Abstracts
Toxoplasmosis is one of the most common parasitic zoonoses throughout the world. Infection in man and animals varies in different geographical areas influenced by many environmental conditions. Seroprevalence of Toxoplasma gondii infection in cattle in Brazil ranges from 1.03 to 71%. A cross-sectional survey was carried out in 58 out of 453 farms in the South Fluminense Paraiba Valley, State of Rio de Janeiro, Brazil. Over 3-year-old cattle (n=589) from dairy herds were selected for blood collection and detection of anti-T. gondii antibodies by indirect immunofluorescence reaction (IFA) with initial titration of 1:16; titers > 64 were considered positive. Univariate analysis of risk factors showed that cats in contact with cattle, cats in contact with drinking water, and number of cats were associated with T. gondii seroprevalence. Logistic regression revealed a two-fold increased risk for infection of cattle (p=0.0138) through larger number of cats (>3) compared with low numbers of cats (1-2) on the farm. In contrast, the presence of chickens was considered a protective factor (p=0.025).
Dairy cattle; toxoplasmosis; antibodies; risk factors
Toxoplasmose é uma das mais comuns zoonoses parasitárias do mundo. Infecções em seres humanos e em animais variam nas diferentes áreas geográficas influenciadas pelas condições ambientais. A soroprevalência da infecção por Toxoplasma gondii em bovinos no Brasil varia de 1,03 a 71,0%. O estudo transversal foi realizado em 58 de um total de 453 propriedades na região Sul Fluminense do estado do Rio de Janeiro. Vacas leiteiras acima de 3 anos de idade (n=589) foram selecionadas para coleta de sangue e a detecção de anticorpos anti-T. gondii foi feita pelo teste de imunofluorescência indireta (IFI) com titulação inicial de 1:16 e títulos > 64 foram considerados positivos. Após análise univariada dos fatores de risco, gatos em contato com bovinos, em contato com a água de beber dos animais e o número de gatos foram associados com a soroprevalência de T. gondii. A regressão logística demonstrou que o número maior de gatos (>3) teve um risco duas vezes maior (p=0,0138) que propriedades que tinham um número menor de gatos (1-2). Em contraste, a presença de galinhas foi considerada um fator de proteção (p=0,025).
Bovinos leiteiros; toxoplasmose; anticorpos; fatores de risco
LIVESTOCK DISEASES
Risk factors associated with Toxoplasma gondii infection in dairy cattle, state of Rio de Janeiro
Fatores de risco associados à infecção por Toxoplasma gondii em bovinos leiteiros no estado do Rio de Janeiro
George R. AlbuquerqueI; Alexandre D. MunhozI; Marcel TeixeiraII; Walter FlausinoIII; Simoni M. de MedeirosIV; Carlos Wilson G. LopesIII,* * Supported by Fundação de Amparo à Pesquisa no Estado do Rio de Janeiro (FAPERJ). Corresponding author: lopescwg@ufrrj.br
IDepartamento de Ciências Agrárias e Ambientais, Universidade Estadual de Santa Cruz, Rodovia Ilhéus-Itabuna Km 16, Salobrinho, Ilhéus, BA 45662-000, Brazil
IIDepto Ciências Agrárias e Ambientais, Universidade Estadual de Santa Cruz, Rodovia Ilhéus-Itabuna Km 16, Salobrinho, Ilhéus, BA 45662-000, Brazil
IIIDepartamento de Parasitologia Animal, Instituto de Veterinária, Universidade Federal Rural do Rio de Janeiro (UFRRJ), Seropédica, RJ 23890-000, Brazil. CNPq fellowship
IVCurso de Enfermagem, Centro Universitário Uniabeu, Rua Itaiara 301, Centro, Belford Roxo, RJ 26113-400, Brazil
ABSTRACT
Toxoplasmosis is one of the most common parasitic zoonoses throughout the world. Infection in man and animals varies in different geographical areas influenced by many environmental conditions. Seroprevalence of Toxoplasma gondii infection in cattle in Brazil ranges from 1.03 to 71%. A cross-sectional survey was carried out in 58 out of 453 farms in the South Fluminense Paraiba Valley, State of Rio de Janeiro, Brazil. Over 3-year-old cattle (n=589) from dairy herds were selected for blood collection and detection of anti-T. gondii antibodies by indirect immunofluorescence reaction (IFA) with initial titration of 1:16; titers > 64 were considered positive. Univariate analysis of risk factors showed that cats in contact with cattle, cats in contact with drinking water, and number of cats were associated with T. gondii seroprevalence. Logistic regression revealed a two-fold increased risk for infection of cattle (p=0.0138) through larger number of cats (>3) compared with low numbers of cats (1-2) on the farm. In contrast, the presence of chickens was considered a protective factor (p=0.025).
