ABSTRACT
The objectives of this study were to monitor the thermal environment of different hatchery locations during the transfer of fertile eggs from the setter and to the hatcher, to measure egg heat loss, and to determine its effects on hatchery results. In total, 1,728 fertile eggs of Cobb broiler breeders were divided into two treatments. In treatment 1 (T1), after 19 days of incubation, eggs were removed from the incubator and transferred to the hatcher in aninsulated box, and in treatment 0 (T0), eggs were transferred with no thermal insulation (T0). The duration of egg transfer was 10 minutes. Eggs were photographed using a thermographic camera at the exit of the setter, arrival at and exit from the candling room, and arrival at the hatcher. Based on the thermographic images, egg heat loss between these locations was calculated. At hatch, total hatchability, hatchability of fertile eggs, and hatchling weight were recorded and compared between T0 and T1. The temperature and relative humidity of the corridor between the setter and the candling room, of the candling room, of the corridor between candling roomand the hatcher were monitored using data loggers. The results indicated that T1 eggs lost 0.15 kJ less heat than T0 eggs during transfer. However, hatchability and hatchling weight were not affected by transfer treatment during the studies period.
Keywords:
Thermographic image; thermal insulation; egg transfer.
INTRODUCTION
During incubation, embryonic development is influenced by the microenvironment around the egg (Swann & Brake, 1990Swann GS, Brake J. Effect of incubation dry-bulb and wet-bulb temperatures on time of hatch and chick weight at hatch. Poultry Science 1990;69(1):887-897.).
The incubation temperature is the most important physical factor affecting hatchability (Decuypere & Michels, 1992Decuypere K, Michels H. Incubation temperature as a management tool: a review. World´s Poultry Science Journal 1992;48(1):27-38.; van Brecht et al. , 2005Van Brecht A, Hens H, Lemaire JL, Aerts JM, Degraeve P, Berckmans, D. Quantification of the heat exchange of chicken eggs. Poultry Science 2005;84(3):353-361.).Wilson (1991Wilson, H.R. Interrelationships of egg size, chick size, posthatching growth and hatchability. World's Poutlry Science Journal 1991;47(2):5-20.) determined that the optimal incubation temperature for chicken eggs is between 37 and 38º C.
Temperature variations in the hatchery locations to which hatching eggs are exposed to may result in different embryonic development rates, affecting hatching window, hatchling quality, and post-hatching performance (Wilson, 1991Wilson, H.R. Interrelationships of egg size, chick size, posthatching growth and hatchability. World's Poutlry Science Journal 1991;47(2):5-20.).
Temperature control of the entire hatchery is essential to prevent egg quality loss and to maintain high hatchability. Although setter and hatcher environmental conditions may be set according to the recommendations, these may be different in other areas of the hatchery through which hatchable eggs are transported, and cause significant production losses.
Most Brazilian hatcheries are concerned only with the thermal environment inside the rooms where the eggs are set and hatch, but not that of the corridors through which the eggs are transferred from one room to another.Therefore, the hypothesis of this study was that, when eggs are transferred on day 19 of incubation from the setter to the candling and vaccination room and then to the hatchers, eggs lose heat to the environment, which may affect total hatchability, hatchability of fertile eggs, and hatchling weight.
The objective of this study was to monitor the temperature and relative humidity in the locations through which eggs are transported when transferred from the setter to the hatcher, to calculate egg heat loss during transfer, and to evaluate the influence of this heat loss on incubation efficiency.
MATERIALS AND METHODS
The experiment was carried out in a commercial hatchery located in Amparo, state of São Paulo, Brazil, between March and April, 2015.
Hatchery facilities are large, with a production capacity of seven million day-old chicks per 30-day production cycle. Figure 1 shows the rooms where the eggs pass from arrival at the hatchery until hatch.
Egg route from reception to chick dispatch. The arrows and the frame in red indicate the locations monitoredduring the experiment.
All hatchery rooms, except for the candling room, are equipped with air-conditioning systems.However, none of the corridors through which eggs are transferred from one room to the other have air conditioning. Therefore, whenever eggs transferred between rooms, they are exposed to inadequate temperature and RH.
The arrows in Figure 1 show the corridors through which eggs are transported between rooms. The arrows and the table represent the locations monitored in the experiment, which are considered the most critical, because embryos are already developing when pass through them and therefore, require adequate temperature and humidity conditions.
