ABSTRACT:
Considering the great economic and sanitarian importance of Haematobia irritans − the development of resistance of this species to the main chemical insecticides used in its control, in several other localities of the world; as well as that different strains of the same species frequently present different types and distribution patterns −, the present aim study was to investigate the typology and distribution of different types of sensillae of H. irritans adults, from populations of this fly present in Brazilian Midwest, with emphasis on olfactory sensillae. This study provides new data on the typology and sensillary distribution of antennal sensilla of this fly. In the antennal segments were found non-innervated spinules and ten subtypes of sensilla: long bristles sensillum, long basiconic sensillum, blunt coeloconic sensillum, clavate coeloconic sensilla − single-tip and double-tip subtypes −, grooved coeloconic sensillum, long grooved coeloconic sensillum, trichoid sensillum, coeloconic sensillum, and smaller basiconic sensillum. A slight sexual dimorphism was observed in the antennal sensillae of H. irritans. These results provide a morphological basis for future investigations on olfactory-mediated behavior of this species, and could assist future studies for the development of alternative measures to the monitoring and control of this fly populations, with less environmental impact.
INDEX TERMS:
Haematobia irritans; antenna; ultrastructure; semiochemical; olfactory sensilla
RESUMO:
Considerando a grande importância econômica e sanitária de Haematobia irritans, o desenvolvimento de resistência desta espécie aos principais inseticidas químicos utilizados em seu controle, em diversas outras localidades do mundo, bem como que diferentes cepas de uma mesma espécie frequentemente apresentam diferentes tipos e padrões de distribuição sensilares, objetivou-se no presente trabalho investigar a tipologia e a distribuição dos diferentes tipos e subtipos sensilares de adulttos de H. irritans, oriunda de populações desta mosca presentes no centro-oeste brasileiro, com ênfase nas sensilas olfatórias. Este estudo apresenta novos dados sobre a tipologia e distribuição sensilar da antenna desta mosca. Em seus segmentos antenais foram observados pilosidades não enervadas e dez subtipos de sensilas, sendo: long bristles sensillum, long basiconic sensillum, blunt coeloconic sensillum, clavate coeloconic sensilla - single-tip and double-tip subtypes -, grooved coeloconic sensillum, long grooved coeloconic sensillum, trichoid sensillum, coeloconic sensillum, e smaller basiconic sensillum. Leve dimorfismo sexual foi observado em relação às sensilas antenais de H. irritans. Estes resultados fornecem uma base morfológica para futuras investigações sobre o comportamento mediado pelo olfato desta espécie, e poderão fomentar futuros estudos para desenvolvimento de medidas alternativas de monitoramento e controle de populações dessa mosca, com menor impacto.
TERMOS DE INDEXAÇÃO:
Haematobia irritans; antena; ultraestrutura; semioquímicos; sensilas olfatórias
Introduction
Haematobia irritans (Linnaeus, 1758) (Diptera: Muscidae: Muscinae), commonly known as the “horn fly”, is considered one of the main problems of animal husbandry in several countries. This ectoparasite occurs commonly in Europe, Asia, Africa, USA and American Continent. This fly was introduced into Brazil in 1978 in Roraima, and in the current days it is disseminated through all the Brazilian states, and also in Argentina, Paraguay and Uruguay. Males and females of H. irritans are obligate blood-feeding ectoparasite, feeding almost exclusively on cattle, but can also parasitize horses and sheep. These flies feed intermittently about 20-40 times a day of bovine blood. While feeding, the “horn fly” inserts and withdraws its proboscis repeatedly into the same puncture, in a pumping motion. Engorgement usually requires 4-10 min but feeding can take as long as 25-30 min. This parasitism causes great stress to the cattle and consequently considerable loss in their production (Honer & Gomes 1990Honer M.R. & Gomes A. 1990. O manejo integrado de mosca-dos-chifres, berne e carrapatos em gado de corte. Embrapa Gado de Corte, Campo Grande, MS. 60p.).
With infestations varying from 50 to 500 individuals per bovine, H. irritans cause very significant losses. It has been estimated that a cow parasitized by about 500 flies per day suffers a blood loss of about 2.6 liters of blood per year, and consequently a reduction in milk production and weight gain. In Brazil the losses caused by this ectoparasite were estimated at about 865 million dollars per year, related to the loss of approximately 10% of the weight of cattle infested, created for slaughter and production of meat (Bianchin et al. 2006Bianchin I., Koller W.W. & Detmann E. 2006. The seasonality of Haematobia irritans in central Brazil. Pesq. Vet. Bras. 26:79-86.). In addition, through its bites and hematophagy, this dipteran can transmit disease pathogens such as anaplasmosis, leucosis and black leg (Almeida et al. 2010Almeida F.A., Basso F.C., Seno M.C.Z. & Valério Filho W.V. 2010. Population dynamics of the horn fly (Haematobia irritans) on Guzera cattle breed and crossbred in Selvíria, MS. Semina, Ciên. Agrárias 31:157-162.). Just in the USA, losses were estimated at between US$700 million and $1 billion, while an additional US$60 million is spent annually on insecticides to reduce horn fly populations (Byford et al. 1992Byford R.L., Craig M.E. & Crosby B.L. 1992. A review of ectoparasites and their effect on cattle production. J. Anim. Sci. 70:597-602., Fitzpatrick & Kaufman 2012Fitzpatrick D. & Kaufman P.E. 2012. Horn fly, Haematobia irritans irritans Linnaeus (Insecta: Diptera: Muscidae). UF/IFAS Extension, Florida. 7p., Benson et al. 2014Benson G., Ho T., Thornton A. & Wilson T. 2014. Population survey of Haematobia irritans (Diptera: Muscidae) on cattle in sale barn in Navasota, Texas. J. Stud. Res. 1:1-6.).
