Abstract:
Amorimia exotropica is an important plant associated with sudden death in cattle in Southern Brazil. In order to understand the mechanisms by which A. exotropica causes acute lesions in the heart and kidney of intoxicated animals, an experiment was conducted to determine the histopathology and ultrastructure of myocardial and renal lesions of intoxicated rabbits. After receiving 18g/kg of dried plant, six rabbits died suddenly. At necropsy, the liver was swollen and no other macroscopic lesions were observed. Histologically, centrolobular and midzonal hepatocytes were vacuolated. These vacuoles were strong PAS stained positive, suggesting that they corresponded to glycogen accumulations. In some regions of the ventricular septum and ventricles were found vacuoles of different sizes and the kidneys of two rabbits showed vacuolar degeneration on distal convoluted tubules. Ultrastructurally, the myocardium had cardiomyocytes swelling with separation of myofibrils bundles and rupture and disorganization of the sarcomeres. The mitochondria displayed swelling, disorganization, disruption of the mitochondrial cristae, and electron-dense matrix. Some mitochondria exhibited eccentric projections of their membranes with disruption of both outer and inner membranes. The sarcoplasmic reticulum had no alterations, whereas the T-tubule system was occasionally dilated and ruptured. The kidneys had mitochondrial swelling with disorganization and disruption of the mitochondrial cristae. The vacuoles result from the swelling of the endoplasmatic reticulum and usually were located between two basolateral infoldings and mitochondria, occurring preferentially around the nucleus. The myocytes and T system damages induced by A. exotropica result in acute heart failure and death. Furthermore, this mechanism of cardiotoxicity may be common to all plant containing monofluoroacetate.
Index Terms:
Poisonous plants; Amorimia exotropica; monofluoroacetate; ultrastructure; cardiotoxicity; kidney; plant poisoning; sudden death
Resumo:
Amorimia exotropica é uma importante planta associada à morte súbita em bovinos no Sul do Brasil. Visando compreender os mecanismos pelos quais a A. exotropica provoca lesões agudas no coração e rins de animais intoxicados, foi conduzido uma intoxicação experimental em coelhos para determinar a histopatologia e ultraestrutura da lesão miocárdica e renal. Depois de receber 18g/kg de planta seca, seis coelhos morreram subitamente. Na necropsia, o fígado apresentava acentuação do padrão lobular. Os demais órgãos não apresentaram alterações macroscópicas. Histologicamente, os hepatócitos centrolobulares e mediozonais estavam vacuolizados e coraram-se fortemente com PAS. Em algumas regiões foram observados vacúolos de diferentes tamanhos no septo ventricular e ventrículos e os rins de dois coelhos mostraram degeneração vacuolar nos túbulos contorcidos distais. Ultraestruturalmente, o miocárdio apresentou cardiomiócitos tumefeitos com separação das bandas de miofibrilas e ruptura e desorganização dos sarcômeros. As mitocôndrias estavam tumefeitas exibindo desorganização das cristas mitocondriais, e a matriz estava eletrodensa. Algumas mitocôndrias exibiam projecções excêntricas das suas membranas com ruptura das membranas externas e internas. O retículo sarcoplasmático não tinha alterações, e os túbulos T estavam ocasionalmente dilatados e rompidos. Os rins apresentavam tumefação mitocondrial com desorganização e ruptura das cristas mitocondriais. Os vacúolos resultam da expansão do retículo endoplasmático e foram localizados geralmente entre duas invaginações basolaterais e as mitocôndrias, ocorrendo preferencialmente ao redor do núcleo. A lesão nos miócitos e o dano no sistema T induzido pela A. exotropica resultam na insuficiência cardíaca aguda e morte. Este mecanismo de cardiotoxicidade pode ser comum a todas as plantas contendo monofluoroacetato.
