ABSTRACT
Catchfly (Silene conoidea), an annual herb, is usually recognized as an emerging weed species in Eurasia and North America. The presence of somatic seed polymorphism might aid in the adaptation of this weed in different climatic conditions. We conducted laboratory and greenhouse experiments to study the seed polymorphism and influence of various environmental factors like temperature, salt stress, osmotic stress and burial depth on the germination and emergence characteristics of catchfly. Optimum germination of seeds of all colors was recorded at a temperature of 15 oC. Germination of catchfly seeds of all colors followed decreasing trend as NaCl concentration increased from 50 mM to 200 mM. Seed germination was maximum (87-96%) at 0 MPa but gradually decreased to 40% as osmotic stress increases up to -0.4 MPa and completely inhibited at 0.6 MPa of all seed colors. A slight increase (from 60 to 95%) in the germination of seeds of black and dark brown colors was observed when seeding depth increased from 0 to 2 cm but decreased when seeding depth increased from 2 to 4 cm in seeds of all colors. There was no emergence of catchfly at seeding depth of 6 cm or greater. Our results concluded that catchfly seeds have the potential to germinate and emerge in various environmental conditions, but germination/emergence percentage of seeds of all colors will be different in different environmental conditions. Soil amendments including deep ploughing may aid for the successful management of this weed in cultivated areas.
Keywords:
polymorphism; catchfly; germination ecology
RESUMO
Silene (Silene conoidea), uma erva anual, é geralmente reconhecida como uma espécie emergente de plantas daninhas na Eurásia e na América do Norte. A presença de polimorfismo somático de sementes pode auxiliar na adaptação dessa planta em diferentes condições climáticas. Foram realizados experimentos em laboratório e em estufa para estudar o polimorfismo da semente e a influência de diversos fatores ambientais, como temperatura, estresse salino, estresse osmótico e profundidade de enterramento, sobre as características de germinação e emergência de silene. A germinação ideal de sementes de todas as cores foi registrada a uma temperatura de 15 oC. A germinação de sementes de silene de todas as cores seguiu tendência decrescente à medida que a concentração de NaCl aumentou de 50 mM para 200 mM. A germinação das sementes foi máxima (87-96%) a 0 MPa, mas diminuiu gradualmente para 40% à medida que o estresse osmótico aumentou até -0,4 MPa, e foi completamente inibida em -0,6 MPa de todas as cores de sementes. Observou-se ligeiro aumento (de 60 a 95%) na germinação de sementes de cores pretas e marrom-escuras quando a profundidade de semeadura aumentou de 0 para 2 cm, porém houve diminuição quando a profundidade de semeadura aumentou de 2 para 4 cm em sementes de todas as cores. Não houve emergência de silene na profundidade de semeadura de 6 cm ou maior. Os resultados mostraram que as sementes de silene têm potencial para germinar e emergir em várias condições ambientais, mas a porcentagem de germinação/emergência de sementes de todas as cores será diferente em distintas condições ambientais. Alterações no solo, incluindo arado profundo, podem ajudar na gestão bem-sucedida dessa planta em áreas cultivadas.