Resistance of the H. irritans to chemical insecticides, the main products used in its control, has been widely diagnosed in the Americas since South America (Argentina) to North America (Canada) (Mwangala & Galloway 1993Mwangala F.S. & Galloway T.D. 1993. Dynamics of pyrethroid resistance in the horn fly, Haematobia irritans (L.) (Diptera: Muscidae), populations on tagged and untagged cattle in Manitoba. Can. Entomol. 125: 839-845.). Also in Brazil resistance of this fly to these products has been verified, arriving at the Northeast, Midwest and South regions (Sabatini et al. 2009Sabatini G.A., Ribolla P.E.M., Barros A.T.M., Guerrero F.D. & Schumaker T.T.S. 2009. Knockdown resistance in pyrethroid-resistant horn fly (Diptera: Muscidae) populations in Brazil. Revta Bras. Parasitol. Vet. 18:8-14., Barros et al. 2013Barros A.T.M., Schumaker T.T.S., Koller W.W., Klafke G.M., Albuquerque T.A. & Gonzalez R. 2013. Mechanisms of pyrethroid resistance in Haematobia irritans (Muscidae) from Mato Grosso do Sul state, Brazil. Revta Bras. Parasitol. Vet. 22:136-142.). The resistance of Horn Fly to many of the insecticides has been demonstrated to occur through several known mechanisms, including target site insensitivity and thorough metabolic detoxification of insecticides (Szalanski et al. 1995Szalanski A.L., Black W.C. & Broce A.B. 1995. Esterase staining activity in pyrethroid-resistant horn flies (Diptera: Muscidae). J. Kans. Entomol. Soc. 68:303-312., Oyarzún et al. 2008Oyarzún M.P., Quiroz A. & Birkett M.A. 2008. Insecticide resistance in the horn fly: alternative control strategies. Med. Vet. Entomol. 22:188-202.). Therefore, studies for the development of new control and monitoring measurements of this fly become appropriate.
Olfactory sensilla, also called chemosensory, are intriguing targets for the development of supplemental control strategies or harmful insects. This is due to the fact that these structures, in hematophagous Diptera, as well as in other arthropod parasites and vectors, are employed in the courtship and mating behavior, in the location of host (nutrients source), as well as in the selection of suitable sites for oviposition. Field monitoring studies have indicated that olfactory system plays an important role in guiding the behaviour of hematophagous winged insects (Zwiebel & Takken 2004Zwiebel L.J. & Takken W. 2004. Olfactory regulation of mosquito-host interactions. Insect. Biochem. Molec. 34:645-652., Mukabana et al. 2012Mukabana W.R., Mweresa C.K., Otieno B., Omusula P., Smallegange R.C., Van Loon J.J. & Takken W. 2012. A novel synthetic odorant blend for trapping of malaria and other African mosquito species. J. Chem. Ecol. 38:235-244.). A promising and low environmental impact alternative would be the development of attractant semiochemicals, to be used in traps (Pomonis et al. 1993Pomonis J.G., Hammak L. & Hakk H. 1993. Identification of compounds in an HPLC fraction from female extracts thar elicit mating responses in male screwworm flies, Cochiomyia hominivorax. J. Chem. Ecol. 19:985-1008., Furukawa et al. 2002Furukawa A., Shibata C. & Mori K. 2002. Syntheses of four methyl-branched secondary acetates and a methyl-branched ketone as possible candidates for the female pheromone of the screwworm fly, Cochliomyia hominivorax. Biosci. Biotechnol. Biochem. 66:1164-1169.). The electrophysiological investigation of odor response using the method of Single-Sensillum Recordings (SSR), important technique for semiochemicals development, becomes a very difficult task in Cyclorrhapha, due to very reduced size of the antenna (Shanbhag et al. 1999Shanbhag S.R., Müller B. & Steinbrecht R.A. 1999. Atlas of olfactory organs of Drosophila melanogaster. 1. Types, external organization, innervation and distribution of olfactory sensilla. Int. J. Insect Morphol. Embryol. 28:377-397.). Therefore, the scanning electronic microscopy (SEM) becomes an important tool for the identification and mapping of olfactory sensilla, based on external morphologic characteristics (Mitchell et al. 1999Mitchell B.K., Itagaki H. & Rivet M.P. 1999. Peripheral and central structure involved in insect gustation. Microsc. Res. Tech. 47:401-415.).
In view of these facts, the purpose of the present work was to expand the knowledge regarding the anatomy and ultrastructure of male and female of H. irritans, and to localize and morphofunctionally classify the differents types of antennal sensilla of H. irritans, comparing with already recognized ones in other insects. with special attention to olfactory sensilla. In addition, the existence of sexual dimorphism on the antenna of this fly was also investigated.
Materials and Methods
Specimens obtained of the horn fly population of the Midwest Region of Brazil were studied in the present work. Males and females without defects or mutilations were selected with the aid of a stereoscope. The flies were obtained in laboratory starting from feces of naturally infested cattle, originating from ranches, and free of insecticidal residues for at least 45 days.