Termos de Indexação:
Plantas tóxicas; Amorimia exotropica; monofluoroacetato; ultra-estrutura; cardiotoxicidade; rins; intoxicação por plantas; morte súbita
Introduction
Several plants species worldwide contain fluoroacetate, which can produce poisoning and sudden death (Marais 1944Marais S.T. 1944. Monofluoroacetic acid, the toxic principle of "Gifblaar" Dichapetalum cymosum (Hook). Onderstepoort J. Vet. Sci. Anim. Ind. 20:67-73., Oelrichs & McEwan 1962Oelrichs P.B. & McEwan T. 1962. The toxic principle of Acacia georginae. Queensl. J. Agric. Sci. 19:1-16., McEwan 1964McEwan T. 1964. Isolation and identification of the principle of Gastrolobium grandiflorum. Queensl. J. Agric. Sci. 21:1-14.). In Brazil, poisonous plants that cause sudden death due to heart failure are among the main causes of cattle losses (Tokarnia et al2002Tokarnia C.H., Döbereiner J.& Peixoto P.V. 2002. Poisonous plants affecting livestock in Brazil. Toxicon 40:1635-1660.). Numerous plants have been listed in this group, Palicourea marcgravii, P. aeneofusca, and Tanaecium bilabiatum (Krebs et al. 1994Krebs H.C., Kemmerling W. & Habermehl G. 1994. Qualitative and quantitative determination of fluoroacetic acid in Arrabidaea bilabiata and Palicourea marcgravii by 19 F-NMR spectroscopy. Toxicon 32:909-913.), Amorimia amazonica, A. camporum, A. exotropica, A. pubiflora, A. rigida, and A. septentrionalis (Lee et al2002Lee S.T., Cook D., Riet-Correa F., Pfister J.A., Anderson W.R., Lima F.G. & Gardner D.R. 2012. Detection of monofluoroacetate in Palicourea and Amorimia species. Toxicon 60:791-796.). Another plant of this genus, A. exotropica, is incriminated in poisoning with sudden death in ruminants in Northern Rio Grande do Sul State (Pavarini et al 2011Pavarini S.P., Soares M.P., Bandarra P.M., Gomes D.C., Bandinelli M.B., Cruz C.E.F. & Driemeier D. 2011. Mortes súbitas causadas por Amorimia exotropica (Malpighiaceae) no Rio Grande do Sul. Pesq. Vet. Bras. 31:291-296., Bandinelli et al. 2014Bandinelli M.B., Bassuino D.M., Fredo G., Mari C., Driemeier D., Sonne L. & Pavarini SP. 2014. Identificação e distribuição de lesões cardíacas em bovinos intoxicados por Amorimia exotropica. Pesq. Vet. Bras. 34:837-844).When poisoned by these plants, cattle die after a rapidly progressive clinical disease triggered by exercise; however, animals may die without showing any clinical signs. Muscular tremors, staggering gait, dyspnea, jugular engorgement, opisthotonus, recumbency, and paddling are immediately followed by death (Tokarnia et al 2012Tokarnia C.H., Brito M.F., Barbosa J.D., Peixoto P.V. & Döbereiner J. 2012. Plantas que afetam o funcionamento do coração, p.27-94. In: Ibid. Eds, Plantas Tóxicas do Brasil para Animais de Produção. 2ª ed. Editora Helianthus, Rio de Janeiro.). No significant changes are observed at necropsy, and the only microscopic lesions, present in approximately one-third of the cases, have been reported to be vacuolar-hydropic degeneration in the epithelial cells of the renal tubules (Tokarnia et al. 1990Tokarnia C.H., Peixoto P.V.& Döbereiner J.1990. Poisonous plants affecting heart function of cattle in Brazil. Pesq. Vet. Bras. 10:1-10.). Intracellular edema and coagulation necrosis of small groups of cardiac fibers were reported in 1985 in poisoning by Amorimia rigida, but it never was investigated as the possible cause of sudden death (Tokarnia et al. 1985Tokarnia C.H., Döbereiner J.