Palavras-chave:
polimorfismo; silene; ecologia da germinação
INTRODUCTION
Somatic seed polymorphism is probably the characteristic that is more common in weed species than in crop plants (Senseman and Lawrence, 1993Senseman S.A., Lawrence R.O. Flowering patterns, seed production, and somatic polymorphism of three weed species. Weed Sci . 1993;41:418-25.). Polymorphic seeds are often produced in the same plant or in the same population by different plants (Harper, 1965Harper J.L. Establishment, aggression and cohabitation in weedy species. In: Baker I.M., Stebbins G.L., editores. The genetics of colonization species. New York: Academic Press, 1965. p.243-68.). This characteristic probably aids in the survival and adaptation of weed plants in different climatic conditions. Seeds variations in color, size and weight may be signaled by the ecological conditions, internal competition for nutrients during the seed development and also by a specific genetic constitution and seed maturation. The climatic conditions under which seeds develop in the mother plant may influence their dormancy and germination (Koller, 1962Koller D.O.V. Preconditioning of germination in lettuce at time of fruit ripening. Am J Bot . 1962;9:841-9. ; Guttermann, 1973Guttermann Y. Differences in the progeny due to day length and hormone treatment of the mother plant. W-Heydeckered. Seed ecology. London: Pennsylvania State University Press, 1973. p.59-80.). Williams and Harper (1965Williams J.T., Harper J.L. Seed polymorphism and germination. I. The influence of nitrates and low temperatures on the germination of Chenopodium album. Weed Res. 1965;5:141-50.) have demonstrated that polymorphic seeds differ in their germination requirements. Bhandari (1977Bhandari D.C. Studies on arid zone legumes with special reference to Indigofera species-their ecology, nitrogen metaholism and role in cropecosystem [thesis] Jodhpur: University of Jodhpur, 1977.) has observed seven different seed coat colors and their varying imbibitions and germination behaviors in Indigofera tinctoria. The effects are attributed mainly to impermeability of the seed coat to water and oxygen, and to the mechanical strength of the seed coat which exerts sufficient restraint to the growth of the embryo (Sharma et al., 1978Sharma N.K. et al. Seed perpetuation in Rhynchosia capitata DC. Biologia Plant. 1978;20:225-8. ). Sometimes, immature seeds have germinated when incubated immediately after removal from the plant, whereas seeds that had matured in the mother plants have not germinated when incubated to the same extent (Morgan and Berrie, 1970Morgan S.F., Berrie A.M.M. Development of dormancy during seed maturation in avena ludoviciana winter wild oat Development of dormancy during the seed maturation in Avena Iudoviciana winter wild oat. Nature. 1970;228:1225.). Such type of seeds develops dormancy during seed development in the mother plants. Knowledge of weed seed germination is critical in an agroecosystem. Germination is the process whereby seeds begin to sprout and grow. This process is affected by such factors as temperature (Widderick et al., 2004Widderick M. et al. Better management of common sowthistle (Sonchus oleraceus) based on weeds ecology. In: Proceedings of the 14th Australian weeds conference. Wagga: New South Wales, 2004. p.535-7.), drought (Chejara et al., 2008Chejara V.K. et al. Factors affecting germination of coolatai grass (Hyparrhnia hirta). Weed Sci . 2008;56:543-8.), salts (Chauhan et al., 2006Chauhan B.S. et al. African mustard (Brassica tournefortii) germination in southern Australia. Weed Sci . 2006;54:891-89.) and burial depth (Koger et al., 2004Koger C.H. et al. Factors affecting seed germination seedling emergence and survival of Texas weed (Caperonia palustris). Weed Sci . 2004;52:989-95.). The present paper is concerned with the germination of catchfly (Silene conoidea) seeds. Catchfly is an annual, erect plant and a problem weed in field crops. There is a shortage of data concerning the germination ecology of catchfly. The objective of the present study was to describe some of the germination characteristics of seeds of different colors and weight collected from catchfly. The findings of this experiment will be helpful in management strategies of catchfly.
MATERIALS AND METHODS
Experiments were conducted under laboratory and greenhouse conditions during 2014. Plants of catchfly were harvested in the first week of May 2014 from plots sown for weed identification. Harvested plants were allowed to dry for a week and seeds were threshed. After threshing, seeds were air-dried for a week, placed in a paper bag and stored in the laboratory at room temperature until used in the experiments. In November 2014, seeds were separated into black, brown and dark brown categories based on their color. To determine seed fresh weight of each category, a random sample of 300 seeds was taken from each category. Fresh weight of seeds of different categories was 1.095 g for black, 0.844 g for brown and 0.937 g for dark brown seeds.
