Abstracts
The Neotropical auchenipterid catfish genus Auchenipterichthys is reviewed and found to include four species. Auchenipterichthys thoracatus, formerly considered to be widely distributed throughout the Amazon River basin, is found to be restricted to the upper Madeira River basin. The widespread Amazonian species that had been misidentified as A. thoracatus is, instead, A. coracoideus; a species that also occurs in the upper Essequibo River. Auchenipterichthys longimanus, the most widely distributed species of the genus, is found through much of the Amazon and Orinoco River basins. The fourth species of the genus, A. punctatus (and its junior synonym A. dantei), is found in the upper portions of the Orinoco and Negro River basins in Venezuela and the central portions of the Amazon River basin in Brazil. All four species of Auchenipterichthys are redescribed and illustrated, and a key to the species is provided.
Auchenipteridae; Neotropical; Auchenipterichthys
O gênero Neotropical Auchenipterichthys de Auchenipteridae é revisado, incluindo quatro espécies. Auchenipterichthys thoracatus, anteriormente considerado como largamente distribuído na bacia do rio Amazonas, é restringido para a região superior da bacia do rio Madeira. A espécie amazônica largamente distribuída e que tem sido identificada erroneamente como A. thoracatus é, ao invés disto, A. coracoideus; uma espécie que ocorre igualmente na região superior do rio Essequibo. Auchenipterichthys longimanus, a espécie de maior distribuição no gênero, é encontrada nas bacias dos rios Amazonas e Orinoco. A quarta espécie do gênero, A. punctatus (e seu sinônimo júnior A. dantei), é encontrada nas porções superiores dos rios Orinoco e Negro na Venezuela e porção central do rio Amazonas no Brasil. Todas as quatro espécies de Auchenipterichthys são redescritas e ilustradas, e fornecida uma chave para a identificação das especies.
Catfishes of the genus Auchenipterichthys (Osteichthyes: Siluriformes: Auchenipteridae); a revisionary study
Carl J. Ferraris Jr.; Richard P. Vari; Sandra J. Raredon
Division of Fishes, Smithsonian Institution, PO Box 37012, National Museum of Natural History, WG-14, MRC 159, Washington, DC 20013-7012, USA (e-mail: carlferraris@comcast.net (CJF), vari.richard@nmnh.si.edu (RPV), raredon.sandra@nmnh.si.edu (SJR) [Send reprint requests to RPV]
ABSTRACT
The Neotropical auchenipterid catfish genus Auchenipterichthys is reviewed and found to include four species. Auchenipterichthys thoracatus, formerly considered to be widely distributed throughout the Amazon River basin, is found to be restricted to the upper Madeira River basin. The widespread Amazonian species that had been misidentified as A. thoracatus is, instead, A. coracoideus; a species that also occurs in the upper Essequibo River. Auchenipterichthys longimanus, the most widely distributed species of the genus, is found through much of the Amazon and Orinoco River basins. The fourth species of the genus, A. punctatus (and its junior synonym A. dantei), is found in the upper portions of the Orinoco and Negro River basins in Venezuela and the central portions of the Amazon River basin in Brazil. All four species of Auchenipterichthys are redescribed and illustrated, and a key to the species is provided.
Key words: Auchenipteridae, Neotropical, Auchenipterichthys.
RESUMO
O gênero Neotropical Auchenipterichthys de Auchenipteridae é revisado, incluindo quatro espécies. Auchenipterichthys thoracatus, anteriormente considerado como largamente distribuído na bacia do rio Amazonas, é restringido para a região superior da bacia do rio Madeira. A espécie amazônica largamente distribuída e que tem sido identificada erroneamente como A. thoracatus é, ao invés disto, A. coracoideus; uma espécie que ocorre igualmente na região superior do rio Essequibo. Auchenipterichthys longimanus, a espécie de maior distribuição no gênero, é encontrada nas bacias dos rios Amazonas e Orinoco. A quarta espécie do gênero, A. punctatus (e seu sinônimo júnior A. dantei), é encontrada nas porções superiores dos rios Orinoco e Negro na Venezuela e porção central do rio Amazonas no Brasil. Todas as quatro espécies de Auchenipterichthys são redescritas e ilustradas, e fornecida uma chave para a identificação das especies.
Introduction
The genus Auchenipterichthys was first proposed in Bleeker (1862-63) for a single species of Brazilian catfish that had been originally placed by Kner (1857) in Auchenipterus, a genus that at that time included many of the Neotropical catfishes that are currently recognized as the Auchenipteridae. Although Bleeker did not recognize a group equivalent to the Auchenipteridae, he clearly recognized that species then placed within Auchenipterus did not belong together in one genus. He created several new genera to accommodate species formerly placed in Auchenipterus among which was Auchenipterichthys, which included only A. thoracatus (Kner).
Shortly after the creation of Auchenipterichthys, Günther (1864) described a second species in the genus. The two species were reported to be widely distributed throughout the Amazon and Orinoco River basins by Soares-Porto (1994), who also named a third species as A. dantei. In a review of the types of auchenipterid catfishes held in MNHN, Royero and Hureau (1996) discovered that the species named by Soares-Porto had been named previously as Auchenipterus punctatus by Valenciennes (in Cuvier & Valenciennes, 1840).
Examination of specimens identified as Auchenipterichthys thoracatus from the Guaporé River basin (the type locality of the species) and from other parts of the Amazon River basin led us to suspect that more than one species was included within that species complex, which prompted this revision.
Material and Methods
Unpaired fin-ray counts and vertebral counts were taken from radiographs. The two posterior most dorsal- and anal-fin rays articulating on the corresponding last pterygiophore of each fin were counted as separate rays. Caudal-fin ray counts report the principal rays (i.e., branched rays and the first unbranched ray of the dorsal and ventral lobes). Paired fin rays were counted under a stereomicroscope and include all elements.
Measurements were taken, point-to-point, as follows: the body depth was taken at the dorsal-fin origin and anal-fin origin; the head length was measured parallel to the body axis, from the tip of the snout to the posterior tip of the fleshy operculum; the cleithral width was measured across the bony cleithrum just anterior to the pectoral spine; the snout length was measured from the tip of the snout to the anterior margin of the eye; the bony interorbital width represents the shortest distance across the bony interorbit; the dorsal-fin spine length was measured from the junction point between the first spine (spinelet) and the second spine to the tip of the bony spine, but not including the fleshy or flexible bony terminal parts of the spine; and the anal-fin base length was measured from the origin of the anal fin to the insertion of the last anal-fin ray.
The following abbreviations are used in the text: SL standard length; TL total length; HL head length; asl above sea level.
Institutional abbreviations are as follows: AMNH, American Museum of Natural History, New York; ANSP, Academy of Natural Sciences of Philadelphia; AUM, Auburn University Museum, Auburn; BMNH, Natural History Museum, London; CAS, California Academy of Sciences, San Francisco; CM, Carnegie Museum, Pittsburgh; FMNH, Field Museum of Natural History, Chicago; INHS, Illinois Natural History Survey, Champaign; IU, Indiana University, Bloomington (now distributed among several institutions); MCNG, Museu de Ciencias Naturales, Guanare; MCZ, Museum of Comparative Zoology, Cambridge; MNHN, Muséum National d'Histoire Naturelle, Paris; MNRJ, Museu Nacional, Rio de Janeiro; MZUSP, Museu de Zoologia, Universidade de São Paulo; NMW, Naturhistorisches Museum, Vienna; NRM, Swedish Museum of Natural History; ROM, Royal Ontario Museum, Toronto; SU, Stanford University, Palo Alto (now at CAS); UF, Florida Museum of Natural History, Gainesville; UMMZ, University of Michigan, Museum of Zoology, Ann Arbor; USNM, National Museum of Natural History, Smithsonian Institution, Washington; and ZMB, Museum für Naturkunde, Berlin.
Results
Auchenipterichthys Bleeker, 1862
Auchenipterichthys Bleeker, 1862 (in Bleeker, 1862-63): 7. Type species: Auchenipterus thoracatus Kner, 1857. Type by original designation. Gender: Masculine.
Diagnosis. A genus of the Auchenipteridae characterized by the following combination of characters: eye large, midlateral, and visible in both dorsal and ventral views; anal fin with long base and at least 18 branched fin rays; lateral surface of body with several vertically-oriented rows of pale spots above the lateral line; caudal fin emarginate or obliquely truncate; pelvic fin with eight or nine branched rays; and adipose fin present.
Remarks. At present, no derived characters have been identified that are unique to the species of Auchenipterichthys (see Ferraris, 1988; Soares-Porto, 1994; de Pinna, 1998). Instead, the characters listed in the diagnosis are derived characters within the Auchenipteridae (except for the presence of the adipose fin, which is primitive), each of which is also present in at least one other genus in the family, but that occur in common only in the species of Auchenipterichthys. In the absence of an identified unique synapomorphy it is possible that Auchenipterichthys is non-monophyletic; however, no evidence has been advanced to date to suggest that the genus is not natural. In the absence of evidence to the contrary we continue to treat the included species as a single, presumably natural, genus.
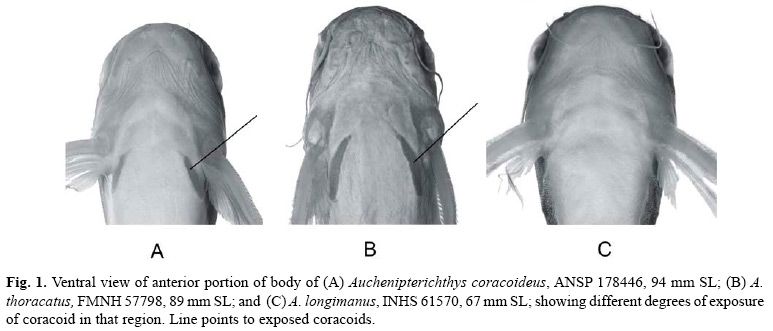
Based on overall similarity, the species of Auchenipterichthys fall into two groups. One group, which consists of A. coracoideus and A. thoracatus, has the ventral surface of the coracoid bone covered only by a thin integument (Figs. 1a, b) and thus appears to be exposed to the body surface, has eight branched pelvic-fin rays, and has an obliquely-truncate caudal-fin margin. The second group, which includes A. longimanus and A. punctatus, has a thick layer of integument superficial to the ventral margin of the coracoid, such that the coracoid is not visible on the body surface (Fig. 1c), has nine branched pelvic-fin rays, and, in most individuals, has a caudal fin that is emarginate and symmetrical (some larger specimens exhibit a truncate fin margin). Because the monophyly of Auchenipterichthys has yet to be established, future studies of the relationships within the Auchenipteridae should include a representative species from each of these two groups so as to determine whether the groups cluster together as a natural unit.
