Abstracts
Cytogenetic analyses were performed in Astyanax jacuhiensis from lago Guaíba, Brazil. The diploid number was 50, with a karyotype composed of 8m+30sm+4st+8a chromosomes, FN = 92. The AgNORs were observed in 2 to 5 chromosomes, with intra- and interindividual variation. The sm pair 8 observed always carried NORs on the short arms, presenting size heteromorphism between homologous. Fluorescence in situ hybridization (FISH) with an 18S rDNA probe only confirmed the location of ribosomal cistrons in the sm pair 8, and heteromorphism of these regions between the homologous chromosomes. C-banding revealed the occurrence of weak C-positive heterochromatin in the pericentromeric regions of several chromosomes, in addition to more evident bands interstitially located on some chromosome pairs and in the terminal region of the short arms in pair 8. C-banding plus CMA3 revealed light fluorescent signals in different chromosomes of the karyotype, with a strong terminal site in pair 8, indicating the occurrence of several GC-rich heterochromatic regions in this species. Our results provide the first description of the Astyanax jacuhiensis karyotype, showing karyotype similarities when compared to various populations of A. altiparanae and A. bimaculatus, indicating that chromosomal features are very similar for these three species.
Chromosomes; Chromomycin A3; 18S rDNA; NOR
Análises citogenéticas foram realizadas em Astyanax jacuhiensis do lago Guaíba, Brasil. O número diplóide foi 50, sendo o cariótipo composto por 8m+30sm+4st+8a cromossomos, NF = 92. As regiões organizadoras de nucléolos (AgNORs) foram observadas em 2 a 5 cromossomos, evidenciando uma variação intra e interindividual nesta espécie. O par sm 8 foi constantemente detectado com NORs nos braços curtos, mostrando um heteromorfismo de tamanho entre os homólogos. Entretanto, a hibridação in situ fluorescente (FISH) com sonda de DNAr 18S, localizou cístrons ribossômicos apenas no par 8, confirmando o heteromorfismo de tamanho entre os homólogos. O bandamento C revelou a presença de bandas discretas de heterocromatina na região pericentromérica da maioria dos cromossomos, além de algumas bandas mais evidentes intersticiais, bem como na região terminal dos braços curtos do par 8. A associação de BC+CMA3 evidenciou marcações fluorescentes mais discretas em diferentes cromossomos e uma forte marcação terminal no par 8, confirmando vários sítios de heterocromatina GC-rica nessa espécie. Nossos resultados fornecem a primeira descrição do cariótipo de Astyanax jacuhiensis, apresentando semelhanças em relação ao cariótipo de diferentes populações de A. altiparanae e A. bimaculatus, indicando que as características cromossômicas são muito semelhantes para estas três espécies.
SCIENTIFIC NOTE
Cytogenetic data on Astyanax jacuhiensis (Characidae) in the lago Guaíba and tributaries, Brazil
Rosiley B. PachecoI; Lucia Giuliano-CaetanoI; Horácio F. Julio JuniorII; Ana L. DiasI
IDepartamento de Biologia Geral, CCB, Universidade Estadual de Londrina. Rodovia Celso Garcia Cid, PR 445, Km 380, Cx. Postal 6001, 86051-970 Londrina, PR, Brazil. rosileypacheco@hotmail.com, giuliano@uel.br, anadias@uel.br
IIDepartamento de Biologia Celular e Genética, Universidade Estadual de Maringá. Av. Colombo, 5790, 87020-900 Maringá, PR, Brazil. julio@nupelia.uem.br
ABSTRACT
Cytogenetic analyses were performed in Astyanax jacuhiensis from lago Guaíba, Brazil. The diploid number was 50, with a karyotype composed of 8m+30sm+4st+8a chromosomes, FN = 92. The AgNORs were observed in 2 to 5 chromosomes, with intra- and interindividual variation. The sm pair 8 observed always carried NORs on the short arms, presenting size heteromorphism between homologous. Fluorescence in situ hybridization (FISH) with an 18S rDNA probe only confirmed the location of ribosomal cistrons in the sm pair 8, and heteromorphism of these regions between the homologous chromosomes. C-banding revealed the occurrence of weak C-positive heterochromatin in the pericentromeric regions of several chromosomes, in addition to more evident bands interstitially located on some chromosome pairs and in the terminal region of the short arms in pair 8. C-banding plus CMA3 revealed light fluorescent signals in different chromosomes of the karyotype, with a strong terminal site in pair 8, indicating the occurrence of several GC-rich heterochromatic regions in this species. Our results provide the first description of the Astyanax jacuhiensis karyotype, showing karyotype similarities when compared to various populations of A. altiparanae and A. bimaculatus, indicating that chromosomal features are very similar for these three species.