In total, 18 setter trays, each with randomly-chosen 96 fertile laid by Cobb broiler breeders reared under similar conditions were used. Three setter trays were evaluated per transfer treatment, with three replicates (trays) each.
The experimental eggs were randomly selected in the egg reception room after cracked, broken, round, and double-yolk eggs were removed.
The objective of the two transfer treatments was to monitor egg heat loss during transfer between the setter and the candling room, in the candling room, and from the candling room and the hatcher.In treatment T0, the experimental eggs followed the typical hatchery flow, that is, they were transferred between rooms in conventional trolleys with no thermal insulation. In treatment T1, the experimental eggs were transferred between rooms in aninsulated box, made of Styrofoam, which provided thermal insulation of the eggs from the external environment, aiming at preventing egg heat loss during transfer.Before T1 egg trays were placed inside the insulated box for transfer, the box remained opened for two minutes inside the setter to allow the heated air of the setter to fill the box, creating a microclimate similar to that of the setter.
All experimental eggs were submitted to identical incubation conditions until day 19 of incubation, at 37.2 °C and 60% relative air humidity (Allcroft, 1964Allcroft WM. Incubation and hatchery practice. 4thed. London: Her Majesty's Stationery Office; 1964.).On day 19, one egg tray per treatment at a time was removed from setter and transferred to the candling room, where infertile eggs were removed, in-ovo vaccination was performed, and eggs were transferred from the setter trays to the hatcher baskets. These procedures were fast, but the room environment was not controlled, exposing the eggs to the risk of experiencing temperature changes.
After the candling room, hatcher baskets with T0 and T1 eggs were transferred to the hatcher, where they remained for two days at 36 ºC and 65% relative humidity.
The time interval of each transfer of T0 and T1 eggs between tray removal from the setter and basket placement in the hatcher was 10 min, during which T0 eggs remained without thermal insulation.
At hatch, hatchlings were transferred to the chick-processing room, where they were weighed and counted to calculate total hatchability (number of chicks/number of incubated eggs) and hatchability of fertile eggs (number of chicks/number of fertile eggs).
Unhatched eggs were opened and submitted to embryo diagnosis. The following causes of hatch failure were determined: infertile eggs; embryo mortality between days 0 to 7, 8 to 18, and 19 to 21 of incubation; pipped and alive; pipped and dead; eggs cracked during incubation; eggs cracked during transfer; contaminated eggs; embryo abnormalities; wing malposition; hemorrhagic embryos; head opposite to the air chamber.
Egg surface temperature was measured using thermographic camera (Testo(r) 875i) for subsequent calculation of egg heat loss. Images of the entire egg tray were recorded.With the aid of TESTO IRSoft(r) software, 16 temperature points of each image were randomly selected (Figure 2).
Thermographic images of each egg tray were recorded four times: immediately before removal from the setter, arrival at the candling room, exit from the candling room, and arrival at the hatcher. Recordings were made at these specific times because these are the most likely moments of heat loss during the transfer from the setter to the hatcher, as the eggs leave a warm room to be processed in a room with no temperature control (candling room).
Actual and thermographic images of an egg tray of treatment T0, third replicate at arrival in the candling room.
Egg heat loss was calculated according to the following equation:
Where: m = average egg mass (kg), c = coefficient of egg specific heat (1.67 kJ/kg), Ti and Tf = initial and final egg temperatures during transfer.
The final and initial temperatures corresponded to:
- Arrival at candling room and removal from the setter (location I);
- Exit from the candling room and arrival at the candling room (location II);
- Arrival at the hatcher and exit from the candling room (location III).
The thermal environment of selected locations (Figure 1) was monitored during the entire experimental period, using data loggers (model Hobo U12-013, Onset(r)).The equipment was set to record the following variables every 30 min: dry bulb temperature (± 0.4 °C) and air relative humidity (± 2.5%).
According to Menezes (2010Menezes AG, Naas IA , Baracho MS . Identification of critical points of thermal environment in broiler production. Brazilian Journal of Poultry Science 2010;12(1):21-29.), temperature and air relative humidity ranges at the evaluated locations should be 35.7-38.2 ºC and 50-65%. These are ranges considered adequate for the setter and the hatcher, and as during transfer, embryos are still developing, the eggs require the same thermal conditions provided in the setter and in the hatcher. The values outside these limits were considered Critical Control Points.
