Abstracts
BACKGROUND AND OBJECTIVES: Several studies have focused on Cannabis sativa (Cs) due to its analgesic potential and its ability to alleviate symptoms of disorders of the central nervous system. However, since marijuana, one of its popular names, is the most common illicit drug throughout the world, it breeds prejudice both among lay people and health care professionals. The objective of this study was to determine the current level of knowledge about this drug and the perspectives for its use, to better understand its actions and effects, both in experimental studies and clinical use, in patients with degenerative neurological disorders or in those who do not have the possibility of cure and are being followed by palliative care programs. CONTENTS: The therapeutic use of Cannabis sativa is not recent. The present study presents the historical background and pharmacology of Cs, the development of its therapeutic use through synthetic cannabinoids, the current scientific knowledge, and its organic and psychological consequences, demonstrating the options for its clinical use and future perspectives. CONCLUSIONS: Pure delta-9-tetrahydrocannabinol (delta9-THC) and its analogues have clinical applicability, being beneficial in selected individuals. The development of pure synthetic substances, in an attempt to attenuate undesirable psychoactive effects, indicates that perspectives for its use in the future are favorable. More detailed studies should be undertaken. Ample debates will be necessary to create standards for its formulation and clinical availability, since it is a substance that generates prejudice, due to its illegal commercialization and use, and also because its use has been attributed to mysticism.
DRUGS; DRUGS; PAIN, Chronic
JUSTIFICATIVA E OBJETIVOS: Muitos estudos têm chamado atenção para a Cannabis sativa (Cs), pelo seu potencial analgésico e pela sua capacidade de aliviar sintomas relacionados com doenças do sistema nervoso central. Porém, a maconha, como é popularmente conhecida, por ser a mais popular das drogas ilegais em todo o mundo, gera preconceito tanto entre leigos como entre profissionais que atuam na área da saúde. Objetivou-se pesquisar o nível de conhecimento atual e suas perspectivas de utilização para compreender melhor suas ações e seus efeitos, na pesquisa experimental e na prática médica, em pacientes com doenças degenerativas neurológicas ou naqueles que não tenham possibilidades de cura, atendidos em programas de cuidados paliativos. CONTEÚDO: Seu uso terapêutico não é recente. O presente estudo fornece uma revisão do histórico e farmacologia da Cs, o desenvolvimento de seu uso terapêutico por meio dos canabinóides sintéticos, o conhecimento científico atual, suas conseqüências orgânicas e psíquicas, demonstrando suas opções de uso clínico e perspectivas futuras. CONCLUSÕES: O delta-9-tetrahidrocanabinol (delta9-THC) puro e seus análogos apresentam aplicabilidade clínica, demonstrando benefícios. O desenvolvimento das substâncias sintéticas puras, buscando a atenuação de efeitos psicoativos indesejáveis aponta para perspectivas favoráveis a sua utilização no futuro. Estudos mais detalhados deverão ser realizados. Debates amplos serão necessários para criar normas de formulação e disponibilização com a finalidade médica, por se tratar de uma substância que gera preconceito pela sua comercialização e utilização ilegal e a seu uso ser atribuído misticismo.
DOR, Crônica; DROGAS; DROGAS
JUSTIFICATIVA Y OBJETIVOS: Muchos estudios han querido destacar la Cannabis sativa (Cs), por su potencial analgésico y por su capacidad de aliviar los síntomas relacionados con las enfermedades del sistema nervioso central. Sin embargo, la marihuana, como popularmente se conoce, por ser la más popular de las drogas ilegales en todo el mundo, genera un prejuicio tanto entre los legos como entre los profesionales que actúan en el área de la salud. Se intentó investigar el nivel de conocimiento actual y sus perspectivas de utilización para comprender mejor sus acciones y efectos en la investigación experimental y en la práctica médica en pacientes con enfermedades degenerativas neurológicas o en aquellos que estén fuera de posibilidades de cura, atendidos en programas de cuidados paliativos. CONTENIDO: Su uso terapéutico no es reciente. El presente estudio suministra una revisión del historial y de la y farmacología de la Cs, el desarrollo de su uso terapéutico a través de los canabinoides sintéticos, el conocimiento científico actual, sus consecuencias orgánicas y psíquicas, demostrando sus opciones de uso clínico y perspectivas futuras. CONCLUSIONES: El delta-9-tetrahidrocanabinol (delta9-THC) puro y sus análogos presentan aplicabilidad clínica, mostrando beneficios. El desarrollo de las sustancias sintéticas puras, buscando la atenuación de efectos psicoactivos no deseados nos muestra perspectivas favorables para su utilización en el futuro. Estudios más detallados deberán ser realizados. Debates amplios serán necesarios para crear normas de formulación y disponibilidad con fines médicos, al tratarse de una sustancia que genera prejuicio por su comercialización y utilización ilegal y porque también a su usoÿse le achaca el misticismo.
