Abstracts
OBJECTIVE: To investigate the ovarian activity before and after gonadal suppression with GnRH-analog in patients with PCO, hyperandrogenism, hyperinsulinism and acanthosis nigricans. DESIGN: Controlled clinical study. SETTING: Tertiary academic medical center. PATIENTS: Six patients with clinical findings of PCO, hirsutism and acanthosis nigricans. INTERVENTIONS: Morning blood samples in the follicular phase to determine the steroid levels, glucose and insulin curve, comparing to a control group. Administration for 2 consecutive months of a GnRH-analog, comparing, in the study group, the free testosterone levels before and after ovarian suppression. MAIN OUTCOME MEASURE: Determination of insulin levels in PCO, hirsutism and acanthotic patients and the free-testosterone levels before and after gonadal suppression. RESULTS: Insulin levels were significantly higher in the study group when compared to normal women during the glycemic test. We also found a significant decrease in the free-testosterone levels after 2 months of gonadal suppression with GnRH-analog when compared to the initial time. CONCLUSIONS: Patients with PCO, hirsutism and acanthosis nigricans present high levels of insulin, suggesting an ovarian hyperesponsiveness, which is not sustained when gonadotrophic blockage was achieved.
PCO2; Hyperandrogenism; Insulin restance; Ovarian suppression
OBJETIVO: Investigar a atividade ovariana antes e após a supressão gonadal com análogo de GnRH em pacientes com síndrome dos ovários policísticos (SOP), hiperandrogenismo, hiperinsulinismo e acantose nigricante. DESENHO: Estudo clínico prospectivo. LOCAL: Centro médico-acadêmico nível terciário. PACIENTES: Seis pacientes com sinais clínicos de SOP, hirsutismo e acantose nigricante. INTERVENÇÕES: Colheita de amostras sanguíneas matinais na fase folicular para determinar os valores séricos de esteróides, curva glicêmica e insulínica, comparando esta última à do grupo controle. Administração por dois meses consecutivos de análogo de GnRH, comparando, nas pacientes do grupo de estudo, os níveis séricos de testosterona livre antes e após a supressão gonadal. PRINCIPAIS DETERMINAÇÕES: Determinação dos níveis séricos de insulina nas pacientes com SOP, hirsutismo e acantose nigricans e avaliar os níveis de testosterona livre antes e após supressão gonadal. RESULTADOS: Os níveis de insulina foram significativamente maiores nas pacientes do grupo de estudo, quando comparados a mulheres normais, durante a curva glicêmica. Também encontramos uma significativa queda nos valores de testosterona livre, após dois meses de supressão gonadal com analógo de GnRH, quando confrontados ao tempo inicial. CONCLUSÕES: Pacientes com SOP, hirsutismo e acantose nigricante apresentam altos níves séricos de insulina, sugerindo uma hiperativação ovariana por este peptídeo, que, entretanto, não é mantida se promovermos o bloqueio gonadotrófico.
SOP; Hiperandrogenismo; Resistência insulínica; Supressão ovariana
Artigo Original
Ovarian activity before and after gonadal suppression by GnRH-a in patients with polycystic ovary syndrome, hyperandrogenism, hyperinsulinism and acanthosis nigricans
E.L.A. Motta, E.C. Baracat, M.A. Haidar, I. Juliano, G.R. Lima
Department of Gynecology, Universidade Federal de São Paulo, São Paulo, SP, Brazil.
SUMMARY OBJECTIVE: To investigate the ovarian activity before and after gonadal suppression with GnRH-analog in patients with PCO, hyperandrogenism, hyperinsulinism and acanthosis nigricans. DESIGN:Controlled clinical study.SETTING:Tertiary academic medical center.PATIENTS: Six patients with clinical findings of PCO, hirsutism and acanthosis nigricans.
INTERVENTIONS: Morning blood samples in the follicular phase to determine the steroid levels, glucose and insulin curve, comparing to a control group. Administration for 2 consecutive months of a GnRH-analog, comparing, in the study group, the free testosterone levels before and after ovarian suppression.
MAIN OUTCOME MEASURE: Determination of insulin levels in PCO, hirsutism and acanthotic patients and the free-testosterone levels before and after gonadal suppression.
RESULTS: Insulin levels were significantly higher in the study group when compared to normal women during the glycemic test. We also found a significant decrease in the free-testosterone levels after 2 months of gonadal suppression with GnRH-analog when compared to the initial time.