A completely randomized experimental design was applied with two treatments. Heat loss, total hatchability, hatchability of fertile eggs, and hatchlingweight data were submitted to analysis of variance at 95% confidence level, using Minitab 17(r) statistical software.
Embryo diagnosis results were not compared between T0 and T1, and were only used for the determination of the main reasons of hatch failure.
RESULTS AND DISCUSSION
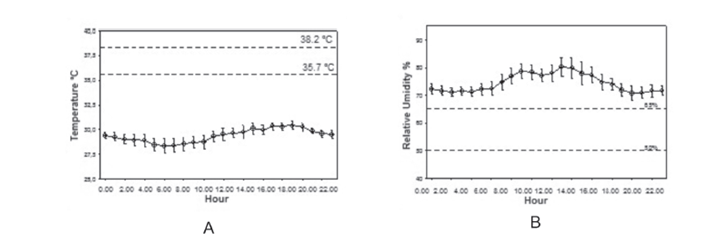
The temperatures measured at the three locations in the hatchery were below the recommendations (35.7 ºC to 38.2 ºC), as shown in Figures 3 (A), 4 (A), and 5 (A). This reduction in temperature in the main rooms of the hatchery where the eggs have already been heated and embryos are developing can be harmful to the embryo. According Decuypere & Michels (1992Decuypere K, Michels H. Incubation temperature as a management tool: a review. World´s Poultry Science Journal 1992;48(1):27-38.), the embryo begins to develop at 24 ºC, and incubation temperatures higher of 39 °C or below 30 °C can be lethal to the embryos.
Variation of temperature (A) and relative humidity (B) in the corridor between the setter and the candling room.
Variation of temperature (A) and relative humidity (B) in the corridor between the candling room and the hatcher.
The relative humidity (RH) recorded at the evaluated environments is shown in Figures 3 (B), 4 (B), and 5 (B) that show that RH was only adequate (50-65%) in corridor between the setter and the candling room. In other locations, relative humidity was higher than that required for good embryonic development. According to Decuypere et al. (2003Decuypere E, Malheiros RD, Moraes VMB, Bruggeman V. Fisiologia do embrião. In: Macari M, Gonzales E, Patrício IS, Naas IA , Martins PC. Manejo da incubação. Campinas: FACTA; 2003. p. 65-94.), low relative humidity levels result in excessive embryo water loss, causing hatch delay and mortality of embryos, which despite being fully developed, are not able to hatch. On the other hand, if the relative humidity is too high, the embryos tend to hatch prematurely, before being fully developed. Decuypere et al. (2003)Decuypere E, Malheiros RD, Moraes VMB, Bruggeman V. Fisiologia do embrião. In: Macari M, Gonzales E, Patrício IS, Naas IA , Martins PC. Manejo da incubação. Campinas: FACTA; 2003. p. 65-94. also point out that although hatchability is not severely affected by much greater RH variations compared with temperature, RH should be maintained within a certain range to ensure successful results.
In all evaluated locations, both the recorded temperature and RH values were characterized as critical control points. When outside the recommended range, corrective actions need to be applied.
The temperature in the evaluated locations was lower than that recommended because no means to ensure adequate environmental conditions during the transfer of eggs from one room to another, and therefore, due to the lack of temperature control, eggs lose heat to the environment.
According to Baracho et al. (2010Baracho MS, Naas IA, Gigli, ACS. Impacto das variáveis ambientais em incubatório de estágio múltiplo de frangos de corte. Engenharia Agrícola 2010;30(4):563-577.), incubation temperature the most important factor affecting embryo development. Relative humidity should be maintained within the recommended range, as deviations affect hatchling quality (Molenaar et al., 2010Molenaar R, Reijrink IAM, Meijerhof R, Van Der Brand H. Meeting embryonic requirements of broilers throughout incubation: a review. Brazilian Journal of Poultry Science 2010;12(3):137-148.). When the RH is above the optimal range during incubation, hatchling weight increases because excessive water is incorporated in the embryonic tissues, resulting in worse performance during the starter rearing phase (Bruzual et al., 2000Bruzual JJ, Peak SD, Brake J, Peebles ED. Effects of relative humidity during incubation on hatchability and body weight of broiler chicks from young broiler breeders. Poultry Science 2000;79(6):827-830. ).
