Abstract
Mastication reduced the molecular weight of natural rubber (NR). This would affect the tensile properties and strain-induced crystallization of the rubber vulcanizates due to the structural changes of the rubber molecules. In this study, influences of mastication time on tensile response, deformation-induced crystallization, and structural effects of crosslinked NR were investigated. The crystallization behavior and structural changes during stretching were studied by means of wide angle X-ray scattering (WAXS) and small angle X-ray scattering (SAXS). Increased mastication time significantly affected modulus at specified strain and upturn point of strain-induced crystallization of the crosslinked samples while the tensile strength was influenced slightly by mastication. During stretching, degree of crystallinity at given strain was found to decrease with increasing mastication time, while the crystallite size was reduced. Moreover, the size of crosslinked network structures induced by crosslinking also decreased slightly with increasing mastication time, as suggested by SAXS measurement.
Keywords:
mastication; natural rubber; strain-induced crystallization; tensile properties
1. Introduction
Although natural rubber (NR) has many attractive properties such as high tensile strength and extensibility, good crack growth resistance, and low heat build-up[11 Smitthipong, W., Suethao, S., Shah, D., & Vollrath, F. (2016). Interesting green elastomeric composites: silk textile reinforced natural rubber. Polymer Testing, 55, 17-24. http://dx.doi.org/10.1016/j.polymertesting.2016.08.007.
http://dx.doi.org/10.1016/j.polymertesti...
], it is never used in its pure form without vulcanization[22 Noordermeer, J. W. M. (1998). Recent developments in rubber processing leading to new applications such as the “Green Tire”. Macromolecular Symposia, 127(1), 131-139. http://dx.doi.org/10.1002/masy.19981270118.
http://dx.doi.org/10.1002/masy.199812701...
]. During the compounding, NR requires proper mastication to reduce its molecular weight before incorporating the other chemical ingredients, because excessive molecular weight would prevent dispersion of the added ingredients[33 Fries, H., & Pandit, R. R. (1982). Mastication of rubber. Rubber Chemistry and Technology, 55(2), 309-327. http://dx.doi.org/10.5254/1.3535880.
http://dx.doi.org/10.5254/1.3535880...
]. Since mastication is usually required, the effects of molecular weight reduction through the mastication on tensile properties and on micro-structure are of great interest for understanding the final properties of vulcanizates. Dependence on molecular weight of the properties of rubber was initially investigated by Flory[44 Flory, P. J. (1946). Effects of molecular structure on physical properties of butyl rubber. Industrial & Engineering Chemistry, 38(4), 417-436. http://dx.doi.org/10.1021/ie50436a023.
http://dx.doi.org/10.1021/ie50436a023...
] in butyl rubber, and he found that the tensile strength was directly proportional to the weight fraction of the active network structure. Later on, Kok[55 Kok, C. M. (1985). The effect of molecular weight on the physical properties of U.V. degraded natural rubber. European Polymer Journal, 21(1), 37-40. http://dx.doi.org/10.1016/0014-3057(85)90062-X.
http://dx.doi.org/10.1016/0014-3057(85)9...
] also reached a similar conclusion for NR. That is, the tensile strength increased with molecular weight. However, in this report, the tensile strength tended to be constant when the molecular weight reached 500,000. Ono et al.[66 Ono, K., Kato, A., & Murakami, K. (1985). Unusual stress-strain properties of natural rubber vulcanizates with high primary molecular weight. Polymer Bulletin, 13(1), 29-33. http://dx.doi.org/10.1007/BF00264237.
http://dx.doi.org/10.1007/BF00264237...
] investigated the stress-strain properties and strain-induced crystallization of NR vulcanizates with different molecular weights, and reported that a higher molecular weight provided a higher level of stress at a given strain and better strain-induced crystallization ability due to the homogeneous network structures of high molecular weight rubbers. However, no evidence of a network structure was offered. Up to now, the effects of different parameters such as crosslink density, filler content, temperature and deformation rate on the final properties, crystallization behavior and micro-structure of NR have been extensively investigated and are well discussed in the report[77 Huneau, B. (2011). Strain-induced crystallization of natural rubber: A review of x-ray diffraction investigations. Rubber Chemistry and Technology, 84(3), 425-452. http://dx.doi.org/10.5254/1.3601131.
http://dx.doi.org/10.5254/1.3601131...
], but how the molecular weight affects strain-induced crystallization and the network structure is still largely unexplored. Thus, further investigation of the mechanical properties and changes of network structure remains interesting, in order to verify the effects of molecular weight.
It is well accepted that mastication affects NR viscosity due to molecular weight reduction[88 Ehabe, E. E., Bonfils, F., Sainte-Beuve, J., Collet, A., & Schue, F. (2006). High-temperature mastication of raw natural rubber: changes in macrostructure and mesostructure. Polymer Engineering and Science, 46(2), 222-227. http://dx.doi.org/10.1002/pen.20433.
http://dx.doi.org/10.1002/pen.20433...
,99 Hayeemasae, N., Waesateh, K., Soontaranon, S., & Masa, A. (2020). The effect of mastication time on the physical properties and structure of natural rubber. Journal of Elastomers and Plastics. http://dx.doi.org/10.1177/0095244320928566.
https://doi.org/10.1177/0095244320928566...
]. Previously, the effect of mastication time on the physical properties and microstructure of uncrosslinked NR was reported[99 Hayeemasae, N., Waesateh, K., Soontaranon, S., & Masa, A. (2020). The effect of mastication time on the physical properties and structure of natural rubber. Journal of Elastomers and Plastics. http://dx.doi.org/10.1177/0095244320928566.
https://doi.org/10.1177/0095244320928566...
]. Unfortunately, the contributions of mastication time to the network structure and the deformation-induced crystallization behavior of NR after vulcanization were not included. Thus, this work aimed to highlight the effects of molecular weight reduction by mastication on microstructure and final properties of the NR vulcanizates. The NR was initially masticated for various times before addition of the other compounding chemicals. The processing properties of NR were investigated by means of the moving die rheometer. The tensile properties, strain-induced crystallization and network structures of the crosslinked NR were analyzed by means of tensile test, wide angle X-ray diffraction (WAXD) and small angle X-ray scattering (SAXS), respectively.
2. Materials and Methods
2.1 Materials
Details of rubber and the various chemical ingredients used in the rubber formulation are summarized in Table 1. The quantities of all ingredients are indicated as part(s) per hundred parts of rubber (phr).
