Summary
Introduction:
Protein-energy malnutrition in Crohn's disease (CD) has been reported in 20 to 92% of patients, and is associated with increased morbidity and mortality and higher costs for the health system. Anti-TNF drugs are a landmark in the clinical management, promoting prolonged remission in patients with CD. It is believed that the remission of this disease leads to nutritional recovery. The effect of biological therapy on body composition and nutritional status is unclear.
Method:
Prospective study of body assessment by bioelectrical impedance method in patients with moderate to severe CD undergoing treatment with infliximab. The main outcome was the body composition before and after 6 months of anti-TNF therapy.
Results:
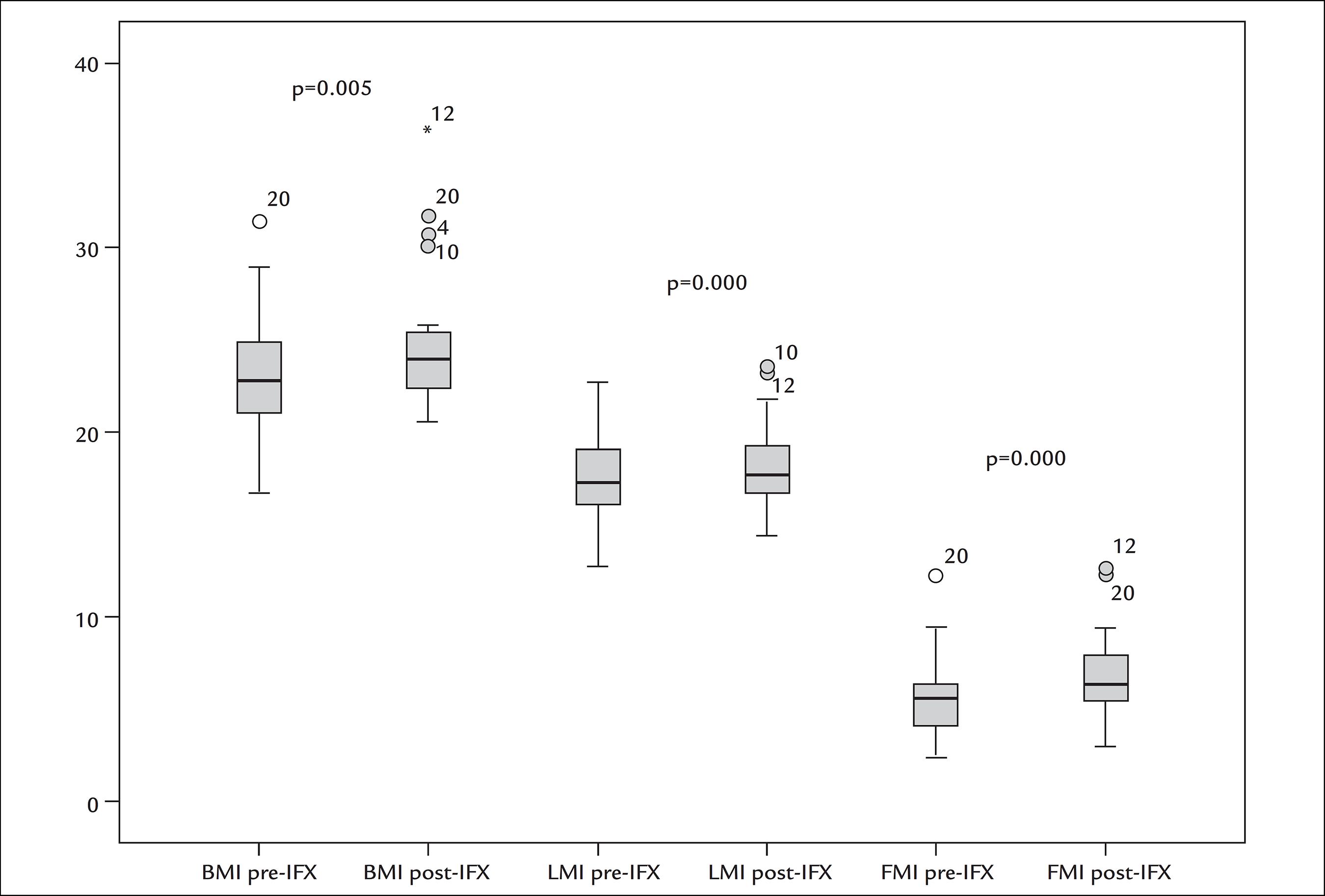
There was a predominance of females (52%) with a mean age of 42±12 years. Most patients were eutrophic at baseline and remained so. There was an increase in all parameters of body composition after anti-TNF treatment: BMI (22.9±3.2 versus 25±3.8; p=0.005), waist circumference (88.1±6.7 versus 93.9±7.7; p=0.002), lean mass index (17.5±2.2 versus 18.2±2.3; p=0.000) and fat mass index (5.5±2.3 versus 6.8±2.3; p=0.000). Phase angle remained unchanged (6.2 versus 6.8; p=0.94).
Conclusion:
After therapy with IFX, all components of body composition increased, except for phase angle. The substantial increase in fat mass index and waist circumference led to concern regarding cardiovascular risk and, thus, to the need for further studies.