Key words: Chromosomes, Chromomycin A3,18S rDNA, NOR.
RESUMO
Análises citogenéticas foram realizadas em Astyanax jacuhiensis do lago Guaíba, Brasil. O número diplóide foi 50, sendo o cariótipo composto por 8m+30sm+4st+8a cromossomos, NF = 92. As regiões organizadoras de nucléolos (AgNORs) foram observadas em 2 a 5 cromossomos, evidenciando uma variação intra e interindividual nesta espécie. O par sm 8 foi constantemente detectado com NORs nos braços curtos, mostrando um heteromorfismo de tamanho entre os homólogos. Entretanto, a hibridação in situ fluorescente (FISH) com sonda de DNAr 18S, localizou cístrons ribossômicos apenas no par 8, confirmando o heteromorfismo de tamanho entre os homólogos. O bandamento C revelou a presença de bandas discretas de heterocromatina na região pericentromérica da maioria dos cromossomos, além de algumas bandas mais evidentes intersticiais, bem como na região terminal dos braços curtos do par 8. A associação de BC+CMA3 evidenciou marcações fluorescentes mais discretas em diferentes cromossomos e uma forte marcação terminal no par 8, confirmando vários sítios de heterocromatina GC-rica nessa espécie. Nossos resultados fornecem a primeira descrição do cariótipo de Astyanax jacuhiensis, apresentando semelhanças em relação ao cariótipo de diferentes populações de A. altiparanae e A. bimaculatus, indicando que as características cromossômicas são muito semelhantes para estas três espécies.
According to Reis et al. (2003), the family Characidae comprises 952 validated species and 400 species not yet described, totaling 1352 species. Among characids, the genus Astyanax corresponds to a complex group, and its taxonomy has been hampered by several similar forms (Melo, 2001).
Until recently, several Characidae genera were included in the subfamily Tetragonopterinae, encompassing heterogeneous groups from small to large-sized fishes. Due to lack of evidence that this subfamily constitutes a monophyletic group, Lima et al. (2003) grouped 88 genera as incertae sedis, including 620 species, among which 399 (64%) are taxonomically little known and probably made up monophyletic groups: Hyphessobrycon (97 species), Astyanax (86 species), Moenkhausia (58 species), Bryconamericus (51 species) and Hemigrammus (43 species). Besides, more than 47 genera included in incertae sedis are monotypic, and 25 genera contain only two or three species (Lima et al., 2003).
Astyanax jacuhiensis was first described as Tetragonopterus jacuhiensis (Cope, 1984). It was later transferred to the genus Astyanax (Fowler, 1906) and considered synonymous with A. bimaculatus (Eigenmann, 1921). This species was recently referred to as A. jacuhiensis (Lima et al., 2003), for the endemic population of the Jacuí River, RS, Brazil, corresponding to its type-locality.
The present study presents the first karyotype description of A. jacuhiensis, using AgNOR conventional and fluorochrome stainings, in addition to C-banding and fluorescence in situ hybridization (FISH) with 18S rDNA probe.
Twenty-four specimens of A. jacuhiensis were collected from March 2006 to November 2007 from three different localities in the lago Guaíba and tributaries, Rio Grande do Sul State (RS), Brazil, and deposited in the Museu de Zoologia da Universidade Estadual de Londrina (MZUEL): three males and two females from the Gasômetro, 30º02'06.39"S 51º14'31.84"W, (MZUEL 4032); three males and one female from the arroio do Ribeiro, 30º18'39.16"S 51º18'56.33"W, (MZUEL 4819), and nine males and six females from the Barra do Ribeiro, 30º17'29.44"S 51º18'09.27"W, (MZUEL 4034), totalizing fifteen males and nine females.
Mitotic chromosomes were obtained from kidney, according to the conventional air drying method (Bertollo et al., 1978). The chromosomes were classified as metacentric (m), submetacentric (sm), subtelocentric (st) and acrocentric (a), according the arm ratio (Levan et al., 1964). Metacentrics and submetacentrics were considered biarmed, and subtelocentric-acrocentrics as uniarmed to determine the fundamental number (FN), i.e., the number of chromosomal arms. Nucleolar organizer regions (NORs) were identified by silver nitrate staining (Ag-NORs), according to Howell & Black (1980). The location of the C-positive heterochromatin on the chromosomes was detected by C-banding, using barium hydroxide (Sumner, 1972). Fluorochrome staining with GC- specific chromomycin-A3 (CMA3) was carried out according to Schweizer (1980). A rDNA 18S probe (1700 pb) obtained from the nuclear DNA of the fish Oreochromis niloticus was used for in situ hybridization, as described by Swarça et al. (2001).