The route between the setter and the hatcher poses a risk due to egg heat loss. Accumulated egg heat loss was observed both in treatments T0 (without thermal insulation) and T1 (with thermal insulation) between removal from the setter and arrival at the candling room (location I), between arrival to and exit from the candling room (location II) and between the exit from the candling room and arrival at the hatcher (location III). This heat loss is shown in Figure 6.
Cumulative heat loss according to treatment, where I corresponds to the exit from the setter to arrival in the candling room; II from the arrival at to the exit from the candling room; III from exit from the candling room to arrival at the hatcher.
Figure 6 shows that the heat loss of T0 eggs between removal from the setter and arrival at the candling room (location I) was about three times higher than that of T1 eggs. During the candling procedure (location II), heat loss, characterized by the slope of the line connecting the two locations evaluated, was similar between treatments. During the transfer from the candling room to the hatcher (location III), T1 eggs lost less heat T0 eggs. These results demonstrate the thermal insulation efficiency of the insulated box used to transport T1 egg trays, which lost less heat than T0 eggs. During candling, heat loss was similar between treatments, which was expected since the eggs were being handled under the same environmental temperature.
The results of the analysis of variance showed that T0 eggs lost more heat than T1 eggs (p<0.01). T1 eggs lost 0.15 kJ during the entire evaluated transfer route.Egg heat loss is dependent on the length of time of egg transfer between the rooms. In this study, the total time spent during the transfer from the setter to the hatcher was 10 minutes. The time usually spent by the company during transfer is longer, which means that, under practical conditions, the eggs lose significantly more heat than that calculated in this experiment (0.15 kJ). According to French (1997French NA. Modeling incubation temperature: the effects of incubator design, embryonic development, and egg size. Poultry Science 1997;76(1):124-133.), broiler embryos are more sensitive to low than high temperatures, and their effect depend both on the degree of deviation and on the length of exposure.
The embryo starts to produce metabolic heat production by the fourth day of incubation. By day 9, embryo temperature is higher than that of the setter.From this day on, the heat excess produced by the embryo is transferred from the eggshell surface to the environment (Lourens, 2007Lourens A, Brand HVD, Heetkamp MJW, Meijerhof R, Kemp B. Effects of eggshell temperature and oxygen concentration on embryo growth and metabolism during incubation. Poultry Science 2007;86(10):2194-2199.), and therefore, embryo heat loss is a natural process (Piaia, 2005Piaia JCZ. Aplicação da inteligência artificial no monitoramento do processo de incubação [dissertation]. Florianópolis (SC): Universidade Federal de Santa Catarina; 2005.). However, adequate temperature and RH should be provided to prevent excessive egg heat loss and consequent damage to the embryo. If during this incubation period, when eggs naturally lose heat, they are exposed to or remain at low temperatures, egg heat loss may cause hatchery production losses. According to Nichelmann & Tzschentke (1999Nichelmann M, Tzschentke B. Thermoregulatory heat production in precocial avian embryos. Ornis Fennica 1999;76(1):177-187.), broilers that experience cold during the prenatal period may have their thermoregulatory set point reduced after hatch, which may negatively affect their live performance.
Although total eggs heat loss was different between treatments, no effect of treatments on total hatchability, hatchability of fertile eggs, or hatchling weight was detected (Table 1).
These results may be explained by the fact that brief episodes of egg cooling during incubation do not affect hatchability, hatchling body weight, or embryonic mortality, as mentioned by Lancaster and Jones (1988Lancaster FM, Jones DR. Cooling of broiler hatching eggs during incubation. British Poultry Science 1988;29(3):597-604.). However, longer periods of exposure of eggs to temperatures outside the adequate range may result in hatchery losses. According to Muraroli & Mendes (2003Muraroli A, Mendes AA. Manejo da incubação , transferência e nascimento do pinto. In: Macari M , Gonzales E . Manejo da incubação . Campinas: FACTA ; 2003. p.180-199.), hatching may be delayed or anticipated when the temperature is outside the acceptable range. Low temperatures delay hatch and increase the incidence of hatchlings with distended abdomen and unhealed navels, and pipped and not hatched embryos (Gustin, 2003Gustin PC. Biossegurança no incubatório. In: Macari M , Gonzales E , Patrício IS , Naas IA , Martins PC . Manejo da Incubação. Campinas: FACTA ; 2003. p.297-352. ). On the other hand, high temperatures anticipate hatch, increasing the number of culls due to dehydration or unhealed navels, dead chicks in the hatcher baskets, and high mortality between 19 and 21 days of incubation.