Sexual dimorphism. Sexual dimorphism in the species of Auchenipterichthys includes the enlarged urogenital orifice in females, whereas the urogenital pore of males is located at the distal tip of an elongated tube that is bound by integument to the anterior margin of the anal fin. Males also have an elongated and enlarged posterior unbranched and anterior branched anal-fin rays and elongated spinules along the anterior and posterior margins of the distal part of the dorsal-fin spine. Nuptial males of at least one species (A. coracoideus) have an elongated dorsal-fin spine. Species of Auchenipterichthys lack the ossification of the maxillary barbel, elongation of the unbranched pelvic-fin ray, and presence of keratinaceous unculi on dorsal surface of the head, abdomen and maxillary barbel that are found in some other genera of auchenipterids (Ferraris and Vari, 1999, Vari and Ferraris, 1998, Akama and Ferraris, 2003).
Key to the species of Auchenipterichthys
Auchenipterichthys coracoideus (Eigenmann & Allen, 1942)Figs. 1-5
Auchenipterichthys thoracatus (not of Kner, 1857), Eigenmann & Eigenmann, 1888: 154 [in listing of South American catfishes; specimens from Coary (=Coari) and Hyavary (=Javari), Brazil]. Eigenmann, 1910: 396 [in listing of South American fresh water fishes; specimens from Coary (=Coari) and Hyavary (=Javari), Brazil]. Eigenmann & Eigenmann, 1890: 282 [redescription of A. thoracatus, erroneously based on specimens of A. coracoideus; Coary (=Coari) and Hyavary (=Javari), Brazil]. Fowler, 1951: 458 [in part, citations of species from outside of upper rio Madeira basin]. Mees, 1974: 35 [in part, not synonymy of Trachycorystes coracoideus into Auchenipterichthys thoracatus; not citations of Auchenipterichthys coracoideus from outside of upper rio Madeira basin; not cited specimen from Peru]. Ortega and Vari, 1986: 114 [in listing of freshwater fishes of Peru].
Trachycorystes coracoideus Eigenmann & Allen, 1942: 44 and 120 [type-locality: Peru, Iquitos; syntypes: CAS 63746 (3)]. Gosline, 1945: 458 [based on Eigenmann & Allen, 1942]. Fowler, 1945: 64 [in listing of species of fishes in Peru]. Fowler, 1951: 473 [literature compilation].
Auchenipterichthys longimanus (not of Günther, 1864), Mees, 1974: 38 [in part, specimen from Nazareth, Peru].
Auchenipterichthys thoracatum (not of Kner, 1857), Merona et al., 1987: 83 [Brazil, lower rio Tocantins; increasing relative abundance following closure of Tucuruí dam].
Auchenipterichthys coracoideus, Ferraris, 2003: 472 [checklist].
Diagnosis. A species of Auchenipterichthys with coracoid bone covered only by thin layer of integument and exposed ventrally (Fig. 1a), an obliquely truncated caudal-fin margin, typically 25 or fewer branched anal-fin rays (rarely 26; Table 1), eight branched pelvic-fin rays, anterior teeth on the premaxilla visible when the mouth is closed, and the body pigmentation dark gray dorsally and lighter (pale in some specimens) laterally and ventrally, body without distinct dark spots. Auchenipterichthys coracoideus is most similar in appearance to A. thoracatus, which typically has 26 or more branched anal-fin rays, and is readily distinguished from its other two congeners, A. longimanus and A. punctatus, which have coracoids that are covered ventrally by a thick layer of integument (Fig. 1c), the anterior teeth on the premaxilla that are not visible when mouth is closed and, typically, nine branched pelvic-fin rays.
Description. Body depth at dorsal-fin origin 0.230.26 of SL and slightly greater than body width at cleithrum. Body depth at anal-fin origin 0.230.25 of SL. Body compressed, with width at anal-fin origin 0.390.41 of body depth at that point. Ventral surface of coracoids exposed on ventral surface of body (see Fig. 1a). Lateral line complete and midlateral. Canal having irregular zigzag pattern, with oblique, posteriorly-directed branches extending off main canal. Lateral line canal extending short distance onto, and obliquely posterodorsally-directed on, caudal-fin base.
Head depressed anteriorly; depth of head at vertical through middle of orbit approximately one-half of head width at middle of orbit. Head length 0.360.39 of SL. Dorsal profile of head broadly convex anteriorly and then straight from vertical running through anterior margin of orbit to dorsal-fin origin. Distance from midpoint of snout to anterior margin of orbit slightly greater than horizontal diameter of orbit. Snout margin broadly rounded from dorsal view. Interorbital width approximately 0.550.58 of HL and approximately equal to distance from middle of eye to posterior margin of opercle. Eye large, lateral, and visible in both dorsal and ventral views. Orbit distinctly ovoid with horizontal axis longer.
Barbels slender and thread-like. Maxillary barbel long, extending posteriorly slightly past margin of opercle. Medial mandibular barbel originating immediately posterior of lower lip; adpressed barbel extending posteriorly to point slightly short of vertical through transverse plane through origin of lateral mandibular barbel. Lateral mandibular barbel originating in plane slightly anterior of vertical through middle of orbit and extending posteriorly approximately to anterior portion of exposed cleithrum.
Branchiostegal membrane broadly attached to isthmus; ventral margin of gill opening extending to anterior margin of exposed portion of cleithrum.
Mouth terminal, but with upper jaw extending slightly beyond margin of lower jaw. Anterior premaxillary teeth visible from ventral view when mouth is closed. Teeth on premaxilla minute and arranged in band. Band consisting of approximately 8 irregular series of teeth at symphysis and of 10 irregular series laterally. Dentary teeth slightly larger than those on premaxilla, with approximately 6 series of teeth at symphysis that progressively decrease to one tooth row posterolaterally.
Dorsal-fin origin at 0.37 of SL. Length of dorsal-fin base approximately one-half of length of first branched dorsal-fin ray. Dorsal-fin spine pungent and slightly curved with convex anterior margin. Length of dorsal-fin spine approximately equal to HL except in nuptial males in which spine may be longer than 1.5 times HL. Basal half of anterior margin of dorsal-fin spine bearing two rows of small, blunt projections. Spine margin smooth or finely serrated distally. Posterior margin of spine with few, short, medial, obliquely distally-directed serrae; serrae proportionally longer in nuptial males. Dorsal-fin rays II,6. Adipose fin relatively small.
Caudal fin obliquely truncate with dorsal most branched ray longest. Principal caudal-fin rays i,7,8,i.
Anal-fin base approximately 0.240.27 of SL. Anal-fin origin located distinctly posterior of middle of SL and at, or slightly posterior of, middle of TL. Anal-fin margin straight, with first ray longest and subsequent rays becoming progressively shorter, but with anterior rays in nuptial males extending beyond margin of rest of fin. Last anal-fin ray without membranous attachment to caudal peduncle. Anal-fin rays iii,20 to iii,26 (Table 1).
Distal margin of pelvic fin broadly convex with third branched ray longest. Pelvic-fin insertion at middle of SL. Tip of adpressed pelvic-fin falling short of anal-fin origin. Pelvic-fin rays i,8 (Table 1).
Pectoral fin with strong spine serrated along entire length of both margins. Serrae antrorse along anterior margin of spine and retrorse along posterior margin. Anterior pectoral-fin rays longest. Fin margin straight anteriorly and convex along posterior rays. Pectoral-fin rays typically I,8, rarely I,7 or I,9 (Table 1).
Pigmentation pattern in alcohol. Overall ground coloration most often grayish, with overall coloration darker on dorsal portion of head and body and in some individuals on midlateral portion of body posterior of head in region overlying swim bladder. Overall pigmentation pattern less intense in some individuals, most notably those from some locations in western portions of species distribution, albeit without any distinct geographic pattern to differences in coloration. Abdomen unpigmented. Snout, upper lip, and region ventral of margin of lower lip very dark in many, but not all, specimens.
Lateral and dorsolateral surface of body with several series of vertically-aligned, unpigmented, rounded spots of size most often approximately equal to one-fifth to one-quarter width of pupil or smaller. These series begin most often under, but in some individuals somewhat anterior of, base of dorsal-fin spine and extend posteriorly to beyond posterior terminus of base of adipose fin. Spots often difficult to discern in specimens of overall light ground pigmentation. Midlateral surface of body with irregular longitudinal series of unpigmented spots extending from rear of head to posterior margin of caudal peduncle. Unpigmented spots coalesce in some individuals into larger spots of irregular form. Region anterior of vertical of pelvic-fin origin and ventral of midlateral series of unpigmented spots with few, scattered, unpigmented spots.
Patch of dark to very dark pigmentation typically present anterior to base of dorsal fin. First and second interradial membranes of dorsal fin dark distally. Adipose fin with dark basal spot continuous with dark pigmentation of body in most individuals, other specimens with diffuse batch of dark chromatophores basally that do not form distinct spot. Caudal fin distinctly dusky basally and continuing pigmentation of body. Region of dark pigmentation on base of caudal fin with distinct, straight to slightly irregular, posterior margin; margin somewhat anteroventrally inclined. Some specimens with small, unpigmented spots within dusky, basal pigmentation field in region overlying central rays.
Basal region of dark caudal-fin pigmentation followed by hyaline region and then vertical band of less intense dark pigmentation along distal margin of fin. Anal fin with variably developed dark pigmentation. Some individuals with distinctly darker basal band on caudal fin formed of dark chromatophores comparable in size and form to those on adjoining region of body; when present, darker field of pigmentation often extending more distally on dorsal rays of mature males. Other individuals with less developed dark pigmentation continuous across fin. Most specimens with distal portions of fin darker than middle sections of rays. Dorsal surface of pelvic fin with diffuse dark pigmentation in some individuals, but without distinct patch of dark pigmentation. Distal portion of fin with dark pigmentation in most individuals. Margins and sometimes dorsal surface of pectoral-fin spine dark. Pectoral-fin rays variably outlined with dark chromatophores.
Maxillary barbel somewhat to darkly pigmented dorsally; barbel pale ventrally. Mandibular barbels unpigmented.
Color pattern in life. Dark pigmentation as in preserved specimens. Lateral and posterodorsal surface of head along with lateral surface of anterior portion of body, dorsolateral surface of body and basal one-half of caudal fin with variably intense yellowish coloration. Yellow coloration also apparent on basal one-half of dorsal-fin spine and rays and on adipose fin (Fig. 2).