CONCLUSIONS: Patients with PCO, hirsutism and acanthosis nigricans present high levels of insulin, suggesting an ovarian hyperesponsiveness, which is not sustained when gonadotrophic blockage was achieved.
KEY WORDS: PCO2. Hyperandrogenism. Insulin restance. Ovarian suppression.
INTRODUCTION
Sclerotic modifications of the human ovary have been described for more than 100 years1, and the association between menstrual alterations, hyperandrogenism (HA) and infertility was acknowledged by Stein and Leventhal in 19352. Since that first report, many studies have followed and despite gains in the knowledge, the pathophisiology of the polycystic ovary syndrome (PCOS) remains unclear.
Ovarian steroidogenesis is regulated chiefly by the hypophyseal gonadotrophins. However, it is also influenced by a number of peptides, so-called growth factors, which modulate follicular growth3-6. Among these, insulin and the insulin-like growth factor-1 (IGF-1) should be highlighted7. The presence of raised serum insulin levels in patients with PCOS and hyperandrogenism was initially elucidated by Burghen et al.8 and Taylor et al.9. Nevertheless it was Barbieri and Ryan10, who, in 1983, described a group of patients with PCOS, HA, peripheral insulin resistance (IR) and acanthosis nigricans, naming it as HAIR-AN syndrome. According to the authors, the insulin resistance raised circulating insulin levels, which, in turn, excess would act in the ovaries and epidermis. Thus, there would be an interaction between insulin receptors and the receptors of IGF-1 in the ovary and skin, leading to androgen production and epidermal proliferation, resulting in hirsutism and acanthosis nigricans respectively. Since these early descriptions, many reports of a correlation between hyperinsulinism and hyperandrogenism in PCOS11 have followed.
We hypothesized that ovarian hyperandrogenism was critically dependent upon pulsatile gonadotropin release and would not be sustained by hyperinsulinemia alone. So, to test this hypothesis, we compare the response of women with PCOS after two months of GnRH-analog (GnRH-a) to eumenorrheic women.
METHODS
Twelve women were studied at the Endocrinologic Gynecology Section of the Discipline of Gynecology of Escola Paulista de Medicina, São Paulo, Brazil. These were classified into two study groups, I and II. The first was composed by six eumenorrheic women, without previous gynecological and/or endocrine alterations. The second study group consisted of another six women with polycystic ovary syndrome, hyperandrogenism, peripheral resistance to insulin and acanthosis nigricans. All women were between the age of 15 and 35 years, have not been taking hormonal medications in the previous six months.
All underwent thorough gynecological examination. The clinical history of those patients in Group II revealed primary oligomenorrhea, hirsutism and infertility. The diagnosis of acanthosis nigricans was based upon the presence of raised hyperpigmented and papillary lesions in the axillary region, nape of the neck, as inframammary sulcus. All were obese with body mass index (BMI) over 2512. Clinical features are displayed in table.
In all cases the following procedural routine was adapted.
Laboratory study
Biochemical profile whereby hemogram, proteinogram, urea and creatinine were determined since there are raised insulin levels in some renal diseases11. The hormonal profile consisted in the dosage of levels of prolactin (PRL), growth hormone (GH), cortisol (C), thyroid-stimulating hormone (TSH), and thyroxine (T4) assessed by the conventional radioimmunoassay (RIA) technique.
Furthermore, levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), free testosterone (free T), dehydroepiandrosterone sulphate (DHEA-S), and estradiol (E2) were also measured.
All of these tests were carried out by immuno-fluorimetric technique, except for free T, which was done by RIA. All of the hormone level assessments were carried out in duplicate and thevariation coefficients were less than 10% intra-assay and less than 12% inter-assay. Table shows the hormonal profile of the patients in the Study Group.
Oral glucose tolerance test
The oral glucose tolerance text (OGTT) was carried out to determine glycemic and insulinemic curves. The patients in both groups were told to ingest a diet rich in carbohydrates (more than 150g) for 3 days prior to the test13. After an overnight fast of 10-12h, basal blood sampling was started, followed by administration of 75g of glucose solution. Samples were taken at times 0, 15, 30, 60, 90 and 120 minutes.
Control of ovarian suppression by analogue agonist of the gonadotropin releasing hormone (GnRH-a)
The patients in Group II received two deep intramuscular injections of GnRH-a (D-Trp6-GnRH) for gonadal suppression at 30 day intervals. Assays were carried out 30 days after each injection to measure levels of free T, E2, FSH, and LH14.