MISCELLANEOUS
Laura BonfáI; Ronaldo Contreiras de Oliveira Vinagre, TSAII; Núbia Verçosa de FigueiredoIII
IAnestesiologista do Hospital da Lagoa Ministério da Saúde RJ; Especialização em Dor e Cuidados Paliativos no HUCFF/UFRJ; Mestranda em Medicina (setor Anestesiologia Farmacologia)
IICo-Responsável pelo CET/SBA Prof. Bento Gonçalves HUCFF/UFRJ
IIIProfessora-Associada, Mestre e Doutora em Medicina pela FM/UFRJ; Coordenadora da Graduação da Disciplina de Anestesiologia do Departamento de Cirurgia da FM/UFRJ; Coordenadora da Pós-Graduação em Cirurgia Geral Área de Concentração: Anestesia e Analgesia; Responsável pelo Ambulatório de Anestesiologia do Hospital Universitário Clementino Fraga Filho (HUCFF/UFRJ); Certificado de Área de Atuação em Dor SBA/AMB
Correspondence to
SUMMARY
BACKGROUND AND OBJECTIVES: Several studies have focused on Cannabis sativa (Cs) due to its analgesic potential and its ability to alleviate symptoms of disorders of the central nervous system. However, since marijuana, one of its popular names, is the most common illicit drug throughout the world, it breeds prejudice both among lay people and health care professionals. The objective of this study was to determine the current level of knowledge about this drug and the perspectives for its use, to better understand its actions and effects, both in experimental studies and clinical use, in patients with degenerative neurological disorders or in those who do not have the possibility of cure and are being followed by palliative care programs.
CONTENTS: The therapeutic use of Cannabis sativa is not recent. The present study presents the historical background and pharmacology of Cs, the development of its therapeutic use through synthetic cannabinoids, the current scientific knowledge, and its organic and psychological consequences, demonstrating the options for its clinical use and future perspectives.
CONCLUSIONS: Pure delta-9-tetrahydrocannabinol (D9-THC) and its analogues have clinical applicability, being beneficial in selected individuals. The development of pure synthetic substances, in an attempt to attenuate undesirable psychoactive effects, indicates that perspectives for its use in the future are favorable. More detailed studies should be undertaken. Ample debates will be necessary to create standards for its formulation and clinical availability, since it is a substance that generates prejudice, due to its illegal commercialization and use, and also because its use has been attributed to mysticism.
Key Words: DRUGS: Cannabis sativa, cannabinoids; PAIN: Chronic: palliative care.
RESUMEN
JUSTIFICATIVA Y OBJETIVOS: Muchos estudios han querido destacar la Cannabis sativa (Cs), por su potencial analgésico y por su capacidad de aliviar los síntomas relacionados con las enfermedades del sistema nervioso central. Sin embargo, la marihuana, como popularmente se conoce, por ser la más popular de las drogas ilegales en todo el mundo, genera un prejuicio tanto entre los legos como entre los profesionales que actúan en el área de la salud. Se intentó investigar el nivel de conocimiento actual y sus perspectivas de utilización para comprender mejor sus acciones y efectos en la investigación experimental y en la práctica médica en pacientes con enfermedades degenerativas neurológicas o en aquellos que estén fuera de posibilidades de cura, atendidos en programas de cuidados paliativos.
CONTENIDO: Su uso terapéutico no es reciente. El presente estudio suministra una revisión del historial y de la y farmacología de la Cs, el desarrollo de su uso terapéutico a través de los canabinoides sintéticos, el conocimiento científico actual, sus consecuencias orgánicas y psíquicas, demostrando sus opciones de uso clínico y perspectivas futuras.
CONCLUSIONES: El delta-9-tetrahidrocanabinol (D9THC) puro y sus análogos presentan aplicabilidad clínica, mostrando beneficios. El desarrollo de las sustancias sintéticas puras, buscando la atenuación de efectos psicoactivos no deseados nos muestra perspectivas favorables para su utilización en el futuro. Estudios más detallados deberán ser realizados. Debates amplios serán necesarios para crear normas de formulación y disponibilidad con fines médicos, al tratarse de una sustancia que genera prejuicio por su comercialización y utilización ilegal y porque también a su usoÿse le achaca el misticismo.
INTRODUCTION
The history of the medical use of Cannabis sativa (Cs) is long, and it has been known in several parts of the world since Ancient times. There are reports that in China, in 2737 BC, the emperor Shen-Nung prescribed it to treat beriberi, malaria, gout, rheumatism, constipation, and fatigue 1. The knowledge of its use seems to have arisen in the region of the Himalayas and India. It was used in Traditional Indian Medicine to treat conditions similar to those observed nowadays in several medical prescriptions, for analgesia and sedation, as neuromuscular relaxant, anticonvulsant, as an appetite stimulant, anti-pyretic, and in alcohol and opioids detoxification.