2.2 Sample preparation
NR was initially masticated for different mastication times (0, 5 and 10 min) prior to adding the other chemicals. The mastication and compounding were performed in a laboratory-sized internal mixer (Brabender® GmbH & Co. KG, Duisburg, Germany) with an initial mixing temperature of 40 °C, a fill factor of 0.8, and a rotor speed of 60 rpm. After the initial mastication, the rubber was further mixed for another 30 sec before incorporating stearic acid and ZnO. When the mixing time reached 1.5 min, an accelerator, CBS, was added and the mixing was continued for another 1 min. Finally, sulfur was incorporated and the compound was further mixed for another 2.5 min. The semi-efficiency system was chosen in this study. The total mixing time after the mastication was kept constant at 5 min for all compounds. The compounds were left at room temperature for 24 h prior to the tests. The compounds were pressed in a compression mold at 160 °C to obtain 1 mm thick sheets, following their respective curing times (Tc90).
2.3 Characterization
2.3.1 Curing characteristics
A moving die rheometer (Montech MDR 3000 BASIC, Buchen, Germany) was used to assess the curing characteristics of the NR compounds. With the testing temperature set at 160 °C, the curing parameters scorch time (Ts1), cure time (Tc90), minimum torque (ML), maximum torque (MH) and torque difference (MH-ML) were recorded. Further information on the rheometer can be found elsewhere[1010 Dick, J. S. (2003). Basic rubber testing: selection methods for a rubber testing program. Pennsylvania: ASTM International. http://dx.doi.org/10.1520/MNL39-EB.
http://dx.doi.org/10.1520/MNL39-EB...
].
The percentage reversion degree was calculated at 300 sec after reaching the maximum curing torque (Maximum torque), in order to estimate the aging resistance of the vulcanizates at an elevated temperature (R300). The R300 is defined as follows[1111 Khang, T. H., & Ariff, Z. M. (2012). Vulcanization kinetics study of natural rubber compounds having different formulation variables. Journal of Thermal Analysis and Calorimetry, 109(3), 1545-1553. http://dx.doi.org/10.1007/s10973-011-1937-3.
http://dx.doi.org/10.1007/s10973-011-193...
]:
where MH is the maximum torque on the curing curve and M300 is the torque at 300 s after MH.
2.3.2 Equilibrium Swelling
The equilibrium swelling test was performed in order to estimate the crosslink density (ν). The rubber samples were swollen in toluene solvent for 168 h at room temperature, followed by drying at 70 °C until constant weight. The ν was estimated based on the Flory-Rehner equation[1212 Flory, P. J., & Rehner, J. Jr (1943). Statistical mechanics of crossLinked polymer networks II. swelling. The Journal of Chemical Physics, 11(11), 521-526. http://dx.doi.org/10.1063/1.1723792.
http://dx.doi.org/10.1063/1.1723792...
].
where Vr is the volume fraction of rubber in the swollen mass, χ is the polymer-solvent interaction parameter which is equal to 0.39 for the NR-toluene system, ρ is the density of the polymer and Vs is molar volume of the solvent (106.3 cm3/mol).
2.3.3 Tensile properties
The tensile properties of crosslinked NR were investigated by means of a universal tensile testing machine (LLOYD Instruments, LR5K Plus, UK). The test was performed according to ISO37 (type 2). Extensometer was used to measure the strain during tensile testing. Five specimens were used and the results reported are averages.
2.3.4 Crystallization behavior and structural changes during deformation
Variations of crystallinity and crystallite size in the vulcanized NR samples during the tensile stretching were investigated by means of WAXD. The structural parameters such as size and dispersion of crosslinked network structures were estimated by means of SAXS measurements. Both WAXD and SAXS were performed at the Siam Photon Laboratory, Synchrotron Light Research Institute (SLRI), Nakhon-Ratchasima, Thailand. The X-ray energy was 9 keV and the measurements were conducted at room temperature (25 °C) with 500 mm/min extension rate during testing. The tensile machine was in-house developed and a laser system was used to measure the extension between gauges. The exposure time at a fixed strain was 30 seconds and the WAXD and SAXS data were corrected and analyzed by using SAXSIT4.41 software.
From WAXD data, the degree of crystallinity (%) corresponding to the (200) and (120) planes during stretching were estimated for samples with different mastication times by using the following equation[1313 Tosaka, M., Murakami, S., Poompradub, S., Kohjiya, S., Ikeda, Y., Toki, S., Sics, T., & Hsiao, B. S. (2004). Orientation and crystallization of natural rubber network as revealed by WAXD using synchrotron radiation. Macromolecules, 37(9), 3299-3309. http://dx.doi.org/10.1021/ma0355608.
http://dx.doi.org/10.1021/ma0355608...
]:
where Xc is the degree of crystallinity, Ac is the area under crystalline peaks of the (200) or (120) planes, and Aa are the area of the amorphous halo.
The average crystallite sizes corresponding to (200) and (120) planes, (L200 and L120) were estimated from the Scherrer equation[1313 Tosaka, M., Murakami, S., Poompradub, S., Kohjiya, S., Ikeda, Y., Toki, S., Sics, T., & Hsiao, B. S. (2004). Orientation and crystallization of natural rubber network as revealed by WAXD using synchrotron radiation. Macromolecules, 37(9), 3299-3309. http://dx.doi.org/10.1021/ma0355608.
http://dx.doi.org/10.1021/ma0355608...
,1414 Ikeda, Y., Yasuda, Y., Hijikata, K., Tosaka, M., & Kohjiya, S. (2008). Comparative study on strain-induced crystallization behavior of peroxide cross-linked and sulfur cross-linked natural rubber. Macromolecules, 41(15), 5876-5884. http://dx.doi.org/10.1021/ma800144u.
http://dx.doi.org/10.1021/ma800144u...
]:
where, Lhkl is the average crystallite size in the (200) or (120) planes, K is equal to 0.89, λ is the wavelength, β is the half-width at half-height, and θ is the Bragg angle.
Herman’s orientation function (OP) was used to estimate the degree of chain orientation during stretching, and it can be defined as follows[1515 Somani, R. H., Yang, L., Zhu, L., & Hsiao, B. S. (2005). Flow-induced shish-kebab precursor structures in entangled polymer melts. Polymer, 46(20), 8587-8623. http://dx.doi.org/10.1016/j.polymer.2005.06.034.
http://dx.doi.org/10.1016/j.polymer.2005...
,1616 Roe, R. J. (2000). Methods of x-ray and neutron scattering in polymer science. New York: Oxford University Press.]:
Here ϕ is the azimuthal angle and I(ϕ) is the scattered intensity along the angle ϕ.