General germination protocol
Twenty catchfly seeds of each color were placed separately in 9 cm diameter Petri dishes containing filter paper Whatman No.10, moistened with 5 mL of distilled water or a treatment solution. Catchfly seeds were surface sterilized by soaking in 10% sodium hypochlorite (NaOCl) for 5 min, followed by five rinses with distilled water before the start of each germination or emergence experiment. Petri dishes were sealed with Parafilm to reduce the water loss. Germinated seeds with a radicle at least 2 mm long were counted and removed daily for three weeks. In a burial depth experiment, seedlings were considered emerged when a cotyledon was visible at the soil surface.
Effect of temperature
Germination of black, brown and dark brown seeds of catchfly was determined in a seed germinator at constant temperatures of 10, 15, 20 and 25 oC for 12 days. These temperatures were selected due to reflecting the temperature variation during the winter period in Pakistan.
Effect of salt stress
Three colored seeds of catchfly were incubated separately in a sodium chloride (NaCl) solution of 0, 50, 100, 150 and 200 mM to examine the effect of salt stress on germination.
Effect of osmotic stress
Catchfly, each color of three different colors, were germinated in aqueous solution with an osmotic potential of 0, -0.2, -0.4, -0.4, and -0.6 MPa. Osmotic potentials were prepared by using Polyethylene glycol (PEG-6000) in distilled water. The following equation (Michel and Kaufmann, 1973Michel B.E., Kaufmann M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973;51:914-6.) was used to calculate the amount (g) of PEG-6000 from the known water potential (MPa) required.
Water potential = - (1.18 × 10-2) C - (1.18 × 10-4) C2 + (2.67 × 10-4) 18CT + (8.39 × 10-7) C2T
where C is the concentration of PEG (g kg-1 distilled water) and T is the temperature (oC).
Effect of seeding depth
The effect of seeding depth on seedling emergence of different seed colors of catchfly was studied in the green house. Twenty seeds of each color were placed on the soil surface or covered with soil (30% clay, 30% silt and 40% sand) to depths of 2, 4, 6, 8 and 10 cm in 15 cm diameter plastic pots. Pots were left opened and watered as needed to maintain an adequate soil moisture. Seedlings were considered emerged when a cotyledon was visible at the soil surface.
Statistical analysis
A completely randomized design with four replications was used in all experiments. Nonlinear regression analysis was used to determine how NaCl, osmotic stress or seeding depth affected germination percentage or the emergence of catchfly seeds. Germination (%) values at different concentrations of NaCl and osmotic potential were fitted to a functional three-parameter logistic model using Sigma Plot 2008 (version 11.0). The model fitted was G(%) = Gmax/[1+(x/x50 )Grate ], where G is the total germination (%) at concentration x, Gmax is the maximum germination (%), x50 is the NaCl concentration or osmotic potential for 50% inhibition of the maximum germination and Grate indicates the slope. A three-parameter Gaussian model {E (%) = Emax × e[-0.5(x-x0 )/Erate ]} was fitted to three-color catchfly seedling emergence (%) where E is the total seedling emergence (%) at burial depth x, Emax is the maximum seedling emergence (%), x0 is the burial depth at which maximum seedling emergence occurred and Erate indicates the slope.
RESULTS AND DISCUSSION
Effect of temperature
The germination percentage of catchfly was minimum at 10 oC for seeds of all colors. The optimum temperature was 15 oC for seed germination as seeds of all colors showed maximum germination at this temperature (Figure 1). After that, germination of catchfly progressively decreased with increase in temperature. The figure shows the highest germination in dark brown seeds and more decrease in germination of black seeds with temperature variation.