Centuries later, in 1799, it was introduced in Europe when Napoleon returned from Egypt with seedlings of Cs, triggering the interest of the scientific community due to its sedative effects and ability to relieve pain. In 1839, the English professor and physician, William O'Shaughnessy, while working in India, reported the use of high doses of CS to treat spastic and convulsive disorders, such as tetanus, hydrophobia, cholera, and delirium tremens, in the first scientific study on the subject 2. In 1844, O'Shaughnessy returned to England, and introduced Cs in western medicine and pharmacopeia, first in the United Kingdom and later in the United States, a country that adopted it as sedative, hypnotic, and anticonvulsant medication, in the form of an extract 3.
In the western world, use of this substance for its psychoactive effects was, initially, circumscribed to determined ethnic groups or minority professional categories. But, from the XIX Century on, commissions of experts started to express their opinions on the commercialization of this substance and to investigate its impact on the health of individuals. Although several studies had concluded that the substance was relatively safe, public administration in several countries limited its use only for medicinal purposes. In the 1960s, its recreative consumption increased considerably in the United States and Europe associated with a change in young people regarding traditional values and practices. Drugs represented a form of defiance and a way to pose against the capitalist system at that time.
The recreative interest on Cs declined when its use by the youth was no longer a novelty. Its medical use also started too decline with the loss of support by the medical community, society in general, and even because the drug represented defiance to society itself, and due to the development of drugs considered to be superior, with more predictable effects, and whose doses could be better controlled. Therefore, Cs would have to be improved considerably by researchers to recover the interest of the medical community. This happened in the 1990s, with new discoveries involving endogenous cannabinoid receptors, which indicated new therapeutic uses of this drug.
PHARMACOLOGY
The research on Cs and its effects started to gain legitimacy with the identification of its chemical structure, the possibility of obtaining isolated components of the drug, and the knowledge on how it could work in the organism. Besides its active component, delta-9-tetrahydrocannabinol (D9-THC), Cs contains more 65 substances called phytocannabinoids (PC).
Mechoulam, in Israel, as well as Claussen and Korte, in Germany, were able to conclude the complete synthesis of those compounds. After the determination of their structure, they started to study their activities, listing their effects on neurons, and identifying cannabinoid receptors (BC) in those cells, showing an affinity between those receptors and the compounds. Since then, several discoveries have been revolutionizing the pharmacology of cannabinoids. Two types of cannabinoid receptors were discovered: CB1 and CB2. CB1 receptors are located in the central nervous system (CNS), in areas that can mediate most of the effects on cognitive function, pain, and short term memory (cerebral cortex and hippocampus), motor control and coordination (basal ganglia and cerebellum), and hypothermia and hyperphagia (hypothalamus) 4. They are also found in the spinal cord, dorsal spinal cord ganglia, intestinal nervous system, adipocytes, endothelial cells, hepatocytes, muscular tissue, and gastrointestinal tract.
CB2 receptors can be found in the peripheral system, and are related with the immunological system, T cells, B cells, spleen, tonsils, and activated microglial cells 5. Delta-9-tetrahydrocannabinol binds to both receptors equally, while the other cannabinoids have greater affinity for one of the receptors. Since there are no PCs in the brain, the existence of those receptors would imply that some endogenous compound binds them. Thus, a molecule very similar to D9-THC, named anandamide (N-arachidonoylethanolamide) was isolated. The word comes from the Sanskrit word for "bliss". In the Veda religion, Cs was called ananda. Anandamide is less potent than THC and it has a shorter duration of action in the brain 3.
Recently, it was discovered that compounds in chocolate are chemically related to anandamides, and can interact with the cannabinoid system. This could explain the irresistible attraction that some people have for chocolate 6. Three endocannabinoids (EC) have been discovered: anandamide (N-arachidonoylethanolamide), 2-arachidonoylglicerol (2-AG), and 2-arachidonoyl ether, synthesized from membrane phospholipids in post-synaptic neurons, and are related to the prostaglandins 3. Endocannabinoids are not stored in vesicles, and are released immediately after post-synaptic stimulation to modulate pre-synaptic neurons, a process called retrograde neurotransmission. They act "on demand", are triggered when necessary, and work to modulate the function of other modulators. Their action is terminated with their reuptake into pre-synaptic terminations, followed by their metabolism 7.
Cannabinoid receptors are inserted in the cellular membrane, bound to G-proteins, the first components in the signal transduction process, and to the enzyme adenylate cyclase (AC). The binding of EC, PC, or synthetic cannabinoids (CSi) is triggered by the increase in intracellular calcium. Different reactions in several intracellular components, including the inhibition of AC, opening of potassium channels, decrease in signal transmission, and closure of calcium channels, which lead to a decrease in the release of neurotransmitters 5, are triggered by this interaction.