Structural changes, e.g., size of crosslinked network structures, with varied mastication times were analyzed by fitting the SAXS profiles with Guinier approximation defined as follows[1717 Lee, C. H., Saito, H., Inoue, T., & Nojima, S. (1996). Time-resolved small-angle x-ray scattering studies on the crystallization of poly(ethylene terephthalate). Macromolecules, 29(22), 7034-7037. http://dx.doi.org/10.1021/ma951828m.
http://dx.doi.org/10.1021/ma951828m...
,1818 Masa, A., Saito, H., Sakai, T., Kaesaman, A., & Lopattananon, N. (2017). Morphological evolution and mechanical property enhancement of natural rubber/polypropylene blend through compatibilization by nanoclay. Journal of Applied Polymer Science, 134(10), 44574. http://dx.doi.org/10.1002/app.44574.
http://dx.doi.org/10.1002/app.44574...
]:
where Rg is Guinier’s radius, a measure of the size of the scattering object.
3. Results and Discussions
3.1 Curing characteristics
Torque-time curves during vulcanization of the NR vulcanizates prepared with varied mastication times are shown in Figure 1. Curing characteristics such as Ts1, Tc90, ML, MH, and MH-ML are listed in Table 2. The ML, an indication of viscosity and processability of the uncured rubber, decreased with mastication time. The ML is usually proportional to the uncured physical crosslinking or chain entanglement[1010 Dick, J. S. (2003). Basic rubber testing: selection methods for a rubber testing program. Pennsylvania: ASTM International. http://dx.doi.org/10.1520/MNL39-EB.
http://dx.doi.org/10.1520/MNL39-EB...
] and to molecular weight of rubber. It is well-known that mastication mechanically breaks and shortens long rubber molecular chains[88 Ehabe, E. E., Bonfils, F., Sainte-Beuve, J., Collet, A., & Schue, F. (2006). High-temperature mastication of raw natural rubber: changes in macrostructure and mesostructure. Polymer Engineering and Science, 46(2), 222-227. http://dx.doi.org/10.1002/pen.20433.
http://dx.doi.org/10.1002/pen.20433...
,99 Hayeemasae, N., Waesateh, K., Soontaranon, S., & Masa, A. (2020). The effect of mastication time on the physical properties and structure of natural rubber. Journal of Elastomers and Plastics. http://dx.doi.org/10.1177/0095244320928566.
https://doi.org/10.1177/0095244320928566...
,1919 Dimier, F., Vergnes, B., & Vincent, M. (2004). Relationships between mastication conditions and rheological behavior of a natural rubber. Rheologica Acta, 43(2), 196-202. http://dx.doi.org/10.1007/s00397-003-0342-7.
http://dx.doi.org/10.1007/s00397-003-034...
]. A longer mastication then corresponds to greater molecular weight reduction. In contrast, the MH is a measure of the cured compound’s stiffness, and it tended to increase with mastication time. Also, the MH - ML torque difference represents the total crosslinking density of the vulcanizate, and it also increased over mastication time. This was simply due to the radicals formed by chain scissions. Shortening the molecular chains may facilitate the reactions of curing precursors with rubber chains during vulcanization, due to a reduction of steric hindrances from large molecular chains. Consequently, the crosslink density of the samples increased with mastication time. That the crosslink density increased with mastication time was later confirmed by using equilibrium swelling test, and the results agreed well with the variation of the MH - ML.
Considering Ts1 and Tc90, it was found that the Ts1 appeared to be independent of the mastication time, but the shortest curing time (Tc90) was observed when the rubber was initially masticated for 5 min. Previously, it was demonstrated that non-rubber components in the masticated rubber were more homogeneously distributed throughout the rubber matrix when the rubber was masticated for a comparatively short time (e.g., for less than 5 min)[99 Hayeemasae, N., Waesateh, K., Soontaranon, S., & Masa, A. (2020). The effect of mastication time on the physical properties and structure of natural rubber. Journal of Elastomers and Plastics. http://dx.doi.org/10.1177/0095244320928566.
https://doi.org/10.1177/0095244320928566...
]. These impurities may be involved in the crosslinking reactions, possibly acting as natural accelerators[2020 Smitthipong, W., Tantatherdtam, R., Rungsanthien, K., Suwanruji, P., Klanarong, S., Radabutra, S., Thanawan, S., Vallat, M. F., Nardin, M., Mougin, K., & Chollakup, R (2013). Effect of non-rubber components on properties of sulphur crosslinked natural rubbers. Advanced Materials Research, 844, 345-348. http://dx.doi.org/10.4028/www.scientific.net/AMR.844.345.
http://dx.doi.org/10.4028/www.scientific...
,2121 Kongkaew, C., Intiya, W., Loykulnant, S., & Sae-oui, P. (2017). Effect of protein crosslinking by maillard reaction on natural rubber properties. KGK. Kautschuk, Gummi, Kunststoffe, 5, 37-41.]. Better chemical crosslinking is expected when the dispersion of these impurities is homogeneous.
The effects of mastication time on aging behavior of the rubber were assessed from the percentage reversion of the vulcanizates at elevated temperatures, by determining R300[1111 Khang, T. H., & Ariff, Z. M. (2012). Vulcanization kinetics study of natural rubber compounds having different formulation variables. Journal of Thermal Analysis and Calorimetry, 109(3), 1545-1553. http://dx.doi.org/10.1007/s10973-011-1937-3.
http://dx.doi.org/10.1007/s10973-011-193...
], and the results are included in Table 2. A larger R300 usually indicates lesser reversion resistance. As the mastication time increased, R300 increased accordingly, suggesting that the ability of rubber molecules to withstand reversion at an elevated temperature diminished. This was clearly due to molecular chain scission. The lower molecular weight rubber was more susceptible to heat degradation[2222 Karaagac, B., Cengiz, S. C., Bayram, T., & Sen, M. (2018). Identification of temperature scanning stress relaxation behaviors of new grade ethylene propylene diene elastomers. Advances in Polymer Technology, 37(8), 3027-3037. http://dx.doi.org/10.1002/adv.21973.
http://dx.doi.org/10.1002/adv.21973...
,2323 Ray, S., & Cooney, R. P. (2018). Thermal degradation of polymer and polymer composites. In M. Kutz (Ed.), Handbook of environmental degradation of materials (pp. 185-206). Oxford: William Andrew Publishing. http://dx.doi.org/10.1016/B978-0-323-52472-8.00009-5.
http://dx.doi.org/10.1016/B978-0-323-524...