Keywords:
Crohn's disease; body composition; biologics
Resumo
Introdução:
Desnutrição proteico-calórica em pacientes de doença de Crohn (DC) tem sido relatada em 20 a 92% dos casos associando-se a maior morbimortalidade e maiores custos para o sistema de saúde. Agentes anti-TNF são um marco no controle clínico, promovendo remissão prolongada em portadores de DC. Acredita-se que a remissão da doença leve à recuperação nutricional desses pacientes. O efeito da terapia biológica na composição corporal e no estado nutricional é pouco conhecido.
Método:
Estudo prospectivo de avaliação corporal por método de bioimpedância em portadores de DC moderada a grave submetidos a terapia com infliximabe (IFX). O desfecho principal foi a composição corporal antes e após 6 meses de terapia anti-TNF.
Resultados:
Houve predomínio do sexo feminino (52%), com média de idades de 42±12 anos. A maioria dos pacientes era eutrófica na inclusão do estudo e assim permaneceu. Houve aumento de todos os parâmetros da composição corporal após o tratamento anti-TNF: IMC (22,9±3,2 versus 25±3,8; p=0,005), circunferência abdominal (88,1±6,7 versus 93,9±7,7; p=0,002), índice de massa magra (17,5±2,2 versus 18,2±2,3; p=0,000) e índice de massa gorda (5,5±2,3 versus 6,8±2,3; p=0,000). O ângulo de fase manteve-se inalterado (6,2 versus 6,8; p=0,94).
Conclusão:
Após terapia com IFX, observou-se aumento de todos os componentes da composição corporal, exceto no ângulo de fase. O aumento substancial do índice de massa gorda e da circunferência abdominal levantam a preocupação de aumento nos riscos cardiovasculares e necessidade de estudos complementares.
Palavras-chave:
doença de Crohn; composição corporal; biológicos
Introduction
Crohn's disease (CD) is a chronic, transmural, immune-mediated condition that affects the gastrointestinal tract with a potential for involvement of various organs with extra-intestinal manifestations. The etiology of CD remains unclear, but it is believed to be multifactorial with associated environmental, genetic and immunological factors. The overall prevalence is 5 to 50 cases per 1,000 inhabitants, with a predominance of Caucasians.11 Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002; 347(6):417-29. An estimate of the prevalence in the city of São Paulo, Brazil, reported the occurrence of 14.8 cases per 100,000 inhabitants.22 Victoria CR, Sassak LY, Nunes HRC. Incidence and prevalence rates of inflammatory bowel diseases, in midwestern of São Paulo State, Brazil. Arq Gastroenterol. 2009; 46(1):20-5.
Protein-calorie malnutrition (PCM) has been reported in about 20 to 92% of patients with CD.33 Han PD, Burke A, Baldassano RN, Rombeau JL, Lichtenstein GR. Nutrition and inflammatory bowel disease. Gastroenterol Clin North Am. 1999; 28(2):423-43.
4 Harries AD, Rhodes J. Undernutrition in Crohn's disease: an anthropometric assessment. Clin Nutr. 1985; 4(2):87-9.
5 Gassull MA, Cabré E. Nutrition in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care. 2001; 4(6):561-9.
6 Gassull MA, Abad A, Cabré E, González-Huix F, Giné JJ, Dolz C. Enteral nutrition in inflammatory bowel disease. Gut. 1986; 27(Suppl 1):76-80.-77 Cabré E, Gassull MA. Nutrition in inflammatory bowel disease: impact on disease and therapy. Curr Opin Gastroenterol. 2001; 17(4):342-9. Many factors contribute to PCM and deficiency of macro-nutrients, vitamins and minerals in this group of patients. Changes directly related to disease activity, such as inflammatory changes in the intestinal epithelium, as well as previous intestinal resections, may be responsible for malabsorption of nutrients and vitamins (such as vitamins A, D, E, K and B12). Pain, present at times of disease exacerbation, and increased inflammatory mediators, such as tumor necrosis factor, are associated with anorexia and cachexia.88 Warne JP. Tumour necrosis factor alpha: a key regulator of adipose tissue mass. J Endocrinol. 2003; 177(3):351-5. In addition to these factors, the therapy used in the treatment of CD can be associated with certain side effects that negatively affect the nutritional profile of these patients.99 Montgomery SC, Williams CM, Maxwell PJ. Nutritional support of patient with inflammatory bowel disease. Surg Clin North Am. 2015; 95(6):1271-9. Also, increased nutritional requirements as a consequence of inflammatory activity and complications of the disease may corroborate for weight loss.1010 O'Sullivan M, O'Morain C. Nutrition in inflammatory bowel disease. Best Pract Res Clin Gastroenterol. 2006; 20(3):561-73.
It is well-known that weight loss, low body mass index (BMI) and PCM predominate in patients with moderate to severe CD, especially those admitted to hospital, being associated with higher mortality, longer hospitalization and increased health costs.1111 Pirlich M, Schütz T, Kemps M, Luhman N, Burmester G-R, Baumann G, et al. Prevalence of malnutrition in hospitalized medical patients: impact of underlying disease. Dig Dis. 2003; 21(3):245-51. Zhang et al. showed that the BMI is inversely related to intra-abdominal infectious complications in patients with CD undergoing an elective surgical procedure.1212 Zhang M, Gao X, Chen Y, Zhi M, Chen H, Tang J, et al. Body mass index is a marker of nutrition preparation sufficiency before surgery for Crohn's disease from the perspective of intra-abdominal septic complications: a retrospective cohort study. Medicine (Baltimore). 2015; 94(35):e1455.
