Abstract
Dung beetle assemblages (Coleoptera, Scarabaeinae) in Atlantic forest fragments in southern Brazil. The beetles of the subfamily Scarabaeinae are important organisms that participate in the cycle of decomposition, especially in tropical ecosystems. Most species feed on feces (dung) or carcasses (carrion) and are associated with animals that produce their food resources. Dung beetles are divided into three functional groups: rollers, tunnelers and dwellers. This present work aims to study the diversity of dung beetle communities inhabiting fragments of the Atlantic Forest, with the purpose of describing the ecology of the species in southern Brazil. This study was conducted in the region of Campos Novos, in Santa Catarina, where twenty sites of Atlantic forest fragments were sampled. Samplings of dung beetles were conducted using 200 pitfall traps, of which 100 were baited with human feces and another 100 with carrion. Size and environmental complexity were also measured for each forest fragment. A total of 1,502 dung beetles, belonging to six tribes, 12 genera and 33 species, were collected. Results of the Levin's index of niche breadth indicated that 11 species were categorized as being coprophagous, ten as generalists, and two as necrophagous. Most species are tunnelers (19), nine of rollers and four of dwellers. The great diversity of Scarabaeinae in the region of Campos Novos, including several rare species, adds important data to the Scarabaeinae fauna in the central-western region of Santa Catarina. It may also help choosing priority areas for conservation in the region, where human impact, with large areas of monoculture, increasingly threatens the fragments of Mixed Ombrophilous Forest.
Cycle of decomposition; diversity; ecology; feeding guild; Insecta
BIOLOGY, ECOLOGY AND DIVERSITY
Dung beetle assemblages (Coleoptera, Scarabaeinae) in Atlantic forest fragments in southern Brazil
Renata C. CamposI,II; Malva I. Medina HernándezI,III
IDepartamento de Ecologia e Zoologia, Centro de Ciências Biológicas, Universidade Federal de Santa Catarina, 88040-900 Florianópolis-SC
ABSTRACT
Dung beetle assemblages (Coleoptera, Scarabaeinae) in Atlantic forest fragments in southern Brazil. The beetles of the subfamily Scarabaeinae are important organisms that participate in the cycle of decomposition, especially in tropical ecosystems. Most species feed on feces (dung) or carcasses (carrion) and are associated with animals that produce their food resources. Dung beetles are divided into three functional groups: rollers, tunnelers and dwellers. This present work aims to study the diversity of dung beetle communities inhabiting fragments of the Atlantic Forest, with the purpose of describing the ecology of the species in southern Brazil. This study was conducted in the region of Campos Novos, in Santa Catarina, where twenty sites of Atlantic forest fragments were sampled. Samplings of dung beetles were conducted using 200 pitfall traps, of which 100 were baited with human feces and another 100 with carrion. Size and environmental complexity were also measured for each forest fragment. A total of 1,502 dung beetles, belonging to six tribes, 12 genera and 33 species, were collected. Results of the Levin's index of niche breadth indicated that 11 species were categorized as being coprophagous, ten as generalists, and two as necrophagous. Most species are tunnelers (19), nine of rollers and four of dwellers. The great diversity of Scarabaeinae in the region of Campos Novos, including several rare species, adds important data to the Scarabaeinae fauna in the central-western region of Santa Catarina. It may also help choosing priority areas for conservation in the region, where human impact, with large areas of monoculture, increasingly threatens the fragments of Mixed Ombrophilous Forest.
Keywords: Cycle of decomposition; diversity; ecology; feeding guild; Insecta.
The subfamily Scarabaeinae (Coleoptera, Scarabaeidae) comprises about 6,000 species (ScarabNet 2011) of beetles extremely important in the functioning of tropical ecosystems, as they actively participate in the cycling of nutrients using decaying organic matter as food for both larvae and adults (Halffter & Matthews 1966; Halffter & Edmonds 1982; Hanski & Cambefort 1991 and Simmons & Ridsdill-Smith 2011). Most species feed on feces (coprophagous) or carcasses (scavengers) and are thus intrinsically linked to animals that produce their food resources (Halffter & Matthews 1966; Halffter & Edmonds 1982; Gill 1991; Hanski 1991; Estrada et al. 1993; Morelli & González-Vainer 1997; Estrada et al. 1999; Nichols et al. 2009; Filgueiras et al. 2009). The main food resources used by Scarabaeinae beetles are droppings of large mammals (Halffter & Matthews 1966; Halffter & Edmonds 1982; Hanski & Cambefort 1991; Davis et al. 2002; Simmons & Ridsdill-Smith 2011). In Neotropical forests the presence of large mammals is reduced and necrophagy is more relevant when compared to open areas where there is almost complete absence of necrophagous species (Halffter & Matthews 1966).
Dung beetles are detritivores and promote the removal of soil and incorporation of organic matter in nutrient cycling, helping to clean the environment and to regulate the physical and chemical properties of soil (Halffter & Edmonds 1982; Hanski & Cambefort 1991; Slade et.al 2007; Nichols et al. 2008; Simmons & Ridsdill-Smith 2011). Furthermore, the building of tunnels by some of these beetles allows aeration and hydration of the soil, as well as the incorporation of nutrients present in feces, animal carcasses and fruits that are buried in these spaces (Halffter & Matthews 1966; Halffter & Edmonds 1982; Hanski & Cambefort 1991; Slade et.al 2007; Nichols et al. 2008).