& Peixoto P.V. 1985. Intoxicação por Mascagnia aff. rigida (Malpighiaceae) em bovinos no Norte do Estado Espírito Santo, Brazil. Pesq. Vet. Bras. 5:77-91.). Goats chronically intoxicated with Palicourea marcgravii exhibit vacuolization of cardiomyocytes, necrosis of cardiac fibers and mononuclear cell infiltration (Barbosa et al. 2015Barbosa E.F.G., Cardoso S.P., Cabral Filho S.L.S., Borges J.R.J., Lima E.M.M., Riet-Correa F. & Castro M.B. 2015. Sinais clínicos e patologia da intoxicação crônica experimental de caprinos por Palicourea marcgravii . Pesq. Vet. Bras. 35:209-215.). Myocardial coagulation necrosis in groups of cardiomyocytes has recently been reported as a frequent finding in cases involving two others plants that induce sudden death in ruminants, Pseudocalymma elegans (Helayel et al. 2009Helayel M.A., França T.N., Seixas J.N., Nogueira V.A., Caldas S.A. & Peixoto P. 2009. Morte súbita em bovinos causada pela ingestão de Pseudocalymma elegans (Bignoniaceae) no município de Rio Bonito, RJ. Pesq. Vet. Bras. 29:498-508.) and Amorimia spp (Schons et al. 2011Schons S.V., Mello T.L., Riet-Correa F.& Schild A.L. 2011. Poisoning by Amorimia (Mascagnia) septium in sheep in northern Brazil. Toxicon 57:781-86.). In electron microscopy of the myocardium in an experimental chronic poisoning by A. exotropica in rabbits, these lesions were not observed (Soares et al. 2011Soares M.P., Pavarini S.P., Adrien M.L., Quevedo P.S., Schild A.L., Peixoto P.V., Cruz C.E.F. & Driemeir D. 2011. Amorimia exotropica poisoning as a presumptive cause of myocardial fibrosis in cattle. J. Vet. Diagn. Invest. 23:1226-1229.). The term fluoroacetate (FA) refers to a large series of chemical compounds of the general formula CH2FCOOR, and it was first synthesized in 1896 (Goncharov et al. 2006Goncharov N.V., Jenkins R.O. & Radilov A.S. 2006. Toxicology of fluoroacetate: a review, with possible directions for therapy research. J. Appl. Toxicol. 26:148-161.). The sodium salt of FA is known as 'compound 1080' and it is used in some countries for controlling the population of certain vertebrate species: in the USA and UK, rodents are controlled in ships, sewers and warehouses; also, coyotes are controlled by the use of FA-impregnated carcasses or collars on livestock; in Australia and New Zealand rabbits, wallabies, goats, wild pigs, deer and opossums are controlled with the use of baits based on apple, carrot or grain; aerial sowing is used to control large or remote areas (Schultz et al. 1982Schultz R., Coetzer J.A., Kellerman T.S. & Naudé T.W. 1982. Observations on the clinical, cardiac, and histopathological effects of fluoroacetate in sheep. Onderstepoort J. Vet. Res. 49:237-245., Twigg & King 1991Twigg L.E. & King D.R. 1991. The impact of fluoroacetate-bearing vegetation on native Australian fauna: a review. Oikos 61:412-430., Proudfoot et al. 2006Proudfoot A.T., Bradberry S.M. & Vale J.A. 2006. Sodium fluoroacetate poisoning. Toxicol. Rev. 25(4):213-9.). The 1080 use is prohibited in Brazil, so it occurs exclusively as a natural product in plants (Gooneratne et al. 2008Gooneratne S.R., Eason C.T., Milne L., Arthur D.G., Cook C., Wickstrom M. 2008. Acute and long-term effects of exposure to sodium monofluoroacetate (1080) in sheep. Onderstepoort J. Vet. Sci. Res. 75:127-139., Nogueira et al 2010Nogueira V.A., França T.N., Peixoto T.C., Caldas A.S., Armién A.G. & Peixoto P.V. 2010. Intoxicação experimental por monofluoroacetato de sódio em bovinos: aspectos clínicos e patológicos. Pesq. Vet. Bras. 30:533-540.). The present study reports the histological and ultrastructural alterations of the myocardium and kidneys of rabbits in experimental acute A. exotropica poisoning and suggests a pathogeny for the intoxication.