Location, phenotype, severity and disease activity appear to be directly related to impairment of nutritional status. Disease activity is related to increased basal energy expenditure, weight loss and anemia.1313 Capristo E, Mingrone G, Addolorato G, Greco A V, Gasbarrini G. Metabolic features of inflammatory bowel disease in a remission phase of the disease activity. J Intern Med. 1998; 243(5):339-47.,1414 Gong J, Zuo L, Guo Z, Zhang L, Li Y, Gu L, et al. Impact of disease activity on resting energy expenditure and body composition in adult Crohn's disease: a prospective longitudinal assessment. JPEN J Parenter Enteral Nutr. 2015; 39(6):713-8. In addition to malnutrition, changes in body composition such as the occurrence of sarcopenia have been described in patients with CD.1515 Shanahan F. Crohn's disease. Lancet. 2002; 359(9300):62-9.
16 Andrade MI, Maio R, Dourado KF, Macêdo PFC, Barreto Neto AC. Excessive weight - muscle depletion paradox and cardiovascular risk factors in outpatients with inflammatory bowel disease. Arq Gastroenterol. 2015; 52(1):37-45.
17 Bryant R V, Ooi S, Schultz CG, Goess C, Grafton R, Hughes J, et al. Low muscle mass and sarcopenia: common and predictive of osteopenia in inflammatory bowel disease. Aliment Pharmacol Ther. 2015; 41(9):895-906.-1818 Schneider SM, Al-Jaouni R, Filippi J, Wiroth J-B, Zeanandin G, Arab K, et al. Sarcopenia is prevalent in patients with Crohn's disease in clinical remission. Inflamm Bowel Dis. 2008; 14(11):1562-8. Schneider et al. describe the occurrence of sarcopenia in 60% of patients with CD even during clinical remission.1818 Schneider SM, Al-Jaouni R, Filippi J, Wiroth J-B, Zeanandin G, Arab K, et al. Sarcopenia is prevalent in patients with Crohn's disease in clinical remission. Inflamm Bowel Dis. 2008; 14(11):1562-8. Loss of lean mass (LM) has been associated with worsening of bone mineral density, increased morbidity, loss of muscle strength, and increased risk of infectious complications.
Traditionally, nutritional status has been evaluated based on objective parameters (anthropometric measures, skinfolds, clinical questionnaires, laboratory tests) and complementary methods already well-established for the evaluation not only of nutritional status, but also of body composition (dilution methods, dual-energy absorptiometry and electrical bioimpedance). Bioelectrical impedance analysis (BIA) is a method widely used to estimate body composition and nutritional status in several clinical populations because it is a simple, fast, noninvasive method with reproducible results.1919 Barbosa-Silva MCG, Barros AJ, Wang J, Heymsfield SB, Pierson RN Jr. Bioelectrical impedance analysis: population reference values for phase angle by age and sex. Am J Clin Nutr. 2005; 82(1):49-52. Not only does it determine the fat mass (FM) and the fat-free mass (LM), the four-part model also allows the evaluation of the degree of cellular damage by determining a phase angle (PA), which has been considered a promising tool to assess the nutritional status and indicate prognosis.1919 Barbosa-Silva MCG, Barros AJ, Wang J, Heymsfield SB, Pierson RN Jr. Bioelectrical impedance analysis: population reference values for phase angle by age and sex. Am J Clin Nutr. 2005; 82(1):49-52.
20 Nagano M, Suita S, Yamanouchi T. The validity of bioelectrical impedance phase angle for nutritional assessment in children. J Pediatr Surg. 2000; 35(7):1035-9.-2121 Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gómez J, et al. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004; 23(6):1430-53.
Biological therapy has dramatically changed the treatment of inflammatory bowel diseases (IBDs). This therapy is based on a monoclonal antibody that acts directly by blocking tumor necrosis factor alpha (TNF-α) and is effective in reducing inflammation, improving symptoms and inducing intestinal mucosa healing in a significant portion of patients.2222 Lichtenstein GR, Hanauer SB, Sandborn WJ. Management of Crohn's disease in adults. Am J Gastroenterol. 2009; 104(2):465-83; quiz 464, 484.
23 Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997; 337(15):1029-35.-2424 Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis. 2010; 4(1):28-62. Anti-TNF therapy is associated with a lower rate of complications of CD in the short and long term, reduction of rates of hospitalization and surgery, and a significant improvement in quality of life.2222 Lichtenstein GR, Hanauer SB, Sandborn WJ. Management of Crohn's disease in adults. Am J Gastroenterol. 2009; 104(2):465-83; quiz 464, 484.,2424 Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis. 2010; 4(1):28-62. On the other hand, some studies have demonstrated an association between biological therapy and changes in metabolic profile (e.g. hypertriglyceridemia, change in LDL composition, increase in HDL), and weight gain, mainly at the expense of increased abdominal and visceral fat.2525 Bryant R V, Trott MJ, Bartholomeusz FD, Andrews JM. Systematic review: body composition in adults with inflammatory bowel disease. Aliment Pharmacol Ther. 2013; 38(3):213-25.