Index terms: Dairy cattle, toxoplasmosis, antibodies, risk factors.
RESUMO
Toxoplasmose é uma das mais comuns zoonoses parasitárias do mundo. Infecções em seres humanos e em animais variam nas diferentes áreas geográficas influenciadas pelas condições ambientais. A soroprevalência da infecção por Toxoplasma gondii em bovinos no Brasil varia de 1,03 a 71,0%. O estudo transversal foi realizado em 58 de um total de 453 propriedades na região Sul Fluminense do estado do Rio de Janeiro. Vacas leiteiras acima de 3 anos de idade (n=589) foram selecionadas para coleta de sangue e a detecção de anticorpos anti-T. gondii foi feita pelo teste de imunofluorescência indireta (IFI) com titulação inicial de 1:16 e títulos > 64 foram considerados positivos. Após análise univariada dos fatores de risco, gatos em contato com bovinos, em contato com a água de beber dos animais e o número de gatos foram associados com a soroprevalência de T. gondii. A regressão logística demonstrou que o número maior de gatos (>3) teve um risco duas vezes maior (p=0,0138) que propriedades que tinham um número menor de gatos (1-2). Em contraste, a presença de galinhas foi considerada um fator de proteção (p=0,025).
Termos de indexação: Bovinos leiteiros, toxoplasmose, anticorpos, fatores de risco.
INTRODUCTION
Toxoplasmosis is one of the most common parasitic zoonoses throughout the world. Infection in man and animals varies in different geographical areas influenced by many environment conditions. The infection is more prevalent in warm climates and in low lying areas than in cold climates and mountain regions and in humid areas than dry areas. This is probably related to conditions favoring sporulation and survivor of oocysts in the environment (Dubey & Beattie 1988). Cultural habits and hygiene may play a role because of food borne transmission. The tissue form of the parasite, a microscopic cyst consisting of bradyzoites, can be transmitted to humans by eating undercooked, contaminated meat (Tenter et al. 2000).
The importance of transmission from cattle to humans is still a controversy. Some studies show that consumption of undercooked meat is considered of great risk to humans (Baril et al., 1999; Cook et al. 2000). In an experimental infection in calves and pregnant cows, Dubey (1983) observed viable cysts of Toxoplasma gondii in animal tissues until the age of slaughter. Lord et al. (1975) and Kean et al. (1969) related outbreaks of human toxoplasmosis due to meat consumption, although Dubey & Jones (2008) found this information not valid. Also many attempts of parasite isolation from cattle tissues failed (Dubey & Streitel 1976, Dubey 1992, Dubey et al. 2005) even in experimental design (Dubey 1986). More recently Dubey & Jones (2008) and Kijlstra & Jongert (2008) considered transmission from cattle not important for human infection.