Materials and Methods
Amorimia exotropica was collected in farms of northern Rio Grande do Sul, Brazil, where outbreaks of sudden death in cattle have been associated with the consumption of this plant (Soares et al. 2011Soares M.P., Pavarini S.P., Adrien M.L., Quevedo P.S., Schild A.L., Peixoto P.V., Cruz C.E.F. & Driemeir D. 2011. Amorimia exotropica poisoning as a presumptive cause of myocardial fibrosis in cattle. J. Vet. Diagn. Invest. 23:1226-1229.). The plant was dried, grinded, and mixed in water immediately before dosing the rabbits by oral administration. Previously, it was established that the lethal dose was 18g/kg (Soares et al. 2011Soares M.P., Pavarini S.P., Adrien M.L., Quevedo P.S., Schild A.L., Peixoto P.V., Cruz C.E.F. & Driemeir D. 2011. Amorimia exotropica poisoning as a presumptive cause of myocardial fibrosis in cattle. J. Vet. Diagn. Invest. 23:1226-1229.). Six rabbits received 18g/kg of the plant fractionated in four dosages of 4.5g/kg at 4-hour intervals. Two additional rabbits were used as controls. The rabbits were kept in individual cages, where they received a commercial diet and had free water access. Tissue samples of all organs were collected and fixed in 10% buffered formalin. For histopathology, fixed tissues were processed routinely, cut at 5μm and stained with hematoxylin and eosin (HE). Selected hepatic sections were stained with periodic acid Schiff (PAS). Immediately after the death of the treated rabbits or the euthanasia of the controls by decapitation under ether anesthesia, samples of the left ventricle, cardiac septum and kidneys were collected and fixed in 2% glutaraldehyde, 2% paraformaldehyde in sodium cacodylate-buffered solution. The samples were dehydrated in ethanol and embedded in Epon 812. Semi-thin sections were stained with methylene blue, and ultra-thin sections from selected areas were stained with uranyl acetate and lead citrate, and observed using a Zeiss EM 109 transmission electron microscope West Germany operated at 80 kilovolts.
Results
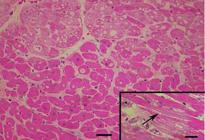
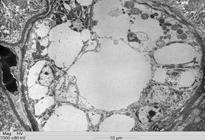
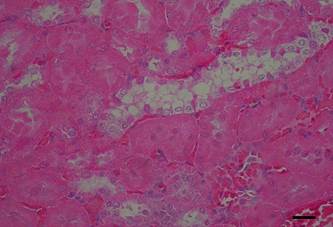
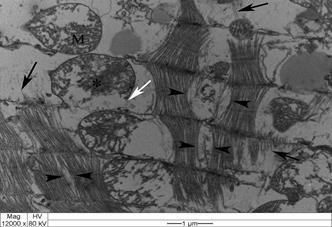
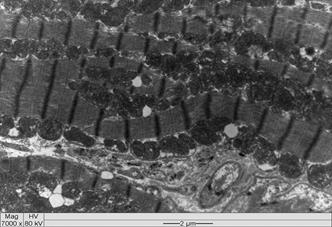
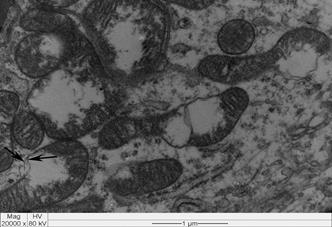
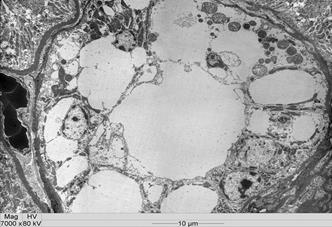
All intoxicated rabbits died approximately 14 hours after receiving the first dosage of A. exotropica. The rabbits had no prior clinical signs, however, a few moments before death, they showed suddenly struggling, falling, some emitted cries, some had paddling movements, difficulty to breath,and generalized contractions and death. At necropsy, the liver showed accentuation of the lobular pattern. No other lesions were observed. In light microscopy, the liver had vacuolated centrolobular and midzonal hepatocytes. These vacuoles were strongly PAS stained suggesting glycogen deposits. In some regions of the ventricular septum and ventricles were found vacuoles of different sizes (Fig.1). In fragments of these areas cut longitudinally can be observed swelling of cardiomyocytes with separation of