With the exception of the assays carried out after the control of ovarian suppression, all the others, including OGTT were carried out during early follicular phase (2nd to 5th day), or randomly in the amenorrheic patients.
Data were analyzed statistically by nonparametric tests by the test of Friedman15 rank analyses of variance and the Mann-Whitney test for two independent samples16. Significance levels was considered at P < 0.05.
RESULTS
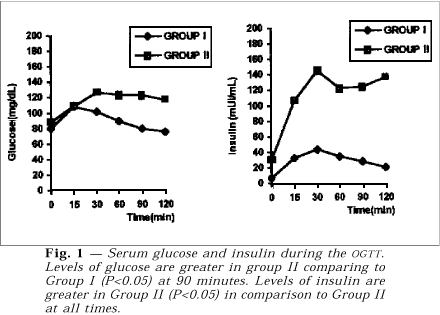
Figure 1 shows the average of glucose and insulin results respectively for each group during OGTT. On analyzing each group separately, one sees that the glycemic levels rose rapidly after 15 minutes, i.e., soon after caloric intake. These levels are significantly higher than the ones observed at 90 and 120 minutes (P<0.05). In Group II, however, despite the same sudden initial rise after 15 minutes, the glycemic values stayed high at 90 and 120 minutes. The comparison of glycemic behavior between the two groups, made by the Mann-Whitney test, revealed to be statistically higher at 90 minutes in Group II.
The second part of the figure shows the insulin determinations in the two groups during the same test. When each group is examined individually one notes that in Group I, the insulin variation took place later, i.e., after 30 and 60 minutes (P<0.05). These levels are considerably higher than the initial ones. As for Group II, the insulin levels rose significantly after 30, 60 and 90 minutes (P<0.05). The Mann-Whitney test shows that all levels of insulin in Group II are statistically higher, at the different times, if compared to those of the control group.
Figure 2 shows simultaneous curves for glucose and insulin in both groups. In Group I the average of insulin levels corresponds to one third of those of glucose although in Group II they are similars. In Figure 3 the serum levels of testosterone and estradiol prior to and after one and two months following administration of GnRH-a. One can note that, in comparison to the basal levels, there has been a marked fall in the respective levels.
DISCUSSION
It has become evident, since the 80's, that ovarian function is not regulated only by the hypophyseal gonadotrophins, but is also influenced by action of other hormones and growth factors17. Among these, insulin and IGF-1 have been the most extensively studied.
The first data about this association have been known since 1921 with the so-called Archard-Thiers Syndrome, or bearded diabetes, where raised serum insulin levels and hyperandrogenism have been associated.
There have also been frequent reports associating conditions of marked insulin resistance, and therefore hyperinsulinism, with exacerbated gonadal activity, such as premature puberty and hirsutism. Conversely, insulin-dependent diabetic patients, i.e., hypoinsulinemic, frequently present late menarche and sexual infantilism7.
The association between hyperinsulinism and PCOS was clarified by Burghen et al.8 These researchers suggested that raised testosterone levels were a consequence of the direct stimulatory effect of insulin on the female gonad. Barbieri and Ryan10, when describing the HAIR-AN Syndrome, highlighted a "nature's experiment", in which these patients were characterized by chronically raised insulin levels, thus denoting ovarian and epidermic hyperactivity.
It is known that acanthosis nigricans is related to various hyperinsulinemic conditions, such as non-insulin-dependent diabetes, Addison's disease, obesity and PCOS17. The factors which promote epidermal proliferation have not been completely elucidated, but the main hypothesis is based upon stimulation through growth factors such as melanocyte-stimulant hormone, growth hormone, adrenocorticotrophic hormone and insulin, among others10. In the human ovary, current evidence, both in vitro and in vivo allow us to state that insulin connects to specific receptors or to IGF-1 receptors, stimulating theca and stromal cells to increase steroidogenesis18.
Thus in this study we set out to evaluate gonadal function in patients with PCOS, hyperandrogenism and insulin resistance. Initially a group of patients with these characteristics and who were obese were selected. Among the various clinical conditions related to peripheral resistance to insulin, obesity seems to be the most prevalent and widely studied19,20.
Between the responsible mechanisms it is accept the decrease number of receptors (down-regulation) and alteration of post-receptor activation21. To date, in spite of populational studies which relate insulin levels to those of glucose22-25, it should be noted that there are no criteria which safely establish hyperinsulinism26. Thus we have attempted to compare the study group to the control group, which consisted of eumenorrheic, non-hirsute and non-obese females with BMI <25 (Group I). During OGTT it was noted that, in Group I, after the stimulus of ingest of carbohydrates there is rapid rise in glycemia, noticeable at 15 minutes, followed by a gradual drop towards the end of the test. These data are concordant with wide populational studies13. This also suggests that after stimulation there would be insulin liberation, which would promote glucose mobilization.
