Abstract
Machilus macrantha Nees, Lauraceae, bark is traditionally used in the treatment of asthma, tuberculosis and rheumatoid arthritis. In order to validate, mechanism based anti-inflammatory activity of fractions M. macrantha bark are investigated for first time. Test materials viz. petroleum ether (PE), alkaloidal fraction (CH), acetone extracts (TAN) and mucilage (MM) (250 and 500 mg/kg, p.o.) obtained from M. macrantha bark were tested for membrane stabilizing, anti-nociceptive; anti inflammatory and Freund's complete adjuvant (FCA) induced arthritis activity. Diclofenac sodium and morphine were used as the reference standards in pharmacological assay. Test materials have significantly (p<0.01) inhibited paw edema after Carrageenan and histamine induction at higher doses. Administration of test materials of M. macrantha (250 and 500 mg/kg b.w.) significantly reduced abdominal writhing, formalin nociception, cotton pellet granuloma and vascular permeability in experimental animal. In addition to this, bark of M. macrantha showed chronic anti-rheumatic effect by suppressing the swelling volume, arthritis index, hematological and biochemical parameters (ESR, RA factor, CRP, liver transferase enzyme) in FCA-induced arthritis. It also significantly inhibited protein denaturation, heat-induced haemolysis of RBC and reduction in total leukocyte migration. Bioassay guided fractionation of the pet. ether extract of bark of M. macrantha led to isolation and characterization of β-sitosterol and stigma sterol confirmed by its HPLC, NMR and GC-MS study. In conclusion, extracts of M. macrantha bark can be explored as a therapeutic agent for the treatment of acute and chronic arthritis.
anti-inflammatory; lauraceae; leucocyte migration; Machilus macrantha; protein denaturation; β-sitosterol
Further studies on membrane stabilizing, anti-inflammatory and FCA induced arthritic activity of various fractions of bark of Machilus macrantha in rats
Anil U Tatiya* * Correspondence: Anil U Tatiya Department of Pharmacognosy R. C. Patel Institute of Pharmaceutical Education and Research, 425405, Shirpur-Maharashtra, Indi. a autatiya@rcpatelpharmacy.co.in. autatiya@rcpatelpharmacy.co.in. Tel.: +91 2563 251808, 255189. Fax: +91 2563 251809 ,I; Ajay K SalujaII
IDepartment of Pharmacognosy, R. C. Patel Institute of Pharmaceutical Education and Research, India
IIA. R. College of Pharmacy and G. H. Patel Institute of Pharmacy, Vallabh Vidyanagar, Gujarat, India
ABSTRACT
Machilus macrantha Nees, Lauraceae, bark is traditionally used in the treatment of asthma, tuberculosis and rheumatoid arthritis. In order to validate, mechanism based anti-inflammatory activity of fractions M. macrantha bark are investigated for first time. Test materials viz. petroleum ether (PE), alkaloidal fraction (CH), acetone extracts (TAN) and mucilage (MM) (250 and 500 mg/kg, p.o.) obtained from M. macrantha bark were tested for membrane stabilizing, anti-nociceptive; anti inflammatory and Freund's complete adjuvant (FCA) induced arthritis activity. Diclofenac sodium and morphine were used as the reference standards in pharmacological assay. Test materials have significantly (p<0.01) inhibited paw edema after Carrageenan and histamine induction at higher doses. Administration of test materials of M. macrantha (250 and 500 mg/kg b.w.) significantly reduced abdominal writhing, formalin nociception, cotton pellet granuloma and vascular permeability in experimental animal. In addition to this, bark of M. macrantha showed chronic anti-rheumatic effect by suppressing the swelling volume, arthritis index, hematological and biochemical parameters (ESR, RA factor, CRP, liver transferase enzyme) in FCA-induced arthritis. It also significantly inhibited protein denaturation, heat-induced haemolysis of RBC and reduction in total leukocyte migration. Bioassay guided fractionation of the pet. ether extract of bark of M. macrantha led to isolation and characterization of β-sitosterol and stigma sterol confirmed by its HPLC, NMR and GC-MS study. In conclusion, extracts of M. macrantha bark can be explored as a therapeutic agent for the treatment of acute and chronic arthritis.
Keywords: anti-inflammatory, lauraceae, leucocyte migration, Machilus macrantha, protein denaturation, β-sitosterol
Introduction
Machilus macrantha Nees, Lauraceae, is a large heighted, shade bearer tree, mainly distributed in Western peninsula, Ceylon and India etc. In India, mainly found in Bihar, Maharashtra and Assam up to an altitude of 2100 m. (Chopra & Nayar, 1992; Satyavati & Gupta, 1987). Traditionally, bark is used in the treatment of asthma, tuberculosis and rheumatoid arthritis (Kirtikar & Basu, 1999).Powdered bark is mixed with egg- white and applied locally to the affected part in form of poultice act as an anti-inflammatory and anti-rheumatic agent by Hallakki Gowda tribes of western ghat region, Karnataka, India (Personal communication).