bundles of myofibrils (Fig.1). The kidneys of two rabbits showed vacuolar degeneration on distal convoluted tubules (Fig.2). There were no lesions in other organs. No lesions were observed in the control rabbits. The ultrastructural findings on heart include cardiomyocyte swelling, disruption of the myofibrils, mitochondrial swelling and disorganization and disruption of the mitochondrial cristae, and more electron-dense mitochondrial matrix (Fig.3). Many mitochondria exhibited eccentric projections of their membranes with disruption of both the outer and inner membranes ( Fig.4). There are cardiomyocyte swelling with separation of the myofibrils, disruption and disorganization of the sarcomeres, the presence of line Z debris, and remains of adhered myofibrils (Fig.4). The sarcolemma and the sarcoplasmic reticulum showed no changes, whereas the T-tubule system was occasionally dilated and ruptured (Fig.5). The intrasarcoplasmic vacuoles found in the cardiomyocytes represented fluid accumulations and organelle debris. These vacuoles were not delimited by a membrane (Fig.6). No changes were observed in the control myocardial samples (Fig.7). The kidneys had mitochondrial swelling with disorganization and disruption of the mitochondrial cristae and disruption of the outer and inner membranes ( Fig.8). The vacuoles result from the swelling of the endoplasmatic reticulum and usually located between two basolateral infoldings and mitochondria (Fig.9). The swollen cisterns of endoplasmatic reticulum forming vacuoles usually located between two basolateral infoldings and mitochondria (Fig.9). The vacuoles occurred preferentially around the nucleus (Fig.10). The coalescence of the various vacuoles occupy the greater area of the cytoplasm and displace the nuclei, most of them picnotic, to the periphery of the cells. The evolution of the lesion occurred, so that all tubule cells were affected (Fig.11) resulting in injury observed in light microscopy. No changes were observed in the control kidney samples ( Fig.12).
Intrasarcoplasmic vacuoles of various sizes in the venricular septum of an Amorimia exotropica-poisoned rabbit. Inset: longitudinal section with swelling of cardiomyocytes and separation of bundles of myofibrils (arrow). HE.
Vacuolar degeneration of epithelial cells of the distal convoluted kidney tubules of an Amorimia exotropica-poisoned rabbit. HE.
Swollen mitochondria and electron-dense deposits in the matrix, associated with disorganization, atrophy, and rupture of the cristae in the left ventricle of an Amorimia exotropica- poisoned rabbit. Note the eccentric membrane projections and breakpoints (arrows). Transmission electron micrograph (TEM).
Swollen cardiomyocytes exhibit bundles of separated myofibrils due to the accumulation of intracellular fluid (between the arrowheads) in the ventricular septum of an Amorimia exotropica-poisoned rabbit, Note the residual myofibrillar fragments (black arrow), occasionally adhered to line Z debris. Swollen mitochondria (M) with electron-dense deposits in the matrix (*) and disrupted mitochondrial membranes (white arrow). TEM.