Effect of salt stress
Germination of catchfly seeds followed an exponential response to increasing salt concentration, with germination decreasing as the NaCl concentration increased from 50 to 200 mM (Figure 2). Black, brown and dark brown seeds of catchfly attained maximum germination percentages of 76, 88 and 91%, respectively, with distilled water. Black seed germination was not significantly affected at a 50 mM NaCl concentration. However, it drastically decreased at a 150 mM NaCl concentration and there was no germination at a 200 mM NaCl concentration (Figure 2). A three-parameter logistic model indicated that 50% inhibition of germination of black, brown and dark brown seeds of catchfly occurred at NaCl concentration of 129, 135 and 134 mM, respectively (Figure 2). These data suggest that seeds of all colors of catchfly may germinate at high salinity.
Effect of sodium chloride (NaCl) on the germination of black, brown and dark brown seeds of catchfly. The line represents the functional three-parameter logistic model {G (%) = Gmax / [1 + (x/x50)Grate]} fitted to the data.
Effect of osmotic stress
A three-parameter logistic model was fitted to the germination % of three colors (black, brown, and dark brown) of catchfly seeds at different osmotic potentials (Figure 3). In dark seeds, maximum germination was 87% with distilled water and it significantly decreased with increasing osmotic stress. Germination of dark seeds of catchfly was completely inhibited at the osmotic potential of -0.6 MPa. The fitted model estimated that 50% inhibition of the maximum germination of catchfly was recorded at the osmotic potential of -0.33 MPa. Germination of brown seeds was 92% at 0 MPa osmotic potential and drastically decreased with increasing osmotic potential. Germination was completely inhibited at the osmotic potential of -0.6 MPa. A three-parameter logistic model estimated that the osmotic potential required for 50% inhibition of the maximum germination of brown seeds of catchfly was -0.35 MPa (Figure 3). Germination of the dark brown seed of catchfly decreased from 96% to 40% when osmotic stress increased from 0 to -0.4 MPa and completely inhibited at -0.6 MPa. A three-parameter logistic model showed that the osmotic potential for 50% inhibition of the maximum germination of catchfly was -0.39 MPa (Figure 3).
Effect of osmotic potential on the germination of black, brown and dark brown seeds of catchfly. The line represents the functional three-parameter logistic model {G (%) = Gmax / [1 + (x/x50)Grate]} fitted to the data.
Effect of seeding depth
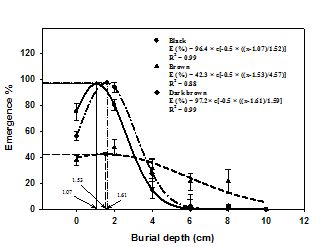
A three-parameter Gaussian model was fitted to the seedling emergence of dark, brown and dark brown seeds of catchfly (Figure 4). The emergence of black and dark brown seeds of catchfly was stimulated from 80% and 60%, respectively to 96% when seeding depth was increased from 0 to 2 cm but declined when seeding depth was further increased to 4 cm. However, there was no emergence of these seeds recorded at a seeding depth of 6 cm or greater. A three-parameter Gaussian model was fitted to the emergence value of dark seeds of catchfly when placed and estimated that the maximum emergence (96%) was recorded at a seeding depth of 1.07 cm. The emergence of brown seeds of catchfly was minimum when compared to those of dark or dark brown seeds. A three-parameter Gaussian model showed that maximum emergence (42%) of brown seeds was at 1.53 cm seeding depth.
Effect of burial depth on the emergence of black, brown and dark brown seeds of catchfly. The line represents the functional three-parameter Gaussian model {E (%) = Emax × e [-0.5(x-x0 )/Erate ]} fitted to the data.