Some bioactive components from leaves of this plant have been reported as three norlignans compounds namely machicendiol, machicenonol and machicenin along with sesamin, (+) pinoresinol dimethyl ether, egonol and homoegonol. (Talapatra et al.,1978; Talapatra et al., 1979). New alkaloids namely machigline, atheroline and β-sitosterol-O-glucoside are also isolated. Two alkaloids (one of which was provisionally named macranthine) and β-sitosterol from the root was reported by Baveja et al., (1968). Bark contains arabinoxylan composed of arabinose (73%) and xylose (27%) (Gowda et al., 1982). Our previous study of M. macrantha bark was preliminary investigated for the acute inflammatory effect by carrageenan-induced hind paw edema and Freund's complete adjuvant (FCA) induced arthritis in rats (Tatiya & Hatapakki, 2003; Kulkarni et.al., 2009). However, in vivo anti-inflammatory, anti-nociceptive and FCA induced arthritis activities have not been reported in depth. Hence, this paper deals with the chronic arthritic (anti-inflammatory and analgesic) effect in adjuvant-induced arthritis in experimental animals and in vitro membrane stabilizing property.
Material and Methods
Plant material and preparation of extracts
The plant material of Machilus macrantha Nees, Lauraceae, bark was collected from Lonavala, Pune, MS, India in the year 2007. The plant material was identified by Director of Botanical Survey of India, Pune, India, in which a voucher specimen (b-12) is deposited at the RCPCOP herbarium for reference purpose.
The bark was air dried at 40 °C, and dried material (1.5 kg) was powdered using a pulverizer and exhaustively extracted by Soxhlet extractor with petroleum ether (60-80 °C) (PE), acetone:water (70:30 v/v) (TAN) and water, which was concentrated under vacuum. Further the mucilage was separated from aqueous extracts by adding solvent acetone to obtained crude mucilage (MM). The total crude alkaloidal fraction was also separated from defatted marc of M. macrantha bark by using standard alkaloid extraction protocol to obtained alkaloid rich chloroform extracts (CH).
Phytochemical analysis of extracts and fractions
The phytochemical analysis of PE, CH, TAN and MM was done using standard methods (Trease & Evans, 1983).
Chromatographic separation and identification of compound MMS 1 and 2
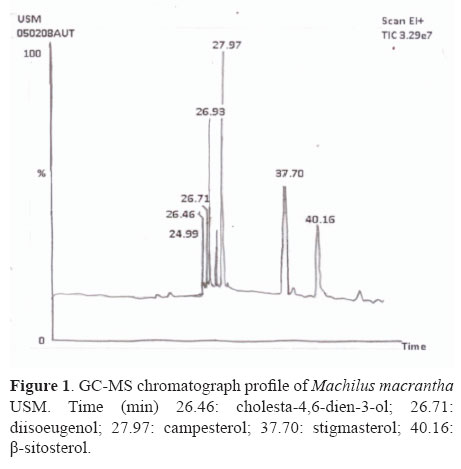
Unsaponifiable matters of pet ether extract (PE) was submitted to repeated column chromatography over 500 g of Si gel (3 cm×75 cm). The elution with pet ether and ethyl acetate in increasing proportions furnished 105 chromatographic fractions of 10 mL each. TLC study of the fractions 41-60 (pet ether:EtOAc, 7.5:2.5) allowed to assemble them into one fraction, which was rechromatographed and separated two constituents MMS 1 and MMS-2 using preparative TLC. Their purity was determined by TLC. The structure of isolated constituents was characterized using 1HNMR and 13CNMR spectroscopy in comparison of spectral data with those of previously published compounds by Saxena & Sosanna (2005); Habib et al., (2007) and also by GC-MS (Perkin Elmer USA model auto system XL).The compounds were identified by comparison with the mass fragmentation pattern of standards available in NIST library, USA.
HPLC analysis
HPLC study of USM was performed using Younglin, ACME 9000 series of HPLC instrument, consisting of photodiode array detector (254 nm).The samples (pet ether) were injected with microlitre syringe in column RP C-18, 250 x 4.6 mm diameter and elute with an isocratic system methanol:water (95:5 v/v) was used as the mobile phase at a flow rate of 1.0 mL/min. An isocratic of HPLC was monitored at 210 nm.
Determination of total phenolics
Total phenolic content of TAN was determined using the Folin-Ciocalteu technique (Christel et al., 2000). Briefly, extract (1 mg/mL) was diluted with 3 mL distilled water. A volume of 0.5 mL of Folin-Ciocalteu reagent was added and the mixture allowed standing for three min at room temperature 2 mL of 20% Na2CO3 was added and the mixture again allowed to stand for 90 min, absorbance was measured at 765 nm after cooling in darkness. The values are presented as means of triplicate analyses.