In the patients with PCOS (Group II), one initially observes similar behavior. However, at the end of the test their levels are discretely higher in comparison to the time of 15 minutes, even comparing a carbohydrate-intolerance state13.These data in themselves suggest that the hypoglycemiant hormone be unable to promote the entry of glucose into the cell.
As for the insulin curve, in Group I, its levels are lower than their respective glycemic levels, i.e., about 1/3, rising significantly at 30 and 60 minutes in relation to the initial time. In Group II the basal levels are also lower than those of glucose, but after the stimulus there is an intense rise, reaching its peak at 30 minutes, when it surpasses the corresponding glycemic value. This characteristic suggests the hyperinsulinic state24,27. When the insulin levels in the two groups are compared, there is statistical difference in all the times studied, thus characterizing hyperinsulinism.
In reference to the use of GnRH-a, these drugs constitute powerful agents, capable of promoting specific ovarian suppression28. In our material the medical castration was proven by the significant drop in estradiol levels even in the first month after administration. As for hyperandrogenism a statistically significant drop in the levels of free testosterone was observed at the end of the second month of treatment, similarly to the findings of Geffner et al.29. Such fact indicates the ovarian source is responsible for the increased production of androgens in patients with PCOS, hyperandrogenism, hyperinsulinism and acanthosis nigricans.
Therefore, the present study enables us to state that the serum insulin levels in normal women correspond to approximately one third of the glycemic levels. However, in the patients with PCOS, hyperandrogenism, hyperinsulinism and acanthosis nigricans the average insulin levels were always high, nearing the average glucose levels. Furthermore, in these patients, after gonadal blockage there is a drop in androgen levels, which allow us to state that the ovaries represent, most likely, the source of these sexual steroids.
RESUMO
Atividade ovariana antes e após supressão gonadal por GnRH-a em pacientes com síndrome dos ovários policísticos, hiperandrogenismo, hiperinsulinismo e acantose nigricante
OBJETIVO: Investigar a atividade ovariana antes e após a supressão gonadal com análogo de GnRH em pacientes com síndrome dos ovários policísticos (SOP), hiperandrogenismo, hiperinsulinismo e acantose nigricante. DESENHO: Estudo clínico prospectivo. LOCAL: Centro médico-acadêmico nível terciário. PACIENTES: Seis pacientes com sinais clínicos de SOP, hirsutismo e acantose nigricante.
INTERVENÇÕES: Colheita de amostras sanguíneas matinais na fase folicular para determinar os valores séricos de esteróides, curva glicêmica e insulínica, comparando esta última à do grupo controle. Administração por dois meses consecutivos de análogo de GnRH, comparando, nas pacientes do grupo de estudo, os níveis séricos de testosterona livre antes e após a supressão gonadal.
PRINCIPAIS DETERMINAÇÕES: Determinação dos níveis séricos de insulina nas pacientes com SOP, hirsutismo e acantose nigricans e avaliar os níveis de testosterona livre antes e após supressão gonadal.
RESULTADOS: Os níveis de insulina foram significativamente maiores nas pacientes do grupo de estudo, quando comparados a mulheres normais, durante a curva glicêmica. Também encontramos uma significativa queda nos valores de testosterona livre, após dois meses de supressão gonadal com analógo de GnRH, quando confrontados ao tempo inicial.
CONCLUSÕES: Pacientes com SOP, hirsutismo e acantose nigricante apresentam altos níves séricos de insulina, sugerindo uma hiperativação ovariana por este peptídeo, que, entretanto, não é mantida se promovermos o bloqueio gonadotrófico. [Rev Ass Med Brasil 1998; 44(2): 94-8.]
UNITERMOS: SOP. Hiperandrogenismo. Resistência insulínica. Supressão ovariana.
- 1. Mahajan DK Polycystic ovarian disease. Endocrinol Metab Clin North Am 1988; 17: preface.
- 2. Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol 1935; 29: 181-91.
- 3. Stuart CA, Prince MJ, Peters EJ, Meyer III WJ. Hyperinsulinemia and hyperandrogenemia: In vivo androgen response to insulin infusion. Obstet Gynecol 1987; 69:921-5.