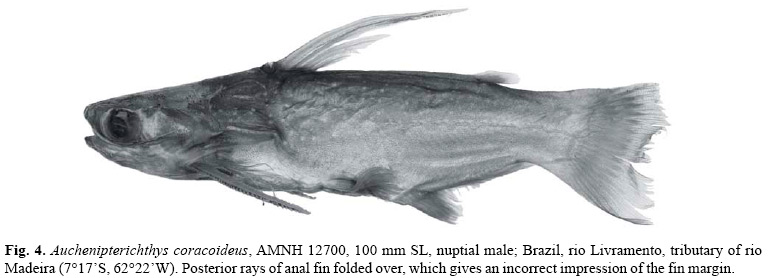
Sexual dimorphism.Auchenipterichthys coracoideus demonstrates sexual dimorphism in the length of the dorsal spine and anterior rays of the anal fin as discussed above (see Figs. 3 and 4).
Distribution. Tocantins River, central and upper portions of Amazon River, and Essequibo River basins (Fig. 5).
Remarks.Auchenipterichthys coracoideus was originally described as a species of Trachycorystes by Eigenmann & Allen (1942: 120) without a discussion of the basis for that generic assignment; however, the species is clearly a species of Auchenipterichthys. Auchenipterichthys coracoideus has been repeatedly misidentified as A. thoracatus by various authors who applied that name to samples of the genus with exposed coracoids that originated across the Amazon basin (see synonymies for those two species). All population samples of A. thoracatus examined in this study originated in a relatively restricted region in the upper portions of the Madeira River in eastern Bolivia and southwestern Brazil (Fig. 10; dots). We consequently treat all reports of A. thoracatus from the portions of the Amazon basin outside of the upper Madeira River system as A. coracoideus, which has a wide distribution across that river basin (Fig. 5).
In addition to the difference in the number of anal-fin rays between Auchenipterichthys coracoideus and A. thoracatus, the two species appear to differ in the shape of the head and the angle of divergence of the exposed portion of the coracoids. The ventral margin of the head of A. coracoideus is nearly horizontal, in contrast to the more oblique aspect of that region in A. thoracatus. The contralateral coracoids diverge more posteriorly in A. coracoideus, whereas they are more nearly parallel in A. thoracatus (see Figs. 1a-b). However, we found it impossible to quantify these differences sufficiently well to include them in the diagnoses of the respective species.
Mees (1974: 36) reported a lot from Nazareth, Peru (FMNH 15502) as Auchenipterichthys longimanus. Examination of that material demonstrates that is it A. coracoideus.
Material examined. BRAZIL. Amazonas: lower rio Madeira, approximately 150 km up from mouth, Borba (4º23'S, 59º35'W), MZUSP 28351, 2 (50-56). Rio Hyavary (=rio Javari), tributary of rio Solimões at the Peruvian-Brazilian border (4º21'S, 70º02'W), MCZ 7347, 4 (4496). Lago do Coari on rio Solimões, at Coari (4º08'S, 63º07'W), MCZ 7386, 1 (102); MCZ 7387, 12 (41-54); MCZ 7353, 1 (102), MCZ 7383, 1 (102), MCZ 7388, 1 (101), MCZ 7372 (plus parts of MCZ 7353, MCZ 7386, MCZ 7388), 15 (80111; some specimens originally in four separate lots now inseparably mixed); CAS 76784, 1 (47); USNM 41529, 2 (88 96). rio Livramento, tributary of rio Madeira (7º17'S, 62º22'W), AMNH 12700, 8 (94112); MCZ 34126, 1 (119). Lago Terra Preta, Janauari (approximately 3º12'36"S, 60º01'56"W), USNM 275525, 5 (6978); USNM 276526, 6 (5365). Goiás: rio Araguaia, lago Ressaca, Luís Alves (approximately 13º14'S, 50º35'W), MZUSP 53869, 1 (67). Rio Araguaia, Lago Preto, Luís Alves (approximately 13º14'S, 50º35'W), MZUSP 54183, 2 (7985). Mato Grosso: rio Araguaia, MZUSP 44073, 3 (6887). Rio Araguaia, lago da Alvoradinha, MZUSP 53568, 1 (69). Rio Araguaia, lago Montaria, MZUSP 53569, 4 (6072). Rio Araguaia, near Ilha do Biratã, MZUSP 62848, 1 (71). Rio Araguaia, lago das Branquinhas, MZUSP 53570, 5 (7381). Pará: matas of igapó do rio Cinzento, tributary of rio Itacaiunas, MZUSP 52971, 1 (89). Rondônia: lago Piauí, mouth of rio Jamari (8º27'S, 63º30'W), MZUSP 28377, 2 (114122). GUYANA. Pirara Creek, Apotorie, USNM 378826, 3 (4149). Guyana, no specific locality, USNM 317963, 1 (40). PERU. Loreto: Iquitos (3º46'S, 73º15'W), CAS 63746, 1 (104; syntype of Trachycorystes coracoideus ), CAS 220574, 2 (104107; syntypes of Trachycorystes coracoideus). Vicinity of Iquitos, río Nanay opposite naval base, backwater pools off Cocha (4 miles above Amazon River, approximately 3º46'S, 73º15'W), ANSP 139052, 13 (4958). Río Nanay (tributary of río Amazon) at Pampa Chica, village 4.54 km W of Iquitos (large beach along N Bank; 3º45'09"S, 73º17'00"W), ANSP 178446, 6 (5594). Yaguas Yacu, near Pebas (3º19'59"S, 71º48'59"W), SU 58655, 4 (4265); SU 58659, 1 (54). Río Nanay drainage, Zúngaro Cocha (3º49'S, 73º22'W), NRM 13516, 2 (3641). Río Samiria drainage, right bank muddy playa, 30 minutes upstream of Pithecia (5º13'S, 74º42'W), NRM 18577, 1 (58). Caño Abico, tributary of río Samiria, approximately 6 to 7 km from mouth in río Marañon (4º55'S, 74º30'W), FMNH 111525, 6 (5772). Río Yavari, San Fernando, 100 m asl (4º09'S, 70º13'59"W), FMNH 96230, 2 (56100). Río Manite system, caño entering Río Manite about 10 km upriver of junction of Río Manite and río Amazonas (3º32'S, 72º40'W), USNM 284865, 1 (60). Río Itaya, main river channel and lower portions of caños, 5 to 20 km upstream of Belen (Iquitos) (3º51'S, 73º12'W), USNM 284856, 1 (51). Ucayali: Pucallpa, río Ucayali, Utuoquinia, USNM 273580, 3 (6082). Pucallpa, río Ucayali, Tacshitea (8º02'48"S, 74º39'19"W), USNM 273595, 5 (47-56). District Coronel Portillo, Yarinacocha, opposite landing for town of Yarinacocha (8º16'S, 74º36'W), USNM 284849, 2 (5980). Inexact locality: Nazareth, FMNH 15502, 2 (42-43).
Auchenipterichthys longimanus (Günther, 1864)Figs. 1, 5-7
Auchenipterus longimanus Günther, 1864: 195 [type locality: River Capin (= rio Capim), Para (Brazil); Syntypes: BMNH 1849.11.8.11 (3), possibly ZMB 5059 (1)].
Auchenipterichthys longimanus, Eigenmann & Eigenmann, 1888: 154 [Manes (=Maués), rio Madeira; Cameta]. Eigenmann & Eigenmann, 1890: 284 [redescription based on specimens from Maués, rio Madeira and Cameta]. Eigenmann & Eigenmann, 1891: 34 [in listing of fresh water fishes of South America; distribution cited as southern tributaries of Amazon]. Eigenmann, 1910: 396 [southern tributaries of the Amazon]. Miranda-Ribeiro, 1911: 372 [description and distribution information based on Günther, 1864, and Eigenmann & Eigenmann, 1891]. Gosline, 1945: 13 [literature summary; tributaries of Amazon]. Fowler, 1951: 458 [literature summary]. Merona, 1987: 122 [Brazil, lower rio Tocantins]. Ferreira, 1995: 52 [Brazil, Pará, rio Trombetas]. Taphorn et al., 1997: 83 [Venezuela]. Ferraris, 2003: 472 [checklist]. Lasso et al., 2003a:240 [checklist, Caura River, Venezuela]. Lasso et al., 2003b:285 [checklist, Caura River, Venezuela]. Rodríguez-Olarte et al., 2003: 199 [checklist, Lower Caura River, Venezuela].
Auchenipterichthys thoracatus (not of Kner, 1857), Fisher, 1917: 424 [specimen from Manáos (=Manaus, Brazil)]. Mees, 1974: 38 [Fisher (1917) record of species from Manaus corrected].
Trachycorystes obscurus (not of Günther, 1863), Steindachner, 1915: 80 [misidentification, see Mees, 1974: 36, 38; Brazil, mouth of rio Negro].
Auchenipterichthys longinmanus, Merona et al., 2001: 389 [Brazil, rio Tocantins; stomach contents analysis; species name misspelled].
Diagnosis. A species of Auchenipterichthys with coracoid bone covered with thick integument and not exposed ventrally (Fig. 1c), an emarginate or truncate caudal-fin margin, 25 or fewer branched anal-fin rays (modally 21; Table 1), nine (rarely eight) branched pelvic-fin rays (Table 1), the anterior teeth on premaxilla not visible when the mouth is closed, and the body pigmentation pattern not consisting of distinct dark spots on a gray background. Auchenipterichthys longimanus is most similar in appearance to A. punctatus, which differs from A. longimanus primarily in having dark spots that cover much of dorsolateral surface of the body. The possession of a coracoid bone that is not exposed ventrally (Fig. 1c), the anterior teeth on the premaxilla that are not visible when the mouth is closed, and nine (rarely eight) branched pelvic-fin rays (Table 1) distinguishes A. longimanus from its other two congeners, A. coracoideus and A. thoracatus, which have coracoids that are exposed ventrally (Figs. 1a-b) rather than covered by a thick layer of integument, the anterior teeth on the premaxilla visible when mouth is closed and, typically eight branched pelvic-fin rays.
Description. Body depth at dorsal-fin origin 0.220.25 of SL and equal to, or slightly greater than, body width at cleithrum. Body depth at anal-fin origin 0.250.27 of SL and nearly equal to HL. Body compressed, with width at anal-fin origin approximately 0.480.51 of body depth at that point. Ventral surface of coracoids not exposed on ventral surface of body (see Fig. 1c). Lateral line complete and midlateral. Canal having irregular zigzag pattern, with oblique posteriorly-directed branches off main canal. Lateral line canal extending short distance onto, and directed posteriorly or obliquelyposterodorsally on, caudal-fin base.