The final result of this interaction depends on the type of cell, binding agent, and other molecules that might compete for the binding sites on this receptor. There are several antagonists of CBs that can be classified according to two factors: the potency of the interaction with CB, which determines the effective dose of the drug, and the efficacy, which determines the maximal extension of the signal that those drugs transmit to cells. The potency and efficacy of D9-THC are relatively small when compared with CSis; in general, CSis are more potent and effective than endogenous agonists. The development of synthetic cannabinoid derivatives with high affinity for each type of receptor was possible after those different types of receptors were isolated 3. The first antagonist specific for the endocannabinoid receptor CB1 was described in 1994, and was called SR141716 or Rimonabant 8. This compound is being studied as appetite suppressant and in smoking cessation 9. The specific antagonist of CB2 receptor, SR144528, is being studied as a modulator of the immunologic response 8 (Chart I).
Cannabinoids have been used in the treatment of pain for many centuries. And, despite pre-clinical studies having demonstrated that they block pain response in the models tested, its use has not propagated, for legal and pharmacologic reasons, such as the psychotropic effects, instability of Cs extracts, unpredictable absorption, and water insolubility. However, in the last decade, scientific research has advanced in the attempt to determine the effects of cannabinoids in nociceptive neurotransmission 10. Those studies improved our knowledge on the basic mechanisms and to develop pharmacological alternatives with more specific effects. Researchers have demonstrated an increase in the expression of CB1 in the contralateral thalamus on a model of neuropathic pain, which might explain the greater efficacy of cannabinoids in chronic cases. Activation of CB1 receptors is associated with the anti-hyperalgic and anti-allodynic properties of cannabinoids 11.
Studies have suggested that CB2 receptors, classically related with immunologic response, are also implicated with antinociception. The administration of low doses of cannabinoids and sub-therapeutic doses of morphine, the nociceptive effect is potentiated by the synergistic action of both drugs. The concomitant administration of both drugs improves the efficacy and safety of pain control, especially since cannabinoids do not cause respiratory depression 12.
PHARMACOKINETICS
Cannabinoids in natura can be administered by several ways. However, due to their high liposolubility, they need a vehicle that would allow its aqueous administration.
The pharmacokinetics of D9-THC varies with the way it is administered. Topical ocular or nasal administration would be possible; however, this preparation tends to be irritating. Cutaneous absorption from adhesives, by impregnation with the herb, could be very slow and, therefore, would not have clinical applications. Oral absorption is slow and varies, with the onset of action usually occurring after 30 to 60 minutes, and reaching a peak 2 to 3 hours after ingestion 13. It can be added to cake or cookie dough to be ingested. The presence of foods and the partial destruction by the gastric acid influence the plasma concentration, increasing its bioavailability. It is metabolized in the liver 12. The rectal administration (suppositories) is irregular, but could have a faster absorption, since it reaches the systemic circulation directly. The intravenous administration, in bolus or infusion, would be possible with a formulation to solubilize it, due to its low water solubility 3.
It can be inhaled, smoked as a cigarette or in special pipes, prepared manually using dry leaves, flowers, and small stems of the plant. Usually, a "joint" (marijuana cigarette) contains between 0.5 to 1 g of the herb, which delivers approximately 20 mg of D9-THC. Smoking is the method better known and the best way to administer Cs. Most of the D9-THC is inhaled in the form of tetrahydrocannabinolic acid, which is converted to free and volatile THC by the combustion of the joint, and inhaled with the smoke, heading straight to the lungs and, from there to the circulation and the brain. Individual differences in smoking technique can cause many variations, for example, in the volume aspirated. Each "draft" has a different depth of inhalation into the lungs and duration of retention of the smoke in the alveoli, which also makes plasma levels unpredictable, dependent on the volume and respiratory rate. This can lead to a fast peak of action of high intensity and short duration. The effects are usually immediate, reaching a peak action in 20 to 30 minutes after smoking, and lasting up to 2 to 3 hours 14.
Delta-9-tetrahydrocannabinol can be inhaled without combustion of Cs 15, through a vaporizer (Volcano®) recommended for debilitated patients who use it for medicinal purposes. Due to its high lipid solubility, it crosses rapidly the alveolar membrane, reaching the blood through pulmonary capillaries and from there it is taken immediately to the heart and then pumped straight into the brain; thus, peak action can be as fast as an intravenous injection. The elimination half-life (T½b) of the D9-THC can be greater than 48 hours; this explains why its metabolites are found in plasma and urine even days after its use 16.