The nesting behavior is closely related to the use of food resources. According to how the resource is used in breeding, dung beetles are divided into three functional groups: the rollers or telecoprids (those that roll balls of food on the surface of soil to some distance from the source of resource, where they bury them); tunnelers or paracoprids (those that carry food resource into the soil, making tunnels on the side or below the resource), and dwellers or endocoprids (which do not reallocate food, using it directly in the source) (Halffter & Mathews 1966; Halffter & Edmonds 1982; Hanski & Cambefort 1991). Tunnelers and rollers may further be divided into several nesting standard types, according to the complexity of their behavior (Doube 1991; Halffter & Matthews 1966; Hanski & Cambefort 1991).
Some species of Scarabaeinae beetles have highly specific habitat preferences (Halffter 1991), many of them being unable to occupy areas with open vegetation (Klein 1989; Spector & Ayzama 2003; Almeida & Louzada 2009). Such species are strongly influenced by habitat loss and fragmentation, which may restrict their distribution or even cause their local extinction (Davis & Philips 2005; Hernández & Vaz-de-Mello 2009).
The structure of the environment is important in determining dung beetle community composition (Estrada et al. 1998; Halffter & Arellano 2002). Davis et al. (2001), when working with dung beetles in Borneo, observed that the distribution of species across different environmental characteristics may show discrete associations typical to particular biotypes within the landscape. In the Amazon Forest, Gardner et al. (2008) showed that the richness, abundance, and total biomass of dung beetles are strongly affected in environments of secondary forests and in eucalyptus plantations. In addition, changes in habitat complexity modify not only the communities of insects, but the whole fauna associated with forests, reducing the richness of some taxonomic groups and increasing others (Barlow et al. 2007).
In the Atlantic Forest of southern and southeastern Brazil, several studies on dung beetles ecology have been carried out recently (e.g. Louzada & Lopes 1997; Medri & Lopes 2001; Hernández & Vaz-de-Mello 2009; Hernández et al. 2011; Lopes et al. 2011; Silva et al., 2011, 2012). Although the state of Santa Catarina has an historical record of 94 species (Vaz-de-Mello 2000), there is only one recent published work in the state on scarabaeid beetles associated with cattle dung, in which only four species were found in Jaragua do Sul (Flechtmann & Rodrigues 1995). Thus, this paper aims to study the diversity of copro-necrophagous beetles that inhabit fragments of Mixed Ombrophilous Forest in the west-central region of the state of Santa Catarina, with the intent to increase knowledge on the ecology of such species in southern Brazil.
MATERIAL AND METHODS
The study was conducted in the municipality of Campos Novos, Santa Catarina (27º23'S, 51º12'W), where there are small fragments of Atlantic Forest in the midst of large crop fields of soybean, maize, and wheat. Sampling sites were located at a mean altitude of 945 m, with mild mesothermal climate, according to the climatic classification of Köppen (Pandolfo et al. 2002) and Mixed Ombrophilous Forest formation (Leite & Klein 1990).
Twenty sampling areas were established, which corresponded to twenty Atlantic Forest fragments in the midst of maize fields. In such fragments various mammalian species of native fauna can be found, and in some of them, there is the presence of cattle. These fragments were spread over an area of approximately 400 km2. Most sampling was carried out from February 7th to 20th, 2011, during the summer. Each area was sampled only once during the period.
For capturing the beetles, pitfall traps were used, because they are the most common method for sampling active invertebrates on soil surface (Southwood 1994). Traps were made with plastic pots with 30 cm in circumference and 20 cm height, corresponding to a volume of 1.5 liters. Such traps were buried in the ground to their top edge and protected by a plastic cap supported by small wooden sticks. Within the traps, a layer of water (200 ml) with detergent was added. Human feces were used as bait (10 g) as well as bits of pork in decomposition (10 g), to attract coprophagous and necrophagous species, respectively, with the bait hanging from the lid of the pot in a small bag of thin cloth.
The sampling protocol for each fragment consisted of five sampling points, 10 m apart from each other, and each point received two traps, one baited with human feces and other with carrion, both five meters away from each other, totaling 10 traps per fragment. After 48 hours of exposure of the traps, the captured insects were fixed in 70% alcohol and taken to the Laboratory of Terrestrial Animal Ecology (Laboratório de Ecologia Terrestre Animal LECOTA/ECZ/UFSC) where they were weighed (dry weight) and identified to genus level using Vaz-de-Mello et al. (2011). Species identification was confirmed by Fernando Zagury Vaz-de-Mello. Individuals were dried, at 40º C, for at least 72 hours, the weighting being conducted in an analytical balance QUIMIS MOD Q-500L210C. The collected material is deposited at the Entomological Collection of the Center for Biological Sciences, Universidade Federal de Santa Catarina (UFSC) and at the Entomological Collection of the Universidade Federal do Mato Grosso.
Species accumulation curve (Mao Tau) was built to evaluate sample sufficiency and calculations for the estimators Jackknife 1 and Chao 1 were carried out to estimate the richness in the region. Both analyzes were made using EstimateS v.7.5.2 (Colwell 2005).
In order to classify the species according to their ecological characteristics,they were classified according to body weight: those weighing over 100 mg were rated as large (L), those weighing 10100 mg as medium (M), and those with less than 10 mg, as small (S). Moreover, the species were classified into functional groups according to the literature (Cambefort & Hanski 1991; Doube 1991; Gill 1991). Species feeding niche breadth was calculated using Levin's standardized index (Ba), in the Ecological Methodology software (Krebs 1999) which is calculated as follows: B = 1/Σ P2j, where: Pj is the proportion of individuals that use the type j resource. For greater confidence in this analysis, only species with abundance greater than 10 individuals were considered. After the calculation, all measurements were standardized in a scale 01 using the expression: Ba = (B1)/(n1), where Ba refers to the standardized index value of Levins, B is the index without standardization and n is the possible number of resources. The species that presented Levins index values up to 0.2 were treated as specialists (coprophagous or necrophagous) and those with values above 0.2 as generalists.