Twenty samples of Haematobia irritans heads of both sexes were selected, dissected (were separated from the rest of the body with a scalpel blade) and cleaned, as described in Fernandes et al. (2004)Fernandes F.F. , Pimenta P.F.P. & Linardi P.M. 2004. Antennal sensilla of the new world screwworm fly, Cochliomyia hominivorax (Diptera: Calliphoridae). J. Med. Entomol. 41:545-551.: particles of the rearing medium were carefully removed from the specimens using air jet. These were lightly agitated in saline for 1min to remove water-soluble impurities. This procedure was repeated five times, changing the saline on each occasion. The specimens were then placed in saline in an ultrasound apparatus for 1h. After this, the samples were fixed overnight with 2.5% glutaraldehyde in 0.1M caccodylate buffer, pH 7.2, at room temperature and posfixed in 1% osmium tetroxide (Fernandes et al. 2004Fernandes F.F. , Pimenta P.F.P. & Linardi P.M. 2004. Antennal sensilla of the new world screwworm fly, Cochliomyia hominivorax (Diptera: Calliphoridae). J. Med. Entomol. 41:545-551., 2005Fernandes F.F., Freitas E.P., Linardi P.M. & Pimenta P.F.P. 2005. Ultrastructure of contact-chemoreceptor sensilla found among the genae of female Gasterophilus nasalis. J. Parasitol. 91:1218-1220., 2008Fernandes F.F., Bahia-Nascimento A.C., Pinto L.C., Leal C.S., Secundino N.F.C. & Pimenta P.F.P. 2008. Fine structure and distribution pattern of antennal sensilla of Lutzomyia longipalpis (Diptera: Psychodidae) sand flies. J. Med. Entomol. 45:982-990.). Afterwards, it was washed in buffer, and dehydrated in an increasing series of concentrations of ethanol, alternating the concentrations of alcohol at 6h intervals, using 100% ethanol three times. After this, the samples were dried by the critical point method device with CO2 (Fernandes et al. 2002Fernandes F.F., Linardi P.M. & Chiarini-Garcia H. 2002. Morphology of the antenna of Dermatobia hominis (Diptera, Cuterebridae) based on scanning electron microscopy. J. Med. Entomol. 39:36-43.).
Whole heads, and also dissected antennae from other heads, were mounted on the Jeol cylinder SEM specimen stub (Ø12.2 x 10mm, brass), using conductive silver paint. There samples were mounted at different inclinations in order to allow the visualization of the entire external surface of the antennae, including its ventral part, which usually remain hidden over the ventral sulcus of the face of the fly, when in its natural position. The samples were then sputtered with platinum in K550X Sputter Coater (Quorum Emitech, Ashford, UK) to be analyze the samples in the JSM 5600 JEOL scanning electron microscope (Jeol Ltd., Tokyo, Japan), at an accelerating voltage of 5.0-25Kv (Fernandes et al. 2004Fernandes F.F. , Pimenta P.F.P. & Linardi P.M. 2004. Antennal sensilla of the new world screwworm fly, Cochliomyia hominivorax (Diptera: Calliphoridae). J. Med. Entomol. 41:545-551., 2008Fernandes F.F., Bahia-Nascimento A.C., Pinto L.C., Leal C.S., Secundino N.F.C. & Pimenta P.F.P. 2008. Fine structure and distribution pattern of antennal sensilla of Lutzomyia longipalpis (Diptera: Psychodidae) sand flies. J. Med. Entomol. 45:982-990.). In the ultrastructural analysis, the morpho-function types of H. irritans sensilla were classified according their cuticle morphology and observed by SEM (Mitchell et al. 1999Mitchell B.K., Itagaki H. & Rivet M.P. 1999. Peripheral and central structure involved in insect gustation. Microsc. Res. Tech. 47:401-415., Hallberg & Hansson 1999Hallberg E. & Hansson B. 1999. Arthropod sensilla: morphology and phylogenetic considerations. Microsc. Res. Tech. 47:428-439.).
