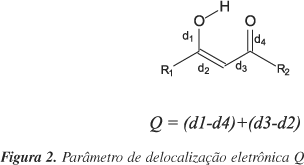
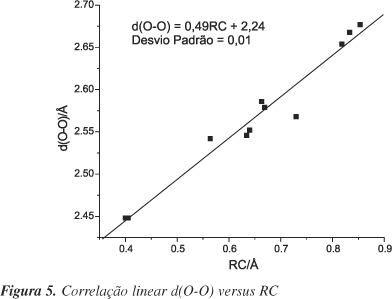
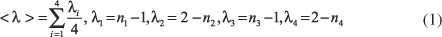The effect of substituents on the energies and geometries of 3-hydroxypropenal was studied using the B3LYP/6-311++G(d,p) model. The hydrogen bond energies indicate that the strongest donors and the weakest acceptors present the highest and the weakest hydrogen bonds, respectively, indicating the validity of the Madsen RAHB model. Geometric parameters indicate that the intensity of the hydrogen bond is proportional to the resonance, as suggested by the RHAB model. The effect of substituents diverges from the model proposed by Gilli et al. Sometimes the results indicate that the donor or acceptor effect is more important than the point of substitution.
3-hydroxypropenal; RAHB; effect of substituents