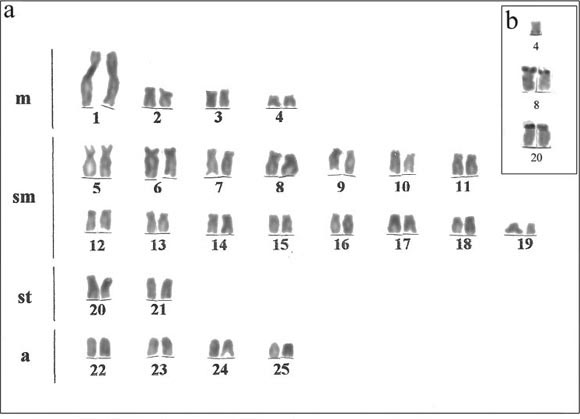
This is the first karyotype description of A. jacuhiensis, which has 2n = 50 chromosomes (8m+30sm+4st+8a), and FN = 92. Males and females showed identical karyotypes, without sex heteromorphism (Fig. 1a). The same diploid number has been observed in other Astyanax species, including A. bimaculatus (Morelli et al., 1983; Alberdi & Fenocchio, 1997; Jorge & Moreira-Filho, 2001), A. altiparanae (Daniel-Silva & Almeida-Toledo, 2001; Pacheco et al., 2001; Fernandes & Martins-Santos, 2004, 2006a) and A. scabripinnis (Mantovani et al., 2000, 2005; Ferro et al., 2001; Fernandes & Martins-Santos, 2005), thus representing a common characteristic in this genus.
Silver nitrate staining showed 2 to 5 chromosomes with probable Ag-NORs sites: on the short arms of sm pair 8 displaying size heteromorphism, on the short arms of st pair 20, and on the long arm of one chromosome of m pair 4, which could correspond to system of multiple NORs. Pair 8 was the most frequent AgNOR-bearing chromosome, being visualized in all metaphases analyzed (Fig. 1b). However, the fluorescence in situ hybridization showed that only sm pair 8 displayed 18S rDNA sites, also confirming the size heteromorphism between homologous (Fig. 1c), thereby characterizing a simple NOR system in A. jacuhiensis. AgNOR heteromorphism is frequently seen in fish, and can be ascribed to variations in the number of copies of ribosomal cistrons between homologous through unequal crossing-overs, transposition or rearrangements, such as deletions and duplications (Galetti-Jr et al., 1995). The other chromosomal sites that were positive for silver nitrate staining, but have proved to be negative after FISH with 18S rDNA probe, may represent only a kind of heterochromatin with some affinity to silver nitrate, irrespective of whether they do or do not carry any site of rDNA.
Although multiple NORs are relatively common in Astyanax (Jorge & Moreira-Filho, 2001; Pacheco et al., 2001; Souza et al., 2001; Almeida-Toledo et al., 2002; Mantovani et al., 2005; Fernandes & Martins-Santos, 2006a, b), a simple NOR system has also been reported in A. bimaculatus from the São Francisco, Doce, and Paraguay Rivers (Paganelli, unpubl. data), and in A. altiparanae from the Paraná River (Fernandes & Martins-Santos, 2004) and Monjolinho River (Peres et al., 2008).
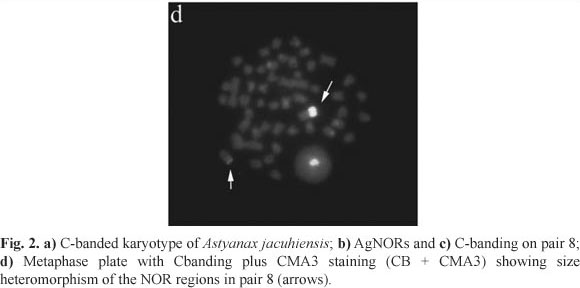
Heterochromatin has been an important marker for the characterization and differentiation of several species and populations. Its distribution pattern in A. jacuhiensis resembled that found in some populations of A. altiparanae (Daniel-Silva & Almeida-Toledo, 2001; Fernandes & Martins-Santos, 2004), and A. bimaculatus (Paganelli, unpubl. data), where interstitial C-positive bands were also observed. In fact, in addition to discrete pericentromeric bands, A. jacuhiensis showed conspicuous interstitial C-positive blocks in several pairs of the karyotype (Fig. 2a), as well as in the terminal region of pair 8, in correspondence with the NOR location (Fig. 2b-c).