Results
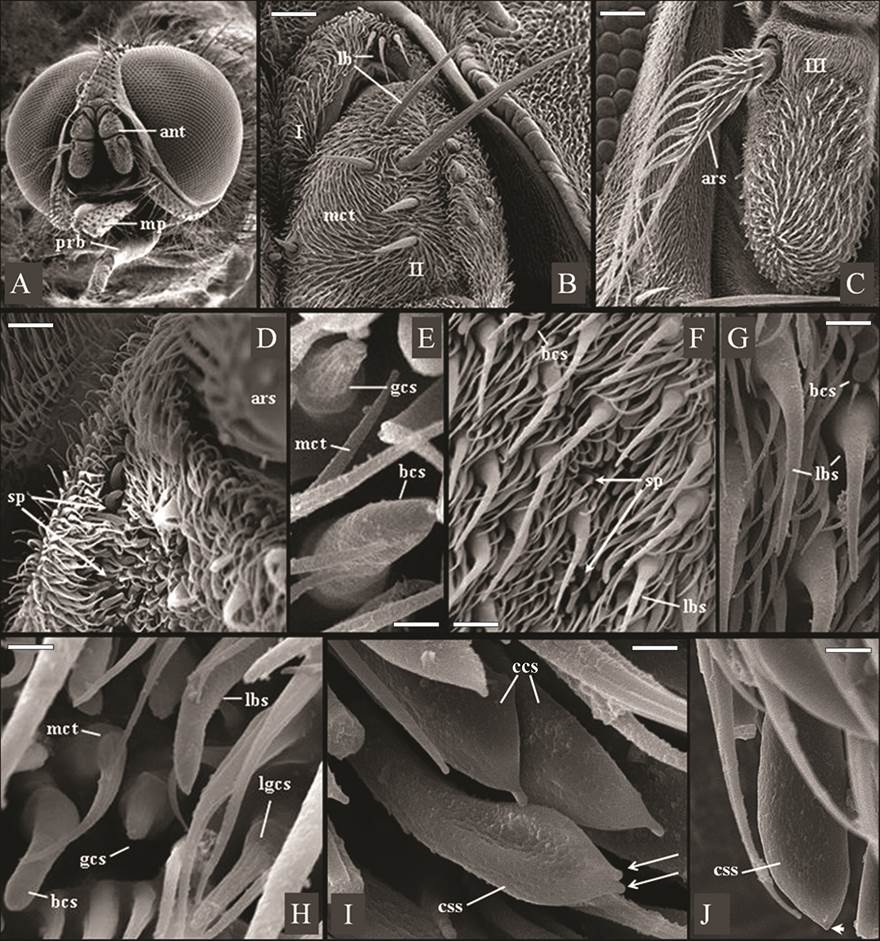
Figure 1A shows the head of Haematobia irritans, with composed of two large dichoptic eyes without hairs and composed of numerous ommatidia, were observed in both sexes (Fig.1A). It also shows its three-articulated antenna, implanted beneath of the ptilineal suture, as well as a relatively shorter proboscis (prb), in contrast to the larger maxillary palps (mp). The scape (1st antennal segment) and the pedicel (2nd antennal segment) of the in both sexes of fly were covered by smaller spinules of microtrichia type and by sensilla of long bristle type, of various sizes (Fig.1B). Figure 1B also shows long bristles sensillum, arranged in a short median tuft (with about three bristles) on the scape, and dispersed in greater numbers on the pedicel.
Eletromicrographs of males and females of Haematobia irritans. (A) Male head showing holoptic eyes, antennae (ant) and a relatively shorter proboscis (prb), in contrast to the larger maxillary palps (mp). (B) Scape (I) and pedicel (II) of the female antenna containing long bristles (lb) and non-innervated spinules of microtrichia type (mct). Scale bar: 42 μm. (C) Frontal view of female flagellum (III) with arista pectinate (ars). Scale bar: 80 μm. (D) Male flagellum with shallow sensory pits (sp) containing distinct sensillar types, beside the base of the arista (ars). Scale bar: 15 μm. (E) Shallow sensory pits in male flagellum containing blunt coeloconic sensillum (bcs) and grooved coeloconic sensillum (gcs, with “fingers”). Scale bar: 1.7 μm. (F,G) Surface of male and female flagellum respectively, showing shallow sensory pits (sp), blunt coeloconic sensillum (bcs), and larger basiconic sensilla (lbs). Scale bar: 10 μm. (H) Surface of male flagellum showing microtrichas (mct) and larger basiconic sensillum (lbs), blunt coeloconic sensillum (bcs), grooved coeloconic sensillum (gcs) and long grooved coeloconic sensillum (lgcs, with long “fingers”). Scale bar: 7 μm. (I,J) Ventral surface of male flagellum containing clavate coeloconic sensilla (ccs), of double-tip (arrows) and single-tip subtypes (arrowhead). Scale bar: 2.0 μm, 1.5 μm and 1.5μm, respectively.
In the antennal segments of H. irritans were found spinules non-innervated spinules of microtrichia type and ten subtypes of sensilla: mechanosensory long bristles sensillum, larger basiconic sensillum, blunt coeloconic sensillum, clavate coeloconic sensilla - single-tip and double-tip subtypes -, grooved coeloconic sensillum, long grooved coeloconic sensillum, trichoid sensillum, coeloconic sensillum and smaller basiconic sensillum (Fig.1).
Table 1 shows the pattern distribution of the different subtypes of sensilla found in the antennal segments of H. irritans.
Figure 2 A and B shows the grooved coeloconic sensilla of the “Horn fly” at higher ampliation. Figure 2C and D shows the female arista, of the pectinate type and with woody aspect, containing trichoid (ts), coeloconic (cs), and basiconic (bs) sensilla.
Eletromicrographs of antennal sensilla of Haematobia irritans. (A) Surface of flagellum of male and (B) female containing grooved coeloconic sensillum (gcs), with “fingers”, blunt coeloconic (bcs) and non-innervated spinules of microtrichia type (mct). Scale bar: 0.8 μm in A and B. (C,D) Female arista, of the pectinate type and with woody aspect, containing trichoid (ts), coeloconic (cs), and basiconic (bs) sensilla. Scale bar: 2.5 μm in C and D.
Discussion
The sensillary typology can be constituted on the basis of morphological criteria, observed by scanning electron microscopy (SEM), such as: distribution; length; grooves on the hairs; presence and location of pore(s), etc. Some opening mechanisms and closing of pores on the chemosensors tips, could also be understood through SEM (Van der Wolk 1978Van der Wolk F.M. 1978. The typology and topography of the tarsal chemoreceptors of the blow flies Calliphora vicina Robineau-Desvoidy and Phormia terranovae Robineau-Desvoidy and the housefly Musca domestica L. J. Morphol. 157:201-210.).