Cattle are commonly resistant to the infection, but signs of fever, anorexia, diarrhea, nasal discharge, and cough could be present (Sanger et al. 1953, Dubey 1983, Oliveira et al. 2001). Toxoplasma gondii was first isolated from bovine tissue by Sanger et al. (1953) and since then has been isolated from many organs (Dubey 1983, Dubey & Thulliez 1993). In Brazil, Jamra et al. (1969) and Spósito Filha et al. (1988) succeeded in the isolation of T. gondii from human food of animal origin and from retina and diaphragm of slaughtered cattle respectively. More recently, Scarpelli et al. (2009) performing bioassays revealed the presence of the parasite in many tissue and semen samples of animals infected with oocysts and tachyzoites on several experimental days between 7 and 84 suggesting the possibility of sexual transmission of T. gondii. In cattle the seroprevalence of antibodies anti-T. gondii ranges from 1.03 to 71% (Gondim et al. 1999, Albuquerque et al. 2005, Santos et al. 2009, Spagnol et al. 2009). Risk factors associated with bovine infection are scarce in the literature, but herd size and farming system has been identified by Klun et al. (2006) and Schoonman et al. (2009).
The aim of this paper was to study possible risk factors for T. gondii infection in cattle from Rio de Janeiro, Brazil.
MATERIALS AND METHODS
A cross-sectional survey was carried out in the municipalities of Resende and Rio Claro in the South Fluminense region of the State of Rio de Janeiro, Brazil. Livestock information, number of farms and animals were obtained from records of the regional Company for Technical Rural Assistance (Emater) from both municipalities. We randomly selected 58 farms among the 453 in the region. Sample sizes were estimated with an expected prevalence of 21.30% and a 5% sample error. Only mature dairy cows over three years of age were used for blood collection. In Resende, 314 blood samples out of 1.556 cows and in Rio Claro 275 blood samples out of 1079 cows were collected, a total of 589 samples.
Blood samples were centrifuged and stored at -20ºC. Detection of anti-T. gondii antibodies were performed using indirect immunofluorescence reaction (IFA) following Camargo (1964). Initial dilution was 1:16 until negative reaction and titers > 1:64 were considered positive.
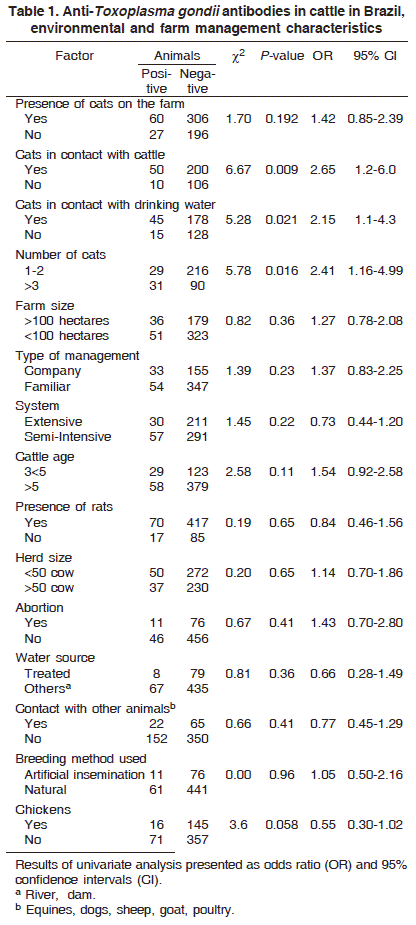
Information about each farm and the animals kept was collected by means of a structured questionnaire, which was administered on all the selected farms through a single visit. The questionnaire was designed to comprise mostly close-ended (categorical) questions to ease data processing, minimize variation, and improve precision of responses (Thrusfield 2004). Important farm and animal level data included type of management, system, breeding method used, water source, contact with other animals, farm size, herd size, presence of cats on the farm, cats in contact with cattle, cats in contact with drinking water, number of cats, presence of rats, abortion.
Differences in the seroprevalence between animals were analyzed by the Chi-square test. The same test was used to analyze the influence of all examined factors as independent categorical variables of T. gondii seroprevalence. Variables significant at p£0.1 with 95% confidence level were tested for colinearity and were selected for inclusion in the multivariate logistic regression model. Overall analyses and fit of the logistic regression models was assessed with the software Epi Info 2000 (Dean & Arnet 2002).
RESULTS
Environmental and farm management characteristics of the examined cattle population are presented in Table 1. The overall seroprevalence in both municipalities was 14.77% (87 from 589), with titers ranging from 1:64 to 1:1024 (Albuquerque et al. 2005).