Up to four CMA3 positive signals were found in A. jacuhiensis (Fig. 1d), where pair 8 was always seen with a strong terminal staining, and demonstrating the same heteromorphism observed with AgNORs and 18S rDNA sites. C-banding plus CMA3 staining (CB+CMA3) evidenced some pericentromeric and interstitial positive sites. However, pair 8 showed strong heteromorphic sites corresponding to the NOR regions (Fig. 2d). Therefore, A. jacuhiensis contains a class of GC-rich heterochromatin in some chromosome pairs and the ribosomal cistrons appear to be interspersed with this kind of heterochromatin.
Astyanax bimaculatus, from the Paraná River, has a karyotypic structure similar to that found in various populations of A. altiparanae (Jorge & Moreira Filho, 2001). In addition, A. jacuhiensis also showed similarities in the diploid number, karyotype formula, and chromosome banding when compared to various populations of A. altiparanae and A. bimaculatus, indicating that these three species are very similar in relation to their chromosomal features. It is important to note that these cytotaxonomic data somehow support Eigenmann's proposal (1921). However, further cytogenetic and taxonomic studies should be conducted to better clarify this question.
Acknowledgements
The authors are grateful to CAPES, CNPq and Fundação Araucária for their financial support and to Luiz R. Malabarba (UFRGS) for the identification of the studied species. We are also thankful to Albert Leyva for his help in the preparation of this manuscript.
Literature Cited
Accepted August 8, 2010
Published September 24, 2010
- Alberdi, A. J. & A. S. Fenocchio. 1997. Karyotypes of five Tetragonopterinae species (Pisces, Characidae) from Argentina. Cytologia, 62: 171-176.
- Almeida-Toledo, L. F., C. Ozouf-Costaz, F. Foresti, C. Bonillo, F. Porto-Foresti & M. F. Z. Daniel-Silva. 2002. Conservation of the 5S-bearing chromosome pair and co-localization with major rDNA clusters in five species of Astyanax (Pisces, Characidae). Cytogenetic and Genome Research, 97: 229-233.
- Bertollo, L. A. C., C. S. Takahashi & O. Moreira-Filho. 1978. Cytotaxonomic considerations on Hoplias lacerdae (Pisces, Erythrinidae). Brazilian Journal of Genetics, 1: 103-120.
- Cope, E. D. 1984. On the fishes obtained by the Naturalist Expedition in Rio Grande do Sul. Proceedings of the American Philosofical Society, 33: 84-104.
- Daniel-Silva, M. F. Z. & L. F. Almeida-Toledo. 2001. Chromosome R-banding pattern and conservation of a marker chromosome in four species, genus Astyanax (Characidae, Tetragonopterinae). Caryologia, 54: 209-215.
- Eigenmann, C. H. 1921. The American Characidae. Memoirs of Museum of Comparative Zoology of Harvard College, 43: 209-310.
- Fowler, H. W. 1906. Further Knowledge of some heterognathous part 2. Proceedings of the Academy of Natural Sciences of Philadelphia, 58: 431-83.
- Fernandes, C. A. & I. C. Martins-Santos. 2004. Cytogenetic studies in two populations of Astyanax altiparanae (Pisces, Characiformes). Hereditas, 141: 328-332.
- Fernandes, C. A. & I. C. Martins-Santos. 2005. Sympatric occurrence of three cytotypes and four morphological types of B chromosomes of Astyanax scabripinnis (Pisces, Characiformes) in the river Ivaí basin, state of Paraná, Brazil. Genetica, 124: 301-306.
- Fernandes, C. A. & I. C. Martins-Santos. 2006a. Mapping of the 18S and 5S ribosomal RNA genes in Astyanax altiparanae Garutti & Britski, 2000 (Teleostei, Characidae) from the upper Paraná river basin, Brazil. Genetics and Molecular Biology, 29: 1-5.
- Fernandes, C. A. & I. C. Martins-Santos. 2006b. Chromosomal location of 5S and 18S rRNA genes in three sympatric cytotypes of Astyanax scabripinnis (Characiformes, Characidae) from the Ivaí river basin, state of Paraná, Brazil. Caryologia, 59: 253-259.
- Ferro, D. A. M., D. M. Néo, O. Moreira-Filho & L. A. C. Bertollo. 2001. Nucleolar organizing regions, 18S and 5S rDNA in Astyanax scabripinnis (Pisces, Characidae): population and functional diversity. Genetica, 110: 55-62.