].
3.2 Tensile properties
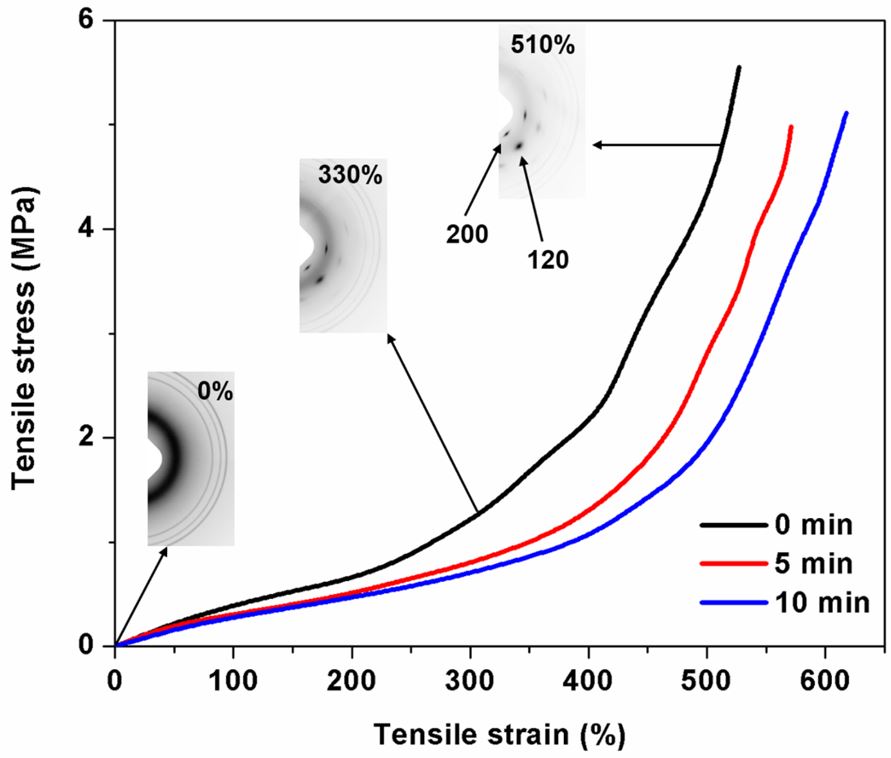
The dependence of stress response during the tensile tests on the mastication time is shown in Figure 2, and the tensile properties in terms of modulus at 100% (M100), 300% (M300) and 500% (M500) strains, tensile strength (TS) and elongation at break (EB) are listed in Table 3. Apparently, the stresses in the vulcanizate sample without mastication (0 min) at all given strains (M100, M300 and M500) were higher than those in rubbers masticated for 5 and 10 min, suggesting that the rubber was stronger without mastication, even though a lower total crosslink density was found for the sample without mastication. The tensile testing results clearly confirm that the effect of molecular weight is more pronounced than that of the total crosslink density. High stress response in the sample without mastication was tentatively attributed to a large proportion of chain entanglements in high molecular weight NR as suggested by the ML value (Table 2). These entanglements are capable of hindering the mobility of rubber chains during deformation, resulting in a higher stress. A reduction of chain entanglements with decreasing rubber molecular weight should be responsible for the decreased tensile stress response[2424 Bueche, F. (1958). Mechanical properties of natural and synthetic rubbers. Rubber Chemistry and Technology, 31(1), 1-18. http://dx.doi.org/10.5254/1.3542259.
http://dx.doi.org/10.5254/1.3542259...
].
Plot of tensile stress versus tensile strain for NR vulcanizates prepared with different mastication times.
Tensile properties in terms of M100, M300, M500, TS and EB for NR vulcanizates prepared with different mastication times.
It was also found that the EB tended to increase with mastication time. Since the molecular weight decreased with mastication, a longer mastication time resulted in a lesser molecular weight. For longer mastication, some molecular chains turned to short chains with low molecular weight. Such chains may act as a plasticizer, facilitating extension of the vulcanized sample.
3.3 Crystallization behavior and structural changes during deformation
Figure 3 shows combination of WAXD images and real-time stress responses as functions of strain, recorded during the WAXD measurement. It can be seen from Figure 3 that the simultaneous stress response during the WAXD test showed similar trend to the stress response obtained from the tensile test (Figure 2). This indicates that the molecular chains responded similarly during tensile testing and WAXD measurement. This provides scientific proof for the similarity of stress response during WAXD and tensile measurements. It is also noted that the stress response during stretching decreased with mastication time, as this caused molecular chain breakdown and lowered molecular weight of the rubber; hence the tensile properties deteriorated. The appearance in inserted WAXD images depends strongly on the strain applied. The image without any reflection spot is for the sample without deformation (0%), suggesting that no crystallization occurs. When the sample was stretched to 330% strain, various reflection spots corresponding to different crystallographic planes are seen. However, the (200) and (120) planes are of main interest in this study. These crystallographic planes were more intense with further increase in deformation, implying that the molecular chain orientation and crystallization increased with strain.
Figure 4 shows the variation of crystallinity corresponding to the (200) and (120) planes for the rubber samples prepared with different mastication times. The crystallinity increased with strain in all cases, because stretching caused chain orientation and reduced the degree of disorder of the NR chains[2525 Tosaka, M. (2007). Strain-induced crystallization of crosslinked natural rubber as revealed by x-ray diffraction using synchrotron radiation. Polymer Journal, 39(12), 1207-1220. http://dx.doi.org/10.1295/polymj.PJ2007059.
http://dx.doi.org/10.1295/polymj.PJ20070...
]. These crystallites provided self-reinforcement to the rubber due to their ability to act as virtual filler or as crosslink[77 Huneau, B. (2011). Strain-induced crystallization of natural rubber: A review of x-ray diffraction investigations. Rubber Chemistry and Technology, 84(3), 425-452. http://dx.doi.org/10.5254/1.3601131.
http://dx.doi.org/10.5254/1.3601131...
,2626 Tosaka, M., Senoo, K., Kohjiya, S., & Ikeda, Y. (2007). Crystallization of stretched network chains in cross-linked natural rubber. Journal of Applied Physics, 101(8), 084909. http://dx.doi.org/10.1063/1.2716382.
http://dx.doi.org/10.1063/1.2716382...