A recent study on the effects of smoked Cs used chromatography and mass spectrometry for qualitative and quantitative determinations of D9-THC and its main metabolites (THC-COOH and THC-OH) in plasma and saliva samples. The inhaled dose of 18.2 ± 2.8 mg was considered low, while 36.5 ± 5.6 mg was considered a high dose. Plasma concentrations shortly after smoking were 47.8 ± 35.0 and 79.1 ± 42.5 µg.L-1, respectively, which decreased to less than 1 µg.L-1 over 6 hours. The T½b of D9-THC was 1.4 ± 0.1 h. The T½b of the metabolites was significantly higher; for THC-OH it was 2.0 ± 0.3 h and for THC-COOH it was 3.4 ± 0.9 h. Concentrations in the saliva were much higher shortly after smoking: 900 ± 589 and 1041 ± 652 µg.L-1 (high and low dose, respectively), but its determination is considered controversial, since the presence of THC could be attributed to contamination of the oral cavity during smoking. The T½b of D9-THC in the saliva was 1.5 ± 0.6 h, which was not significantly different than the plasma concentration. Although the rate of elimination of D9-THC in the plasma and saliva were similar, it was not related to the concentration, and that showed a considerable difference. This demonstrates that the oral compartment and the pharmacokinetics of D9-THC are not completely understood 17.
PHARMACODYNAMICS
The main psychoactive effect of Cs is an increase in mental capacity, making the mind aware of aspects normally inaccessible. It is believed that this is secondary to the disablement of filters that block signals related to several functions in the central nervous system, including the senses, emotions, and memory, besides subconscious functions that reach the conscious mind. Smoked Cs is considered a mild psychotic drug. It can cause hallucinations; euphoria; loquacity; unmotivated laughs; decreased fatigue on exertion; change in time perception; increased color, sounds, and texture perception; and an exaggerated increase in appetite, especially for carbohydrates. Besides the excitatory characteristics, it also has depressor activities, causing physical relaxation, tranquility, and a feeling of well-being. High doses of Cs cause cognitive changes (memory and attention), dysphoria, it can lead to anxiety and panic attacks, and a feeling of losing control ("fear of going crazy"), especially in new users.
Cannabinoids has also several physical effects: tachycardia, conjunctival hyperemia, xerostomia, decreased hearing, increased visual acuity, mydriasis, bronchodilation, reduction in pain perception, hypothermia, dizziness, lack of motor coordination, and orthostatic hypotension 18.
Those effects depend on geographical factors and weather conditions that interfere with the quality of the plant, prior experience and sensitivity of the individual, and the environment it is consumed in. Normally, the effects of Cs last 2 to 3 hours, but can be more prolonged due to increased accumulation in the adipose tissue.
THERAPEUTICAL USE
Synthetic Cannabinoids
The use of pure, active cannabinoids with known composition, stability, and dosages was possible due to the advances in chemical and pharmacological research. In the last several years different cannabinoid compounds have been synthesized, an improvement over the natural herb, in which the potency and composition vary.
The first medication obtained directly from the plant Cannabis sativa was synthesized in the British laboratory GW Pharmaceuticals from its active principles, D9-THC and cannabidiol, a cannabinoid devoid the psychotropic effects. It underwent clinical tests and was approved for medical use as an oral spray (Sativex®), which allows the patient to titrate the dose according to his/her needs. Patients with oncologic and neuropathic pain and multiple sclerosis apply the spray a mean of 8 to 12 times a day, which are equivalent to approximately 2.7 mg of D9-THC (22 to 32 mg/day) and 2.5 mg of cannabidiol (20 to 30 mg/day). It is sold in Canada, where its use was approved 19. A synthetic THC, called dronabinol (Marinol®), which, in the oral dose of 7.5 mg, causes a significant reduction in intraocular pressure and, therefore, it is used in the treatment of glaucoma 20. Clinical studies with 204 patients with acquired immunodeficiency syndrome (AIDS) and 469 patients with terminal cancer and anorexia-cachexia syndrome indicated that dronabinol can be used to increase the appetite and maintain the weight of the patients 11. Trials were done to determine the therapeutic efficacy of oral and sublingual nabilone, another synthetic cannabinoid, in patients with pain secondary to multiple sclerosis, brachial plexus damage, sciatic pain, trigeminal neuralgia, orofacial pain, and peripheral neuropathy. Doses of 0.25 to 3 mg/day were effective in 30% of the patients, who reported improvement in sleep quality, anxiety, and muscle spasms; 25% did not tolerate the treatment, and the most frequent undesirable effects were dysphoria and somnolence. The rest of the patients had previous exposure to Cs and expressed their preference for this substance 11. Nabilone (Cesamet®) is approved for sale in Canada as 1-mg capsules 5. It is indicated for the relief of chronic neuropathic pain refractory to conventional analgesics, and also has antiemetic activity in cancer patients; it can be administered 2 to 3 times a day 22.
CANNABIS FOR THE TREATMENT OF ACUTE PAIN
Based on the results of experimental and clinical studies, it is a consensus that Cs and cannabinoids offer benefits to patients who have no possibility of cure 3, such as those with the AIDS, terminal cancer, and neurological disorders, like amyotrophic lateral sclerosis (ALS) 23. Chart II shows examples of the wide spectrum of medical applications. To use cannabinoids as analgesics in the treatment of acute pain, one should consider the limitations of the therapeutic modality. Besides the variety of compounds available and its use in each study, the medical, ideological, political, and economical debate should be settled 11.