Ventricular septum of an Amorimia exotropica-poisoned rabbit. The T-system is dilated (arrow). The mitochondria exhibit swelling and eccentric membrane projections. TEM.
Ventricular septum of an Amorimia exotropica-poisoned rabbit. Intrasarcoplasmic vacuoles that are not delimited by membranes contain fluid, myofibrillar remnants and degenerated organelles. TEM.
Mitochondrial swelling with disorganization and disruption of the mitochondrial cristae, electron-dense deposits in the matrix and disruption of the outer and inner membranes (arrow) of the distal kidney tubules of an Amorimia exotropica-poisoned rabbit. TEM.
Kidney of an Amorimia exotropica-poisoned rabbit. Distal tubule. Presence of vacuoles those are usually located between two basolateral infoldings (arrow) and mitochondria. The cristae of the mitochondria are shorts and the matrix more electro dense. TEM.
Dilatation of cisterns of endoplasmic reticulum at an advanced stage in the distal kidney tubules of an Amorimia exotropica-poisoned rabbit. TEM.
High degree of hydropic degeneration of epithelial cells of the distal kideney tubules of an Amorimia exotropica-poisoned rabbit. The nuclei are picnotic with chromatin displaced to the periphery. TEM.
Discussion
Mitochondria of cardiomyocytes are recognized as targets of FA-toxicity (Bovis et al. 1966Bovis R., Kasten F.H. & Okigaki T. 1966. Electron microscopic study of the toxic effect of sodium fluoroacetate on rat myocardial cultures. Exp. Cell Res. 43:611-621.). However, the available information has not established an unequivocal mechanism by which FA acts on mitochondria. Biochemical studies have implicated FA in the production of the specific aconitase inhibitor fluorocitrate, interruption of the citric acid cycle, and the accumulation of citrate in the mitochondria, an excess of which could provide the basis for an explanation of mitochondrial abnormalities (Bovis et al. 1966Bovis R., Kasten F.H. & Okigaki T. 1966. Electron microscopic study of the toxic effect of sodium fluoroacetate on rat myocardial cultures. Exp. Cell Res. 43:611-621., Goncharov et al. 2006Goncharov N.V., Jenkins R.O. & Radilov A.S. 2006. Toxicology of fluoroacetate: a review, with possible directions for therapy research. J. Appl. Toxicol. 26:148-161.). Swelling of the mitochondria with disruption of the cristae, fluid accumulation in the sarcoplasm and disorganization of the sarcomeres were the main ultrastructural changes observed in all poisoned rabbits. Similar changes have been associated with the effects of FA on myocardial mitochondria of rats (Bovis et al. 1966Bovis R., Kasten F.H. & Okigaki T. 1966. Electron microscopic study of the toxic effect of sodium fluoroacetate on rat myocardial cultures. Exp. Cell Res. 43:611-621.). The results suggest that A. exotropica induces drastic mitochondrial changes leading to energy depletion, dysfunction of the ionic pumps with consequent osmotic imbalance in the sarcoplasm and fluid intake. The energy depletion and the accumulation of intracellular fluid with separation and disruption of myofibrils result in degradation and loss of sarcomeres followed by dilatation and disruption of T-tubules. This severe myocytes damage strongly suggests the development of cardiac failure and sudden death. Chronic intoxication occurs in pastures where animals consume small quantities of this plant for long periods, which causes slow cardiomyocytes injury and fibrosis (Soares et al. 2011Soares M.P., Pavarini S.P., Adrien M.L., Quevedo P.S., Schild A.L., Peixoto P.V., Cruz C.E.F. & Driemeir D. 2011. Amorimia exotropica poisoning as a presumptive cause of myocardial fibrosis in cattle. J. Vet. Diagn. Invest. 