Univariate analysis of risk factors showed that cats in contact with cattle, cats in contact with drinking water, and number of cats were associated with Toxoplasma gondii seroprevalence (Table 1). These factors were simultaneously analyzed in a logistic regression model to determine their relative contribution to T. gondii seropositivity (Table 2). A two-fold increased risk of infection was shown for dairy cattle (p=0.0138) through a large number of cats (>3) compared with a low number (1-2). In contrast, the presence of chickens was considered a protective factor (p=0.025) compared with the absence of chickens on the farms from Resende.
DISCUSSION
According to the data analyzed there are significant differences (p<0.05) in the association between Toxoplasma gondii specific antibodies and some factors studied. Cats were strongly associated with increased risk of infection. However, the more presence of cats on the farms does not lead to a real risk if they are not in direct contact with cattle, or drinking water, as much as their number on the farms increased the risk of infection. Likewise, Schoonman et al. (2009) did not consider presence of cats without direct contact with cattle as risk factor. This is understandable since the cat is the only definitive host of T. gondii, and hence, oocysts are numerous in the environment. In Brazil, cats are commonly used to avoid rodent infestation on farms, but increased risk is seen with their waste contaminated feed, grain, silage and water sources. Also, cats use to bury their feces away, and T. gondii oocysts can survive two years in the soil and may remain confined to the area for a long time (Dubey & Beattie 1988). In the first infection, cats can shed a high number of oocysts, but only for a short time, then the shedding stops for a long period. Nevertheless, a higher number of cats on the farm raise the chance for a contaminated environment and these results in a high level of positive cattle.
There was no significant association between T. gondii seroprevalence and cattle age, but similar to Arias et al. (1994), there were more positive cases in 3 to 5-year-old cows. Dubey (1986) reported that calves had higher titers than adults and inoculated animals had high titers very soon after the infection. Therefore, within a short time, anti-T. gondii antibodies decreased to negative reaction according to the sensibility of the test. In cattle, results were different from other animal species, including humans, where there is a positive relationship between age and a seropositive population (Weigel et al. 1995, Jones et al. 2001, Ali et al. 2003).
In this study, the herd size was not related to seropositive cattle, similar to the findings of Schoonman et al (2009), but different of Klun et al. (2006). An explanation for this is the point that farms with a higher number of animals used a semi-intensive system for cattle, what means more risk for contact with oocysts. Thus, despite not statistically significant, as intensive was the system of production, higher positive rates were encountered. This may be explained by the more direct contact with cats and oocysts confined cows have. Also, in semi-intensive systems the feed for cattle are much more exposed to environmental contamination (Penkert 1973).
The presence of chickens on the farms seems to be a factor of protection for the cattle. In Resende, farms without chickens had 2.6 times more positive animals (p=0.02). In Rio Claro, although not statistically significant, farms with chickens had 12.9% seropositive cattle, while farms without chickens had 14.6%. Recent investigations have been concerned with behavioral aspects that influence toxoplasmosis. Since it can affect the spread of T. gondii oocysts, food habits of others animals have epidemiological and ecological implicitness. Thus, the protection provided by chicken presence is probably due to forage as chickweed and other plants, seeds, and insects that partially clean the environment from contaminating oocysts. Pastured chickens are often found positive for T. gondii infection (Dubey et al. 2008).
Received on September 30, 2010.
Accepted for publication on November 9, 2010.
- Albuquerque G.R., Munhoz A.D., Flausino W., Silva R.T., Almeida C.R. R., Medeiros S.M. & Lopes C.W.G. 2005. Prevalência de anticorpos anti-Toxoplasma gondii em bovinos leiteiros do Vale do Paraíba Sul Fluminense, Estado do Rio de Janeiro. Revta Bras. Parasitol. Vet. 14:125-128.
- Ali C.N., Harris J.A., Watkins J.D. & Adesiyun A.A. 2003. Sero-epidemiology of Toxoplasma gondii in dogs in Trinidad and Tobago. Vet. Parasitol. 113:179-187.