There is an increase interest on the use of cannabinoids in acute pain, especially postoperative pain, and some authors have already reported the results of their observations. A study with women undergoing abdominal hysterectomy who, postoperatively, were treated with patient controlled anesthesia (PCA) with morphine in the first 24 hours, followed by capsules of THC (5 mg) or placebo, did not demonstrate significant differences on the need of rescue analgesia or pain evaluation between the two study groups (THC and placebo) in the first six hours. But in this study, a fixed, low dose of THC was administered to all patients as a single dose 24. The efficacy of nabilone (Nabilone®), a synthetic oral cannabinoid, was studied by Beaulieu on a double-blind randomized study comparing the effects of the administration of 3 doses in 24 hours in the postoperative period of large surgeries, using different doses (1 and 2 mg), ketoprofen, and placebo. Patients were maintained on PCA with morphine. Differences in morphine consumptions were not observed among the three groups, but pain levels were significantly higher in patients treated with 2 mg of nabilone. Other significant differences, among the groups, in important adverse reactions, were not observed 25. Contradicting the main hypothesis of the study, higher doses of nabilone in the presence of morphine were associated with higher pain levels in patients undergoing large size surgeries. The critics to this study include the small number of patients and inclusion of patients who underwent different surgeries, orthopedic and gynecological, although they were considered large size surgeries. A study by Holdcroft et al. presented partial results on the analgesic and collateral effects of the oral extract of Cs (Cannador®). The authors used increasing doses (5, 10, and 15 mg of THC) in 65 patients after the discontinuation of patient controlled infusion with morphine. The study was interrupted because the dose of 15 mg caused a serious episode of vasovagal response. The authors conclude that 10 mg would be the optimal dose 26.
The last studies published indicate that cannabinoids are still not effective in the treatment of postoperative pain.
HEALTH RISKS AND DEPENDENCY
It is known that besides the therapeutic activity, several types of cannabinoids also possess psychotropic effects that can limit their use as a medication. Inexperienced patients can develop, predominantly, unpleasant effects (bad trip), with increased anxiety, anguish, fear, tremor, and diaphoresis. The abusive use decreases reflexes, acuity for psychomotor tasks, and muscular tonus leading to ataxia. Reduced attention can cause somnolence. Those factors can increase the risk of several accidents, including car accidents. Some cognitive functions are affected, such as speech fluency, attention, and short-term memory; the prolonged use of those substances can slow learning. Those undesirable effects are important factors that trigger and potentiate schizophrenia in individuals predisposed to psychopathies 35. The chronic intoxication syndrome manifests with mental confusion, hallucinations, and paranoia, but it can also cause depression of the CNS, leading to depression and apathy. It is not clear whether cognitive changes associated with the chronic use of those compounds improve after prolonged abstinence or if the neuropsychological changes can be irreversible; studies on those possibilities are necessary for more consistent conclusions 36.
The data available on the respiratory system are controversial. There are indications of the increased risk for the development of chronic bronchitis or lung cancer in chronic users; however, other studies did not show pre-cancerous histological changes on the bronchial epithelium 37. Toxins that produce irritation of the tracheobronchial tree have been attributed to the combustion of smoked Cs, which would not happen by using the vaporizer 38. Another study indicated the development of chronic obstructive pulmonary disease; but this study is not conclusive, since most chronic smokers of Cs are also chronic cigarette smokers, and those effects can be additive 39.
On the cardiovascular system, only users with a history of angina could evolve with chest pain secondary to the increased myocardial demand and tachycardia. Due to its low toxicity, there are no reports of death secondary to the isolated overdose of Cs 37, but as a consequence of the psychoactive effects, such as accidents caused while under the influence of the drug. The lack of cannabinoid receptors in the brain stem could explain the low lethality of delta-9-THC, since the brain stem controls respiration and other vital functions 13. It is estimated that the lethal dose in humans is approximately 1,000 times the dose necessary to produce the psychoactive effects 40.
On the endocrine system, the chronic use of Cs is related with a reduction in testosterone levels and consequent reduction in male libido and in the number of spermatozoids, reduction of luteinizing hormone and prolactin, change in menstrual period and anovulatory cycles 37. Its use during pregnancy results on the birth of small for gestational age babies 35. Studies have demonstrated that the intrauterine exposure to Cs can cause attention deficit hyperactivity disorder in children and predisposition for drug use as adults because of the deleterious actions on the CNS that have been detected in MRIs 41.