Survival of plants may be dependent on various factors such as germination regulating mechanism, defensive mechanisms or dormancy. Seeds of catchfly may utilize the germination regulating mechanism and strategies for survival. Various biological and environmental factors and their interaction may serve as a signal by which germination is triggered in order to take advantage of environmental conditions that maximize the probability for successful establishment. In the absence of such signal, most of the seed population remains dormant. Morphological variations among polymorphic seeds of catchfly are considerably correlated with the germination ability of seeds, with black seeds being less dormant than brown and dark brown seeds. Black seeds showed the highest rate and germination percentage while brown seeds showed the lowest rate and percentage of germination. Brown seeds were more susceptible to change in temperature being more dormant at low and high temperatures as compared to dark brown and black seeds which are more tolerant to variations in temperature for germination. It might be because low temperature probably maintains dormancy in seeds during the winter and early spring. Similarly, Khan and Ungar (1984aKhan M.A., Ungar I.A. The effect of salinity and temperature on germination of polymorphic seeds and growth of Atriplex triangularis Willd. Am J Bot. 1984a;71:481-9.) have recorded a high germination of A. triangularis at a moderate temperature as compared to low and alternating temperature. The maximum rate of germination in all polymorphic seeds of catchfly is recorded at 20 and 25 oC as compared to higher and lower temperatures. This shows that a moderately high temperature appeared to be playing a significant role in promoting germination. This increase in germination rate could be due to an enhanced metabolic activity (Koller and Hadas, 1982Koller D., Hadas A. Water relation in the germination of seeds. In: Lange O.L. et al., editores. Encyclopedia of plant physiology; Physiological plant ecology. Berlin: Springer, 1982. v.2. p.402-31.), increased water uptake by seeds (Uchiyama, 1981Uchiyama Y. Studies on the germination of salt bushes. I. the relationship between temperature and germination of Atriplex nummularia (Lindl). Japanese J Trop Agric. 1981;25:62-7.; Koller and Hadas, 1982) and changes in membrane permeability (Taylorson and Hendricks, 1977Taylorson R.B., Hendricks S.B. Dormancy in seeds. Ann RevPlant Physiol . 1977;28:331-54. ). However, the exact nature of these mechanisms is not properly understood (Ungar, 1982).
Polymorphic seeds of catchfly showed a differential response towards salinity. The germination percentage of brown seeds was significantly inhibited by exposure to salt stress. Dark brown and black seeds showed more tolerance to salt stress. However, an increasing salinity concentration seems to induce dormancy. Seeds of many species remain dormant at a low water potential. These seeds do not lose viability and are going to germinate when subjected to a distilled water treatment (Ungar, 1982Ungar I.A. Germination ecology of halophytes. In: Sen D.N., Rajpurohit K.S., editores. Contribution to ecological of halophytes. The Hague: 1982. v.2. p.143-54.; Khan and Urgar 1984bKhan M.A., Ungar I.A. Seed polymorphism and germination responses to salinity stress in Atriplex triangularis willd. Bot Gazette. 1984b;155:487-94.). This could be of ecological significance since the germination of seeds in early spring, when high soil moisture level reduces salinity stress, probably assumed that some plants would survive until the growing season later in the summer (Ungar, 1982).