Since anti-TNF therapy controls the inflammatory state and induces remission in moderate to severe CD, it is possible that after this intervention an increase in body weight, LM and PA of these patients may be observed. In this context, our study was designed to evaluate the impact of anti-TNFα therapy on body composition and PA in patients with active CD.
Method
In the period from April 2013 to August 2015, patients with moderate to severe CD with indication of anti-TNF therapy (infliximab - IFX) from the Intestinal Inflammatory Disease Outpatient Clinic of the Federal University of Juiz de Fora were included consecutively in our prospective study for the evaluation of body composition using BIA.
The study protocol was defined in accordance with the Declaration of Helsinki and approved by our Institutional Ethics Committee. All patients signed the informed consent form, before being accepted in the study.
The diagnosis of CD was established based on clinical, endoscopic, histopathological or imaging data.2424 Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis. 2010; 4(1):28-62. Patients aged between 18 and 65 years with moderate to severe CD, refractory or dependent on corticosteroids were included. Exclusion criteria were: severe comorbidities, gestation and/or concomitant infection. All patients underwent induction therapy with IFX (5 mg/kg at weeks 0, 2 and 6) followed by maintenance every 8 weeks. Patients who did not respond to anti-TNF therapy were excluded from the analysis.
At baseline, medical history and eligibility criteria were assessed. Relevant data such as age, gender, BMI and smoking habit were noted. Variables associated with the disease included duration, location and phenotype according to the Montreal Classification,2626 Silverberg MS, Satsangi J, Ahmad T, Arnott IDR, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005; 19(Suppl A):5A-36A. therapy and previous history of surgery.
Disease activity was established according to the Harvey-Bradshaw Index (HBI). Moderate to severe cases were considered as active disease if HBI > 8, either associated or not with the presence of extensive and profound ulcers on colonoscopy, complications (e.g., stenosis, fistulas or abscess) and/or complex perianal disease. Biochemical parameters such as complete blood count, ESR, lipid profile, triglycerides, blood glucose, ferritin and albumin were evaluated.
Anthropometry and body composition
Patients should wear light clothing and be barefoot for the measurement of anthropometric data. Height was measured in centimeters (cm) rounded to the nearest 0.5 cm, and weight was measured in kilograms (kg) rounded to the nearest 0.1 kg. LM, FM and PA were obtained by electrical bioimpedance (Quantum BIA-101Q, Detroit, MI). Impedance measurements were performed on the right side, with the patient in the supine position, and the limbs apart from each other. We used a previously validated regression equation to analyze LM.1919 Barbosa-Silva MCG, Barros AJ, Wang J, Heymsfield SB, Pierson RN Jr. Bioelectrical impedance analysis: population reference values for phase angle by age and sex. Am J Clin Nutr. 2005; 82(1):49-52.,2727 Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982; 36(5):936-42. The LM and FM indexes are calculated by dividing the LM and FM by the squared height, respectively. PA is a derived measure obtained from the relation between the direct measures of resistance and reactance. It is calculated directly from reactance and resistance:2828 Florindo AA, Latorre MRDO, Jaime PC, Tanaka T, Zerbini CA de F. Methodology to evaluation the habitual physical activity in men aged 50 years or more. Rev Saúde Pública. 2004; 38(2):307-14.
PA = arc tangent (reactance / resistance) ? 180° / π
PA occurs when a portion of the electric current is stored by the cell membranes, creating a phase shift.2929 Vadan R, Gheorghe LS, Constantinescu A, Gheorghe C. The prevalence of malnutrition and the evolution of nutritional status in patients with moderate to severe forms of Crohn's disease treated with Infliximab. Clin Nutr. 2011; 30(1):86-91. In a healthy individual, PA can range from 4 to 10 degrees.88 Warne JP. Tumour necrosis factor alpha: a key regulator of adipose tissue mass. J Endocrinol. 2003; 177(3):351-5.,3030 Briot K, Gossec L, Kolta S, Dougados M, Roux C. Prospective assessment of body weight, body composition, and bone density changes in patients with spondyloarthropathy receiving anti-tumor necrosis factor-alpha treatment. J Rheumatol. 2008; 35(5):855-61.
Physical Activity Assessment Questionnaire
The level of habitual physical activity (HPA) in daily life was assessed using the questionnaire activity described by Baecke et al.,2727 Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982; 36(5):936-42. and validated for the Brazilian population.2828 Florindo AA, Latorre MRDO, Jaime PC, Tanaka T, Zerbini CA de F. Methodology to evaluation the habitual physical activity in men aged 50 years or more. Rev Saúde Pública. 2004; 38(2):307-14. This instrument is composed of 16 questions that cover three components of physical activity: 1) occupational physical activity score (Q1 to Q8); 2) leisure-time physical activity score (Q9 to Q12); and 3) leisure-time activities and locomotion score (Q13 to Q16). Together, these three domains, work, sports and non-sporting leisure, result in a global physical activity score. Scores ranging from 9 to 16 indicate a moderate level of daily physical activity, while scores < 9 are observed in sedentary individuals.3131 Argilés JM, López-Soriano J, Busquets S, López-Soriano FJ. Journey from cachexia to obesity by TNF. FASEB J. 1997; 11(10):743-51.