To assess the environmental complexity of the vegetation in each sampled fragment, the adapted method of quadrant-section was used (Brower et al. 1997) to evaluated the variables: tree height, tree basal area, shrub height, shrub basal area, minimal distance to tree and to shrub, height of leaf litter, exposed soil, canopy, leaf litter and green area cover. Measurements were conducted in collecting points two and four, in the traps of feces and carrion. By using a cross as reference, four quadrants (northeast, southeast, southwest and northwest) were marked, where measurements were made of vegetation and environment. In each quadrant, for each tree (diameter at breast height > 5 cm) and shrub (DBH < 5 cm and height > 1 m) that were closest, distances to the center of the cross, height, crown diameter and trunk diameter were all measured. This last measure was taken at breast height (DBH = 1.3 m) for the trees and ankle height (DAH = 0.1 m) for shrubs.
Moreover, in each quadrant, in a square of 1 x 1 m marked on the ground with PVC pipe, the height of leaf litter was measured, and through visual estimate, percentages of leaf litter layer, green and exposed soil area (no vegetation or leaf litter) were measured using the following classes: 05%, 625%, 2650%, 5175%, 7695% and 96100%. Using these same classes, the percentage of canopy cover in the four directions was visually estimated, with the aid of a hollow square area of 10 x 10 cm, placed at a distance of 40 cm from the eye of the observer, at an inclination of 20º in relation to the zenith (Ramos 2000).
To test the hypothesis that the spatial distribution of species is related to environmental variables, a Canonical Correspondence Analysis (CCA) was carried out in CANOCO version 4.5 (Ter Braak & Smilauer 2002). In this analysis, the abundance data of each species were transformed (square-root transformation) to be homogenized and was selected the option downweighting of rare species, to give less weight to species with lower abundance. The relation between the matrix species and the values of the vegetation/environment variables was tested through statistical Monte-Carlo (1,000 permutations under the reduced model), under the null hypothesis that there is independence between the response matrix (species) and the matrix of predictor variables. After the preliminary analysis, the auto-correlated variables were excluded. Only those species that had abundance greater than 10 individuals were included in the analyses.
RESULTS
A total of 1,502 Scarabaeinae beetles were collected, belonging to six tribes, 12 genera and 33 species (Table I). Species accumulation curve of dung beetles indicates sample sufficiency in the study (Fig. 1), since the number of species observed was at least 87% of the estimated values of species richness by estimators Jackknife 1 (37.7 species) and Chao 1 (35.3 species).
The most abundant species in the region were Canthon latipes (n = 212, 14.1%), Onthophagus tristis (n = 204, 13.6%), Uroxys sp. (n = 204, 13.6%) and Eurysternus francinae (n = 169, 11.3%), which together represent 52.6% of total captured individuals (Fig. 2). Species with only one collected individual (singletons) were Dichotomius luctuosus, Dichotomius riehli, Malagoniella virens, Eurysternus calligrammus and Eurysternus caribaeus. Species with only two captured individuals (doubletons) were Canthidium aff. breve and Dichotomius fissus (Fig. 2).
The species showed great diversity in size, reflecting the morphological diversity found in this subfamily. Thus, among the ten largest species we may highlight D. fissus, with a mean of 437 mg of dry weight (with about 2.5 cm in length), D. brasiliense with 362 mg and C. saphirinus with 361 mg. There were 15 species of medium size and seven small species. The smallest one was C. aff. breve with a mean weight of 5 mg (0.3 cm in length). Other small species were O. catharinensis, O. aff. hirculus, both weighting 6 mg, and Uroxys sp. and C. cavifrons both with 7 mg (Table I).
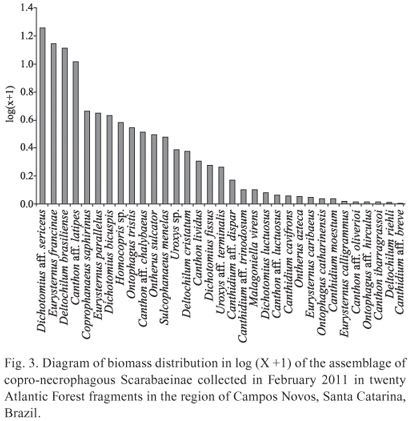
The species that most contributed in terms of biomass, possibly being the most important in the transformation of organic matter in this ecosystem, were two with large size, D. aff. sericeus and D. brasiliense, and two of medium size, E. francinae and C. latipes (Fig. 3).
Among the captured species, nineteen are tunnelers, ten rollers and only four are dwellers (Table I). Regarding food habits, of the 23 species with sufficient abundance for the calculation of the trophic niche width, 11 species were rated as coprophagous, 10 as generalists and two as strictly necrophagous, C. aff. dispar and C. aff. luctuosus (Table I). Is worth mentioning that U. aff. terminalis was most observed in field in fragments where cattle had open access. The only individual of M. virens, captured in traps of feces, was found in a fragment located near a lake, where the presence of nutria (Myocastor coypus (Molina, 1782)) was noted.