- Galetti-Jr, P. M., C. A. Mestriner, P. J. Monaco & E. M. Rash. 1995. Post-zygotic modifications and intra and interindividual nucleolar organizing region variations in fish: report of a case involving Leporinus friderici Chromosome Research, 3: 285-290.
- Howell, W. M. & D. A. Black. 1980. Controlled silver-staining of nucleolus organizer regions with the protective coloidal developer: a 1-step method. Experientia, 36: 1014-1015.
- Jorge, L. C. & O. Moreira-Filho. 2001. Estudios citogenéticos en Astyanax bimaculatus (Pisces, Characidae) del río Paraná, Argentina. Revista de Ictiologia, 9: 21-24.
- Levan, A., K. Fredga & A. A. Sandberg. 1964. Nomenclature for centromeric position on chromosome. Hereditas, 52: 201-220.
- Lima, F. C. T., L. R. Malabarba, P. A. Buckup, J. F. P. Silva, R. P. Vari, A. Harold, R. Benine, O. T. Oyakawa, C. S. Pavanelli, N. A. Menezes, C. A. S. Lucena, M. C. S. L. Malabarba, Z. M. S. Lucena, R. E. Reis, F. Langeani, L. Cassati, V. A. Bertaco, C. Moreira & P. H. F. Lucinda. 2003. Genera incertae sedis in Characidae. Pp. 106-169. In: Reis, R. R., S. O. Kullander, C. J. Ferraris Jr. (Eds.). Check list of the freshwater fishes of South and Central America. Porto Alegre, Edipucrs, 748p.
- Mantovani, M., L. D. S. Abel, C. A. Mestriner & O. Moreira-Filho. 2000. Acentuated polymorfhism of heterochrimatin and nucleolar organizer regions in Astyanax scabripinnis (Pisces, Characidae): tools understanding karyotypic evolution. Genetica, 109: 161-168.
- Mantovani, M., L. D. S. Abel & O. Moreira-Filho. 2005. Conserved 5S and variable 45S rDNA chromosomal localization revealed by FISH in Astyanax scabripinnis (Pisces, Characidae). Genetica, 123: 211-216.
- Melo, F. A. G. 2001. Revisão taxonômica das espécies do gênero Astyanax Baird & Girard, 1854 (Teleostei: Characiformes: Characidae) da região da Serra dos Órgãos. Arquivos do Museu Nacional, 59: 1-46.
- Morelli, S., L. A. C. Bertollo, F. Foresti, O. Moreira-Filho & A. S. Toledo-Filho. 1983. Cytogenetics considerations on the genus Astyanax (Pisces, Characidae), I Karyotypic variability. Caryologia, 36: 235-244.
- Pacheco, R. B., L. Giuliano-Caetano & A. L. Dias. 2001. Occurrence of cytotypes and multiple NORs in an Astyanax altiparanae population (Pisces, Tetragonopterinae). Chromosome Science, 5: 109-114.
- Peres, W. A. M., L. A. C. Bertollo & O. Moreira-Filho. 2008. Physical mapping of the 18S and 5S ribosomal genes in nine Characidae species (Teleostei, Characiformes). Genetics and Molecular Biology, 31: 222-226.
- Reis, R. R., S. O. Kullander & C. J. Ferraris Jr. 2003. Check List of the Freshwater Fishes of South and Central America, Porto Alegre, Edipucrs, 729p.
- Souza, I. L., J. Galiann, P. D. L. Rúa, L. A. C. Bertollo & O. Moreira-Filho. 2001. Non-radom distribution of the GC-rich heterochromatin and nuclear rDNA sites on Astyanax scabripinnis chromosomes. Cytologia, 66: 85-91.
- Sumner, A. T. 1972. A simple technique for demonstrating centromeric heterochromatin. Experimental Cell Research, 75: 304-306.
- Schweizer, D. 1980. Simultaneous fluorescent staining of R-bands and specific heterochromatic regions (DA-DAPI bands) in human chromosomes. Cytogenetic and Cell Genetics, 27: 190-193.
- Swarça, A. C., M. M. Cestari, L. Giuliano-Caetano & A. L. Dias. 2001. Cytogenetic Characterization of the Large South American Siluriform Fish Species Zungaro zungaro (Pisces, Pimelodidae). Chromosome Science, 5: 51-55.
Publication Dates
-
Publication in this collection
04 Nov 2010 -
Date of issue
2010