The classification of the sensilla H. irritans was based on its cuticular ultrastructure, comparing it with sensillary types already recognized in other insects, supported by studies of ultrastructure and electrophysiology. In this way, for the analysis of its ultrastructure, the sensilla may be related to different types of functions, such as chemoreceptor, hygroreceptor, thermoreceptor or mechanoreceptor (Hallberg & Hansson 1999Hallberg E. & Hansson B. 1999. Arthropod sensilla: morphology and phylogenetic considerations. Microsc. Res. Tech. 47:428-439., Mitchell et al. 1999Mitchell B.K., Itagaki H. & Rivet M.P. 1999. Peripheral and central structure involved in insect gustation. Microsc. Res. Tech. 47:401-415., Fernandes et al. 2002Fernandes F.F., Linardi P.M. & Chiarini-Garcia H. 2002. Morphology of the antenna of Dermatobia hominis (Diptera, Cuterebridae) based on scanning electron microscopy. J. Med. Entomol. 39:36-43., 2005Fernandes F.F., Freitas E.P., Linardi P.M. & Pimenta P.F.P. 2005. Ultrastructure of contact-chemoreceptor sensilla found among the genae of female Gasterophilus nasalis. J. Parasitol. 91:1218-1220., 2008Fernandes F.F., Bahia-Nascimento A.C., Pinto L.C., Leal C.S., Secundino N.F.C. & Pimenta P.F.P. 2008. Fine structure and distribution pattern of antennal sensilla of Lutzomyia longipalpis (Diptera: Psychodidae) sand flies. J. Med. Entomol. 45:982-990.).
The scape (1st antennal segment) and the pedicel (2nd antennal segment) of the in both sexes of fly were covered by smaller spinules of microtrichia type and by sensilla of long bristle type, of various sizes. Microtrichias, as observed in H. irritans (Fig.1B), constitute the most abundant pilosity type that covers the entire antennal articles of different species of Muscomorpha, being frequently designated by non-innervated spinules, spines, or trichomes (Shanbhag et al. 1999Shanbhag S.R., Müller B. & Steinbrecht R.A. 1999. Atlas of olfactory organs of Drosophila melanogaster. 1. Types, external organization, innervation and distribution of olfactory sensilla. Int. J. Insect Morphol. Embryol. 28:377-397., Stocker 2001Stocker R.F. 2001. Drosophila as a focus in olfactory research: mapping of olfactory sensilla by fine structure, odor specificity, odorant receptor expression, and central connectivity. Microsc. Res. Tech. 55:284-296., Fernandes et al. 2002Fernandes F.F. & Linardi P.M. 2002. Observations on mouthparts of Dermatobia hominis (Linneaus, 1781) (Diptera: Cuterebridae) by scanning electron microscopy. J. Parasitol. 88:191-194., 2004Fernandes F.F. , Pimenta P.F.P. & Linardi P.M. 2004. Antennal sensilla of the new world screwworm fly, Cochliomyia hominivorax (Diptera: Calliphoridae). J. Med. Entomol. 41:545-551.).
As described here in H. irritans (Fig.1B), mechanosensory long bristles sensillum had been previously observed in the flies Dermatobia hominis (L. Jr., 1781) (Diptera: Oestridae: Cuterebrinae)17 and Cochliomyia hominivorax (Coquerel, 1858) (Diptera: Calliphoridae: Chrysomyinae) (Fernandes et al. 2004Fernandes F.F. , Pimenta P.F.P. & Linardi P.M. 2004. Antennal sensilla of the new world screwworm fly, Cochliomyia hominivorax (Diptera: Calliphoridae). J. Med. Entomol. 41:545-551.). The long bristles sensilla are trichoid sensilla, with articulated base, no surface pores, and mechanosensory function (Chapman 1991Chapman R.F. 1991. General anatomy and function, p.33-67. In: Division of Entomology CSIRO Australia [Ed.], The Insects of Australia: a textbook for students and research workers. 2nd ed. National Library of Australia, Melbourne.). This sensillum type can be present in several parts of the body of the insects (Fernandes & Linardi 2002Fernandes F.F. & Linardi P.M. 2002. Observations on mouthparts of Dermatobia hominis (Linneaus, 1781) (Diptera: Cuterebridae) by scanning electron microscopy. J. Parasitol. 88:191-194.) and have frequently been used in taxonomic keys for species identification, based on light microscopy observations (Chapman 1991Chapman R.F. 1991. General anatomy and function, p.33-67. In: Division of Entomology CSIRO Australia [Ed.], The Insects of Australia: a textbook for students and research workers. 2nd ed. National Library of Australia, Melbourne.).
The long bristles are mechanosensory sensilla that receive stimuli originating from contact with surfaces, air currents or sound waves. Each cuticular projection is associated with a single neurone. Various types of mechanoreceptors also function as propioceptors, that is, they detect mechanical stimuli produced by the antenna itself. The mechanoreceptors are fundamental, because they provide information about position, degree of distension of parts of the body, monitoring of flight and rhythmic movements such as those involved in ventilation (Chapman 1991Chapman R.F. 1991. General anatomy and function, p.33-67. In: Division of Entomology CSIRO Australia [Ed.], The Insects of Australia: a textbook for students and research workers. 2nd ed. National Library of Australia, Melbourne., Catalá 1998Catalá S. 1998. Morfologia externa e anatomia, Antenas e rostro, p.74-83. In: Carcavalho R.U. (Ed.), Atlas dos Vetores da Doença de Chagas nas Américas. Fiocruz, Rio de Janeiro.).