The absence of osmotic stress improved the rate and percentage of germination, particularly of (large) black seeds. Germination was not significantly affected even at the lower level of osmotic stress but with an increase in osmotic stress, rate and percentage of germination was declined to no germination at the highest level of osmotic stress. This might be due to the seeds ability to uptake water. Uchiyama (1981Uchiyama Y. Studies on the germination of salt bushes. I. the relationship between temperature and germination of Atriplex nummularia (Lindl). Japanese J Trop Agric. 1981;25:62-7.) has related seed germination to the rate of water absorption. He believed that irrespective of temperature, when water contents of seeds of A. nummularia reached 70%, seeds would germinate. Springfield (1970Springfield H.W. Germination characteristics of Atriplex canescens seed. Inter Grass Land Congr Proc. 1970;11:586-9.) has revealed that the imbibition in A. canescens depends on whether the seeds are winged or dewinged. The rate of imbibition of dewinged seeds was faster than winged seeds. Nobs and Hagar (1974Nobs M.A., Hagar W.A. Analysis of germination and flowering rates of dimorphic seeds of Atriplex hortensis. Ann Rep Dep Plant Biol Carnegie Inst. 1974;7:859-64.) have shown that during the first 5 hours, the large brown seeds of A. hortensis had a very rapid rate of water imbibition. The small black seeds showed a considerably slower rate of water uptake. Germination of horse-weed decreased from 25 to 2% as osmotic potential decreased from 0 to -0.8 MPa (Nandula et al., 2006Nandula V.K. et al. Factors affecting germination of horse weed (Conyza Canadensis). Weed Sci . 2006;54:898-902.). Germination of catchfly polymorphic seeds was gradually decreased with increase in osmotic stress level, with a pronounced effect on the brown seed, which may be due to its genetic potential. Germination over a broad range of osmotic potential indicated that catchfly seeds could pose a weed threat under both adequate and moisture stress soil conditions. This also implies that (large) black seeds are going to show a nearly complete germination in soil seed bank while (small) brown seeds are going to provide a longer term seed bank. This behavior is most likely related to the differences in physiological responses to polymorphic seeds and fluctuations in soil conditions during the growing season (Wertis, 1982Wertis B.A. Aspects of the population biology of halophytes Atriplex triangularis [dissertation] Athens: Ohio University, 1982.) which limits the periods when seeds can germinate. Rate, as well as percentage emergence of polymorphic seeds of catchfly, dramatically decreased with increasing plant depth. Most of the emergence of all types of seeds occurred from 0-4 cm. Bhowmik and Bekech (1993Bhowmik P.C., Bekech M.M. Horseweed (Conyza canadensis) seed production, emergence, and distribution in no-tillage and conventional-tillage corn (Zea mays)." Agron Trends Agric Sci. 1993;1:67-7.) have reported 80% emergence of horseweed seeds in the top 2 cm soil, but no emergence was found at a depth greater than 6 cm. Brown (small) catchfly seeds showed the lowest emergence at a depth greater than 4 cm, which might be due to the difficulty in the emergence of weak seedlings due to fewer food reserves. The emergence of weeds is affected by the amount of food reserves in seeds and the depth of burial in soil (Nandula et al., 2006). Decreased emergence due to increase in planting depth has been reported in several weed species such as hairy beggar ticks (Reddy and Singh, 1992Reddy K.N., Singh M. Germination and emergence of hairy beggar ticks (Bidens pilosa). Weed Sci . 1992;40:195-99. ) and horse purslane (Balyan and Bhan, 1986Balyan R.S., Bhan V.M. Germination of horse pursulane (Trianthema portulacastrum) in relation to temperature, storage condition and seeding depth. Weed Sci. 1986;34:513-5.). Under field conditions, no-tillage or shallow or minimum tillage practices may encourage germination and emergence of catchfly seeds.
Our study has clearly demonstrated an ecological significance of the polymorphic seeds in catchfly. The various seed sizes and colors have different tolerance limits to temperature, salinity, osmotic stress and planting depth. All of the (large) black seeds may germinate early in the growing season. Brown (small) seeds may germinate throughout the season and are more dormant. These results suggest that catchfly has the ability to germinate under a broad range of ecological conditions. Prevailing conditions could determine the extent of germination, subsequent emergence and overall problem of catchfly.
REFERENCE
- Balyan R.S., Bhan V.M. Germination of horse pursulane (Trianthema portulacastrum) in relation to temperature, storage condition and seeding depth. Weed Sci. 1986;34:513-5.
- Bhandari D.C. Studies on arid zone legumes with special reference to Indigofera species-their ecology, nitrogen metaholism and role in cropecosystem [thesis] Jodhpur: University of Jodhpur, 1977.
- Bhowmik P.C., Bekech M.M. Horseweed (Conyza canadensis) seed production, emergence, and distribution in no-tillage and conventional-tillage corn (Zea mays)." Agron Trends Agric Sci. 1993;1:67-7.
- Chauhan B.S. et al. African mustard (Brassica tournefortii) germination in southern Australia. Weed Sci . 2006;54:891-89.