Baracho et al. (2010Baracho MS, Naas IA, Gigli, ACS. Impacto das variáveis ambientais em incubatório de estágio múltiplo de frangos de corte. Engenharia Agrícola 2010;30(4):563-577.), evaluating the results of two broiler breeder strains (Cobb and Aviagen) in a commercial hatchery, observed that low incubation temperature and high RH reduced egg hatchability in both strains and increased the incidence of embryo abnormalities, embryo malposition, and low-quality chicks. Low temperature increased the incidence of pipped eggs with live and dead Aviagen chicks, where as embryo mortality Cobb eggs between 0-7, 15-18, and 19-21 days of incubation increased.
In order to determine the causes of hatch failure in both treatments, unhatched eggs were submitted to embryo diagnosis.The results are shown in Table 2.
Table 2 shows that the incidences of all causes of hatch failure were very similar between T0 and T1. Infertility of the incubated eggs and early embryo mortality (0-7 days of incubation) were the main causes of embryo mortality. These results are consistent with the findings of Gigli (2007Gigli ACS. Monitoramento do ambiente em incubatório visando melhorias na produção [dissertação]. Campinas (SP): Faculdade deEngenharia Agrícola , Universidade Estadual de Campinas; 2007.), who reported that this early mortality was due egg contamination by fungi. Mauldin et al. (2007) associated this early embryo mortality to a circulatory system deficiency of the embryo, which often is not able to supply oxygen to the embryo tissues.Other causes of early embryo mortality are inadequate egg acclimation in the egg preheating room (Wilson, 1991Wilson, H.R. Interrelationships of egg size, chick size, posthatching growth and hatchability. World's Poutlry Science Journal 1991;47(2):5-20.; Schmidt et al., 2002Schmidt GS, Figueiredo EAP, Avila V.S. Incubação: estocagem dos ovos férteis. Concórdia; Embrapa; 2002.) and incubation in multi-stage setters.This type of setter does not allow temperature regulation as a function of embryo development stage (Gonzales, 2003Gonzales E , Cesário MD. Desenvolvimento embrionário. In: Macari M , Gonzales E , Patrício IS , Naas IA , Martins PC . Manejo da incubação . Campinas: FACTA ; 2003. p. 51-54.), and embryos at different development stages require different temperature and humidity levels. Therefore, eggs may be overheated immediately after setting, resulting in embryo death.
Embryo mortality due to wing malposition at hatch was also frequent in both treatments. According to Gustin (2003Gustin PC. Biossegurança no incubatório. In: Macari M , Gonzales E , Patrício IS , Naas IA , Martins PC . Manejo da Incubação. Campinas: FACTA ; 2003. p.297-352. ), this result may be associated with high incubation temperature, while Baracho et al. (2010Baracho MS, Naas IA, Gigli, ACS. Impacto das variáveis ambientais em incubatório de estágio múltiplo de frangos de corte. Engenharia Agrícola 2010;30(4):563-577.) relate it with the presence of fungi.
The incidence of pipped eggs with live or dead chicks was correlated with air velocity in the setter by Gigli (2007Gigli ACS. Monitoramento do ambiente em incubatório visando melhorias na produção [dissertação]. Campinas (SP): Faculdade deEngenharia Agrícola , Universidade Estadual de Campinas; 2007.), whoobserved that increasing air velocity in the setter increases the incidence of pipped eggs with live or dead embryos, whereas increasing carbon dioxide concentration is strongly associated with a reduction in the number of pipped eggs with live embryos.
Under the conditions of the present experiment, treatment T1 ensured the thermal insulation of the eggs during transfer, as demonstrated by the lower heat loss recorded compared with T0. Therefore, egg thermal insulation may allow egg heat loss to remain within an acceptable range that does not cause hatchability and hatchling weight losses, particularly during transfer times longer than that used in the present experiment. In addition, efficient means to maintain egg temperature in the candling room should be evaluated, because the greatest egg heat loss was recorded in this room due to the long exposure of the eggs to inadequate environmental temperature during handling.