Head depressed anteriorly; depth of head at vertical through middle of orbit greater than distance from middle of eye to dorsal midline of head. Head length 0.250.27 of SL. Dorsal profile of head broadly convex anteriorly and then straight or very slightly convex from vertical running through anterior margin of orbit posteriorly as far as dorsal-fin origin. Distance from midpoint of snout to anterior margin of orbit approximately 1.5 times horizontal diameter of orbit. Snout margin broadly rounded from dorsal view. Interorbital width approximately 0.650.68 of HL and approximately equal to distance from middle of eye to posterior margin of opercle. Eye large, lateral, and visible in both dorsal and ventral views. Orbit distinctly ovoid with horizontal axis longer.
Barbels slender and thread-like. Maxillary barbel very long, extending posteriorly nearly to posterior tip of cleithral spine. Medial mandibular barbel originating immediately posterior of lower lip; adpressed barbel extending posteriorly to point slightly short of vertical through transverse plane through origin of lateral mandibular barbel. Lateral mandibular barbel originating in plane slightly anterior of vertical through middle of orbit and extending posteriorly approximately to transverse line through pectoral-spine origin.
Branchiostegal membrane broadly attached to isthmus; ventral margin of gill opening extending to vertical approximately one orbit length posterior of rear margin of orbit.
Mouth terminal. Anterior teeth on premaxilla not visible from ventral view when mouth closed. Teeth on premaxilla minute and arranged in band. Band consisting of approximately eight irregular series of teeth at symphysis and of ten irregular series laterally. Dentary teeth slightly larger than those on premaxilla, with approximately six series of teeth at symphysis that decrease to one tooth row posterolaterally.
Dorsal-fin origin at 0.310.34 of SL. Length of dorsal-fin base slightly less than one-half length of first branched dorsal-fin ray. Dorsal-fin spine pungent, with slightly curved, convex anterior margin. Length of dorsal-fin spine approximately equal to distance between anterior nares and posterior margin of opercle, without elongation apparent in examined mature males. Anterior surface of dorsal-fin spine with single series of relatively well-developed, antrorse serrae extending nearly to tip of spine. Posterior margin of spine with medial row of retrorse serrae extending nearly to tip of spine. Serrae on posterior surface of spine shorter than those on anterior surface in juveniles and females; posterior serrae on distal portion of spine proportionally longer in mature males. Dorsal-fin rays II,6. Adipose fin relatively small.
Caudal fin emarginate to obliquely truncate, with dorsal lobe longer than ventral lobe. Principal caudal-fin rays i,7,8,i.
Anal-fin base approximately 0.220.24 of SL and slightly shorter than HL. Anal-fin origin located distinctly posterior of middle of SL and slightly posterior of middle of TL. Anal-fin margin straight, with first ray longest and subsequent rays becoming progressively shorter, but with anterior rays in mature males extending slightly beyond margin of rest of fin. Last anal-fin ray without membranous attachment to caudal peduncle. Anal-fin rays typically iii,18 to iii,23 (iii,25 in one of 87 specimens; Table 1).
Distal margin of pelvic fin broadly convex, with third branched ray longest. Pelvic-fin insertion slightly posterior of middle of SL. Tip of adpressed pelvic fin barely reaching anal-fin origin. Pelvic-fin rays typically i,9, rarely i,8 (Table 1).
Pectoral fin with strong spine serrated along entire length of both margins with antrorse serrae along anterior margin and retrorse serrae along posterior margin. Anterior pectoral-fin rays longest. Fin margin straight anteriorly and convex along posterior rays. Pectoral-fin rays I,7, or I,8, rarely I,9 (Table 1).
Pigmentation pattern in alcohol. Overall ground coloration ranging from light to dark brown, with overall ground coloration darker on dorsal portion of head and body and in some individuals on midlateral portion of body posterior of head in region overlying swimbladder. Abdomen unpigmented. Snout and upper lip darkly pigmented to some degree in all specimens. Lower lip with curved patch of dark coloration along margin in more darkly pigmented individuals.
Lateral surface of body with small, unpigmented, rounded spots of approximately one-fifth width of pupil or sometimes smaller. Number and distribution of unpigmented spots varies greatly in different population samples, with spots and their arrangement difficult to discern in some individuals, particularly those of fainter overall coloration. Unpigmented spots in some individuals form variably-developed row or sometimes narrow band along midlateral region of body from rear of head to hypural joint, but with anterior and posterior portions of such row or band variably present in different specimens. Individual spots or series of vertically-aligned spots present on dorsolateral portion of body in region from vertical through base of rear of dorsal fin, or posterior of that point, to area under base of adipose fin. Some specimens, often those with very dark overall pigmentation, with individual spots or series of vertically-aligned spots present on dorsolateral portion of body from vertical through base of rear of dorsal fin, or posterior of that point, to area under base of adipose fin. Number of such unpigmented spots highly variable in different specimens, with spots nearly completely absent in some individuals.
Dorsal fin with dark pigmentation on distal portion of first (lightly pigmented individuals) or first and second (more darkly pigmented individuals) interradial membranes. Adipose fin dusky to dark. Caudal fin ranges from dusky basally and lighter posteriorly to dark overall with little decrease in degree of pigmentation posteriorly. Some specimens with small white spots within dark, basal pigmentation field in region overlying central rays. Anal fin with variably developed dark pigmentation basally. Such pigmentation more obvious in overall more darkly pigmented individuals. Dorsal surface of pelvic fin hyaline or with diffuse field of small, dark chromatophores in darker individuals with chromatophores not, however, forming distinct spot. Margins and sometimes dorsal surface of pectoral-fin spine dark; interradial membranes with diffuse fields of small, dark chromatophores.
Maxillary and mandibular barbels typically hyaline, but with few small, dark chromatophores present in overall darkly pigmented individuals.
Sexual dimorphism. Although a number of mature males of 126 to 140 mm SL (as indicated by the possession of an elongated urogenital tube adhering to the anterior margin of the anal fin) were examined, none demonstrated an elongation of the dorsal-fin spine beyond the condition present in females of comparable sizes. It is uncertain whether this observation reflects the absence of sexual dimorphism in the species or the lack of nuptial males in the available samples. Serrae on the posterior surface of the distal one-half of the dorsal-fin spine are proportionally longer in mature males than they are in juveniles and females.
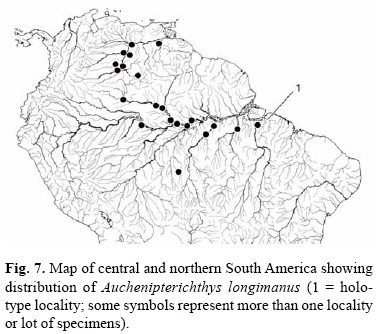
Distribution. Orinoco River basin in Venezuela, lower and middle portions of Amazon and Tocantins Rivers in Brazil (Fig. 7).
Biology. Merona et al. (2001: 389) report that Auchenipterichthys longinmanus (sic) in the lower portions of the Tocantins River, feeds primarily on terrestrial invertebrates (81.88 % of diet) and also decapods (10.94%) and aquatic invertebrates (7.19%).
Remarks. The catalog number for the syntype series of Auchenipterus longimanus, BMNH 1849.11.8.11, was misreported in Eschmeyer (1998: 931) as BMNH 1849.121.8.? and BMNH 1849.11.8.?, and in Ferraris (2003: 472) as BMNH 1849.121.8.11. The original description indicated that the description was based on "fine specimens"; however the BMNH register provides no further information as to the number of specimens actually examined by Günther. Three specimens are currently found in the syntype lot (BMNH 1849.11.8.11). Eschmeyer (1998: 981) cited a specimen from ZMB as a syntype of this species, without comment except that the specimen was from BMNH. We have not investigated further to see if there is justification for labeling the ZMB specimen as part of the type series.