]. As a result, the tensile stress steeply increased after strain-induced crystallization began. It is also seen that the degree of crystallinity of both crystallographic planes had rank order 0 min > 5 min > 10 min. The result clearly suggests that strain-induced crystallization decreased with reduction of molecular weight, achieved via mastication. The decreased crystallinity with increasing mastication time can be explained by increased total crosslink density, as previously shown in Figure 1 and Table 2. Enhanced crosslinking could reduce the mobility or NR chains and delay the orientation of the crystallites during stretching. It is well accepted that the ability for strain-induced crystallization decreases when crosslink density increases, because the molecular chain spans between crosslinks (Mc) are shortened according to υ = 1/Mc, where υ is crosslink density. Consequently, the ability to produce large crystallites is lowered[2727 Trabelsi, S., Albouy, P. A., & Rault, J. (2003). Crystallization and melting processes in vulcanized stretched natural rubber. Macromolecules, 36(20), 7624-7639. http://dx.doi.org/10.1021/ma030224c.
http://dx.doi.org/10.1021/ma030224c...
,2828 Chenal, M., Chazeau, L., Guy, L., Bomal, Y., & Gauthier, C. (2007). Molecular weight between physical entanglements in natural rubber: A critical parameter during strain-induced crystallization. Polymer, 48(4), 1042-1046. http://dx.doi.org/10.1016/j.polymer.2006.12.031.
http://dx.doi.org/10.1016/j.polymer.2006...
]. In this case, increased mastication time reduced the molecular chain weight and increased crosslink density of the NR. As a result, the Mc was smallish and limited the crystallization during stretching. One may argue that ploting the crystallinity data as a function of stress might be useful. However, the reinforcement of rubber is often defined by an improvement in properties at a given strain, i.e., modulus at a certain strain, and tensile strength. Futhermore, the changes of crystallization during stretching are involved with the rubber deformation particularly at large strains, and reflect the reinforcement of rubber, appearing as a function of stretching level. Thus, plotting the crystallization data as a function of stress might be inappropiate due to the different phenomena in the progression of strain-induced crystallization.
Degree of crystallinity at various strains in the NR vulcanizates prepared with different mastication times.
Figure 5 shows variation of the average crystallite size in NR vulcanizates. The size of crystallites appeared to decrease with strain due to the increased number of crystallites with strain (Figure 4), and a reduction of mean distance between the stretched chains which acted as crystallite precursors[2929 Klug, H. P., & Alexander, L. E. (1974). X-ray diffraction procedures: For polycrystalline and amorphous materials. New York: Wiley.]. It is also observed that the average size of crystallite during stretching of the sample without mastication (0 min) was slightly smaller than in the samples with 5 and 10 min of mastication. This is attributed to the large number of crystallites formed in the samples with mastication, resulting in a small average size of crystallite.
Variation of crystallite size at various strains in the NR vulcanizates prepared with different mastication times.
To gain a deeper understanding regarding the orientation of crystallites during stretching, Herman’s orientation function (OP) was used to estimate the degree of crystallite orientation. The degree of crystallite orientation was calculated from the azimuthal intensity distribution of the (120) plane. The OP approaches 1 when the crystallites are completely aligned along the stretching direction, while the value is 0 for crystals that are randomly oriented, and 0.5 when the crystals are aligned perpendicular to the stretching direction[1313 Tosaka, M., Murakami, S., Poompradub, S., Kohjiya, S., Ikeda, Y., Toki, S., Sics, T., & Hsiao, B. S. (2004). Orientation and crystallization of natural rubber network as revealed by WAXD using synchrotron radiation. Macromolecules, 37(9), 3299-3309. http://dx.doi.org/10.1021/ma0355608.
http://dx.doi.org/10.1021/ma0355608...
]. Figure 6 shows the OP for NR vulcanizates prepared with different mastication times, as functions of strain. The OP slightly increased with strain and approached 1, implying that the alignment of crystallites was almost parallel to the stretching direction. The results obtained in recent studies align well with these observations[3030 Che, J., Burger, C., Toki, S., Rong, L., Hsiao, B. S., Amnuaypornsri, S., & Sakdapipanich, J. (2013). Crystal and crystallites structure of natural rubber and synthetic cis-1,4-polyisoprene by a new two dimensional wide angle x-ray diffraction simulation method, I, strain-induced crystallization. Macromolecules, 46(11), 4520-4528. http://dx.doi.org/10.1021/ma400420k.
http://dx.doi.org/10.1021/ma400420k...
,3131 Che, J., Burger, C., Toki, S., Rong, L., Hsiao, B. S., Amnuaypornsri, S., & Sakdapipanich, J. (2013). Crystal and crystallites structure of natural rubber and peroxide-vulcanized natural rubber by a two-dimensional wide-angle x-ray diffraction simulation method, II, strain-induced crystallization versus temperature-Induced crystallization. Macromolecules, 46(24), 9712-9721. http://dx.doi.org/10.1021/ma401812s.
http://dx.doi.org/10.1021/ma401812s...
].
Orientation of crystallinity at various strains in the NR vulcanizates prepared with different mastication times.
Since the SAXS intensity patterns of the vulcanized rubber samples were attributed to the presence of crosslinked networks[3232 Salgueiro, W., Somoza, A., Torriani, I. L., & Marzocca, A. J. (2007). Cure temperature influence on natural rubber - a small angle x-ray scattering study. Journal of Polymer Science. Part B, Polymer Physics, 45(21), 2966-2971. http://dx.doi.org/10.1002/polb.21293.
http://dx.doi.org/10.1002/polb.21293...
,3333 Masa, A., Soontaranon, S., & Hayeemasae, N. (2020). Influence of sulfur/accelerator ratio on tensile properties and structural inhomogeneity of natural rubber. Polymer, 44(4), 519-526. http://dx.doi.org/10.7317/pk.2020.44.4.519.
https://doi.org/10.7317/pk.2020.44.4.519...
], the variations of crosslinked networks were investigated. Effects of the mastication time on dispersion and size of crosslinked network structures were investigated by using the SAXS measurement, and the results are shown in Figures 7 and 8.
Correlation of scattering intensity I(q) with scattering vector q for NR vulcanizates prepared with different mastication times.