Statistical analysis
Sample size was calculated considering a type I error set at 0.05 and a sample power at 0.9 to detect a 1.5 kg LM difference in patients with CD before and after treatment with anti-TNFα. Thus, 25 patients would be needed for the study.
The collected data were analyzed using specific statistical software (SPSS- Statistical Package for the Social SciencesTM, v. 13.0). For intra-group comparison in the active disease (baseline) and remission phases (in the 6th month), paired Student's t-test or Wilcoxon test were performed. Pearson or Spearman correlation was used to assess the degree of association between variables. The type I error probability was assumed to be 5% in all tests.
Results
Of the 26 patients selected for the study, three were excluded: one did not attend the reevaluation visit, one had morbid obesity and one did not respond to IFX induction therapy.
Table 1 shows the demographic and clinical characteristics of patients with CD.
Six months after IFX therapy, there was a significant reduction in HBI score (7 versus 2; p<0.0001), as well as complete withdrawal of corticosteroids (35% versus 0%; p<0.0018).
Table 2 shows the laboratory data, anthropometric values and body composition. Based on BMI, most patients were classified as having normal weight both at baseline and after 6 months, despite an increase in BMI observed after treatment with IFX (22.9 versus 24.9; p=0.000).
Anthropometric and body composition indicators at baseline and 6 months after treatment with IFX in patients with Crohn's disease.
In the analysis of body composition, we observed an increase in all parameters, except PA (Figure 1). Although baseline and post-intervention PA values were similar, they were significantly lower than the predicted values (predicted PA: 6.8°; baseline PA: 5.6° and post-intervention PA: 6.1°).
In relation to the level of habitual physical activity of the patients, low levels of habitual physical activity were observed in the baseline evaluation and after the intervention, even after achieving disease remission (6.2 vs. 6.8; p=0.94).
Discussion
Biological therapy is a milestone in the treatment of IBDs. Anti-TNF agents have become drugs of choice in the treatment of patients with moderate to severe CD who do not tolerate the usual therapy. TNF-α is an inflammatory cytokine with catabolic power and a lipolytic and apoptotic effect that plays a significant role in lipid and glucose metabolism.
The efficacy of anti-TNFα agents in controlling the inflammatory process of IBD is indisputable. However, its effects on the body composition of patients with CD are still controversial. In our study, 20 (87%) of 23 patients had an increase in total body weight and, in 9 (39.1%), BMI values increased above 24.9, which is indicative of overweight/obesity. With regard to body composition, an increase in the LM (17.5±1.2 versus 18.1±2.3; p=0.000) and FM indexes (5.5±2.3 versus 6.8±2.3; p=0.000) was observed. Vandan et al. evaluated 30 patients with CD undergoing IFX therapy and observed weight gain and change in nutritional status, the latter associated with clinical remission of the disease.2929 Vadan R, Gheorghe LS, Constantinescu A, Gheorghe C. The prevalence of malnutrition and the evolution of nutritional status in patients with moderate to severe forms of Crohn's disease treated with Infliximab. Clin Nutr. 2011; 30(1):86-91. Weight gain after biologic therapy has also been observed in other studies in patients with autoimmune diseases, suggesting that this therapy, and not simply the remission of CD, is associated with these changes in weight.3030 Briot K, Gossec L, Kolta S, Dougados M, Roux C. Prospective assessment of body weight, body composition, and bone density changes in patients with spondyloarthropathy receiving anti-tumor necrosis factor-alpha treatment. J Rheumatol. 2008; 35(5):855-61.,3232 Nic Suibhne T, Raftery TC, McMahon O, Walsh C, O'Morain C, O'Sullivan M. High prevalence of overweight and obesity in adults with Crohn's disease: associations with disease and lifestyle factors. J Crohns Colitis. 2013; 7(7):e241-8.,3333 Brown RA, Spina D, Butt S, Summers GD. Long-term effects of anti-tumour necrosis factor therapy on weight in patients with rheumatoid arthritis. Clin Rheumatol. 2012; 31(3):455-61.
Our results demonstrated that there was a substantial increase in FM after the use of IFX. It should be noted that all patients had the body water percentage within normal values, so that FM and LM could not be affected by disturbances in the patients' hydration status.2121 Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gómez J, et al. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004; 23(6):1430-53. Increased FM after treatment with anti-TNF in patients with CD has also been reported in the study by Wiese et al.3434 Wiese D, Lashner B, Seidner D. Measurement of nutrition status in Crohn's disease patients receiving infliximab therapy. Nutr Clin Pract. 2008; 23(5):551-6. The gain of FM in patients who achieved clinical remission after biological therapy can be explained by factors such as improvement in the quantity and quality of food intake due to absence of symptoms, less catabolism secondary to reduction of inflammation and, last, TNF-α blockade. TNF is a mediator of cachexia induced by inflammation.3131 Argilés JM, López-Soriano J, Busquets S, López-Soriano FJ. Journey from cachexia to obesity by TNF. FASEB J. 1997; 11(10):743-51. In addition, inhibition of TNF-α may trigger an increase in the number of adipocytes by facilitating adipogenesis, resulting in increased FM.88 Warne JP. Tumour necrosis factor alpha: a key regulator of adipose tissue mass. J Endocrinol. 2003; 177(3):351-5.