Material examined. BRAZIL. Amazonas: Alto rio Negro, at mouth of igarapé do Ibará, MZUSP 31094, 1 (135); MZUSP 52104, 14 (135167). rio Daraá, Cachoeira do Aracu (1º40'S, 64º40'W), MZUSP 52097, 10 (127173); MZUSP 59039, 1 (146). Rio Negro, São Gabriel da Cachoeira, MZUSP 52098, 7 (131144). Rio Negro, Anavilhanas (2º40'S, 60º40'W), MZUSP 52099, 10 (94-140); MZUSP 52102, 1 (126); MZUSP 59036, 2 (123136); MZUSP 59037, 1 (107). Rio Negro, Ilha Mari-Mari, MZUSP 52100, 3 (126146); MZUSP 59040, 1 (153). Blackwater igapó at confluence of rio Cuiuni and rio Negro (0º40'S, 63º10'W), MZUSP 52101, 8 (117162). Blackwater igapó at confluence of rio Marauiá and rio Negro (approximately 0º20'S, 65º20'W), MZUSP 52103, 23 (137182). rio Arirará, near its mouth (0º30'S, 63º35'W), MZUSP 31078, 2 (124131). Rio Negro, 6.5 km below Jufari (1º17'37"S, 61º56' 57"W), MZUSP 55909, 1 (50). Igarapé Jaraqui, left bank of rio Negro, above Manaus (latter locality at 3º06'47"S, 60º01'30"W), MZUSP 6206, 12 (116141). Rio Negro above Manaus (latter locality at 3º06'47"S, 60º01'30"W), MZUSP 6161, 4 (124137). Manaos (=Manaus, 3º06'47"S, 60º01'30"W), FMNH 57799, 1 (129; formerly CM 6782). Rio Negro, Anavilhanas, igapó, MZUSP 63298, 1 (123). Rio Maués at Maués (3º22'S, 57º38'W), MCZ 7374, 1 (54). Lago Saracá (2º52'S, 58º22'W), MZUSP 5814, 2 (114118). Igarapé of rio Maraú (3º24'S, 57º42'W), MZUSP 7300, 2 (115119). Rio Preto da Eva, near Manaus (2º46'S, 59º10'W), MZUSP 57104, 13 (108137). Lago Puraquequara, mouth of rio Puraquequara (2º56'S, 59º49'W), MZUSP 6104, 16 (139167). Rio Tefé, Supi?-Pucu (approximately 3º40'S, 65º50'W), MZUSP 52074, 1 (145). Pará: rio Xingu, edge of river channel (3º10' S, 51º50'W), MZUSP 52096, 86 (109162). Rio Xingu, Belo Monte (3º10' S, 51º50'W), MZUSP 59043, 2 (124129). Beach near mouth of lago Jacaré, Reserva Biológica de Trombetas, rio Trombetas (approximately 1º11'S, 56º40'W), MZUSP 15827, 2 (135-143). Lago do Comprido (opposite mouth of lago Leonardo), Reserva Biológica de Trombetas, rio Trombetas (approximately 1º11'S, 56º40'W), MZUSP 15913-15915, 3 (133-146). rio Trombetas, MZUSP 5475, 3 (92132). Rio Trombetas, Cuminá, river margin (1º21'20"S, 56º00'W), MZUSP 57108, 3 (116136). Island at the mouth of lago do Erepecu, Reserva Biológica de Trombetas, rio Trombetas (approximately 1º11'S, 56º40'W), MZUSP 15969, 1 (33). Rio Trombetas, 20 km above the mouth, shore of the river (1º40'S, 56º00'W), MZUSP 59061, 1 (128). Rio Capin (=rio Capim), BMNH 1849.11.8.11, 3 (110140; syntypes of Auchenipterus longimanus). Rio Capim, Praia de Caranandéua, MZUSP 57103, 3 (104134). Rio Capim, Vila Santana, MZUSP 57109, 16 (118135); FMNH 95542, 2 (115118); ROM 37954, 2 (120129). Rio Capim, MZUSP 57106, 1 (124). Igarapé Pacuí, km 97 on highway between Belém and Brasília, MZUSP 57107, 1 (124). Paraná Samuuma, mouth of rio Tocantins, MZUSP 57105, 2 (110126). Rio Tapajós (3º35'S, 55º20'W), MZUSP 57102, 3 (129132). Rio Tapajós, between Itaituba and São Luís, shore of river (4º27'S, 56º15'W), MZUSP 63301, 1 (136). Município de Cametá, Cametá, rio Tocantins (2º14'S, 49º30'W), MCZ 8158, 1 (140). Mato Grosso: rio Aripuanã, upstream of Porto de Balsa, road joining Distrito de Colniza to Panelas, km 18 (9º34'45"S, 59º25'19"W), MZUSP 60387, 1 (89). VENEZUELA. Amazonas: Río Orinoco, Kiratare, SU 58754,1 (69). Rocks and rapids in Río Orinoco at Isla Cupaven, FMNH 103482, 28 (3265). Caño Guasuriapana at Guasuriapana; caño and backwater approximately 7 minutes from San Fernando de Atabapo, tributary of río Atabapo (4º00'N, 67º42'W), FMNH 105177, 2 (7476). Río Atabapo, at San Fernando de Atabapo (4º02'N, 67º42'W), MCNG 22361, 2 (4344). Río Mavaca, at base camp, AMNH 91384, 2 (107118). Río Emoni, approximately 2 km upriver from its mouth, Río Siapa basin, Caño Emoni (2º07'N, 66º20'W), MCNG 12308, 1 (77). Río Manipitare, approximately 5 to 8 km by water above its confluence with río Siapa, MCNG 38209, 10 (6683). Backwater of río Orinoco behind sand playa approximately 0.5 hr upstream from Isla Tremblador (3º04'N, 66º28'W), ANSP 165794, 1 (117). Caño Pozo Azul, río Orinoco drainage (5º45'49"N, 67º29'21"W), INHS, 61570, 8 (60119). Anzoategui: río Orinoco basin, Soledad, lago Terecaya (8º11'30"N, 63º27'20"W), ANSP 166377, 1 (66). Apure: caño La Pica, río Orinoco drainage, approximately 75 km N of Puerto Paez on road to San Fernando (6º53'28"N, 67º30'46"W), AUM 22446, 1 (44). Caño Potrerito, río Orinoco drainage, approximately 15 km N of Puerto Paez on road to San Fernando (6º24'43"N, 67º31'55"W), AUM 22624, 1 (106). Río Claro, río Orinoco drainage, 102 road kms N of Puerto Paez on road to San Fernando (7º09'08"N, 67º38'06"W), AUM 224494, 5 (5095). Río Capanaparo, backwater lagoon at mouth of caño Las Varitas, near San Fernando de Apure to Puerto Paez Highway (7º02'N, 67º25'W), ANSP 165494, 1 (92). Bolivar: río Orinoco, caño de Quiribana near Caicara (approximately 7º38'44"N, 66º10'23"W), SU 58687, 2 (5759). Río Chaviripa, río Orinoco drainage, on Caicara to Puerto Ayacucho road (7º07'57"N, 66º29'56"W), AUM 22290, 1 (65). Confluence of río Orinoco and río Caura (7º38'36"N, 64º50'00"W), ANSP 160362, 1 (54). Small stream crossing Caicara to Puerto Ayacucho Highway 18 km N of Maniapure (latter locality at 7º11'59"N, 66º31'59"W), ANSP 160824, 1 (75). Río Caura, E side of ferry crossing on Caicara to Ciudad Bolivar Highway (7º27'00"N, 65º12'00"W), ANSP 160990, 11 (35114). Guarico: Hato San Jose del Aguaro, río Aguaro (7º46'59"N, 66º24'59"W), UF 36170, 2 (6168). Parque Nacional Aguaro-Guariquito, río Aguas Muertas (8º06'30"N, 66º40'19"W), MCNG 31766, 8 (4985). Río San Bartolo (río Guariquito-río Orinoco drainage) at Bartolena Ranch (7º59'20"N, 66º39'00"W), INHS 61930, 1 (45). Río Bartolo (río Orinoco drainage), Parque Nacional Aguaro-Guariquito, río Aguas Muertas (8º06'30"N, 66º40'19"W), INHS 34874, 7 (48112). Río Bartolo (río Orinoco drainage), Parque Nacional Aguaro-Guariquito, río Aguas Muertas (8º08'01"N, 66º40'53"W), INHS 34076, 2 (4894). Río Bartolo (río Orinoco drainage), Parque Nacional Aguaro-Guariquito, río Aguas Muertas (8º04'14"N, 66º40'50"W), INHS 34499, 10 (5 087).
Auchenipterichthys punctatus (Valenciennes, 1840)Figs. 8-10
Auchenipterus punctatus Valenciennes, in Cuvier & Valenciennes, 1840: 219 (163 in Strasbourg deluxe edition); [type locality: probably Brazil (translated from original description); holotype: MNHN b-0216]. Royero & Hureau, 1996: 374, fig. 3 [comments on holotype and transfer to Auchenipterichthys].
Auchenipterichthys dantei Soares-Porto, 1994: 282, fig. 3 [type locality: Brazil, Amazonas, Paricatuba, rio Negro (3º07'S, 60º26'W); holotype: MZUSP 43332].
Auchenipterichthys punctatus, Royero & Hureau, 1996: 374, fig. 3 [transfer of species to Auchenipterichthys]. Taphorn et al., 1997: 83 [Venezuela]. Wallace, 2002: 296, figs. 117118 [rio Negro]. Ferraris, 2003: 472 [checklist].
Diagnosis. A species of Auchenipterichthys with coracoid bone overlain by thick layer of skin ventrally and not visible ventrally (see Fig. 1c), an emarginate or obliquely truncate caudal-fin margin, 21 to 24 branched anal-fin rays (Table 1), nine branched pelvic-fin rays (Table 1), the anterior teeth on the premaxilla not visible in the closed mouth, and a pattern of body pigmentation consisting of variably-sized spots of dark pigmentation scattered over the dorsal and lateral surface of the body and fins. Auchenipterichthys punctatus is most similar in appearance to A. longimanus, which differs from A. punctatus primarily in lacking distinct, dark spots covering the head or body. Auchenipterichthys punctatus is readily distinguished from its other two congeners, A. coracoideus and A. thoracatus, which have coracoids that are covered ventrally only by a thin layer of integument and appear to be exposed to the surface (Figs. 1a-b), the anterior teeth on premaxilla are visible in the closed mouth and, typically, eight (rarely nine) branched pelvic-fin rays (Table 1).
Description. Body depth at dorsal-fin origin 0.250.27 of SL and equal to, or slightly greater than body width at cleithrum. Body depth at anal-fin origin approximately 0.25 of SL and equal to HL. Body compressed, with width at anal-fin origin slightly less than one-half of body depth at that point. Ventral surface of coracoids not exposed on ventral surface of body (Fig. 1c). Lateral line complete and midlateral. Canal having irregular zigzag pattern, with oblique posteriorly-directed branches off main canal. Lateral line canal extending short distance onto caudal fin base and branched with both obliquely posterodorsal and obliquely posteroventral branches.
Head depressed anteriorly; height of head at vertical through middle of orbit greater than distance from middle of eye to dorsal midline of head. Head length 0.230.26 of SL. Dorsal profile of head broadly convex anteriorly and then straight or very slightly convex from vertical running through anterior margin of orbit to dorsal-fin origin. Distance from midpoint of snout to anterior margin of orbit approximately 1.5 times horizontal diameter of orbit. Snout margin broadly rounded from dorsal view. Interorbital width approximately 0.570.65 of HL and approximately equal to distance from middle of eye to posterior margin of opercle. Eye large, lateral, and visible in both dorsal and ventral views. Orbit distinctly ovoid with horizontal axis longer.
Barbels slender and thread-like. Maxillary barbel long, extending posteriorly slightly past middle of length of cleithral spine. Medial mandibular barbel originating immediately posterior of lower lip; adpressed barbel extending posteriorly to point slightly past transverse plane through origin of lateral mandibular barbel. Lateral mandibular barbel originating in plane slightly anterior of vertical through middle of orbit and extending posteriorly approximately to transverse line through pectoral-spine origin.
Branchiostegal membrane broadly attached to isthmus; ventral margin of gill opening extending to vertical approximately one orbit length posterior of rear margin of orbit.
Mouth terminal. Anterior teeth on premaxilla not visible from ventral view of closed mouth. Teeth on premaxilla minute and arranged in band. Band consisting of approximately eight irregular series of teeth at symphysis and of ten irregular series laterally. Dentary teeth slightly larger than those on premaxilla, with approximately six series of teeth at symphysis that progressively decrease to one tooth row posterolaterally.
Dorsal-fin origin at approximately 0.300.34 of SL. Length of dorsal-fin base slightly less than one-half of length of first branched dorsal-fin ray. Dorsal-fin spine pungent, with slightly curved, convex anterior margin. Length of dorsal-fin spine approximately equal to distance between anterior margin of orbit and posterior margin of opercle. Anterior surface of dorsal-fin spine with single series of relatively feeble, antrorse serrae extending nearly to tip of spine; serrae acute in smaller individuals and blunt in larger individuals. Posterior margin of spine with medial row of relatively feeble, irregularly-directed serrae extending nearly to tip of spine. Serrae on anterior and posterior surfaces of spine approximately of equivalent size. Dorsal fin with spinelet, spine, and six slender branched rays. Adipose fin relatively small.
Caudal fin emarginate to obliquely truncate with dorsal lobe longer than ventral lobe. Principal caudal-fin rays i,7,8,i.
