi K, Saitoh K, Nishida M. Evolutionary history of Otophysi (Teleostei), a major clade of the modern freshwater fishes: Pangaea origin and Mesozoic radiation. BMC Evol Biol. 2011; 11:177. Available from: https://doi.org/10.1186/1471-2148-11-177
» https://doi.org/10.1186/1471-2148-11-177 - Nores M. The implications of Tertiary and Quaternary sea level rise events for avian distribution patterns in the lowlands of northern South America. Global Ecol Biogeogr . 2004; 13(2):149-61.
- NUGEO - Núcleo Geoambiental/Universidade Estadual do Maranhão. Bacias hidrográficas e climatologia no Maranhão. São Luís: UEMA; 2016.
- Oliveira D. River capture as relief evolution evidences: a review. Rev Dep Geo. 2010; 20:37-50.
- Paradis E, Claude J, Strimmer K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics. 2004; 20(2):289-90.
- Pavoine S, Bonsall MB. Measuring biodiversity to explain community assembly: a unified approach. Biol Rev. 2011; 86(4):792-812.
- Pereira EHL. Relações filogenéticas de Neoplecostominae Regan, 1904 (Siluriformes: Loricariidae). [PhD Thesis]. Porto Alegre, RS: Pontifícia Universidade Católica do Rio Grande do Sul; 2008.
- Petri AC, Fúlfaro VJ. Geologia do Brasil. São Paulo: Editora da Universidade Federal de São Paulo; 1983.
- Piorski NM. Diversidade genética e filogeografia das espécies Hoplias malabaricus (Bloch, 1794) e Prochilodus lacustris Steindachner, 1907 no Nordeste do Brasil. [PhD Thesis]. São Carlos, SP: Universidade Federal de São Carlos; 2010.
- Piorski NM, Castro ACL, Pereira LG, Muniz MEL. Ictiofauna do trecho inferior do rio Itapecuru, Nordeste do Brasil. B Lab Hidro. 1998; 11:15-24.
- Piorski NM, Castro ACL, Pinheiro CUB. A prática da pesca entre os grupos indígenas das bacias dos rios Pindaré e Turiaçu, no Estado do Maranhão, Nordeste do Brasil. B Lab Hidro . 2003; 16:67-74.
- Piorski NM, Castro ACL, Sousa Neto AM. Peixes do cerrado da região sul maranhense. In: Barreto LN, editor. Cerrado Norte do Brasil. São Luís: USEB; 2007. p.177-212.
- Piorski NM, Ferreira BRA, Guimarães EC, Ottoni FP, Nunes JLS, Brito PS. Peixes do Parque Nacional dos Lençóis Maranhenses. São Luis: Café & Lápis/Edufma; 2017.
- Proches S, Wilson JRU, Cowling RM. How much evolutionary history in a 10x10m plot? Proc R Soc B. 2006; 273(1590):1143-48.
- Ramos RTC, Ramos TPA, Rosa RS, Beltrão GBM, Groth F. Diversidade de peixes (Ictiofauna) da bacia do rio Curimatau, Paraíba. In: Araujo FS, Rodal MJN, Barbosa MRV, editores. Análise das variações da biodiversidade do bioma caatinga: suporte das estratégias regionais de conservação. Brasília: Ministério do Meio Ambiente; 2005. p.291-318.
- Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba River basin, northeastern Brazil. Biota Neotrop . 2014; 14(1):1-8.
- Reis RE, Kullander SO, Ferraris J, Carl J. Check List of the freshwater fishes of South and Central America. Porto Alegre: EDIPUCRS; 2003.
- Rosa R. Diversidade e conservação dos peixes da Caatinga. In: Silva JMC, Tabarelli M, Fonseca MT, Lins LV, editors. Biodiversidade da caatinga: áreas e ações prioritárias para a conservação. Brasilia: Ministério do Meio Ambiente; 2003. p.149-162.
- Rosenzweig ML. Reconciliation ecology and the future of species diversity. Oryx. 2003; 37(2):194-205.
- Rosenzweig ML. Applying species-area relationships to the conservation of species diversity. In Lomolino MV, Heaney LR, editors. Frontiers of biogeography. New directions in the geography of nature. Sunderland, MA: Sinauer Associates Inc.; 2004. p.325-343.
- Soares EC. Peixes do Mearim. São Luís: Instituto GEIA; 2005.
- Soares AB, Pelicice FM, Lucinda PHF, Akama A. Diversidade de peixes na área de influência da barragem de Peixe Angical, antes e após a formação do reservatório. In: Agostinho CS, Pelicice FM, Marques EE, editores. Reservatório de Peixe Angical: bases ecológicas para o manejo da ictiofauna. São Carlos: RiMa; 2009. p.15-27.
- Soares Júnior AV, Hasui Y, Costa JBS, Machado FB. Evolução do rifteamento e paleogeografia da margem atlântica equatorial do Brasil: Triássico ao Holoceno. Geociências. 2011; 30(4):669-92.
- Sullivan JP, Lundberg JG, Hardman M. A phylogenetic analysis of the major groups of catfishes (Teleostei: Siluriformes) using rag1 and rag2 nuclear gene sequences. Mol Phylogenet Evol . 2006; 41(3):636-62.
- Tagliacollo VA, Bernt MJ, Craig JM, Oliviera C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol . 2016; 95:20-33.
- Thomaz AT, Malabarba LR, Bonatto SL, Knowles LL. Testing the effect of palaeodrainages versus habitat stability on genetic divergence in riverine systems: study of a Neotropical fish of the Brazilian coastal Atlantic Forest. J Biogeogr . 2015; 42(12):2389-401.
- Vari RP. The Curimatidae, a lowland neotropical fish family (Pisces: Characiformes): distribution, endemism and phylogenetic biogeography. In: Vanzolini PE, Heyer WR, editors. Proceedings of a workshop on Neotropical distribution patterns. Rio de Janeiro: Academia Brasileira de Ciências; 1988. p.343-377.
- Vicente IST, Elias F, Fonseca-Alves CE. Perspectivas da produção de tilápia do Nilo (Oreochromis niloticus) no Brasil. Revista de Ciências Agrárias. 2014; 37:392-98.
- Webb CO. Exploring the phylogenetic structure of ecological communities: an example for rain forest trees. Am Nat . 2000; 156(2):145 -55.
- Webb CO, Ackerly DD, Kembel SW. Phylocom: Software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics. 2008; 24(18):2098-100.
- Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. Phylogenies and community ecology. Annu Rev Ecol Syst. 2002; 33(1):475-505.
- Wiens JJ, Donoghue MJ. Historical biogeography, ecology and species richness. Trends Ecol Evol. 2004; 19(12):639- 44.
- Wilkinson MJ, Marshall LG, Lundberg JG. River behavior on megafans and potential influences on diversification and distribution of aquatic organisms.J South Am. Earth Sci. 2006; 21(1-2):151-72.
Edited by
Publication Dates
-
Publication in this collection
19 June 2019 -
Date of issue
2019
History
-
Received
03 Oct 2018 -
Accepted
04 Apr 2019