It should be noted that IFX therapy resulted in increased waist circumference (88.1±6.7 vs. 93.9±7.7 cm; p<0.05) in our patients with CD. This finding makes us speculate whether the increase in waist circumference should be attributed to visceral or subcutaneous adiposity. Some studies using magnetic resonance imaging have shown an increase in abdominal fat volume in patients treated for 8 weeks with IFX, with homogeneous fat distribution between the visceral and subcutaneous compartments.3535 Parmentier-Decrucq E, Duhamel A, Ernst O, Fermont C, Louvet A, Vernier-Massouille G,et al. Effects of infliximab therapy on abdominal fat and metabolic profile in patients with Crohn's disease. Inflamm Bowel Dis. 2009; 15(10):1476-84.,3636 Desreumaux P, Ernst O, Geboes K, Gambiez L, Berrebi D, Müller-Alouf H, et al. Inflammatory alterations in mesenteric adipose tissue in Crohn's disease. Gastroenterology. 1999; 117(1):73-81. Interestingly, the accumulation of mesenteric fat in patients with CD has been observed and demonstrated to be independent of steroid use.2929 Vadan R, Gheorghe LS, Constantinescu A, Gheorghe C. The prevalence of malnutrition and the evolution of nutritional status in patients with moderate to severe forms of Crohn's disease treated with Infliximab. Clin Nutr. 2011; 30(1):86-91.,3737 Valentini L, Schaper L, Buning C, Hengstermann S, Koernicke T, Tillinger W, et al. Malnutrition and impaired muscle strength in patients with Crohn's disease and ulcerative colitis in remission. Nutrition. 2008; 24(7-8):694-702.
A low level of physical activity leads to the disuse of skeletal muscle and consequent atrophy, which may be a factor present in patients with CD. All of our patients had a low level of habitual physical activity at baseline, with no change after induction and maintenance of CD remission.
Data regarding the metabolic profile of patients with CD undergoing anti-TNF therapy are controversial.3535 Parmentier-Decrucq E, Duhamel A, Ernst O, Fermont C, Louvet A, Vernier-Massouille G,et al. Effects of infliximab therapy on abdominal fat and metabolic profile in patients with Crohn's disease. Inflamm Bowel Dis. 2009; 15(10):1476-84.,3838 Popa C, van den Hoogen FHJ, Radstake TRDJ, Netea MG, Eijsbouts AE, den Heijer M, et al. Modulation of lipoprotein plasma concentrations during long-term anti-TNF therapy in patients with active rheumatoid arthritis. Ann Rheum Dis. 2007; 66(11):1503-7.
39 Soubrier M, Jouanel P, Mathieu S, Poujol D, Claus D, Dubost JJ, Ristori JM. Effects of anti-tumor necrosis factor therapy on lipid profile in patients with rheumatoid arthritis. Joint Bone Spine. 2008; 75(1):22-4.-4040 Kiortsis DN, Mavridis AK, Filippatos TD, Vasakos S, Nikas SN, Drosos AA. Effects of infliximab treatment on lipoprotein profile in patients with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 2006; 33(5):921-3. Popa et al. observed reduced levels of triglycerides after six months of anti-TNFα therapy in patients with rheumatoid arthritis.3838 Popa C, van den Hoogen FHJ, Radstake TRDJ, Netea MG, Eijsbouts AE, den Heijer M, et al. Modulation of lipoprotein plasma concentrations during long-term anti-TNF therapy in patients with active rheumatoid arthritis. Ann Rheum Dis. 2007; 66(11):1503-7. Similar to the findings of Parmantier-Decreucq et al., despite weight gain, we did not observe changes in our patients' profile after anti-TNF therapy.3535 Parmentier-Decrucq E, Duhamel A, Ernst O, Fermont C, Louvet A, Vernier-Massouille G,et al. Effects of infliximab therapy on abdominal fat and metabolic profile in patients with Crohn's disease. Inflamm Bowel Dis. 2009; 15(10):1476-84. It is believed that a good control of the inflammatory state can positively influence the lipid concentration.4141 Cacciapaglia F, Anelli MG, Rinaldi A, Serafino L, Covelli M, Scioscia C, et al. Lipid profile of rheumatoid arthritis patients treated with anti-tumor necrosis factor-alpha drugs changes according to disease activity and predicts clinical response. Drug Dev Res. 2014; 75(Suppl 1):S77-80.
Some studies have suggested that in different chronic clinical conditions, the PA can be considered a marker of general health, nutritional status and prognosis.4242 Norman K, Stobäus N, Pirlich M, Bosy-Westphal A. Bioelectrical phase angle and impedance vector analysis--clinical relevance and applicability of impedance parameters. Clin Nutr. 2012; 31(6):854-61.,4343 Gonzalez MC, Barbosa-Silva TG, Bielemann RM, Gallagher D, Heymsfield SB. Phase angle and its determinants in healthy subjects: influence of body composition. Am J Clin Nutr. 2016; 103(3):712-6. Reduced PA values are associated with unfavorable disease progression and poor prognosis. In our study, the values found for PA were significantly lower than the reference values, even after treatment with IFX. This lack of improvement after 24 weeks of IFX therapy may be explained by the time of intervention, which may not have been sufficient to produce substantial gain in the cell compartments of which PA is representative. Another factor to be considered is that PA decreases the greater the FM and the lower the LM. Although our patients gained LM, FM gain was even more substantial.