- Arias L.M., Reyes L., Chinchilla M. & Linder E. 1994. Seroepidemiology of Toxoplasma gondii (Apicomplexa) in meat producing animals in Costa Rica. Revta Biol. Trop. 42:15-20.
- Baril L., Ancelle T., Goulet V., Thulliez P., Tirard-Fleury V. & Carme B. 1999. Risk factors for Toxoplasma infection in pregnancy: A case-control study in France. Scand. J. Infect. Dis. 31:305-309.
- Camargo M.E. 1964. Improved technique of indirect immunofluorescence for serological diagnosis of toxoplasmosis. Revta Inst. Med. Trop. São Paulo 6:117-118.
- Cook A.J.C., Gilbert R.E., Buffolano W., Zufferey J., Petersen E., Jenum P.A., Foulon W., Semprini A.E. & Dunn D.T. 2000. Sources of Toxoplasma infection in pregnant women: European multicentre case-control study. Brit. Med. J. 321:142-147.
- Dean A.G. & Arner T. 2002. Epi Info: Epidemiology of program office. Disponible on <http://www. cdc.gov/epiinfo/index.html>. Acessed on Apr. 20, 2002.
- Dubey J.P. 1983. Distribuition of cysts and tachyzoites in calves and preagnant cows inoculated with Toxoplasma gondii oocysts. Vet. Parasitol. 13:199-211.
- Dubey J.P. 1986. A review of toxoplasmosis in cattle. Vet. Parasitol. 22:177-202.
- Dubey J.P. 1992. Isolation of Toxoplasma gondii from a naturally infected beef cow. J. Parasitol. 78:151-153.
- Dubey J.P. & Beattie C.P. 1988. Toxoplasmosis of Animals and Man. CRC Press, Boca Raton. 75p.
- Dubey J.P. & Jones J.L. 2008. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 38:1257-1278.
- Dubey J.P. & Thulliez P.H. 1993. Persistence of tissue cysts in edible tissues of cattle fed Toxoplasma gondii oocysts. Am. J. Vet. Res. 54:270-273.
- Dubey J.P., Hill D.E., Jones J.L., Hightower A.W., Kirkland E., Roberts J.M., Marcet P.L., Lehmann T., Vianna M.C.B., Miska K., Sreekumar C., Kwok O.C.H., Shen S.K. & Gamble H.R. 2005. Prevalence of viable Toxoplasma gondii in beef, chicken and pork from retail meat stores in the United States: Risk assessment to consumers. J. Parasitol. 91:1082-1093.
- Dubey J.P. & Streitel R.H. 1976. Prevalence of Toxoplasma infection in cattle slaughtered at an Ohio abattoir. J. Am. Vet. Med. Assoc. 169:1197-1199.
- Dubey J.P., Velmurugan G.V., Chockalingam A., Pena H.F., Oliveira L.N., Leifer C.A., Gennari S.M., Bahia Oliveira L.M. & Su C. 2008. Genetic diversity of Toxoplasma gondii isolates from chickens from Brazil. Vet. Parasitol. 157:299-305.
- Gondim L.F.P., Barbosa Jr H.V., Ribeiro Filho C.H. & Saeki H. 1999. Serological survey of antibodies to Toxoplasma gondii in goats, sheep, cattle and water buffaloes in Bahia State, Brazil. Vet. Parasitol. 82:273-276.
- Jamra L.F., Deane M.P. & Guimarães E.C. 1969. On the isolation of Toxoplasma gondii from human food of animal origin: Partial results in the city of São Paulo (Brazil). Revta Inst. Med. Trop. São Paulo 11:169-176.
- Jones J.L., Kruszon-Moran D., Wilson M., McQuillan G., Navin T. & McAuley J.B. 2001. Toxoplasma gondii infection in the United States: Seroprevalence and risk factors. Am. J. Epidemiol. 154:357-365.