Anal-fin base approximately 0.230.26 of SL and approximately equal to HL. Anal-fin origin located distinctly posterior of middle of SL and slightly posterior of middle of TL. Anal-fin margin straight, with first ray longest and subsequent rays becoming progressively shorter. Last anal-fin ray without membranous attachment to caudal peduncle. Anal-fin rays iii,21 to iii,24 (Table 1).
Distal margin of pelvic fin broadly convex, with third branched ray longest. Pelvic-fin insertion situated at middle of SL. Tip of adpressed pelvic fin extending posterior of anal-fin origin and approximately to base of first branched anal-fin ray. Pelvic-fin rays i,9 (Table 1).
Pectoral fin with strong spine serrated along entire length of both margins with antrorse serrae along anterior margin and retrorse serrae along posterior margin. Anterior pectoral-fin rays longest. Fin margin straight anteriorly and convex along posterior rays. Pectoral-fin rays I,8 (Table 1).
Pigmentation pattern in alcohol. Overall ground coloration of adults tan to brown, universally dark on dorsal portion of head and body other than in smaller individuals that may have darker spots scattered over dorsal one-half of body. Midlateral region along lateral line pale other than in smaller individuals. Pale region in such specimens form ing narrow, irregularly-margined, horizontal stripe. Lateral and dorsolateral surface of body with series of unpigmented, rounded spots of size most often approximately equal to one-fifth of width of pupil or smaller. Lateral surface of body ventral of lateral line tan to light brown and overlain by variably sized and distributed spots of dark pigmentation. Dark spots proportionally larger in small specimens. Abdomen and lower portion of head posterior of lateral mandibular barbel pale. Margin of lower lip darkly pigmented.
First, second, and sometimes third interradial membranes of dorsal fin dark distally with remainder of fin in larger individuals with scattered, dark spots similar in size to those on lateral surface of body. Adipose fin generally dark with single darker subterminal spot within field of dark pigmentation, but with pale margin. Caudal fin typically dark on basal portion of rays and membranes, with dark basal region bordered by lighter, near vertical band, and distal tip of fin somewhat darker than coloration of vertical band. Spots of darker pigmentation scattered over all of fin; spots on basal one-half of fin larger than those on remainder of fin. Ground coloration of anal fin comparable to that of adjoining ventral portion of body, but with broad, darker, distal margin on fin. Anal fin with scattered, small, darker spots over entire surface. Ground coloration of pelvic fin comparable to that of adjoining portions of body; with small, scattered, darker spots throughout. Pectoral fin darkly pigmented on distal portions of first two interradial membranes and generally lighter across remainder of fin. Pectoral spine dark along entire dorsal surface.
Maxillary and lateral mandibular barbels darkly pigmented throughout. Medial mandibular barbel more lightly pigmented.
Color pattern of juvenile specimens similar to that described above, but patterning bolder and spots proportionally larger than those found in adults (Fig. 8).
Sexual dimorphism. No nuptial males were examined in the course of this study and as such it is uncertain whether Auchenipterichthys punctatus demonstrates the sexually-dimorphic features occurring in some congeneric species. Soares-Porto (1994: 285) noted that the males she examined in her description of A. dantei did not demonstrate any sexual differences relative to females other that for their urogenital modifications.
Distribution. Examined specimens originated in the upper portions of the Orinoco and Negro River basins in Venezuela and the central portions of the Amazon River basin in Brazil (Fig. 10; triangles). Soares-Porto (1994, fig. 6) also examined material of the species that originated along the length of the Brazilian portion of the Negro River.
Remarks.Auchenipterus punctatus appears not to have been cited in the literature following its description by Valenciennes (in Cuvier & Valenciennes, 1840) until it was discussed by Royero & Hureau (1996), who first recognized that it represented a species of Auchenipterichthys. Perhaps as a consequence of that situation, this species was subsequently redescribed as A. dantei by Soares-Porto (1994).
Material examined. BRAZIL. Amazonas: rio Riozinho, right bank of rio Jutaí (approximately 2º58'S, 66º58'W), MZUSP 43333, 2 (99116). rio Tefé, lago, MZUSP 52105, 1 (120). Rio Negro, lago on island, MZUSP 31076, 1 (51). Município Santa Isabel do Rio Negro, MZUSP 84736, 1 (111). VENEZUELA. Amazonas: rocks in río Atabapo at shore and inlet of Isla de Sapo, approximately 1.2 hours above San Fernando de Atabapo (latter locality at 4º02'25"N, 67º42'08"W), FMNH 103481, 1 (76). Caño Cuchaken, approximately 7 km from its confluence with río Atabapo (3º31'N, 67º24'W), MCNG 23085, 1 (137). Vicinity of "Puerto Esperanza" (4º42'37"N, 67º44'58"W), MCNG 35949, 2 (111112). Along river bank at la Comunidad de "Maraya" (3º59'24"N, 66º57'08"W), MCNG 46391, 1 (68). Río Siapa approximately 124 km from mouth of Río Casiquiare (1º49'N, 65º48'W), MCNG 25981, 1 (149). Caño Candela, near its confluence with río Pasimoni (1º32'06"N, 66º34'34"W), MCNG 42191, 8 (5886).
Auchenipterichthys thoracatus (Kner, 1857)Figs. 10-11
Auchenipterus thoracatus Kner, 1857: 425, pl. 7, fig. 22 [type locality: rio Guaporé; syntypes: NMW 47454 (2)].
Auchenipterus thoracicus Günther, 1864: 194 [based on Kner, 1857; unjustified emendation of Auchenipterus thoracatus].
Auchenipterichthys thoracatus, Eigenmann & Eigenmann, 1888: 154 [in listing of South American catfishes; not cited presence at Coary (=Coari) and Hyavary (=Javari), Brazil]. Eigenmann & Eigenmann, 1891: 34 [in listing of fresh water fishes of South America; not cited occurrence in Solimoens (=Solimões) and tributaries]. Eigenmann, 1910: 396 [in listing of South American fresh water fishes; not cited presence at Coary (=Coari) and Hyavary (=Javari), Brazil]. Miranda-Ribeiro, 1911: 372 [description and distribution information based on Günther, 1864, and Eigenmann & Eigenmann, 1891; not citations of species at Coary (=Coari) and Hyavary (=Javari), Brazil]. Fisher, 1917: 424 [Maciél, rio Guaporé; San Joaquin, Bastos, rio Alegre into R. Guaporé; not cited specimen from Manáos (=Manaus)]. Pearson, 1937: 110 [Río Mamoré basin]. Fowler, 1940: 96 [Bolivia, San Joaquin; based on Fisher, 1917]. Fowler, 1951: 458 [in part, citations of species in the upper rio Madeira]. Miranda-Ribeiro, 1968: 8, pl. 7 [body, head, and anal-fin form illustrated]. Mees, 1974: 34 [literature in part, citations of species from upper rio Madeira basin; not synonymy of Trachycorystes coracoideus into Auchenipterichthys thoracatus; not cited specimen from Peru]. Lauzanne et al., 1991: 68 [Bolivia: Trinidad, Itenez (Guaporé)]. Sarmiento, 1998: 364 [Bolivia, Parque Nacional Noel Kempff Mercado]. Sarmiento et al., 1999: 91 [Bolivia, Pando, upper Río Othon basin]. Chernoff et al., 1999: 59 [Bolivia, riverine habitats]. Chernoff et al., 2000: 281 [Bolivia, Amazon]. Ferraris, 2003: 473 [checklist].
Auchenipterichthys cf. thoracatus, Mendes dos Santos et al., 1984: 78 [Brazil, rio Tocantins].
Diagnosis. A species of Auchenipterichthys with the coracoid bone covered with a thin layer of integument and exposed ventrally (Fig. 1b), an obliquely truncated caudal-fin margin, typically 26 or more (rarely 25) branched anal-fin rays (Table 1), eight (rarely nine or ten) branched pel-vic-fin rays (Table 1), the anterior teeth on the premaxilla visible when the mouth is closed, and a body pigmentation not consisting of distinct dark spots on a gray background. Auchenipterichthys thoracatus is most similar in appearance to A. coracoideus, which typically has fewer than 25 (26 in one of 63 specimens) branched anal-fin rays (Table 1). Furthermore, it appears not to have the elongated dorsal-fin spine that is found in nuptial males of A. coracoideus. Auchenipterichthys thoracatus is readily distinguished from its other two congeners, A. longimanus and A. punctatus, which have coracoids that are covered ventrally by a thick layer of integument (Fig. 1c), the anterior teeth on the premaxilla are not visible in the closed mouth and, typically, have nine (very rarely eight) branched pelvic-fin rays (Table 1).
Description. Body depth at dorsal-fin origin 0.250.29 of SL and slightly greater than width at cleithrum. Body depth at anal-fin origin 0.27 of SL. Body compressed, with width at anal-fin origin 0.380.40 of body depth at that point. Ventral surface of coracoids exposed on ventral surface of body (see Fig. 1b). Lateral line complete and midlateral. Canal having irregular zigzag pattern, with oblique, posteriorly-directed branches off main canal. Lateral line canal extending short distance onto, and directed obliquely-posterodorsally on, caudal-fin base.
Head depressed anteriorly; height of head at vertical through middle of orbit approximately equal to distance from middle of eye to dorsal midline of head. Dorsal profile of head broadly convex anteriorly and then slightly concave from vertical running through anterior margin of orbit to dorsal-fin origin. Distance from midpoint of snout to anterior margin of orbit approximately equal to horizontal diameter of orbit. Snout margin broadly rounded from dorsal view. Interorbital width approximately 0.650.70 of HL and approximately equal to distance from middle of eye to posterior margin of opercle. Eye large, lateral, and visible in both dorsal and ventral views. Orbit distinctly ovoid with horizontal axis longest.
Barbels slender and thread-like. Maxillary barbel long, extending posteriorly slightly past margin of opercle. Medial mandibular barbel originating immediately posterior of lower lip; adpressed barbel extending posteriorly only to vertical through transverse plane through lateral mandibular barbel. Lateral mandibular barbel originating in plane slightly anterior of vertical through middle of orbit and extending posteriorly approximately to anterior portion of exposed cleithrum.
Branchiostegal membrane broadly attached to isthmus; ventral margin of gill opening extending to anterior margin of exposed portion of cleithrum.
Mouth terminal, but with upper jaw extending very slightly beyond margin of lower jaw. Anterior teeth on premaxilla visible from ventral view in closed mouth. Teeth on premaxilla minute and arranged in band. Band consisting of approximately eight irregular series of teeth at symphysis and of ten irregular series laterally. Dentary teeth slightly larger than those on premaxilla, with approximately six series of teeth at symphysis that progressively decrease to one tooth row posterolaterally.