- Chejara V.K. et al. Factors affecting germination of coolatai grass (Hyparrhnia hirta). Weed Sci . 2008;56:543-8.
- Guttermann Y. Differences in the progeny due to day length and hormone treatment of the mother plant. W-Heydeckered. Seed ecology. London: Pennsylvania State University Press, 1973. p.59-80.
- Harper J.L. Establishment, aggression and cohabitation in weedy species. In: Baker I.M., Stebbins G.L., editores. The genetics of colonization species. New York: Academic Press, 1965. p.243-68.
- Khan M.A., Ungar I.A. Seed polymorphism and germination responses to salinity stress in Atriplex triangularis willd. Bot Gazette. 1984b;155:487-94.
- Khan M.A., Ungar I.A. The effect of salinity and temperature on germination of polymorphic seeds and growth of Atriplex triangularis Willd. Am J Bot. 1984a;71:481-9.
- Koger C.H. et al. Factors affecting seed germination seedling emergence and survival of Texas weed (Caperonia palustris). Weed Sci . 2004;52:989-95.
- Koller D., Hadas A. Water relation in the germination of seeds. In: Lange O.L. et al., editores. Encyclopedia of plant physiology; Physiological plant ecology. Berlin: Springer, 1982. v.2. p.402-31.
- Koller D.O.V. Preconditioning of germination in lettuce at time of fruit ripening. Am J Bot . 1962;9:841-9.
- Michel B.E., Kaufmann M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973;51:914-6.
- Morgan S.F., Berrie A.M.M. Development of dormancy during seed maturation in avena ludoviciana winter wild oat Development of dormancy during the seed maturation in Avena Iudoviciana winter wild oat. Nature. 1970;228:1225.
- Nandula V.K. et al. Factors affecting germination of horse weed (Conyza Canadensis). Weed Sci . 2006;54:898-902.
- Nobs M.A., Hagar W.A. Analysis of germination and flowering rates of dimorphic seeds of Atriplex hortensis Ann Rep Dep Plant Biol Carnegie Inst. 1974;7:859-64.
- Reddy K.N., Singh M. Germination and emergence of hairy beggar ticks (Bidens pilosa). Weed Sci . 1992;40:195-99.
- Senseman S.A., Lawrence R.O. Flowering patterns, seed production, and somatic polymorphism of three weed species. Weed Sci . 1993;41:418-25.
- Sharma N.K. et al. Seed perpetuation in Rhynchosia capitata DC. Biologia Plant. 1978;20:225-8.
- Springfield H.W. Germination characteristics of Atriplex canescens seed. Inter Grass Land Congr Proc. 1970;11:586-9.
- Taylorson R.B., Hendricks S.B. Dormancy in seeds. Ann RevPlant Physiol . 1977;28:331-54.
- Uchiyama Y. Studies on the germination of salt bushes. I. the relationship between temperature and germination of Atriplex nummularia (Lindl). Japanese J Trop Agric. 1981;25:62-7.
- Ungar I.A. Germination ecology of halophytes. In: Sen D.N., Rajpurohit K.S., editores. Contribution to ecological of halophytes. The Hague: 1982. v.2. p.143-54.
- Wertis B.A. Aspects of the population biology of halophytes Atriplex triangularis [dissertation] Athens: Ohio University, 1982.
- Widderick M. et al. Better management of common sowthistle (Sonchus oleraceus) based on weeds ecology. In: Proceedings of the 14th Australian weeds conference. Wagga: New South Wales, 2004. p.535-7.
- Williams J.T., Harper J.L. Seed polymorphism and germination. I. The influence of nitrates and low temperatures on the germination of Chenopodium album Weed Res. 1965;5:141-50.
Publication Dates
-
Publication in this collection
18 May 2017 -
Date of issue
2017
History
-
Received
23 June 2016 -
Accepted
01 Sept 2016