Figure 7 shows a plot of the scattering intensity I(q) against scattering vector q for the NR vulcanizates prepared with different mastication times. The q is defined as: q = (4πsinθ)/λ; λ is the wavelength and 2θ is the scattering angle. From Figure 7, a reduction of molecular weight with increasing mastication time slightly affected the SAXS profile of the vulcanizate. This was attributed to the fact that there was only reorientation of scattering bodies such as a crosslinked network[3434 Weng, G., Huang, G., Lei, H., Qu, L., Nie, Y., & Wu, J. (2011). Crack initiation and evolution in vulcanized natural rubber under high temperature fatigue. Polymer Degradation & Stability, 96(12), 2221-2228. http://dx.doi.org/10.1016/j.polymdegradstab.2011.09.004.
http://dx.doi.org/10.1016/j.polymdegrads...
]. The I(q) in all cases decreased continuously with increasing q and the I(q) was almost constant when the value of q approached 0.2. However, the lowest intensity was observed for the sample with 5 min of mastication, suggesting a homogeneous distribution of the crosslinked networks throughout the rubber matrix[99 Hayeemasae, N., Waesateh, K., Soontaranon, S., & Masa, A. (2020). The effect of mastication time on the physical properties and structure of natural rubber. Journal of Elastomers and Plastics. http://dx.doi.org/10.1177/0095244320928566.
https://doi.org/10.1177/0095244320928566...
]. The homogeneity of crosslinked networks clearly contributed to the tensile properties and thus the highest tensile strength was obtained for the sample with 5 min mastication. This clearly confirms the importance of crosslinked networks to tensile properties.
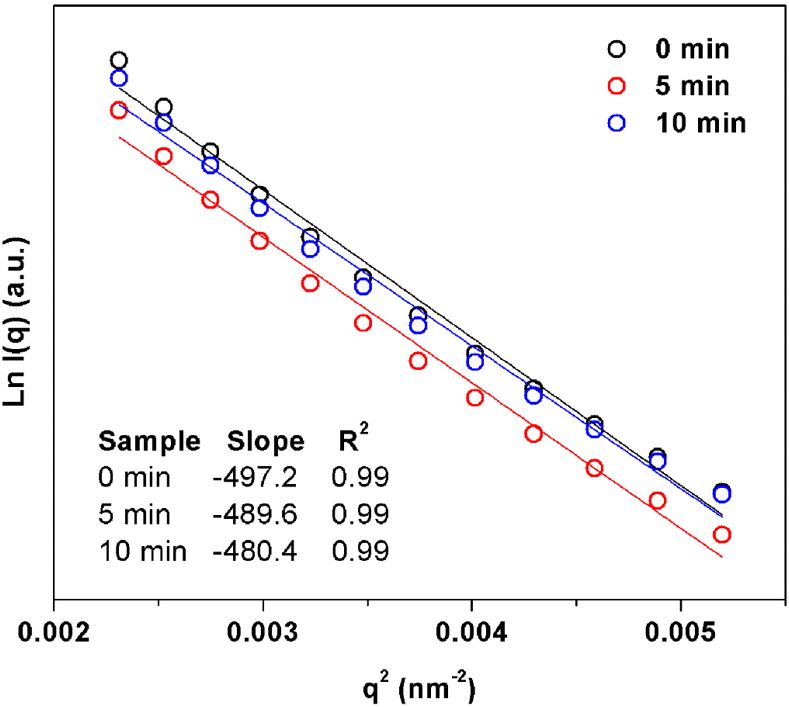
To gain further details of network structural differences in vulcanizate samples prepared with varied mastication times, the SAXS profiles were then fitted with the Guinier equation. Figure 8 shows typical Guinier plots (Ln I(q) versus q2) for the crosslinked NR samples. The data in Figure 8 were fitted with straight lines with R-squared of 0.99. It can be seen that the slope decreased with mastication time. From the slopes obtained from Figure 8, the Rg can be estimated, and the results are shown in Figure 9.
Figure 9 shows changes in size of crosslinked network structures in the NR vulcanizates prepared with different mastication times. The Rg slightly decreased with mastication time, implying that the size of crosslinked networks in the vulcanizates was reduced with decreasing rubber molecular weight. A reduction of Rg would be attributed to the decreased number of high molecular weight chains that participated in crosslinking. This study suggests not only the variation of crosslinked structures with decreasing molecular weight caused by mastication, but also suggests reduction in size of crosslinked structures with decreasing molecular weight. As compared to NR without vulcanization[99 Hayeemasae, N., Waesateh, K., Soontaranon, S., & Masa, A. (2020). The effect of mastication time on the physical properties and structure of natural rubber. Journal of Elastomers and Plastics. http://dx.doi.org/10.1177/0095244320928566.
https://doi.org/10.1177/0095244320928566...
], the size of Rg for the vulcanized rubber was larger. This is due to the fact that the Rg in unvulcanized NR was attributed to the aggregation of impurities (non-rubber components)[3535 Karino, T., Ikeda, Y., Yasuda, Y., Kohjiya, S., & Shibayama, M. (2007). Nonuniformity in natural rubber as revealed by small-angle neutron scattering, small-angle X-ray scattering, and atomic force microscopy. Biomacromolecules, 8(2), 693-699. http://dx.doi.org/10.1021/bm060983d. PMid:17243766.
http://dx.doi.org/10.1021/bm060983d...
], but the Rg in the vulcanized sample was due to crosslinking of clusters by all impurities, activating agents and crosslinking agents[3636 Ikeda, Y., Higashitani, N., Hijikata, K., Kokubo, Y., Morita, Y., Shibayama, M., Osaka, N., Suzuki, T., Endo, H., & Kohjiya, S. (2009). Vulcanization: new focus on a traditional technology by small-angle neutron scattering. Macromolecules, 42(7), 2741-2748. http://dx.doi.org/10.1021/ma802730z.
http://dx.doi.org/10.1021/ma802730z...
]. Therefore, a larger Rg was achieved for samples after vulcanization.
4. Conclusions
In this study, influences of mastication times on the tensile response, the deformation-induced crystallization and the molecular structure of NR vulcanizates were investigated. Increased mastication time slightly enhanced the crosslink density in rubber vulcanizates. The stress response to tensile deformation tended to decrease with increasing mastication time, which agrees well with the deformation-induced crystallization behavior. Increased mastication time significantly affected modulus at specified strain and upturn point of strain induced-crystallization of the crosslinked samples. Prolonged mastication decreased chain crystallization during stretching and size of crosslinked networks due to shorter rubber polymer chains. Based on the results obtained in this study, it is suggested that prolonged mastication influenced the deformation induced crystallization and structural homogeneity of the crosslinked NR, which in turn affected the tensile properties.