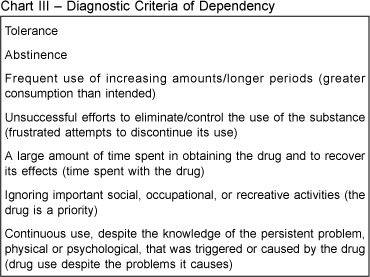
Among all those risks, the development of Dependency Syndrome is more prevalent. It is known that this risk is increased with the cumulative use, but, due to the difficulty in quantifying the dose that reaches the blood stream, doses of THC that generate or are precursors of dependency have not been formally defined. Psychotropic effects responsible for the development of this syndrome are not well known despite several studies on the characteristics and abuse of Cs 18. However, its occurrence is scientifically recognized on the user, who develops clinically significant deterioration over a 12-month period manifested by three (or more) of the items listed in chart III, which was elaborated by the American Association of Psychiatry and published in its manual, Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 42.
FUTURE PERSPECTIVES
The development of new vehicles that allow the solubility of Cs preparations makes the topical ocular administration possible, besides the greater use of the inhalational administration to obtain fast systemic effects without the dangers associated with smoking.
It is likely that new synthetic analogues, with better separation between therapeutic and collateral effects, such as the undesirable psychoactivity, will be developed.
It is important to undertake more studies, through systematic observations, to make its use possible for the treatment of acute pain, including postoperative pain 43.
Throughout history, Cs has always created, and will still create, controversies. Currently, since it is considered an illegal drug, worldwide data does not rule out or support its effective medicinal use, probably out of fear of an increase in the illegal use of this drug. The tension generated among those who defend its prohibition, legalization, or medicinal consumption has not ended and, it is certain that in a few more years we will have those answers. Scientific perspectives indicate that it is a treatment option, providing a more dignifying end of life for some patients.
CONCLUSIONS
It has been demonstrated that pure THC, and its analogues, have significant therapeutic benefits in the relief of nausea and vomiting, and as an appetite stimulant in inappetent patients 3.
Studies undertaken in several countries have also demonstrated its utility in clinical practice due to its analgesic and anti-spasticity effects 3.
The anticonvulsant effect o cannabidiol is sufficiently promising to justify new clinical assays 3.
Use of the herb in natura, by smoking, could be justified on humanitarian grounds by patients without possibility of cure who are accustomed to use it; studies have demonstrated that it is effective. But it is still premature to recommend its use in chronic patients due to the risk of chronic inflammatory disorders or cancer of the airways associated with its use 2.
Its effects in the reduction of intraocular pressure in glaucoma, and the bronchodilation in patients with asthma or severe emphysema have not been shown to be strong enough, long-acting, and reliable to validate its therapeutic use; but some studies are gaining ground on this field 34.
Better understanding on the pharmacokinetics of the prolonged use and mechanisms of action of cannabinoids and their derivatives are necessary to introduce them in the therapeutic armamentarium.
We would like to thank Dr. Peter Spiegel and Dr. Lilian Hennemann for their incentive.
REFERENCES
- 01. Bloomquist ER Marijuana. Beverly Hills, California, Glencoe Press, 1968;19.
- 02. Zuardi AW History of cannabis as a medicine: a review. Rev Bras Psiquiatr, 2006;28:153-157.
- 03. Kalant H Medicinal use of cannabis: history and current status. Pain Res Manag, 2001;6:80-91.
- 04. Pertwee, RG Pharmacology of cannabinoids CB1 and CB2 receptors. Pharmacol Ther, 1997;74:129-180.
- 05. Honório KM, Arroio A, Silva ABF Therapeutical aspects of compounds of the plant Cannabis sativa. Quím Nova, 2006;29: 318-325.
- 06. Iversen L Cannabis and the brain. Brain, 2003;126:1252-1270.
- 07. Hillard CJ, Jarrahian A Cellular accumulation of anandamide: consensus and controversy. Br J Pharmacol, 2003;140:802-808.
- 08. Howlett AC, Breivogel CS, Childers SR et al. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacol, 2004;47:345-358.
- 09. Cox SL Rimonabant hydrochloride: an investigational agent for the management of cardiovascular risk factors. Drugs Today, 2005;41:499-508.
- 10. Walker JM, Huang SM Cannabinoid analgesia. Pharmacol Ther, 2002;95:127-35.
- 11. Duran M, Laporte JR, Capellà D Novedades sobre las potencialidades terapéuticas del Cannabis y el sistema cannabinoide. Med Clin, 2004;122:390-398.
- 12. Siegling A, Hofmann HA, Denzer D Cannabinoid CB1 receptor upregulation in a rat model of chronic neuropathic pain. Eur J Pharmacol, 2001;415:5-7.
- 13. Julien RM A Primer of Drug Action: a Concise, Nontechnical Guide to the Actions, Uses, and Side Effects of Psychoactive Drugs, 8th Ed., New York, Henry Holt, 1997;548.
- 14. Cerro JCL Drogodependencias Cannabis Madri, Panamericana, 1998;191-214.
- 15. MacRae EJBN Redução de Danos para o Uso da Cannabis: Panorama Atual de Drogas e Dependências. 1Ş Ed., São Paulo, Atheneu, 2006;361-370.