Dorsal-fin origin at 0.360.39 of SL. Length of dorsal-fin base approximately one-half of length of first branched dorsal-fin ray. Dorsal-fin spine pungent with slightly convexly curved anterior margin. Length of dorsal spine approximately equal to HL in specimens of both sexes. Basal one-half of anterior margin of dorsal-fin spine bearing two rows of small, blunt projections with spine margin smooth or finely serrated distally. Posterior margin of spine with few, short, medial, obliquely distally-directed serrae. Dorsal-fin rays II,6. Adipose fin relatively small.
Caudal fin obliquely truncate with dorsal most branched ray longest. Principal caudal-fin rays i,7,8,i.
Anal-fin base approximately 0.280.31 of SL. Anal-fin origin located distinctly posterior of middle of SL and slightly anterior of middle of TL. Anal-fin margin straight, with first ray longest and subsequent rays becoming progressively shorter. Last anal-fin ray without membranous attachment to caudal peduncle. Anal-fin rays iii,25 to iii,28 (Table 1).
Distal margin of pelvic fin broadly convex with middle branched ray longest. Pelvic-fin insertion situated anterior of middle of SL. Tip of adpressed anal fin falling short of anal-fin origin. Pelvic-fin rays typically i,8, rarely i,9 or i,10 (Table 1).
Pectoral fin with strong spine serrated along entire length of both margins with antrorse serrae along anterior margin and retrorse serrae along posterior margin. Anterior pectoral-fin rays longest. Fin margin straight anteriorly and convex along posterior rays. Pectoral-fin rays typically I,8, infrequently I,7 (Table 1).
Pigmentation pattern in alcohol. Overall ground coloration ranging from brown to dark brown, with coloration darker on dorsal portion of head and body and in some individuals on midlateral portion of body posterior of head in region overlapping swimbladder. Abdomen unpigmented. Snout, upper lip, and region ventral of margin of lower lip very dark. Patch of very dark pigmentation present anterior to base of dorsal fin. Lateral and dorsolateral surface of body with series of unpigmented spots of size approximately equal to one-quarter width of pupil or smaller. Unpigmented spots arranged in several series. Midlateral surface of body with irregular longitudinal series of light spots extending from rear of head to posterior margin of caudal peduncle. Anterior portion of this midlateral series overlies several series of approximately vertically-aligned pairs of unpigmented spots. Dorsolateral surface of body with irregularly spaced, vertically-aligned series of unpigmented spots; these series begin under, or posterior of, base of dorsal fin and typically extend posteriorly to beyond base of adipose fin, but in some individuals continue posteriorly to rear of caudal peduncle.
Dorsal fin-rays dark distally. Adipose fin with dark basal spot continuous with dark pigmentation of body. Caudal fin dark basally with pigmentation continuing that of body. Some specimens with small, unpigmented spots within dark basal pigmentation field in region overlying central caudal-fin rays. Darkly pigmented region with distinct, straight to slightly irregular, posterior margin; margin of dark pigmentation ranges from approximately vertical to somewhat anteroventrally inclined. Dark region at base of caudal fin followed posteriorly by hyaline region and then by band of less intense dark pigmentation along distal margin of fin. Anal fin with variably developed dark pigmentation basally. Distal margin of dark pigmentation usually of irregular form, but sometimes smoothly convex. Dorsal surface of basal portion of pelvic fin with variably dark patch of pigmentation. Distal portion of fin with dark pigmentation in most individuals; pigmentation intense in well pigmented specimens. Margins and sometimes dorsal surface of pectoral fin spine dark; fin rays variably outlined with dark chromatophores.
Maxillary barbel dark. Mandibular barbels pale, with scattered, dark chromatophores.
Sexual dimorphism. Although a number of mature males of Auchenipterichthys thoracatus of 94 to 109 mm SL (as indicated by the possession of an elongated urogenital tube adhering to the anterior margin of the anal fin) were examined, none demonstrated an elongation of the dorsal-fin spine beyond the condition present in females of comparable sizes. It is uncertain whether this observation reflects the absence of sexual dimorphism in the species or the lack of nuptial males in the available samples. Serrae on the posterior surface of the distal one-half of the dorsal-fin spine are proportionally longer in mature males than they are in juveniles and females of the species.
Distribution. Upper portions of the Madeira River basin in Bolivia and Brazil (Fig. 10; dots).
Remarks. The catalog number for the syntype series of Auchenipterus thoracatus, NMW 47454, was incorrectly reported as NMW 47452 in Eschmeyer (1998: 1674) and Ferraris (2003: 473).
Various authors have misidentified samples of Auchenipterichthys coracoideus as A. thoracatus as is well exemplified in the synonymies for both of these species. One of the major consequences of the failure to discriminate these two species was the erroneous broad purported range for A. thoracatus that was thought to range across major portions of the Amazon River basin. Our results rather indicate that A. thoracatus is limited to the upper portions of the Madeira River basin in southwestern Brazil and eastern Bolivia (Fig. 10, dots); with A. coracoideus having an extensive distribution along the length of the Amazon River basin into the Tocantins River system and upper portions of the Essequibo River basin in Guyana (Fig. 5).
As noted under Remarks for Auchenipterichthys coracoideus, that species and A. thoracatus differ in the shape of the head and angle of the divergence of the exposed portion of the coracoids (see Figs. 1a-b). We have, however, been unable to unambiguously quantify those differences.
Material examined. BRAZIL. "Guaporé" (= rio Guaporé), NMW 47454, 2 (91108; syntypes of Auchenipterus thoracatus). Mato Grosso: rio Alegre, tributary of rio Guaporé, approximately 30 km from Vila Bela da Santíssima Trindade (15º30'S, 59º20'W), MZUSP 37485, 186 (91109); MZUSP 36934, 22 (8698). rio Guaporé, Vila Bela da Santíssima Trindade, MZUSP 37518, 11 (9098). Rio Guaporé, Vila Bela da Santíssima Trindade, near bairro do Aeroporto (15º01'17"S, 59º57'90"W), MZUSP 63034, 23 (71100). Bastos, rio Alegre, upper rio Guaporé basin (approximately 15º06' S, 59º57'W), FMNH 58011, 1 (105). Rondônia: rio Jaciparana, approximately 3 km upstream from town of Jaci Paraná (9º15'S, 64º23'W), rio Madeira basin, UF 100659, 1 (91). Inexact locality: Maciel, rio Guaporé basin, FMNH 57798, 8 (4593). BOLIVIA. Beni: San Joaquín (= San Joaquín on Río Machupo; see Eigenmann, 1911: 311; approximately 13º03'59"S, 64º48'59"W), FMNH 58016, 2 (6170). Río Itenez at mouth of dry run, 2 km SE of Costa Marques, tributary of río Mamoré, río Madeira basin (approximately 12º28'S, 64º16'W), UMMZ 204300, 1 (52). Pando: río Manuripi, approximately 13 km by river above Puerto Rico (latter locality at 11º04'59"S, 67º37'59"W), FMNH 106716, 2 (3639). Pando, approximately 9 km from Puerto Rico (latter locality at 11º04'59"S, 67º37'59"W), FMNH 106717, 1 (39).
Acknowledgments
Research associated with this study was supported by the Neotropical Lowland Research Program of the National Museum of Natural History, Smithsonian Institution, the All Catfish Species Inventory (NSF DEB-0315963), and the Herbert R. and Evelyn Axelrod Chair in Systematic Ichthyology in the Division of Fishes of the National Museum of Natural History, Smithsonian Institution. The successful completion of this project was made possible by the assistance of individuals who arranged for the loan and exchange of specimens, provided diverse forms of information, hospitality during visits, and myriad other types of assistance. We thank Melanie L. J. Stiassny and Barbara Brown (AMNH); John G. Lundberg and Mark H. Sabaj (ANSP); Jonathan W. Armbruster (AUM); David Catania (CAS); Barry Chernoff, Mary Anne Rogers, and Kevin Swagle (FMNH); Michael E. Retzer (INHS); Donald C. Taphorn (MCNG), Karsten E. Hartel (MCZ); Ramiro Barriga (MEPN); Claude Weber and Sonia Fisch-Muller (MHNG); Paulo A. Buckup (MNRJ); Mário C. C. de Pinna, José L. de Figueiredo, Osvaldo T. Oyakawa, and Flávio C. T. Lima (MZUSP); Sven O. Kullander and Erik Ahlander (NRM); Erling Holm (ROM); George H. Burgess (UF); and Douglas W. Nelson (UMMZ). The photographs of fishes in this paper were prepared by T. Britt Griswold (NMNH) and Mark Sabaj (ANSP).
Literature Cited
Received October 2004
Accepted February 2005
- Akama, A. & C. J. Ferraris, Jr. 2003. Entomocorus melaphareus, a new species of auchenipterid catfish (Osteichthyes: Siluriformes) from the lower and middle reaches of the Amazon River. Neotropical Ichthyology, 1 (2): 7782.
- Boeseman, M. 1983. Introduction. Pp. 112, in: P. Bleeker, Atlas Ichthyologique des Indes Orientales Néêrlandaises; plates originally prepared for planned tomes XI-XIV. Smithsonian Institution Press, Washington. 22 p., 152 pl.
- Bleeker, P. 1862-63. Atlas ichthyologique des Indes Orientales Néêrlandaises, publié sous les auspices du Gouvernement colonial néêrlandais. Tome II Siluroïdes, Chacoïdes et Hétérobranchoïdes. Amsterdam. 112 p., pls. 49101. [Issued in installments. Text pages 1-32 and plates 4972 date to 1862; pages 33112 and plates 73101 date to 1863. See Boeseman (1983: 4) for details.]
- Bleeker, P. 1863. Systema Silurorum revisum. Nederlandsch Tijdschrift voor de Dierkunde, 1: 77122.
- Chernoff, B., A. Machado-Allison, P. Willink, J. Sarmiento, S. Barrera, N. Menezes & H. Ortega. 2000. Fishes of three Bolivian rivers: diversity, distribution and conservation. Interciencia, 25: 273283.
- Chernoff, B., P. Willink, J. Sarmiento, A. Machado-Allison, N. Menezes & H. Ortega. 1999. Geographic and macrohabitat partitioning of fishes in the Tahuamany-Manuripi region, upper Río Orthon basin, Bolivia. Chapter 5, in B. Chernoff & P. Willink (eds.), A Biological assessment of the aquatic ecosystems of the upper Río Orthon basin, Pando, Bolivia. Bulletin of Biological Assessment 15. 145 p.