23:1226-1229.). Unlikely, acute poisoning occurs with the ingestion of large amounts of this plant in a short period of time causing extensive and severe cardiomyocytes injury, myocardial collapse and sudden death. The kidneys had mitochondrial swelling with disorganization and disruption of the mitochondrial cristae. The ultrastructural findings in the kidney of the rabbits are similar to those observed in experimental poisoning FA mice (McDowell 1972McDowell M.C. 1972. Light and Electron Microscopic studies of the rat kidney after administration of inhibitors of the citric acid cycle in vivo. Am. J. Pathol. 66:513-42.). The hydropic degeneration observed in the distal convoluted tubules does not occur in all cases of poisoning probably due to the amount of FA contained in the plant ingested, and the time necessary to eliminate this substance in the kidney. The ultrastructural findings in this study demonstrated that the death occurred from heart failure, and the renal injury was a secondary effect of the elimination of MFA in the kidney. It is proposed that an increase in the amount of glycogen in the cell cytoplasm is an indicator of diminished usage and accumulation, rather than a sign of increased metabolic activity (Ghadially 1988Ghadially F.N. 1988. Ultrastructural Pathology of the Cell and Matrix. Vol.2, 3rd ed. Butterworths, London, p.1022-26.). The FA interferes in the Krebs cycle and impairs the production of energy by the hepatocytes (Goncharov et al. 2006Goncharov N.V., Jenkins R.O. & Radilov A.S. 2006. Toxicology of fluoroacetate: a review, with possible directions for therapy research. J. Appl. Toxicol. 26:148-161.). This impairment could explain the accumulation of glycogen in the liver cells. Most likely, the accumulation plays no role in the mechanism of sudden death. The findings presented here provide a reasonable basis for a cause-effect relationship between the cardiomyocytes lesions and the clinical disease defined by acute heart failure followed by sudden death associated with A. exotropica poisoning. Moreover, these results suggest that similar degenerative mitochondrial changes should be involved in the disease caused by other FA containing plants.
References
- Bandinelli M.B., Bassuino D.M., Fredo G., Mari C., Driemeier D., Sonne L. & Pavarini SP. 2014. Identificação e distribuição de lesões cardíacas em bovinos intoxicados por Amorimia exotropica Pesq. Vet. Bras. 34:837-844
- Barbosa E.F.G., Cardoso S.P., Cabral Filho S.L.S., Borges J.R.J., Lima E.M.M., Riet-Correa F. & Castro M.B. 2015. Sinais clínicos e patologia da intoxicação crônica experimental de caprinos por Palicourea marcgravii . Pesq. Vet. Bras. 35:209-215.
- Bovis R., Kasten F.H. & Okigaki T. 1966. Electron microscopic study of the toxic effect of sodium fluoroacetate on rat myocardial cultures. Exp. Cell Res. 43:611-621.
- Ghadially F.N. 1988. Ultrastructural Pathology of the Cell and Matrix. Vol.2, 3rd ed. Butterworths, London, p.1022-26.
- Goncharov N.V., Jenkins R.O. & Radilov A.S. 2006. Toxicology of fluoroacetate: a review, with possible directions for therapy research. J. Appl. Toxicol. 26:148-161.
- Gooneratne S.R., Eason C.T., Milne L., Arthur D.G., Cook C., Wickstrom M. 2008. Acute and long-term effects of exposure to sodium monofluoroacetate (1080) in sheep. Onderstepoort J. Vet. Sci. Res. 75:127-139.
- Helayel M.A., França T.N., Seixas J.N., Nogueira V.A., Caldas S.A. & Peixoto P. 2009. Morte súbita em bovinos causada pela ingestão de Pseudocalymma elegans (Bignoniaceae) no município de Rio Bonito, RJ. Pesq. Vet. Bras. 29:498-508.
- Krebs H.C., Kemmerling W. & Habermehl G. 1994. Qualitative and quantitative determination of fluoroacetic acid in Arrabidaea bilabiata and Palicourea marcgravii by 19 F-NMR spectroscopy. Toxicon 32:909-913.