- Kean B.H., Kimball A.C. & Christenson W.N. 1969. An epidemic of acute toxoplasmosis. J. Am. Med. Assoc. 208:1002-1004.
- Kijlstra A. & Jongert E. 2008. Control of the risk of human toxoplasmosis transmitted by meat. Int. J. Parasitol. 38:1359-1370.
- Klun I., Djurkoviæ-Djakoviæ O., Katiæ-Radivojeviæ S. & Nikoliæ A. 2006. Cross-sectional survey on Toxoplasma gondii infection in cattle, sheep and pigs in Serbia: Seroprevalence and risk factors. Vet. Parasitol. 30:121-131.
- Lord W.G., Boni F., Bodek A., Hilberg R.W., Rosini R. & Clack F. 1975. Toxoplasmosis in Pennsylvania. Morb. Mortal. 24:285-287.
- Oliveira F.C.R., Costa A.J. & Sabatini G.A. 2001. Clínica e hematologia de Bos indicus, Bos taurus, e Bubalus bubalis inoculados com oocistos de Toxoplasma gondii (Apicomplexa: Toxoplasmatinae). Ciência Rural 31:621-626.
- Penkert R.A. 1973. Possible spread of toxoplasmosis by feed contaminated for cats. J. Am. Vet. Med. Assoc. 162:924.
- Sanger V.L., Chamberlain D.M., Chamberlain K.W., Cole C.R. & Farrell R.L. 1953. Toxoplasmosis. V. Isolation of Toxoplasma from cattle. J. Am. Vet. Med. Assoc. 123:87-91.
- Santos T.R., Costa A.J., Toniollo G.H., Luvizotto M.C.R., Benetti A.H., Santos R.R., Matta D.H., Lopes W.D.Z., Oliveira J.A. & Oliveira G.P. 2009. Prevalence of anti-Toxoplasma gondii antibodies in dairy cattle, dogs and humans from the Jauru micro-region, Mato Grosso state, Brazil. Vet. Parasitol. 161:324-326.
- Scarpelli L., Lopes W.D.Z., Migani M., Bresciani K.D.S. & Costa A.J. 2009. Toxoplasma gondii in experimentally infected Bos taurus and Bos indicus semen and tissues. Pesq. Vet. Bras. 29:59-64.
- Schoonman L.B., Wilsmore T. & Swai E.S. 2009. Sero-epidemiological investigation of bovine toxoplasmosis in traditional and smallholder cattle production systems of Tanga Region, Tanzania. Trop. Anim. Health Prod., DOI 10.1007/s11250-009-9460-2
- Spagnol F.H., Paranhos E.B., Oliveira L.L., Medeiros S.M., Lopes C.W.G. & Albuquerque G.R. 2009. Prevalência de anticorpos anti-Toxoplasma gondii em bovinos abatidos em matadouros do estado da Bahia, Brasil. Revta Bras. Parasitol. Vet. 18:42-45.
- Spósito Filha E., Amaral V., Macruz R., Barci L.A.G., Rebouças M.M., Santos S.M. & Rinald C.A.S. 1988. Toxoplasma gondii em bovinos: evidenciação do parasita a partir de retina e diafragma de animais abatidos em matadouros do Estado de São Paulo, Brasil. Arqs Inst. Biológico, São Paulo, 55:43-47.
- Tenter A.M., Heckeroth A.R. & Weiss L.M. 2000. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 30:1217-1258.
- Thrusfield M. 2004. Epidemiologia Veterinária. Editora Roca, São Paulo. 572p.
- Weigel R.M., Dubey J.P., Siegel A.M., Kitron U.D., Mannelli A., Mitchell M.A., Mateus-Pinilla N.E., Thulliez P., Shen S.K., Kwok O.C.H. & Todd K.S. 1995. Risk factor for transmission of Toxoplasma gondii on swine farms in Illinois. J. Parasitol. 81:736-741.
Publication Dates
-
Publication in this collection
25 Apr 2011 -
Date of issue
Apr 2011
History
-
Accepted
09 Nov 2010 -
Received
30 Sept 2010