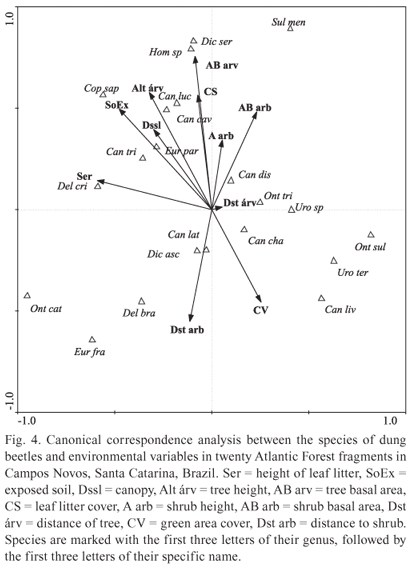
The spatial distribution of dung beetle abundance according to the structure of the environment has shown that some beetle species relate to certain characteristics of their habitat. Canonical correlation analysis was significant (F = 1.627, P = 0.008), and the first axis explained 30% and the second axis 29% of the variability of data (Fig. 4). The difference in the distribution of some species of beetles in the forest fragments thus shows a relation: D. cristatum was mainly associated with the areas of taller layers of leaf litter; C. saphirinus occurred mostly in areas with exposed soil and taller trees and D. aff. sericeus and Homocopris sp. occurred in areas of thicker tree forest and large percentage of leaf litter cover. Conversely, O. sulcator, U. aff. terminalis and C. lividus occurred in areas of soil with higher percentage of green cover and smaller trees in height. Onthophagus catharinensis, D. brasiliense and E. francinae occurred in more open areas, with greater distance and lower basal area of shrubs, however, S. menelas occurred in areas with thicker and closer shrubs (Fig. 4).
DISCUSSION
The great richness of dung beetles (n = 33) shows that the diversity of such insects in the forest fragments studied is significantly high, and it underscores the importance of research studies in regions where the fauna is still poorly known. This shows the importance of forest fragments within agricultural landscapes for the maintenance of diversity, enabling the conservation of species that otherwise would likely be locally extinct (Estrada et al. 1998; Halffter & Arellano 2002; Díaz et al. 2010).
In the fragments examined, there were a greater number of tunnelers species compared to the other functional groups. This pattern is common in tropical forests and seems to be related to the diversity of Scarabaeinae beetles in the Neotropical region (Halffter et al. 1992; Louzada & Lopes 1997).
The attractiveness of different types of resources is a pattern known for several species (Halffter & Matthews 1966; Vaz-de-Mello et al. 1998; Hernández 2007). With regard to resource utilization, almost half of the species collected in this study (11 of 23 species) were considered coprophagous. Scarabaeinae beetles are high specialized in coprophagy, (Halffter & Matthews 1966; Halffter & Edmonds 1982; Hanski & Cambefort 1991), a pattern that seems to be related to increased availability of mammal excrement in the ecosystem, since carcasses are less frequent and are spatially limited (Halffter & Matthews 1966). In addition, ten species were considered to be generalists. It is known that the use of more than one type of food resources (trophic generality) decreases the competition for scarce and ephemeral food such as feces, carcasses and rotten fruits (Halffter & Halffter 2009) and can also provide the species with a wider use of the environment, which would have contributed to the great diversity of Scarabaeinae beetles in the Neotropical region (Halffter & Halffter 2009), whereas specificity tends to restrict the occupation of new ecosystems in which their resource is not available.
Necrophagy in Scarabaeinae is considered important in Neotropical forests, where there are reduced numbers of large mammals (Halffter & Matthews 1966). Southeast Asia, where large mammals are scarce, is the only comparable biogeographic region, considering the presence of many necrophagous beetles (Halffter & Matthews 1966; Gill 1991; Halffter 1991). In this study, we have found two scavengers (necrophagous), C. aff. dispar and C. aff. luctuosus.
Within the tribe Coprini, C. moestum was generalist, which supports the works of Silva et al. (2008, 2009, 2011). This species is distributed throughout the southern region of Brazil, in Argentina and Uruguay (Martínez 1959; Martínez & Halffter 1986; González-Vainer & Morelli 2008). Homocopris sp., which is coprophagous, belongs to a genus recently revalidated. This genus is distributed in Chile and Brazil. In Brazil it can be found in the southern and southeastern Atlantic Forest (Vaz-de-Mello et al. 2010). Ontherus sulcator, which is coprophagous, is a common species and widely distributed in the Neotropical region, mostly found in herbivore dung and human feces (Martínez 1959), may also be attracted to carcasses and artificial light (Génier 1996).
As for the species of the tribe Deltochilini, C. chalybaeus was generalist, agreeing with the results of Silva et al. (2007). Luederwaldt (1911) and Martínez (1987) have claimed that this species is found in carcasses from early stages to advanced decomposition. Martínez (1959) states that this species is found in excrement in the early stages of decomposition, and is widely distributed across South America. The species C. latipes was rated as coprophagous and Martínez (1959) claims that it can be found in herbivore and human feces. Pereira & Martínez (1956) have also found C. latipes behaving as saprophagous beetles, at ripe jelly palm fruit (Butia sp.). This dung beetle is distributed in mountain forest environments in southern and southeastern Brazil, Argentina and Uruguay (Vulcano & Pereira 1964; Martínez 1987). Canthon lividus had generalist feeding habits, as reported in the works of Martínez (1959), Halffter & Matthews (1966) and Silva et al. (2011). It can be found in Brazil, Argentina, Paraguay and Uruguay (Martínez, 1959). Deltochilum brasiliense was also generalist, in accordance with Almeida & Louzada (2009) and Silva et al. (2011). It can be found in the center-south region of Brazil and Argentina.