- Lee S.T., Cook D., Riet-Correa F., Pfister J.A., Anderson W.R., Lima F.G. & Gardner D.R. 2012. Detection of monofluoroacetate in Palicourea and Amorimia species. Toxicon 60:791-796.
- Marais S.T. 1944. Monofluoroacetic acid, the toxic principle of "Gifblaar" Dichapetalum cymosum (Hook). Onderstepoort J. Vet. Sci. Anim. Ind. 20:67-73.
- McDowell M.C. 1972. Light and Electron Microscopic studies of the rat kidney after administration of inhibitors of the citric acid cycle in vivo. Am. J. Pathol. 66:513-42.
- McEwan T. 1964. Isolation and identification of the principle of Gastrolobium grandiflorum Queensl. J. Agric. Sci. 21:1-14.
- Oelrichs P.B. & McEwan T. 1962. The toxic principle of Acacia georginae Queensl. J. Agric. Sci. 19:1-16.
- Nogueira V.A., França T.N., Peixoto T.C., Caldas A.S., Armién A.G. & Peixoto P.V. 2010. Intoxicação experimental por monofluoroacetato de sódio em bovinos: aspectos clínicos e patológicos. Pesq. Vet. Bras. 30:533-540.
- Pavarini S.P., Soares M.P., Bandarra P.M., Gomes D.C., Bandinelli M.B., Cruz C.E.F. & Driemeier D. 2011. Mortes súbitas causadas por Amorimia exotropica (Malpighiaceae) no Rio Grande do Sul. Pesq. Vet. Bras. 31:291-296.
- Proudfoot A.T., Bradberry S.M. & Vale J.A. 2006. Sodium fluoroacetate poisoning. Toxicol. Rev. 25(4):213-9.
- Schultz R., Coetzer J.A., Kellerman T.S. & Naudé T.W. 1982. Observations on the clinical, cardiac, and histopathological effects of fluoroacetate in sheep. Onderstepoort J. Vet. Res. 49:237-245.
- Schons S.V., Mello T.L., Riet-Correa F.& Schild A.L. 2011. Poisoning by Amorimia (Mascagnia) septium in sheep in northern Brazil. Toxicon 57:781-86.
- Soares M.P., Pavarini S.P., Adrien M.L., Quevedo P.S., Schild A.L., Peixoto P.V., Cruz C.E.F. & Driemeir D. 2011. Amorimia exotropica poisoning as a presumptive cause of myocardial fibrosis in cattle. J. Vet. Diagn. Invest. 23:1226-1229.
- Tokarnia C.H., Brito M.F., Barbosa J.D., Peixoto P.V. & Döbereiner J. 2012. Plantas que afetam o funcionamento do coração, p.27-94. In: Ibid. Eds, Plantas Tóxicas do Brasil para Animais de Produção. 2ª ed. Editora Helianthus, Rio de Janeiro.
- Tokarnia C.H., Döbereiner J.& Peixoto P.V. 1985. Intoxicação por Mascagnia aff. rigida (Malpighiaceae) em bovinos no Norte do Estado Espírito Santo, Brazil. Pesq. Vet. Bras. 5:77-91.
- Tokarnia C.H., Döbereiner J.& Peixoto P.V. 2002. Poisonous plants affecting livestock in Brazil. Toxicon 40:1635-1660.
- Tokarnia C.H., Peixoto P.V.& Döbereiner J.1990. Poisonous plants affecting heart function of cattle in Brazil. Pesq. Vet. Bras. 10:1-10.
- Twigg L.E. & King D.R. 1991. The impact of fluoroacetate-bearing vegetation on native Australian fauna: a review. Oikos 61:412-430.
Publication Dates
-
Publication in this collection
Mar 2016
History
-
Received
03 July 2015 -
Accepted
06 Jan 2016