-
How to cite: Hayeemasae, N., Soontaranon, S., Rasidi, M. S. M., & Masa, A. (2021). Tensile and Structural Properties of Natural Rubber Vulcanizates with Different Mastication Times. Polímeros: Ciência e Tecnologia, 31(1), e2021003. https://doi.org/10.1590/0104-1428.09120
-
5. Acknowledgements Financial support from the Prince of Songkla University (Grant No. RDO6202102S) to the first author is gratefully acknowledged. Research and Development Office (RDO), Prince of Songkla University and Assoc. Prof. Dr. Seppo Karrila are acknowledged for assistance in editing the English language in this manuscript. The SCG chemicals is acknowledged for in-house developed extensometer during WAXD and SAXS measurements.
6. References
-
1Smitthipong, W., Suethao, S., Shah, D., & Vollrath, F. (2016). Interesting green elastomeric composites: silk textile reinforced natural rubber. Polymer Testing, 55, 17-24. http://dx.doi.org/10.1016/j.polymertesting.2016.08.007
» http://dx.doi.org/10.1016/j.polymertesting.2016.08.007 -
2Noordermeer, J. W. M. (1998). Recent developments in rubber processing leading to new applications such as the “Green Tire”. Macromolecular Symposia, 127(1), 131-139. http://dx.doi.org/10.1002/masy.19981270118
» http://dx.doi.org/10.1002/masy.19981270118 -
3Fries, H., & Pandit, R. R. (1982). Mastication of rubber. Rubber Chemistry and Technology, 55(2), 309-327. http://dx.doi.org/10.5254/1.3535880
» http://dx.doi.org/10.5254/1.3535880 -
4Flory, P. J. (1946). Effects of molecular structure on physical properties of butyl rubber. Industrial & Engineering Chemistry, 38(4), 417-436. http://dx.doi.org/10.1021/ie50436a023
» http://dx.doi.org/10.1021/ie50436a023 -
5Kok, C. M. (1985). The effect of molecular weight on the physical properties of U.V. degraded natural rubber. European Polymer Journal, 21(1), 37-40. http://dx.doi.org/10.1016/0014-3057(85)90062-X
» http://dx.doi.org/10.1016/0014-3057(85)90062-X -
6Ono, K., Kato, A., & Murakami, K. (1985). Unusual stress-strain properties of natural rubber vulcanizates with high primary molecular weight. Polymer Bulletin, 13(1), 29-33. http://dx.doi.org/10.1007/BF00264237
» http://dx.doi.org/10.1007/BF00264237 -
7Huneau, B. (2011). Strain-induced crystallization of natural rubber: A review of x-ray diffraction investigations. Rubber Chemistry and Technology, 84(3), 425-452. http://dx.doi.org/10.5254/1.3601131
» http://dx.doi.org/10.5254/1.3601131 -
8Ehabe, E. E., Bonfils, F., Sainte-Beuve, J., Collet, A., & Schue, F. (2006). High-temperature mastication of raw natural rubber: changes in macrostructure and mesostructure. Polymer Engineering and Science, 46(2), 222-227. http://dx.doi.org/10.1002/pen.20433
» http://dx.doi.org/10.1002/pen.20433 -
9Hayeemasae, N., Waesateh, K., Soontaranon, S., & Masa, A. (2020). The effect of mastication time on the physical properties and structure of natural rubber. Journal of Elastomers and Plastics http://dx.doi.org/10.1177/0095244320928566.
» https://doi.org/10.1177/0095244320928566 -
10Dick, J. S. (2003). Basic rubber testing: selection methods for a rubber testing program Pennsylvania: ASTM International. http://dx.doi.org/10.1520/MNL39-EB
» http://dx.doi.org/10.1520/MNL39-EB -
11Khang, T. H., & Ariff, Z. M. (2012). Vulcanization kinetics study of natural rubber compounds having different formulation variables. Journal of Thermal Analysis and Calorimetry, 109(3), 1545-1553. http://dx.doi.org/10.1007/s10973-011-1937-3
» http://dx.doi.org/10.1007/s10973-011-1937-3 -
12Flory, P. J., & Rehner, J. Jr (1943). Statistical mechanics of crossLinked polymer networks II. swelling. The Journal of Chemical Physics, 11(11), 521-526. http://dx.doi.org/10.1063/1.1723792
» http://dx.doi.org/10.1063/1.1723792 -
13Tosaka, M., Murakami, S., Poompradub, S., Kohjiya, S., Ikeda, Y., Toki, S., Sics, T., & Hsiao, B. S. (2004). Orientation and crystallization of natural rubber network as revealed by WAXD using synchrotron radiation. Macromolecules, 37(9), 3299-3309. http://dx.doi.org/10.1021/ma0355608
» http://dx.doi.org/10.1021/ma0355608 -
14Ikeda, Y., Yasuda, Y., Hijikata, K., Tosaka, M., & Kohjiya, S. (2008). Comparative study on strain-induced crystallization behavior of peroxide cross-linked and sulfur cross-linked natural rubber. Macromolecules, 41(15), 5876-5884. http://dx.doi.org/10.1021/ma800144u
» http://dx.doi.org/10.1021/ma800144u -
15Somani, R. H., Yang, L., Zhu, L., & Hsiao, B. S. (2005). Flow-induced shish-kebab precursor structures in entangled polymer melts. Polymer, 46(20), 8587-8623. http://dx.doi.org/10.1016/j.polymer.2005.06.034
» http://dx.doi.org/10.1016/j.polymer.2005.06.034 -
16Roe, R. J. (2000). Methods of x-ray and neutron scattering in polymer science New York: Oxford University Press.
-
17Lee, C. H., Saito, H., Inoue, T., & Nojima, S. (1996). Time-resolved small-angle x-ray scattering studies on the crystallization of poly(ethylene terephthalate). Macromolecules, 29(22), 7034-7037. http://dx.doi.org/10.1021/ma951828m
» http://dx.doi.org/10.1021/ma951828m -
18Masa, A., Saito, H., Sakai, T., Kaesaman, A., & Lopattananon, N. (2017). Morphological evolution and mechanical property enhancement of natural rubber/polypropylene blend through compatibilization by nanoclay. Journal of Applied Polymer Science, 134(10), 44574. http://dx.doi.org/10.1002/app.44574
» http://dx.doi.org/10.1002/app.44574 -
19Dimier, F., Vergnes, B., & Vincent, M. (2004). Relationships between mastication conditions and rheological behavior of a natural rubber. Rheologica Acta, 43(2), 196-202. http://dx.doi.org/10.1007/s00397-003-0342-7
» http://dx.doi.org/10.1007/s00397-003-0342-7 -
20Smitthipong, W., Tantatherdtam, R., Rungsanthien, K., Suwanruji, P., Klanarong, S., Radabutra, S., Thanawan, S., Vallat, M. F., Nardin, M., Mougin, K., & Chollakup, R (2013). Effect of non-rubber components on properties of sulphur crosslinked natural rubbers. Advanced Materials Research, 844, 345-348. http://dx.doi.org/10.4028/www.scientific.net/AMR.844.345
» http://dx.doi.org/10.4028/www.scientific.net/AMR.844.345 -
21Kongkaew, C., Intiya, W., Loykulnant, S., & Sae-oui, P. (2017). Effect of protein crosslinking by maillard reaction on natural rubber properties. KGK. Kautschuk, Gummi, Kunststoffe, 5, 37-41.