Sensilla on the antennal funiculus similar to the long basiconic sensillum (Fig.1F-H) and to the grooved coeloconic sensilla (Fig.1E and H), largest and smallest, observed in this work, was also previously evidenced in Drosophila melanogaster (Fallén, 1823) (Diptera: Drosophilidae: Drosophilinae). They all present wall pores, in different structure and arrangement, allowing access of volatile substances, and therefore possessing an olfactory function (Shanbhag et al. 1999Shanbhag S.R., Müller B. & Steinbrecht R.A. 1999. Atlas of olfactory organs of Drosophila melanogaster. 1. Types, external organization, innervation and distribution of olfactory sensilla. Int. J. Insect Morphol. Embryol. 28:377-397., Stocker 2001Stocker R.F. 2001. Drosophila as a focus in olfactory research: mapping of olfactory sensilla by fine structure, odor specificity, odorant receptor expression, and central connectivity. Microsc. Res. Tech. 55:284-296., Fernandes et al. 2004Fernandes F.F. , Pimenta P.F.P. & Linardi P.M. 2004. Antennal sensilla of the new world screwworm fly, Cochliomyia hominivorax (Diptera: Calliphoridae). J. Med. Entomol. 41:545-551.). The grooved coeloconic sensilla was shown to be an elevation, emerging from a pit, presenting longitudinal grooves and apparently formed by “cuticular fingers-like projections” (Fig.2 C and D). Sensilla, similar to this grooved coeloconic sensilla were also evidenced in the “sphinx moth”, Manduca sexta (Linn., 1763) (Lepidoptera: Sphingidae). Using field-emission scanning electron microscopy, very small cuticular pores could be found at the bottom of each groove, connecting the inside of the sensillum via cuticular spoke channels rather than pore tubules (Shields & Hildebrand 2001Shields V.D.C. & Hildebrand J.G. 2001. Recent advances in insect olfaction, specifically regarding the morphology and sensory physiology of antennal sensilla of the female sphinx moth Manduca sexta. Microsc. Res. Tech. 55:307-329.).
There was also a subtype of coeloconic sensillum, in the flagellum “horny fly” of both sexes, with wall pores, but presenting a blunted apex, called blunt coeloconic sensillum (Fig. 1G-H). Another subtype of coeloconic sensillum was also observed in the present work, as a cylindrical stalk, emerging from a pit and presenting an apical dilation (bulbous tip). For this reason, it was denominated clavate coeloconic sensillum (Fig. 1I and J). It also presented wall pores, homogeneously distributed on the surface.
Sensilla, presenting wall pores and similar to the clavate coeloconic sensillum and the blunt coeloconic sensillum observed in the present work were also previously identified in “stable fly” Stomoxys calcitrans (Linn., 1758) (Diptera: Muscidae: Stomoxydinae) (Tangtrakulwanich et al. 2011Tangtrakulwanich K., Chen H., Baxendale F., Brewer G. & Zhu J.J. 2011. Characterization of olfactory sensilla of Stomoxys calcitrans and electrophysiological responses to odorant compounds associated with hosts and oviposition media. Med. Vet. Entomol. 25:327-336., Zhang et al. 2013Zhang D., Wang Q.K., Yang Y.Z., Chen Y.O. & Li K. 2013. Sensory organs of the antenna of two Fannia species (Diptera: Fanniidae). Parasitol. Res. 112:2177-2185.).
The present study reveals for the first time the existence of a sensillum with characteristics of sensila olfactory in the aristae of Muscomorpha ? This was evidenced only in females of the species and classified as sensillum of the type basiconic and coeloconic type (Fig. 2C and D).
The trichoid sensilla observed in the present work in the in male and female arista of horn fly (Fig. 2C and D) is similar to the sensilla previously observed in the aristae of others flies, such as for D. hominis, C. hominivorax, S. calcitrans and F. hirticeps (Fernandes et al. 2002Fernandes F.F., Linardi P.M. & Chiarini-Garcia H. 2002. Morphology of the antenna of Dermatobia hominis (Diptera, Cuterebridae) based on scanning electron microscopy. J. Med. Entomol. 39:36-43., 2004Fernandes F.F. , Pimenta P.F.P. & Linardi P.M. 2004. Antennal sensilla of the new world screwworm fly, Cochliomyia hominivorax (Diptera: Calliphoridae). J. Med. Entomol. 41:545-551.; Wang et al. 2012Wang Q.K., Zhang M., Li K. & Zhang D. 2012. Olfactory sensilla on antennae and maxillary palps of Fannia hirticeps (Stein, 1892) (Diptera: Fanniidae). Microsc. Res. Tech. 75:1313-1320.). The absence of pores in this sensilla suggests that this types of sensillum may function as mechanoreceptors.