- 4. Barbieri RL, Hornstein MD Hyperinsulinemia and ovarian hyperandrogenism: cause and effect. Endocrinol Metab Clin North Am 1988; 17: 685-703.
- 5. Stuart CA, Nagamani M. Insulin infusion acutely augments ovarian androgen production in normal women. Fertil Steril 1990; 54: 788-92.
- 6. Adashi EY. Intraovarian peptides. Stimulators and inhibitors of follicular growth and differentiation. Endocrinol Metab Clin North Am 1992; 21: 1-17.
- 7. Poretsky L, Kalin MF. The gonadotrofic function of insulin. Endocrinol Rev 1987; 8: 132-41.
- 8. Burgen GA, Givens JR, Kitabchi. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab 1980; 50: 113-6.
- 9. Taylor SI, Dons RF, Hernandez, E, Roth J, Gorden P. Insulin resistance associated with androgen excess in women with autoantibodies to the insulin receptor. Ann Intern Med 1982; 97: 851-5.
- 10. Barbieri RL, Ryan KJ. Hyperandrogenism, insulin resistance, and acanthosis nigricans syndrome: A common endocrinopathy with distinct pathophysiologic features. Am J Obstet Gynecol 1983; 147: 90-101.
- 11. Barbieri RL. Effects of insulin on ovarian steroidogenesis. In: Dunaif A, Givens JR, Haseltime FP, Merrian GR (eds). Polycystic ovary syndrome Boston, Blackwell Scient. Publ., 1992; 249-63.
- 12. Bray GA. Pathophisiology of obesity. Am J Clin Nutr 1992; 55: 488S-494S.
- 13. National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979; 28: 1.039-57.
- 14. Adashi EY. Potential utility of gonadotropin-releasing hormone agonists in the management of ovarian hyperandrogenism. Fertil Steril 1990; 53: 765-79.
- 15. Siegel S. Estadistica no parametrica. México, Trillas, 1975.
- 16. Hollander M, Wolfe DA. Nonparametric statistical methods. New York, John Wiley & Sons, 1973.
- 17. Geffner ME, Golde DW. Selective insulin action on skin, ovary, heart in insulin-resistant states. Diabetes Care 1988; 111: 500-5.
- 18. Poretsky L, Kalin MF. The gonadotrofic function of insulin. Endocrinol Rev 1987; 8: 132-41.
- 19. Nestler JE, Clore JN, Blackard WG The central role of obesity (hyperinsulinemia) in the pathogenesis of the polycystic ovary syndrome. Am J Obstet Gynecol 1989; 161: 1.095-7.
- 20. Kolterman OG, Insel J, Saekow M, Olefsky JM. Mechanisms of insulin resistance in human obesity. J Clin Invest 1979; 65: 1.272-84.
- 21. Ciaraldi TP, El-Roeiy A, Madar Z et al. Cellular mechanisms of insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab 1992; 75: 577-83.
- 22. Reaven GM, Miller R. Study of the relantionship between glucose and insulin responses to an oral glucose load in man. Diabetes 1968; 17: 560-9.
- 23. Zimmet P, Whitehouse S, Alford F, Chisholm D. The relantionship of insulin response to a glucose stimulus over a wide range of glucose tolerance. Diabetologia 1978; 15: 23-7.
- 24. Savage PJ, Flock EV, Mako ME et al. C-peptide and insulin secretion in pima indians and caucasian: Constant fractional hepatic extraction over a wide range of insulin concentrations and in obesity. J Clin Endocrinol Metab 1979; 48: 594-8.
- 25. Reaven GM. Role of insulin resistance in human disease. Diabetes 1988; 37: 1.595-607.
- 26. Barbieri RL, Hornstein MD. Hyperinsulinemia and ovarian hyperandrogenism: cause and effect. Endocrinol Metab Clin North Am 1988; 17: 685-703.
- 27. Reaven G.M. Insulin-independent diabetes mellitus: Metabolic characteristics. Metabolism 1980; 29: 445-54.
- 28. Chang RJ, Laufer LR, Meldrum DR et al. Steroid secretion in polycystic ovarian disease after ovarian supression by a long-acting gonadotropin-releasing hormone agonist. J Clin Endocrinol Metab 1983; 56: 897-903.
- 29. Geffner ME, Kaplan SA, Bersch N et al. Persistence of insulin resistance in polycystic ovary disease after inhibition of ovarian steroid secretion. Fertil Steril 1986; 45: 327-33.
Publication Dates
-
Publication in this collection
28 July 2000 -
Date of issue
June 1998