Of the species of Oniticellini, E. francinae was coprophagous, agreeing with the work of Génier (2009), who has examined specimens collected in human feces, with the exception of one specimen collected in cattle dung. This species can be found throughout the Atlantic Forest at altitudes above 1,000 m, except in southern Brazil, where the latitude seems to compensate for the altitude (Génier 2009). Eurysternus parallelus was rated as coprophagous, agreeing with the results of Silva et al. (2011), but differing from those of Louzada & Lopes (1997), who have also captured it in carrion traps. This species is distributed throughout southern and southeastern Brazil, Argentina and Paraguay (Martínez 1959; Génier 2009).
Of the species of Onthophagini, O. catharinensis was coprophagous, in accordance with the results of Silva et al. (2011). The distribution of this species includes the state of Santa Catarina, from where it was originally described (Paulian 1936), Rio Grande do Sul and Paraná. Lopes et al. (2011) propose that this species may be an indicator of preserved areas. Onthophagus tristis was coprophagous, as also found by Silva et al. (2011).
As for the species of Phanaeini, C. saphirinus was rated as generalist. According to Martínez (1959) it is coprophagous and found in the droppings of herbivores. It occurs in southeastern and southern Brazil, Argentina and Paraguay (Martínez 1959; Arnaud 2002; Edmonds & Zidek 2010), having a color variation among different populations (Edmonds & Zidek 2010). Sulcophanaeus menelas was rated as coprophagous, agreeing with Edmonds (2000), who states that it has strict coprophagous feeding habits, and it can be found in different types of droppings. It can be found in Bolivia, Argentina, Uruguay and southern Brazil, and prefers open areas to forested areas (Edmonds 2000).
Habitat structural complexity and resource availability are important factors in determing the dung beetle community (Gardner et al. 2008; Almeida & Louzada 2009; Neves et al. 2010, and for revision see Nichols et al. 2007). The forest coverage and type of vegetation are factors that influence the assemblage of dung beetles in different environments (Halffter & Arellano 2002; Hernández & Vaz-de-Mello 2009). Estrada et al. (1998) observed that the diversity of dung beetles had a positive relationship with measures of vertical and horizontal diversity of vegetation. Davis et al. (2001), working with dung beetles in Borneo, observed that the distribution of species across different environmental characteristics might show discrete associations that are typical to particular biotypes within the landscape. When comparing different environments with varying degrees of disturbance in Mexico, Halffter & Arellano (2002) proposed that the structure of the environment is more important in determining community composition of dung beetles than the allocation of resources in areas occupied by livestock.
Factors such as sunlight and humidity are important, since reproductive aspects would be affected (Martínez & Vásquez 1995). In a study conducted in Brazil, in the Amazon rainforest, Gardner et al. (2008) showed that assemblage of Scarabaeinae beetles are strongly and negatively affected in secondary forest environments. The microclimatic differences due to low, relatively open canopies with hot and dry understory environments, could help explain the observed impoverishment of dung beetle communities.
Knowledge of the species and studies on the ecological and behavioral characteristics of each species are the first steps in finding species indicators to assess the conservation status of a particular ecosystem (Brown 1997). Changes in habitat complexity can alter not only the communities of insects, but also the whole fauna associated with forests, reducing the richness of some taxonomic groups and increasing others (Barlow et al. 2007; Noriega et al. 2007).
In order to preserve the community of dung beetles and their ecosystem services there is, therefore, a need for landscape conservation planning, with special attention to habitat structure (Barlow et al. 2010), reduction of isolation and increased connectivity between fragments (Numa et al. 2009).
ACKNOWLEDGEMENTS
We would like to thank Elena Rocca, Rubens Nodari and Genok for supporting the project, the farmers of Campos Novos for the permission to collect, Gilmar Espanhol and Gabriela Corso for help during field work, Fernando Vaz-de-Mello for confirming the identification of dung beetles and CNPq for funding the project (Process 553880/20101: the Masters Scholarship to the first author, Process 303800/20100: Research Productivity Grant to the second author and Process 479203/20105: Research Assistance)
Received 30 May 2012; accepted 15 October 2012
Associate Editor: Rodrigo F. Krüger
- Almeida, S.S P. & Louzada, J.N.C. 2009. Estrutura da comunidade de Scarabaeinae (Scarabaeidae: Coleoptera) em fitofisionomias do Cerrado e sua importância para a conservação. Neotropical Entomology 38: 3243.
- Arnaud, P. 2002. Les Coléoptères du Monde, Phanaeini. vol. 28, Canterbury, Hillside Books, 151 p.
- Barlow, J., Gardner, T.A., Araújo I.S., Ávila-Pires, T.C., Bonaldo, A.B., Costa, J.E., Esposito, M.C., Ferreira, L.V., Hawes, J., Hernández, M.I.M., Hoogmoed, M.S., Leite, R.N, Lo-Man-Hung, N.F., Malcom, J.R. Martins, M.B., Mestre, L.A.M., Miranda-Santos, R, Nunes-Gutjahr, W.L, Overal, A.L., Parry, L., Peters, S.L., Ribeiro-Junior, M.A., Da Silva, M.N.F., Da Silva Motta, C. & Peres, C.A. 2007. Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proceedings of National Academy of Sciences of the United States of America, USA 104: 1855518560.
- Barlow, J., Louzada, J., Parry, L., Hernández, M. I. M., Hawes, J., Peres, C. A., Vaz-de-Mello, F. Z. & Gardner, T. A. 2010. Improving the design and management of forest strips in human-dominated tropical landscapes: a field test on Amazonian dung beetles. Journal of Applied Ecology 47: 779788.