An slight sexual dimorphism in the sensilla patterns was observed in H. irritans (Table 1). Analyses showed that the sensilla have a similar distribution on the antennae of male and female H. irritans, but present a few differences, in relation to the sensillary types, described along this manuscript. This subtle differences probably is due to the need to process different types of stimuli received during the life cycle, involved in mating and oviposition e.g. Differences between the antennal sensilla of males and females have been also noted in others Muscomorpha, as in “Fruit-fly” D. melanogaster (Shanbhag et al. 1999Shanbhag S.R., Müller B. & Steinbrecht R.A. 1999. Atlas of olfactory organs of Drosophila melanogaster. 1. Types, external organization, innervation and distribution of olfactory sensilla. Int. J. Insect Morphol. Embryol. 28:377-397.), “American botfly” D. hominis (Fernandes et al. 2002Fernandes F.F., Linardi P.M. & Chiarini-Garcia H. 2002. Morphology of the antenna of Dermatobia hominis (Diptera, Cuterebridae) based on scanning electron microscopy. J. Med. Entomol. 39:36-43.), “New World Screwworm fly” C. hominivorax (Fernandes et al. 2004Fernandes F.F. , Pimenta P.F.P. & Linardi P.M. 2004. Antennal sensilla of the new world screwworm fly, Cochliomyia hominivorax (Diptera: Calliphoridae). J. Med. Entomol. 41:545-551.), as well as in Nematocera, as in “Sand fly” Lutzomyia longipalpis (Lutz and Neiva, 1912) (Diptera: Psychodidae: Plebotominae) (Fernandes et al. 2008Fernandes F.F., Bahia-Nascimento A.C., Pinto L.C., Leal C.S., Secundino N.F.C. & Pimenta P.F.P. 2008. Fine structure and distribution pattern of antennal sensilla of Lutzomyia longipalpis (Diptera: Psychodidae) sand flies. J. Med. Entomol. 45:982-990.). We believe that the observation of few differences between the distribution of sensillary types of males and females is due to the fact that both sexes of the “horn fly” have the same feeding habits, the same hosts, feeding mainly on bovine blood. Differently, in the Diptera species in which the feeding stimuli (and the food itself) are different between the sexes, a greater sexual dimorphism has been observed in relation to the patterns of distribution and sensory types. As an example may be mentioned “sand flies”, in which only females feed on blood, that is, males do not bite men or animals.
Conclusions
This study provides new data on the typology and sensillary distribution of antennal sensilla of Haematobia irritans. The present study reveals for the first time the existence of a sensillum with characteristics of olfactory sensilla in the aristae of Muscomorpha.
The presence of different sensorial subtypes with olfactory sensory characteristics found in the antennae (flagellum and arista) of H. irritans indicates that the olfaction should play an important role in this species behavior and communication.
The results of this study could assist future electrophysiological analysis to identify chemosensors, combined with behavioral studies to determine their significance.
Such studies would in turn contribute to the development of attractants semiochemicals for monitoring and control of the “horn fly”.
Acknowledgements
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazilian government entity which promotes Scientific and Technological Development; to Fundação de Amparo a Pesquisas do Estado de Minas Gerais (FAPEMIG); to Programa de Apoio a Núcleos de Excelência (PRONEX), Secretaria de Ciência e Tecnologia (SECTEC), and to Fundação Oswaldo Cruz (Fiocruz).
References
- Almeida F.A., Basso F.C., Seno M.C.Z. & Valério Filho W.V. 2010. Population dynamics of the horn fly (Haematobia irritans) on Guzera cattle breed and crossbred in Selvíria, MS. Semina, Ciên. Agrárias 31:157-162.
- Barros A.T.M., Schumaker T.T.S., Koller W.W., Klafke G.M., Albuquerque T.A. & Gonzalez R. 2013. Mechanisms of pyrethroid resistance in Haematobia irritans (Muscidae) from Mato Grosso do Sul state, Brazil. Revta Bras. Parasitol. Vet. 22:136-142.
- Benson G., Ho T., Thornton A. & Wilson T. 2014. Population survey of Haematobia irritans (Diptera: Muscidae) on cattle in sale barn in Navasota, Texas. J. Stud. Res. 1:1-6.
- Bianchin I., Koller W.W. & Detmann E. 2006. The seasonality of Haematobia irritans in central Brazil. Pesq. Vet. Bras. 26:79-86.
- Byford R.L., Craig M.E. & Crosby B.L. 1992. A review of ectoparasites and their effect on cattle production. J. Anim. Sci. 70:597-602.
- Catalá S. 1998. Morfologia externa e anatomia, Antenas e rostro, p.74-83. In: Carcavalho R.U. (Ed.), Atlas dos Vetores da Doença de Chagas nas Américas. Fiocruz, Rio de Janeiro.
- Chapman R.F. 1991. General anatomy and function, p.33-67. In: Division of Entomology CSIRO Australia [Ed.], The Insects of Australia: a textbook for students and research workers. 2nd ed. National Library of Australia, Melbourne.
- Fernandes F.F., Bahia-Nascimento A.C., Pinto L.C., Leal C.S., Secundino N.F.C. & Pimenta P.F.P. 2008. Fine structure and distribution pattern of antennal sensilla of Lutzomyia longipalpis (Diptera: Psychodidae) sand flies. J. Med. Entomol. 45:982-990.
- Fernandes F.F., Freitas E.P., Linardi P.M. & Pimenta P.F.P. 2005. Ultrastructure of contact-chemoreceptor sensilla found among the genae of female Gasterophilus nasalis J. Parasitol. 91:1218-1220.