Our study has some limitations. First, although the electrical bioimpedance method is valid and reliable for body composition analysis, it consists of a bi-compartmental model, unable to measure visceral fat and subcutaneous fat. Although the method was influenced by the state of hydration (e.g., chronic or cirrhotic renal patients), our patients maintained healthy hydration and, therefore, there was no impact of this factor on CD. Second, the sample size, although modest, was based on sample calculations, and was able to detect significant differences. And finally, the lack of a control group with healthy individuals did not allow comparisons with the patients.
Important clinical implications can be highlighted in the present study. In patients with active CD who achieved remission, there was a gain in LM and FM. However, fat gain was more significant with consequent nutritional disorder despite CD remission. In addition, considerable fat gain, the persistence of sedentary lifestyle even with disease in remission, and a significant increase in waist circumference suggest a possible increase in cardiovascular risk in these patients.
In conclusion, in patients with moderate to severe CD who achieved remission after 24 weeks of anti-TNF therapy, body weight gain was observed, attributed mainly to gain of FM, increased waist circumference and unchanged PA. Future studies should seek to assess the impact of fat gain on cardiovascular outcomes and overall health, as well as evaluate the effects of nutritional and physical activity interventions simultaneously with anti-TNFα treatment.
-
Study conducted at the Inflammatory Bowel Diseases Center, University Hospital, Faculdade de Medicina da Universidade Federal de Juiz de Fora, Juiz de Fora, MG, Brazil
References
-
1Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002; 347(6):417-29.
-
2Victoria CR, Sassak LY, Nunes HRC. Incidence and prevalence rates of inflammatory bowel diseases, in midwestern of São Paulo State, Brazil. Arq Gastroenterol. 2009; 46(1):20-5.
-
3Han PD, Burke A, Baldassano RN, Rombeau JL, Lichtenstein GR. Nutrition and inflammatory bowel disease. Gastroenterol Clin North Am. 1999; 28(2):423-43.
-
4Harries AD, Rhodes J. Undernutrition in Crohn's disease: an anthropometric assessment. Clin Nutr. 1985; 4(2):87-9.
-
5Gassull MA, Cabré E. Nutrition in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care. 2001; 4(6):561-9.
-
6Gassull MA, Abad A, Cabré E, González-Huix F, Giné JJ, Dolz C. Enteral nutrition in inflammatory bowel disease. Gut. 1986; 27(Suppl 1):76-80.
-
7Cabré E, Gassull MA. Nutrition in inflammatory bowel disease: impact on disease and therapy. Curr Opin Gastroenterol. 2001; 17(4):342-9.
-
8Warne JP. Tumour necrosis factor alpha: a key regulator of adipose tissue mass. J Endocrinol. 2003; 177(3):351-5.
-
9Montgomery SC, Williams CM, Maxwell PJ. Nutritional support of patient with inflammatory bowel disease. Surg Clin North Am. 2015; 95(6):1271-9.
-
10O'Sullivan M, O'Morain C. Nutrition in inflammatory bowel disease. Best Pract Res Clin Gastroenterol. 2006; 20(3):561-73.
-
11Pirlich M, Schütz T, Kemps M, Luhman N, Burmester G-R, Baumann G, et al. Prevalence of malnutrition in hospitalized medical patients: impact of underlying disease. Dig Dis. 2003; 21(3):245-51.
-
12Zhang M, Gao X, Chen Y, Zhi M, Chen H, Tang J, et al. Body mass index is a marker of nutrition preparation sufficiency before surgery for Crohn's disease from the perspective of intra-abdominal septic complications: a retrospective cohort study. Medicine (Baltimore). 2015; 94(35):e1455.
-
13Capristo E, Mingrone G, Addolorato G, Greco A V, Gasbarrini G. Metabolic features of inflammatory bowel disease in a remission phase of the disease activity. J Intern Med. 1998; 243(5):339-47.
-
14Gong J, Zuo L, Guo Z, Zhang L, Li Y, Gu L, et al. Impact of disease activity on resting energy expenditure and body composition in adult Crohn's disease: a prospective longitudinal assessment. JPEN J Parenter Enteral Nutr. 2015; 39(6):713-8.
-
15Shanahan F. Crohn's disease. Lancet. 2002; 359(9300):62-9.
-
16Andrade MI, Maio R, Dourado KF, Macêdo PFC, Barreto Neto AC. Excessive weight - muscle depletion paradox and cardiovascular risk factors in outpatients with inflammatory bowel disease. Arq Gastroenterol. 2015; 52(1):37-45.
-
17Bryant R V, Ooi S, Schultz CG, Goess C, Grafton R, Hughes J, et al. Low muscle mass and sarcopenia: common and predictive of osteopenia in inflammatory bowel disease. Aliment Pharmacol Ther. 2015; 41(9):895-906.
-
18Schneider SM, Al-Jaouni R, Filippi J, Wiroth J-B, Zeanandin G, Arab K, et al. Sarcopenia is prevalent in patients with Crohn's disease in clinical remission. Inflamm Bowel Dis. 2008; 14(11):1562-8.
-
19Barbosa-Silva MCG, Barros AJ, Wang J, Heymsfield SB, Pierson RN Jr. Bioelectrical impedance analysis: population reference values for phase angle by age and sex. Am J Clin Nutr. 2005; 82(1):49-52.