- 16. Mercolini L, Musenga A, Comin I Determination of plasma and urine levels of Delta (9)-tetrahydrocannabinol and its main metabolite by liquid chromatography after solid-phase extraction. J Pharm Biomed Anal, 2007;47:156-163.
- 17. Kauert GF, Ramaekers JG, Schneider E Pharmacokinetic properties of D9-Tetrahydrocannabinol in serum and oral fluid. J Anal Toxicol, 2007;31:288-293.
- 18. Ribeiro M, Marques ACPR, Laranjeira R Abuso e dependência da maconha. Rev Assoc Med Bras, 2005;51:247-249.
- 19. Barnes MP Sativex®: Clinical efficacy and tolerability in the treatment of symptoms of multiple sclerosis and neuropathic pain. Exp Opin Pharm, 2006;7:607-615.
- 20. Plange N, Arend KO, Kaup M Dronabinol and retinal hemodynamics in humans. Am J Ophthalmol 2007;143:173-174.
- 21. Beal JE, Olson R, Laubenstein I Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Sympton Manage, 1995;10:89-97.
- 22. Berlach DM, Shir Y, Ware MA Experience with the synthetic cannabinoid nabilone in chronic noncancer pain. Pain Med, 2006;7:25-29.
- 23. Chochinov HM, Breitbart W Handbook of Psychiatry in Palliative Medicine, New York, Oxford Univ Press, 2000;124-125.
- 24. Buggy DJ, Toogood L, Maric S Lack of analgesic efficacy of oral delta-9-tetrahydrocannabinol in postoperative pain. Pain, 2003;106:169-172.
- 25. Beaulieu P Effects of nabilone, a synthetic cannabinoid on postoperative pain. Can J Anaesth, 2006;53:769-775.
- 26. Holdcroft A, Maze M, Doré C A muticenter dose-escalation study of the analgesic and adverse effects of an oral cannabis extract (Cannador) for postoperative pain management. Anesthesiology, 2006;104:1040-1046.
- 27. Russo E, Guy GW A tail of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses, 2006;66:234-246.
- 28. Karst M, Salim K, Burstein S Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: a randomized controlled trial. JAMA, 2003;290:1757-1762.
- 29. Woolridge E, Barton S, Samuel J Cannabis use in HIV for pain and other medical symptoms. J Pain Symptom Manage, 2005; 29:358-367.
- 30. Mackie K Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol, 2006;46:101-122.
- 31. Carter G, Rosen BS Marijuana in the management of amyotrophic lateral sclerosis. Am J Hosp Pall Care, 2001;18:264-269.
- 32. Wade DT, Makela PM, House H Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult Scler, 2006;12:639-647.
- 33. Tomida I, Azuara-Blanco A, House H Effect of sublingual application of cannabinoids on intraocular pressure: a pilot study. J Glaucoma, 2006;15:349-353.
- 34. De Petrocellis L, Melck D, Bisogno T et al. Endocannabinoids and fatty acid amides in cancer, inflammation and related disorders. Chem Phys Lipids, 2000;108:191-209.
- 35. Kalant H, Corrigal WA, Hall W The Health Effects of Cannabis, Toronto, Centre for Addiction and Mental Health Research Foundation,1999;145.
- 36. Jungerman FS, Laranjeira R, Bressan RA Maconha: Qual a amplitude de seus prejuízos? Rev Bras Psiquiatr, 2005;27:5-6.
- 37. Kalant H Adverse effects of cannabis on health: an update of the literature since 1996. Prog Neuropsychopharmacol Biol Psychiatry, 2004;28:849-863.
- 38. Hazekamp A, Ruhaak R, Zuurman L Evaluation of a vaporizing device (Volcano) for the pulmonary administration of tetrahydrocannabinol. J Pharm Sci, 2006;95:1308-1317.
- 39. Taylor DR, Poulton R, Moffitt TE The respiratory effects of cannabis dependence in young adults. Addiction, 2000;95:1669-1677.
- 40. Abel, EL Marihuana, Tabaco, Alcohol y Reproduccion. Madrid, Ed. Diaz de Santos, 1986;8.
- 41. Smith AM, Fried PA, Hogan MJ Effects of prenatal marijuana on response inhibition: an MRI study of young adults. Neurotoxicol Teratol, 2004;26:533-542.
- 42. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, DSM-IV, 4a ed., Washington, American Psychiatric Association, 1994.
- 43. Holdcroft A, Maze M, Doré C A multicenter dose-escalation study of the analgesic and adverse effects of an oral cannabis extract (Cannador) for postoperative pain management. Anesthesiology, 2006;104:1040-1046.
Cannabinoids in chronic pain and palliative care
Publication Dates
-
Publication in this collection
15 Sept 2008 -
Date of issue
June 2008
History
-
Accepted
25 Feb 2008 -
Received
04 Apr 2007