-
22Karaagac, B., Cengiz, S. C., Bayram, T., & Sen, M. (2018). Identification of temperature scanning stress relaxation behaviors of new grade ethylene propylene diene elastomers. Advances in Polymer Technology, 37(8), 3027-3037. http://dx.doi.org/10.1002/adv.21973
» http://dx.doi.org/10.1002/adv.21973 -
23Ray, S., & Cooney, R. P. (2018). Thermal degradation of polymer and polymer composites. In M. Kutz (Ed.), Handbook of environmental degradation of materials (pp. 185-206). Oxford: William Andrew Publishing. http://dx.doi.org/10.1016/B978-0-323-52472-8.00009-5
» http://dx.doi.org/10.1016/B978-0-323-52472-8.00009-5 -
24Bueche, F. (1958). Mechanical properties of natural and synthetic rubbers. Rubber Chemistry and Technology, 31(1), 1-18. http://dx.doi.org/10.5254/1.3542259
» http://dx.doi.org/10.5254/1.3542259 -
25Tosaka, M. (2007). Strain-induced crystallization of crosslinked natural rubber as revealed by x-ray diffraction using synchrotron radiation. Polymer Journal, 39(12), 1207-1220. http://dx.doi.org/10.1295/polymj.PJ2007059
» http://dx.doi.org/10.1295/polymj.PJ2007059 -
26Tosaka, M., Senoo, K., Kohjiya, S., & Ikeda, Y. (2007). Crystallization of stretched network chains in cross-linked natural rubber. Journal of Applied Physics, 101(8), 084909. http://dx.doi.org/10.1063/1.2716382
» http://dx.doi.org/10.1063/1.2716382 -
27Trabelsi, S., Albouy, P. A., & Rault, J. (2003). Crystallization and melting processes in vulcanized stretched natural rubber. Macromolecules, 36(20), 7624-7639. http://dx.doi.org/10.1021/ma030224c
» http://dx.doi.org/10.1021/ma030224c -
28Chenal, M., Chazeau, L., Guy, L., Bomal, Y., & Gauthier, C. (2007). Molecular weight between physical entanglements in natural rubber: A critical parameter during strain-induced crystallization. Polymer, 48(4), 1042-1046. http://dx.doi.org/10.1016/j.polymer.2006.12.031
» http://dx.doi.org/10.1016/j.polymer.2006.12.031 -
29Klug, H. P., & Alexander, L. E. (1974). X-ray diffraction procedures: For polycrystalline and amorphous materials New York: Wiley.
-
30Che, J., Burger, C., Toki, S., Rong, L., Hsiao, B. S., Amnuaypornsri, S., & Sakdapipanich, J. (2013). Crystal and crystallites structure of natural rubber and synthetic cis-1,4-polyisoprene by a new two dimensional wide angle x-ray diffraction simulation method, I, strain-induced crystallization. Macromolecules, 46(11), 4520-4528. http://dx.doi.org/10.1021/ma400420k
» http://dx.doi.org/10.1021/ma400420k -
31Che, J., Burger, C., Toki, S., Rong, L., Hsiao, B. S., Amnuaypornsri, S., & Sakdapipanich, J. (2013). Crystal and crystallites structure of natural rubber and peroxide-vulcanized natural rubber by a two-dimensional wide-angle x-ray diffraction simulation method, II, strain-induced crystallization versus temperature-Induced crystallization. Macromolecules, 46(24), 9712-9721. http://dx.doi.org/10.1021/ma401812s
» http://dx.doi.org/10.1021/ma401812s -
32Salgueiro, W., Somoza, A., Torriani, I. L., & Marzocca, A. J. (2007). Cure temperature influence on natural rubber - a small angle x-ray scattering study. Journal of Polymer Science. Part B, Polymer Physics, 45(21), 2966-2971. http://dx.doi.org/10.1002/polb.21293
» http://dx.doi.org/10.1002/polb.21293 -
33Masa, A., Soontaranon, S., & Hayeemasae, N. (2020). Influence of sulfur/accelerator ratio on tensile properties and structural inhomogeneity of natural rubber. Polymer, 44(4), 519-526. http://dx.doi.org/10.7317/pk.2020.44.4.519.
» https://doi.org/10.7317/pk.2020.44.4.519 -
34Weng, G., Huang, G., Lei, H., Qu, L., Nie, Y., & Wu, J. (2011). Crack initiation and evolution in vulcanized natural rubber under high temperature fatigue. Polymer Degradation & Stability, 96(12), 2221-2228. http://dx.doi.org/10.1016/j.polymdegradstab.2011.09.004
» http://dx.doi.org/10.1016/j.polymdegradstab.2011.09.004 -
35Karino, T., Ikeda, Y., Yasuda, Y., Kohjiya, S., & Shibayama, M. (2007). Nonuniformity in natural rubber as revealed by small-angle neutron scattering, small-angle X-ray scattering, and atomic force microscopy. Biomacromolecules, 8(2), 693-699. http://dx.doi.org/10.1021/bm060983d PMid:17243766.
» http://dx.doi.org/10.1021/bm060983d -
36Ikeda, Y., Higashitani, N., Hijikata, K., Kokubo, Y., Morita, Y., Shibayama, M., Osaka, N., Suzuki, T., Endo, H., & Kohjiya, S. (2009). Vulcanization: new focus on a traditional technology by small-angle neutron scattering. Macromolecules, 42(7), 2741-2748. http://dx.doi.org/10.1021/ma802730z
» http://dx.doi.org/10.1021/ma802730z
Publication Dates
-
Publication in this collection
10 May 2021 -
Date of issue
2021
History
-
Received
07 Oct 2020 -
Reviewed
25 Jan 2021 -
Accepted
15 Feb 2021