- Brower, J.E, Zar, J.H, & Von Ende, C. 1997. Field and laboratory methods for general ecology. 4th Edition, Boston, McGraw-Hill, 288 p.
- Brown, K.S.J. 1997. Diversity, disturbance, and sustainable use of Neotropical forests: insects as indicators for conservation monitoring. Journal of Insect Conservation 1: 2542.
- Cambefort, Y. & Hanski, I. 1991. Dung beetle population biology, p. 3650. In: Hanski, I. & Cambefort, Y. (eds.). Dung beetle ecology. Princeton, Princeton University Press, 481 p.
- Colwell, R.K. 2005. EstimateS: Statistical estimation of species richness and shared species from samples. Version 7.5.1. University of Connecticut. 22 p.
- Davis, A.L.V, Scholtz, C.H &. Philips, T.K. 2002. Historical biogeography of Scarabaeinae dung beetle. Journal of Biogeography 29: 12171256.
- Davis, A.J., Holloway, J.D, Huijbregts, H., Krikken, J., Kirk-Spriggs, A.H. & Sutton, S.L. 2001. Dung beetles as indicators of change in the forests of northern Borneo. Journal of Applied Ecology 38: 593616.
- Davis, A.L.V. & Philips, T.K. 2005. Effect of deforestation on a southwest Ghana dung beetle assemblage (Coleoptera: Scarabaeidae) at the periphery of Ankasa Conservation Area. Environmental Entomology 34: 10811088.
- Díaz, A., Galante, E. & Favila, M.E. 2010. The effect of the landscape matrix on the distribution of dung and carrion beetles in a fragmented tropical rain forest. Journal of Insect Science 10 (81):1216.
- Doube, B.M. 1991. Dung beetles of Southern Africa, p. 133155. In: Hanski, I. & Cambefort, Y. (eds.). Dung beetle ecology Princeton, Princeton University Press, 481 p.
- Edmonds, W.D. 2000. Revision of the Neotropical dung beetle genus Sulcophanaeus (Coleoptera: Scarabaeidae: Scarabaeinae). Folia Heyrovskyana, Suppl. 6: 160.
- Edmonds, W.D. & Zídek, J. 2010. A taxonomic review of the genus Coprophanaeus Olsoufieff, 1924 (Coleoptera: Scarabaeidae: Scarabaeinae). Insecta Mundi 129: 1111.
- Estrada, A., Halffter, G., Coates-Estrada, R. & Merritt, D.A.J. 1993. Dung beetles attracted to mammalian herbivore (Alouatta palliata) and omnivore (Nasua narica) dung in the tropical rain forest of Los Tuxtlas, Mexico. Journal of Tropical Ecology 9: 4554.
- Estrada A., Coates-Estrada, R., Dadda, A. & Cammarano, P. 1998. Dung and carrion beetles in tropical rain forest fragments and agricultural habitats at Los Tuxtlas, Mexico. Journal of Tropical Ecology 14: 577593.
- Estrada, A., Anzures, A.D. & Coates-Estrada, R. 1999. Tropical rain forest fragmentation, howler monkeys (Alouatta palliata) and dung beetles at Los Tuxtlas, Mexico. American Journal of Primatology 48: 253262.
- Filgueiras, B.K.C., Liberal, C.N., Aguiar, C.D.M., Hernández, M.I.M. & Ianuzzi, L. 2009. Attractivity of omnivore, carnivore and herbivore mammalian dung to Scarabaeinae (Coleoptera, Scarabaeidae) in a Tropical Atlantic Rainforest remnant. Revista Brasileira de Entomologia 53: 422427.
- Flechtmann, C.A.H. & Rodrigues, S.R. 1995. Insetos fimícolas associados a fezes bovinas em Jaraguá do Sul/SC Besouros coprófagos (Coleoptera, Scarabaeidae). Revista Brasileira de Entomologia 39: 303309.
- Gardner, T.A., Hernández, M.I.M., Barlow, J. & Peres, C.A. 2008. Understanding the biodiversity consequences of habitat change: the value of secondary and plantation forests for neotropical dung beetles. Journal of Applied Ecology 45: 883-893.
- Génier, F. 1996. A revision of the Neotropical genus Ontherus Erichson (Coleoptera, Scarabaeidae, Scarabaeinae). Memoirs of the Entomological Society of Canada 170: 1169.
- Génier, F. 2009. Le genre Eurysternus Dalman, 1824 (Scarabaeidae: Scarabaeinae: Oniticellini), revision taxonomique et clés de determination illustrées Sofia, Pensoft, 430 p.
- Gill, B.D. 1991. Dung beetles in American Tropical Forest, p.211-229. In: Hanski, I. & Cambefort, Y. (Eds.) Dung Beetle Ecology. Princeton, Princeton University Press, 481p.
- González-Vainer, P. & Morelli, E. 2008. Relevamiento de los coleópteros coprófilos y necrófilos de Sierra de Minas, Uruguay (Insecta: Coleoptera). Boletin de la Sociedad Zoológica del Uruguay 17: 20-33.
- Halffter, G. & Matthews, E.G. 1966. The natural history of dung beetles of the subfamily Scarabaeinae (Coleoptera: Scarabaeidae). Folia Entomologica Mexicana 12/14: 1-312.
- Halffter, G. & Edmonds, W.D. 1982. The nesting behavior of dung beetles (Scarabaeinae): An ecological and evolutive approach México D.F., Man and the Biosphere Program UNESCO, 177 p.
- Halffter, G. 1991. Historical and ecological factors determining the geographical distribution of beetles (Coleoptera: Scarabaeidae: Scarabaeinae). Folia Entomologica Mexicana 82: 195-238.