CONCLUSIONS
The corridor between the setter and the candling room, the candling room, and the corridor between this room and the hatcher are considered as critical control points for egg heat loss during transfer because both their temperature and relative humidity remained outside the recommended range during the entire experimental period. Egg thermal insulation during a 10-min transfer time reduced egg heat loss; however, it did not affect total hatchability, hatchability of fertile eggs, or hatchling weight.
ACKNOWLEDGEMENTS
The authors thank Granja São José for allowing us to carry out this study in its facilities, its employee, Mr. Vitor, for helping to collect the data, and CAPES for M.Sc. grant to the first author.
REFERENCES
- Allcroft WM. Incubation and hatchery practice. 4thed. London: Her Majesty's Stationery Office; 1964.
- Baracho MS, Naas IA, Gigli, ACS. Impacto das variáveis ambientais em incubatório de estágio múltiplo de frangos de corte. Engenharia Agrícola 2010;30(4):563-577.
- Bruzual JJ, Peak SD, Brake J, Peebles ED. Effects of relative humidity during incubation on hatchability and body weight of broiler chicks from young broiler breeders. Poultry Science 2000;79(6):827-830.
- Decuypere E, Malheiros RD, Moraes VMB, Bruggeman V. Fisiologia do embrião. In: Macari M, Gonzales E, Patrício IS, Naas IA , Martins PC. Manejo da incubação. Campinas: FACTA; 2003. p. 65-94.
- Decuypere K, Michels H. Incubation temperature as a management tool: a review. World´s Poultry Science Journal 1992;48(1):27-38.
- French NA. Modeling incubation temperature: the effects of incubator design, embryonic development, and egg size. Poultry Science 1997;76(1):124-133.
- Gigli ACS. Monitoramento do ambiente em incubatório visando melhorias na produção [dissertação]. Campinas (SP): Faculdade deEngenharia Agrícola , Universidade Estadual de Campinas; 2007.
- Gonzales E , Cesário MD. Desenvolvimento embrionário. In: Macari M , Gonzales E , Patrício IS , Naas IA , Martins PC . Manejo da incubação . Campinas: FACTA ; 2003. p. 51-54.
- Gustin PC. Biossegurança no incubatório. In: Macari M , Gonzales E , Patrício IS , Naas IA , Martins PC . Manejo da Incubação. Campinas: FACTA ; 2003. p.297-352.
- Lancaster FM, Jones DR. Cooling of broiler hatching eggs during incubation. British Poultry Science 1988;29(3):597-604.
- Lourens A, Brand HVD, Heetkamp MJW, Meijerhof R, Kemp B. Effects of eggshell temperature and oxygen concentration on embryo growth and metabolism during incubation. Poultry Science 2007;86(10):2194-2199.
- Menezes AG, Naas IA , Baracho MS . Identification of critical points of thermal environment in broiler production. Brazilian Journal of Poultry Science 2010;12(1):21-29.
- Molenaar R, Reijrink IAM, Meijerhof R, Van Der Brand H. Meeting embryonic requirements of broilers throughout incubation: a review. Brazilian Journal of Poultry Science 2010;12(3):137-148.
- Muraroli A, Mendes AA. Manejo da incubação , transferência e nascimento do pinto. In: Macari M , Gonzales E . Manejo da incubação . Campinas: FACTA ; 2003. p.180-199.
- Nichelmann M, Tzschentke B. Thermoregulatory heat production in precocial avian embryos. Ornis Fennica 1999;76(1):177-187.
- Piaia JCZ. Aplicação da inteligência artificial no monitoramento do processo de incubação [dissertation]. Florianópolis (SC): Universidade Federal de Santa Catarina; 2005.
- Schmidt GS, Figueiredo EAP, Avila V.S. Incubação: estocagem dos ovos férteis. Concórdia; Embrapa; 2002.
- Swann GS, Brake J. Effect of incubation dry-bulb and wet-bulb temperatures on time of hatch and chick weight at hatch. Poultry Science 1990;69(1):887-897.
- Van Brecht A, Hens H, Lemaire JL, Aerts JM, Degraeve P, Berckmans, D. Quantification of the heat exchange of chicken eggs. Poultry Science 2005;84(3):353-361.
- Wilson, H.R. Interrelationships of egg size, chick size, posthatching growth and hatchability. World's Poutlry Science Journal 1991;47(2):5-20.
Publication Dates
-
Publication in this collection
Oct-Dec 2016
History
-
Received
July 2016 -
Accepted
Aug 2016