- Fernandes F.F., Linardi P.M. & Chiarini-Garcia H. 2002. Morphology of the antenna of Dermatobia hominis (Diptera, Cuterebridae) based on scanning electron microscopy. J. Med. Entomol. 39:36-43.
- Fernandes F.F. & Linardi P.M. 2002. Observations on mouthparts of Dermatobia hominis (Linneaus, 1781) (Diptera: Cuterebridae) by scanning electron microscopy. J. Parasitol. 88:191-194.
- Fernandes F.F. , Pimenta P.F.P. & Linardi P.M. 2004. Antennal sensilla of the new world screwworm fly, Cochliomyia hominivorax (Diptera: Calliphoridae). J. Med. Entomol. 41:545-551.
- Fitzpatrick D. & Kaufman P.E. 2012. Horn fly, Haematobia irritans irritans Linnaeus (Insecta: Diptera: Muscidae). UF/IFAS Extension, Florida. 7p.
- Furukawa A., Shibata C. & Mori K. 2002. Syntheses of four methyl-branched secondary acetates and a methyl-branched ketone as possible candidates for the female pheromone of the screwworm fly, Cochliomyia hominivorax Biosci. Biotechnol. Biochem. 66:1164-1169.
- Hallberg E. & Hansson B. 1999. Arthropod sensilla: morphology and phylogenetic considerations. Microsc. Res. Tech. 47:428-439.
- Honer M.R. & Gomes A. 1990. O manejo integrado de mosca-dos-chifres, berne e carrapatos em gado de corte. Embrapa Gado de Corte, Campo Grande, MS. 60p.
- Mitchell B.K., Itagaki H. & Rivet M.P. 1999. Peripheral and central structure involved in insect gustation. Microsc. Res. Tech. 47:401-415.
- Mukabana W.R., Mweresa C.K., Otieno B., Omusula P., Smallegange R.C., Van Loon J.J. & Takken W. 2012. A novel synthetic odorant blend for trapping of malaria and other African mosquito species. J. Chem. Ecol. 38:235-244.
- Mwangala F.S. & Galloway T.D. 1993. Dynamics of pyrethroid resistance in the horn fly, Haematobia irritans (L.) (Diptera: Muscidae), populations on tagged and untagged cattle in Manitoba. Can. Entomol. 125: 839-845.
- Oyarzún M.P., Quiroz A. & Birkett M.A. 2008. Insecticide resistance in the horn fly: alternative control strategies. Med. Vet. Entomol. 22:188-202.
- Pomonis J.G., Hammak L. & Hakk H. 1993. Identification of compounds in an HPLC fraction from female extracts thar elicit mating responses in male screwworm flies, Cochiomyia hominivorax J. Chem. Ecol. 19:985-1008.
- Sabatini G.A., Ribolla P.E.M., Barros A.T.M., Guerrero F.D. & Schumaker T.T.S. 2009. Knockdown resistance in pyrethroid-resistant horn fly (Diptera: Muscidae) populations in Brazil. Revta Bras. Parasitol. Vet. 18:8-14.
- Shanbhag S.R., Müller B. & Steinbrecht R.A. 1999. Atlas of olfactory organs of Drosophila melanogaster 1. Types, external organization, innervation and distribution of olfactory sensilla. Int. J. Insect Morphol. Embryol. 28:377-397.
- Shields V.D.C. & Hildebrand J.G. 2001. Recent advances in insect olfaction, specifically regarding the morphology and sensory physiology of antennal sensilla of the female sphinx moth Manduca sexta Microsc. Res. Tech. 55:307-329.
- Stocker R.F. 2001. Drosophila as a focus in olfactory research: mapping of olfactory sensilla by fine structure, odor specificity, odorant receptor expression, and central connectivity. Microsc. Res. Tech. 55:284-296.
- Szalanski A.L., Black W.C. & Broce A.B. 1995. Esterase staining activity in pyrethroid-resistant horn flies (Diptera: Muscidae). J. Kans. Entomol. Soc. 68:303-312.
- Tangtrakulwanich K., Chen H., Baxendale F., Brewer G. & Zhu J.J. 2011. Characterization of olfactory sensilla of Stomoxys calcitrans and electrophysiological responses to odorant compounds associated with hosts and oviposition media. Med. Vet. Entomol. 25:327-336.
- Van der Wolk F.M. 1978. The typology and topography of the tarsal chemoreceptors of the blow flies Calliphora vicina Robineau-Desvoidy and Phormia terranovae Robineau-Desvoidy and the housefly Musca domestica L. J. Morphol. 157:201-210.
- Wang Q.K., Zhang M., Li K. & Zhang D. 2012. Olfactory sensilla on antennae and maxillary palps of Fannia hirticeps (Stein, 1892) (Diptera: Fanniidae). Microsc. Res. Tech. 75:1313-1320.
- Zhang D., Wang Q.K., Yang Y.Z., Chen Y.O. & Li K. 2013. Sensory organs of the antenna of two Fannia species (Diptera: Fanniidae). Parasitol. Res. 112:2177-2185.
- Zwiebel L.J. & Takken W. 2004. Olfactory regulation of mosquito-host interactions. Insect. Biochem. Molec. 34:645-652.
Publication Dates
-
Publication in this collection
Jan 2018
History
-
Received
20 June 2017 -
Accepted
03 Aug 2017