- Cuvier, G. & A. Valenciennes. 1840. Histoire naturelle des poissons. Tome quinzième. Suite du livre dix-septième. Siluroïdes. Ch. Pitois & Ve Levrault, Paris & Strasbourg. xxxi + 540 p., pls. 421455.
- Eigenmann, C. H. 1910. Catalogue of the fresh-water fishes of tropical and south temperate America. Pp. 375511, in: Reports of the Princeton University expeditions to Patagonia 1896-1899, Zoology, vol. 3.
- Eigenmann, C. H. 1911. A brief report upon the expedition of the Carnegie Museum to central South America (by John D. Haseman) together with a list of localities at which Mr. Haseman collected. Annals of the Carnegie Museum, 7 (3-4): 287314.
- Eigenmann, C. H., & W. R. Allen. 1942. Fishes of western South America. I. The intercordilleran and Amazonian lowlands of Peru. II. The high pampas of Peru, Bolivia, and northern Chile; with a revision of the Peruvian Gymnotidae, and of the genus Orestias. University of Kentucky. xv + 494 p., 22 pl.
- Eigenmann, C. H., & R. S. Eigenmann. 1888. Preliminary notes on South American Nematognathi, I. Proceedings of the California Academy of Sciences (Ser. 2), 1 (pt 2): 119172.
- Eigenmann, C. H., & R. S. Eigenmann. 1890. A revision of the South American Nematognathi or cat-fishes. Occasional Papers of the California Academy of Sciences, no. 1: 1 508 + errata and map.
- Eigenmann, C. H. & R. S. Eigenmann. 1891. A catalogue of the fresh-water fishes of South America. Proceedings of the United States National Museum, 14: 181.
- Eschmeyer, W. N. (ed.). 1998. Catalog of fishes. California Academy of Sciences, San Francisco. 3 volumes: 2905 pp.
- Ferraris, C. J., Jr. 1988. The Auchenipteridae: Putative monophyly and systematics, with a classification of the Neotropical doradoid catfishes (Ostariophysi: Siluriformes). Unpublished Ph.D. Dissertation. City University of New York.
- Ferraris, C. J., Jr. 2003. Auchenipteridae. Pp. 470482, in: R. E. Reis, S. O. Kullander, and C. J. Ferraris, Jr. (eds.), Check list of the freshwater fishes of South and Central America. Edipucrs, Porto Alegre, Brazil. 729 p.
- Ferraris, C. J., Jr. & R. P. Vari. 1999. The South American catfish genus Auchenipterus Valenciennes, 1840 (Ostariophysi: Siluriformes: Auchenipteridae): monophyly and relationships, with a revisionary study. Zoological Journal of the Linnean Society, 126: 387450.
- Ferreira, E. J. G. 1995. Composição, distribuição e aspectos ecológicos da ictiofauna de um trecho do rio Trombetas, na área de influência da futura UHE Cachoeira Porteira, Estado do Pará, Brasil. Acta Amazonica, 23 (14, Suplemento): 188. [Issue for 1993, actual publication date 1995.]
- Fisher, H. G. 1917. A list of the Hypophthalmidae, the Diplomystidae and of some unrecorded species of Siluridae in the collections of the Carnegie Museum. Annals of the Carnegie Museum, 11 (34): 405427, pl. 42.
- Fowler, H. W. 1940. Zoological results of the second Bolivian expedition for the Academy of Natural Sciences of Philadelphia, 19361937. Part I. The fishes. Proceedings of the Academy of Natural Sciences, Philadelphia, 92: 43103.
- Fowler, H. W. 1945. Los peces del Perú. Catálogo sistematico de los peces que habitan en aguas peruanas. 298 pages. Lima: Museo de Historia Natural "Javier Prado."
- Fowler, H. W. 1951. Os peixes de água doce do Brasil, 3.Şentrega. Arquivos de Zoologia do Estado de São Paulo, 6: 405624.
- Gosline, W. A. 1945. Catálogo dos nematognatos de água-doce da América do Sul e Central. Boletim do Museu Nacional. Nova série, Zoologia, no. 33: 1138.
- Günther, A. 1864. Catalogue of the fishes in the British Museum, vol. 5: Catalogue of the Physostomi, containing the families Siluridae, Characinidae, Haplochitonidae, Sternoptychidae, Scopelidae, Stomiatidae in the collection of the British Museum. British Museum Trustees, London. xxii + 455 p.
- Kner, R. 1857. Ichthyologische Beiträge. II. Abtheilung. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften, Mathematisch- Naturwissenschaftlichen Classe, Wien, 26: 373448, pls. 19.
- Lasso, C. A., A. Machado-Allison, D. Taphorn, D. Rodríguez-Olarte, C. R. Vispo, B. Chernoff, F. Provenzano, O. Lasso-Alcalá, A. Cervó, K. Nakamura, N. González, J. Meri, C. Silvera, Al Bonilla, H. López-Rojas & D. Machado-Aranda. 2003. The fishes of the Caura River basin, Orinoco drainage, Venezuela: annotated checklist. Scientia Guianae, 12: 223245.
- Lasso, C. A., C. R. Vispo & O. Lasso-Alcalá. 2003. Floodplain fishes of the Caura River, southern Venezuela. Scientia Guianae, 12: 273295.
- Lauzanne, L., G. Loubens & B. le Guennec. 1991. Liste commentée des poissons de l'Amazonie bolivienne. Revue de Hydrobiologie Tropicale, 24(1): 6176.
- Mees, G. F. 1974. The Auchenipteridae and Pimelodidae of Suriname (Pisces, Nematognathi). Zoologische Verhandelingen (Leiden), no. 132: 1256, pls. 115.
- Mendes dos Santos, G., M. Jegú & B. de Merona. 1984. Catálogo de peixes comerciais do baixo rio Tocantins. 83 pages. Manaus, Brazil: Contrais Eletricas do Norte do Brasil, S.A. (Electronorte), Choselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), & Instituto Nacional de Perquisas da Amazonia (INPA).
- Merona, B. de. 1987. Aspectos ecológicos da ictiofauna no baixo Tocantins. Acta Amazonica 16-17: 109124
- Merona, B. de., J. L. de Carvalho & M. M. Bittencourt. 1987. Les effets immédiats de la fermeture du barrage de Tucurui (Brésil) sur l'ichtyofaune en aval. Revue de Hydrobiologie Tropicale, 20(1): 7384.
- Merona, B. de, G. Mendes dos Santos & R. Gonçalves de Almeida. 2001. Short term effects of Tucuruí Dam (Amazonia, Brazil) on the trophic organization of fish communities. Environmental Biology of Fishes, 60: 377392.
- Miranda Ribeiro, A. 1911. Fauna brasiliense. Peixes. Tomo IV (A) [Eleutherobranchios Aspirophoros]. Arquivos do Museu Nacional do Rio de Janeiro, 16: 1504, pls. 2254.
- Miranda Ribeiro, P. 1968. Apontamentos ictiológicos. Boletim do Museu Nacional, nova série, Zoologia, 263: 114
- Ortega, H., & R. P. Vari. 1986. Annotated checklist of the freshwater fishes of Peru. Smithsonian Contributions to Zoology, 437: 125.
- Pearson, N. A. 1937. The fishes of the Beni-Mamoré and Paraguay basins, and a discussion of the Paraguayan fauna. Proceedings of the California Academy of Sciences, series 4, 23 (8): 99114.
- Pinna, M. C. C. de. 1998. Phylogenetic relationships of Neotropical Siluriformes (Teleostei: Ostariophysi): historical overview and synthesis of hypotheses. Pp. 279330, in: L. R. Malabarba, R. E. Reis, R. P. Vari, Z. M. Lucena & C.A.S. Lucena (eds.), Phylogeny and classification of Neotropical fishes. Edipucrs, Porto Alegre. 603 p.
- Rodríguez-Olarte, D., D. Taphorn, C. A. Lasso & C. R. Vispo. 2003. Fishes of the lower Caura River, Orinoco basin, Venezuela. Scientia Guianae, 12: 181221.
- Royero, R. & J.-C. Hureau. 1996. The type specimens of authenipterid catfishes (Siluriformes: Auchenipteridae) in the Muséum National d'Histoire Naturelle, Paris. Cybium, 20 (4): 369377.
- Sarmiento, J. 1998. Ichthyology of Parque Nacional Noel Kempff Mercado. Appendix 5, in: T. J. Killeen & T. S. Schulenberg (eds). A biological assessment of Parque Nacional Noel Kempff Mercado, Bolivia. RAP Working Papers 10. Conservation International, Washington, D.C. 372 p.
- Sarmiento, J. B. Chernoff, S. Barrera, A. Machado-Allison, N. Menezes & H. Ortega. 1999. Fishes collected during the AquaRAP Expedition to Pando, Bolivia in September 1996, in: B. Chernoff & P. Willink (eds). A Biological assessment of the aquatic ecosystems of the upper Río Orthon basin, Pando, Bolivia. Bulletin of Biological Assessment 15. 145 p.
- Soares-Porto, L. M. 1994. Auchenipterichthys dantei, a new species of catfish from the Amazon basin (Siluriformes: Auchenipteridae). Ichthyological Exploration of Freshwaters, 5 (3): 281287.
- Steindachner, F. 1915. Beiträge zur Kenntniss der Flussfische Südamerikas, V. Denkschriften der Mathematisch-Naturwissenschaftlichen Classe der Kaiserlichen Akademie der Wissenschaften in Wien, 93 (for 1917): 15 106, pls. 113. [Issued first as a separate in 1915, with dual pagination.]
- Taphorn, D. C., R. Royero, A. Machado-Allison & F. Mago-Leccia. 1997. Lista actualizada de los peces de agua dulce de Venezuela. In: E. La Marca, editor, Vertebratos actuales y fósiles de Venezuela, pages 55100. Serie Catálogo Zoológico de Venezuela. Volume 1. Merida, Venezuela: Museo de Ciencia y Tecnología de Mérida. 298 p.
- Vari, R. P. & C. J. Ferraris, Jr. 1998. The neotropical catfish genus Epapterus Cope (Siluriformes: Auchenipteridae): a reappraisal. Proceedings of the Biological Society of Washington, 111 (4): 9921007.
- Wallace, A. R. 2002. Peixes do Rio Negro [Fishes of the Rio Negro]. Organization, introductory text and translation by Mônica de Toledo-Piza Ragazzo. Editoria de Universidade de São Paulo, São Paulo, Brazil. 517 p.
Publication Dates
-
Publication in this collection
20 Dec 2007 -
Date of issue
Mar 2005
History
-
Accepted
Feb 2005 -
Received
Oct 2004