- Halffter, G., Favila, M.E. & Halffter, V. 1992. A comparative study of the structure of the scarab guild in mexican tropical rain forest and derived ecosystems. Folia Entomologica Mexicana 84: 131-156.
- Halffter, G. & Arellano, L. 2002. Response of dung beetle diversity to human-induced changes in a tropical landscape. Biotropica 34: 144-154.
- Halffter, G. & Halffter, V. 2009. Why and where coprophagous beetles (Coleoptera: Scarabaeinae) eat seeds, fruits or vegetable detritus. Boletín de la Sociedad Entomologica Aragonesa 45: 1-22.
- Halffter, G., Favila, M.E. & Halffter, V. 1992. A comparative study of the structure of the scarab guild in Mexican tropical rain forests and derived ecosystems. Folia Entomologica Mexicana 84: 131-156.
- Hanski, I. 1991. The Dung Insect Community, p. 521. In: Hanski, I. & Cambefort, Y. (Eds.). Dung Beetle Ecology Princeton, Princeton University Press, 481p.
- Hanski, I. & Cambefort, Y. 1991. Dung Beetle Ecology. New Jersey, Princeton University Press, 481 p.
- Hernández, M.I.M. 2007. Besouros escarabeíneos (Coleoptera: Scarabaeidae) da Caatinga paraibana, Brasil. Oecologia Brasiliensis 11: 356-364.
- Hernández, M.I.M. & Vaz-de-Mello, F. Z. 2009. Seasonal and spatial species richness variation of dung beetle (Coleoptera, Scarabaeidae s.str.) in the Atlantic Forest of southeastern Brazil. Revista Brasileira de Entomologia 53: 607-613.
- Hernández, M.I.M., Monteiro, L.R, Favila, M.E. 2011. The role of body size and body shape in understanding competitive interactions within a community of Neotropical dung beetles. Journal of Insect Science 11:1-14.
- Klein, B.C. 1989. Effects of forest fragmentation on dung and carrion beetle communities in Central Amazonia. Ecology 70: 1715-1725.
- Krebs, C.J. 1999. Ecological Methodology. 2nd ed., New York, Addison-Wesley Longman, 620 p.
- Leite, P.F. & Klein, R.M. 1990. Geografia do Brasil. Rio de Janeiro, Fundação Instituto Brasileiro de Geografia e Estatística, Diretoria de Geociência: IBGE, 420 p.
- Lopes, J., Korasaki V., Catelli, L.L, Marçal, V.V.M. & Nunes, M.P.B.P. 2011. A comparison of dung beetle assemblage structure (Coleoptera: Scarabaeidae: Scarabaeinae) between an Atlantic forest fragment and adjacent abandoned pasture in Paraná, Brazil. Zoologia 28: 72-79.
- Louzada, J.N. C. & Lopes, F.S. 1997. A comunidade de Scarabaeidae copronecrófagos (Coleoptera) de um fragmento de Mata Atlântica. Revista Brasileira de Entomologia 41: 117-121.
- Luederwaldt, H. 1911. Os insectos necróphagos Paulistas. Revista do Museu Paulista 8: 414-433.
- Martínez, A. 1959. Catálogo de los Scarabaeidae Argentinos (Coleoptera). Revista del Museo Argentino de Ciencias Naturales "Bernardino Rivadavia" 5: 1-126.
- Martínez, A. & Halffter, G. 1986. Situación del género Canthidium Erichson (Coleoptera: Scarabaeidae: Scarabaeinae). Acta Zoologica Mexicana 17: 19-40.
- Martínez, A. 1987. La entomofauna de Scarabaeinae de la provincia de Salta (Col. Scarabaeoidea). Anales de la Sociedad Científica Argentina 216: 45-69.
- Martínez, I.M, Vásquez, A.A. 1995. Influencia de algunos factores ambientales sobre la reproducion em Canthon cyanellus cyanellus Le Conte (Coleoptera: Scarabaeidae: Scarabaeinae). Elytron 9: 5-13.
- Medri, I.M. & Lopes, J. 2001. Scarabaeidae (Coleoptera) do Parque Estadual Mata dos Godoy e de área de pastagem, no norte do Paraná, Brasil. Revista Brasileira de Zoologia 18: 135-141.
- Morelli, E. & González-Vainer, P. 1997. Dung beetles (Coleoptera: Scarabaeidae) inhabiting bovine and ovine dropping in Uruguayan prairies. The Coleopterists Bulletin 51: 197.
- Neves, F.D., Oliveira, V.H.F., Do Espírito-Santo, M.M., Vaz-De-Mello, F.Z., Louzada, J., Sanchez-Azofeifa, A., & Fernandes, G.W. 2010. Successional and seasonal changes in a community of dung beetles (Coleoptera: Scarabaeinae) in a Brazilian Tropical Dry Forest. Natureza & Conservação 8: 160-164.
- Nichols, E., Larsen, T., Spector, S., Davis, A. L., Escobar, F., Favila, M. & Vulinec, K. 2007. Global dung beetle response to tropical forest modification and fragmentation: A quantitative literature review and meta-analysis. Biological Conservation 137: 1-19.
- Nichols, E., Spector, S., Louzada, J., Larsen, T., Amezquita, S. & Favila, M.E. 2008. Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biological Conservation 141:1461-1474.
- Nichols, E., Gardner, T.A., Peres C.A. & Spector, S. 2009. Co-declining mammals and dung beetles: an impending ecological cascade. Oikos 118: 481-487.