-
20Nagano M, Suita S, Yamanouchi T. The validity of bioelectrical impedance phase angle for nutritional assessment in children. J Pediatr Surg. 2000; 35(7):1035-9.
-
21Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gómez J, et al. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004; 23(6):1430-53.
-
22Lichtenstein GR, Hanauer SB, Sandborn WJ. Management of Crohn's disease in adults. Am J Gastroenterol. 2009; 104(2):465-83; quiz 464, 484.
-
23Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997; 337(15):1029-35.
-
24Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis. 2010; 4(1):28-62.
-
25Bryant R V, Trott MJ, Bartholomeusz FD, Andrews JM. Systematic review: body composition in adults with inflammatory bowel disease. Aliment Pharmacol Ther. 2013; 38(3):213-25.
-
26Silverberg MS, Satsangi J, Ahmad T, Arnott IDR, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005; 19(Suppl A):5A-36A.
-
27Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982; 36(5):936-42.
-
28Florindo AA, Latorre MRDO, Jaime PC, Tanaka T, Zerbini CA de F. Methodology to evaluation the habitual physical activity in men aged 50 years or more. Rev Saúde Pública. 2004; 38(2):307-14.
-
29Vadan R, Gheorghe LS, Constantinescu A, Gheorghe C. The prevalence of malnutrition and the evolution of nutritional status in patients with moderate to severe forms of Crohn's disease treated with Infliximab. Clin Nutr. 2011; 30(1):86-91.
-
30Briot K, Gossec L, Kolta S, Dougados M, Roux C. Prospective assessment of body weight, body composition, and bone density changes in patients with spondyloarthropathy receiving anti-tumor necrosis factor-alpha treatment. J Rheumatol. 2008; 35(5):855-61.
-
31Argilés JM, López-Soriano J, Busquets S, López-Soriano FJ. Journey from cachexia to obesity by TNF. FASEB J. 1997; 11(10):743-51.
-
32Nic Suibhne T, Raftery TC, McMahon O, Walsh C, O'Morain C, O'Sullivan M. High prevalence of overweight and obesity in adults with Crohn's disease: associations with disease and lifestyle factors. J Crohns Colitis. 2013; 7(7):e241-8.
-
33Brown RA, Spina D, Butt S, Summers GD. Long-term effects of anti-tumour necrosis factor therapy on weight in patients with rheumatoid arthritis. Clin Rheumatol. 2012; 31(3):455-61.
-
34Wiese D, Lashner B, Seidner D. Measurement of nutrition status in Crohn's disease patients receiving infliximab therapy. Nutr Clin Pract. 2008; 23(5):551-6.
-
35Parmentier-Decrucq E, Duhamel A, Ernst O, Fermont C, Louvet A, Vernier-Massouille G,et al. Effects of infliximab therapy on abdominal fat and metabolic profile in patients with Crohn's disease. Inflamm Bowel Dis. 2009; 15(10):1476-84.
-
36Desreumaux P, Ernst O, Geboes K, Gambiez L, Berrebi D, Müller-Alouf H, et al. Inflammatory alterations in mesenteric adipose tissue in Crohn's disease. Gastroenterology. 1999; 117(1):73-81.
-
37Valentini L, Schaper L, Buning C, Hengstermann S, Koernicke T, Tillinger W, et al. Malnutrition and impaired muscle strength in patients with Crohn's disease and ulcerative colitis in remission. Nutrition. 2008; 24(7-8):694-702.
-
38Popa C, van den Hoogen FHJ, Radstake TRDJ, Netea MG, Eijsbouts AE, den Heijer M, et al. Modulation of lipoprotein plasma concentrations during long-term anti-TNF therapy in patients with active rheumatoid arthritis. Ann Rheum Dis. 2007; 66(11):1503-7.
-
39Soubrier M, Jouanel P, Mathieu S, Poujol D, Claus D, Dubost JJ, Ristori JM. Effects of anti-tumor necrosis factor therapy on lipid profile in patients with rheumatoid arthritis. Joint Bone Spine. 2008; 75(1):22-4.
-
40Kiortsis DN, Mavridis AK, Filippatos TD, Vasakos S, Nikas SN, Drosos AA. Effects of infliximab treatment on lipoprotein profile in patients with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 2006; 33(5):921-3.
-
41Cacciapaglia F, Anelli MG, Rinaldi A, Serafino L, Covelli M, Scioscia C, et al. Lipid profile of rheumatoid arthritis patients treated with anti-tumor necrosis factor-alpha drugs changes according to disease activity and predicts clinical response. Drug Dev Res. 2014; 75(Suppl 1):S77-80.
-
42Norman K, Stobäus N, Pirlich M, Bosy-Westphal A. Bioelectrical phase angle and impedance vector analysis--clinical relevance and applicability of impedance parameters. Clin Nutr. 2012; 31(6):854-61.
-
43Gonzalez MC, Barbosa-Silva TG, Bielemann RM, Gallagher D, Heymsfield SB. Phase angle and its determinants in healthy subjects: influence of body composition. Am J Clin Nutr. 2016; 103(3):712-6.
Publication Dates
-
Publication in this collection
May 2017
History
-
Received
28 Oct 2016 -
Accepted
20 Nov 2016