- Noriega, J.A., Realpe, E &. Fagua, G. 2007. Diversidad de escarabajos coprófagos (Coleoptera: Scarabaeidae) en un bosque de galería con tres estádios de alteración. Universitas Scientiarum 12: 51-63.
- Numa, C., Verdú, J.R., Sánchez, A. & Galante, E. 2009. Effect of landscape structure on the spatial distribution of Mediterranean dung beetle diversity. Diversity and Distributions 15: 489-501.
- Pandolfo, C., Braga, H.J., Silva Júnior, V.P., Massignam, A.M., Pereira, E.S. & Thomé, V.M.R. 2002. Atlas Climático digital do estado de Santa Catarina. Florianópolis. Epagri (CD-ROM).
- Paulian, R. 1936. Sur quelques Onthophagus américains nouveaux ou peu connus (Col. Lamellimaizees). Festschrift zum 60. Geburtstage von Professor Dr. Embrik Strand 1: 506-509.
- Pereira, F.S. & Martínez, A. 1956. Os gêneros de Canthonini americanos (Col. Scarabaeidae). Revista Brasileira de Entomologia 6: 91-192.
- Ramos, F.A. 2000. Nymphalid butterfly communities in an Amazonian forest fragment. Journal of Research on the Lepidoptera 35: 29-41.
- SCARABNET. [data 2011] Global Taxon Database. Available at: http://216.73.243.70/scarabnet/results.htm (accessed 11 August 2011).
- Silva, F.A.B., Hernández, M.I.M., Ide, S. & Moura, R.C. 2007. Comunidade de escarabeíneos (Coleoptera, Scarabaeidae) copronecrófagos da região de Brejo Novo, Caruaru, Pernambuco, Brasil. Revista Brasileira de Entomologia 51: 228-233.
- Silva, P.G.; Garcia, M.A.R. & Vidal, M.B. 2008. Besouros copro-necrófagos (Coleoptera: Scarabaeidae stricto sensu) coletados em ecótono natural de campo e mata em Bagé, RS. Ciência e Natura 30: 71-91.
- Silva, P.G.; Garcia, M.A. R. & Vidal, M.B. 2009. Besouros copro-necrófagos (Coleoptera: Scarabaeidae sensu stricto) do município de Bagé, RS (Bioma Campos Sulinos). Biociências 17: 33-43.
- Silva, P.G., Vaz-de-Mello, F.Z. & Di Mare, R.A. 2011. Guia de identificação das espécies de Scarabaeinae (Coleoptera: Scarabaeidae) do município de Santa Maria, Rio Grande do Sul, Brasil. Biota Neotropica 14: 329-345.
- Silva, P.G., Vaz-de-Mello, F.Z. & Di Mare, R.A. 2012.Attractiveness of different baits to Scarabaeinae (Coleoptera: Scarabaeidae) in forest fragments in the extreme south of Brazil. Zoological Studies 51: 429-441.
- Simmons, L.W & Ridsdill-Smith, J. 2011. Ecology and Evolution of Dung Beetles. Oxford, Wiley-Blackwell, 368 p.
- Slade, E.M., Mann, D.J., Villanueva, J.F, & Lewis, O.T. 2007. Experimental evidence for the effects of dung beetle functional group richness and composition on ecosystem function in a tropical forest. Journal of Animal Ecology 76: 1094-1104.
- Southwood, T.R.E. 1994. Ecological methods: with particular reference to the study of insect populations. London, Chapman & Hall, 575 p.
- Spector, S. & Ayzama, S. 2003. Rapid turnover and edge effects in dung beetle assemblages (Scarabaeidae) at a Bolivian Neotropical Forest-Savanna Ecotone. Biotropica 35: 394-404.
- Ter Braak, C.J.F. & Smilauer. P. 2002. CANOCO Reference manual and CanoDraw for Windows user's guide: Software for Canonical Community Ordination (version 4.5). Ithaca, Microcomputer Power, 500 p.
- Vaz-de-Mello, F.Z., Louzada, J.N.C. & Schoereder, J.H. 1998. New data and comments on Scarabaeidae (Coleoptera: Scarabaeoidea) associated with Attini (Hymenoptera: Formicidae). The Coleopterists Bulletin 52: 209-216.
- Vaz-de-Mello, F.Z. 2000. Estado atual de conhecimento dos Scarabaeidae s. str (Coleoptera: Scarabaeoidea) do Brasil. In: Martín-Piera, F., Morrone, J. J. & Melic, A. (Comp.). Hacia un Proyecto CYTED para el Inventario y Estimación de la Diversidad Entomológica en Iberoamérica. Zaragoza, Sociedad Entomológica Aragonesa, 183-195.
- Vaz-de-Mello, F.Z., Génier, F. & Smith, S.B.T. 2010. Reclassification of Homocopris Burmeister as a valid genus to accomodate three species formerly in Dichotomius Hope (Scarabaeidae: Scarabaeinae: Coprini). The Coleopterists Bulletin 64: 192-192.
- Vaz-de-Mello, F.Z., Edmonds, W.D., Ocampo, F. & Schoolmeesters, P. 2011. A multilingual key to the genera and subgenera of the subfamily Scarabaeinae of the New World. Zootaxa 2854: 1-73.
- Vulcano, M.A. & Pereira, F.S. 1964. Catalogue of the Canthonini (Col. Scarab.) inhabiting the Western Hemisphere. Entomologische Arbeiten aus dem Museum G. Frey 15: 570-685.
Publication Dates
-
Publication in this collection
01 Apr 2013 -
Date of issue
Mar 2013
History
-
Received
30 May 2012 -
Accepted
15 Oct 2012