Determination of total proanthocyanidin
Initially, proanthocyanidin was detected by chemical test. Therefore it was determined using vanillin-H2SO4 method (Price et al., 1978 Tebourbi et al., 2006). Briefly, 1 mL of extract (50 and 100 µg/mL) was mixed with 2 mL of freshly prepared vanillin solution (1% vanillin in 70% of H2SO4 v/v) and maintained for 15 min at 20 °C. The absorbance was measured at 500 nm for epicatechin (8-40 µg/mL). Calibration curved was drawn. Spectrophotometric determinations were performed using on Shimadzu UV-2450 spectrophotometer.
Pharmacological study
Experimental animals
Adult albino wistar rats and swiss albino mice of either sex weighing between 150-250 and 25-40 g, respectively were used for the study. Animals were procured from R. C. Patel Institute of Pharmaceutical Education and Research Centre. The animals were housed in well ventilated colony cages in the departmental animal house at (25±2 °C, 12:12 h L:D (light and dark cycle). The animals were fed with standard pellet diet and water ad libitum. All the experimental protocols used in the study were approved by Institutional animal Ethical committee (Protocol number 045/2005) and committee for the purpose of Control and supervision on experiments on Animals (CPCSEA), guidelines, Chennai, India.
Acute oral toxicity study
Acute oral toxicity studies were performed according to OECD-423 guidelines (acute toxic class method). Swiss mice of either sex selected by random sampling technique were employed in this study. Mortality was observed in 2/3 or 3/3 animals and then the dose administered was considered as toxic dose.
Anti-inflammatory activity
Carrageenan and histamine induced rat paw edema
According to a modification of method Winter et al. (1962), male wistar rats (150-200 g each) divided into groups of six animals each were used for acute inflammation, induced in the right hind paw of rats by subcutaneous injection of a freshly prepared Carrageenan (0.1 mL 1%) and histamine (0.1mL of 0.1%) separately. Group of animal were treated with M. macrantha crude extracts (250 and 500 mg/kg, bw) orally 1 h before the carrageenan injection. The control group received only saline. Following the administration of phlogistic agent, the volume of the injected paws was measured every hour for 6 h using a plethysmometer (Ugo Basile, Italy). The percentage increase in paw volume or swelling was calculated based on the paw volume prior to injection. Positive control group was treated with Diclofenac (10 mg/kg, p.o.) in both tests (Mani et al., 2007).
Acetic acid induced vascular permeability
According to a modification of method Whittle (1964), mice were divided into groups of seven. One hour after oral administration of the extract, 0.1 mL/10 g body weight of 1% Evans blue solution was injected intravenously into group of mouse (n=6) immediately followed by an intra peritoneal injection of 0.1 mL/10 g of 0.7% acetic acid. Thirty minutes after the administration of acetic acid, the mice were killed by cervical dislocation. After injecting 10 mL of saline into the peritoneal cavity, the washing solutions were collected. The concentration of evans blue in the peritoneal cavity was measured by absorbance at 630 nm using an ELISA Analyzer (Biotek, USA). The vascular permeability was represented in terms of the absorbance (A630) that leaked into the cavity (Hong et al., 2007).
Granulomatous tissue induction
The method has been described by Winter & Porter (1957). Male wistar rats with an average weight of 200 g were anaesthetized with ether. The back skin was shaved and disinfected with 70% ethanol. An incision was made in the lumbar region. Sterilized cotton pellet (15±1 mg) was placed on both sides in the scapular region. The animals were treated with test samples (250 and 500 mg/kg) for seven days orally while the control group received vehicle only (1%, w/v, carboxymethylcellulose, 5 mL/kg).The rats were sacrificed on the eighth day. The implanted pellets were removed surgically free from extraneous tissues incubated at 37 °C for 24 h and dried at 60 °C overnight and recorded the weight. The average weight of the pellets of the control group as well as of the test group was calculated. (Vanu et al., 2004).
Analgesic activity
Acetic acid induced writhing method
The method used in this test has been described by Koster et al. (1959). The total number of writhings response was produced by intra peritoneal administration of acetic acid solution (1%, 10 mL/kg). The mice were treated with PE, CH, MM and TAN Fraction of M. macrantha (250 and 500 mg/kg) and standard drug (aspirin, 300 mg/kg), 30 min before administration of acetic acid. The number of writhes, a response consisting of contraction of the abdominal wall and pelvic rotation followed by hind limb extension, was counted during continuous observation for 20 min beginning at 5 min after the acetic acid injection. Percentage of protection was calculated by using the following ratio: (control mean treated-mean)×100/control mean.
Formalin induced nociception
The antinociceptive activity of M. macrantha test materials were determined using the formalin test according to Hunskar et al. (1985). The dried extract was dissolved in a mixture of suspending agent and water (1:4) and ethyl morphine hydrochloride(10 mg/kg) were administered i.p. in a volume of 1.5-2 mL. Control group received only vehicle (1.5-2 mL). Fifteen minutes after treatment, 50 μL 2.5% formalin was injected to the dorsal surface of the left hind paw. The rat was observed for 30 min after the injection of formalin, and duration of paw licking was measured for 0-5 min (early phase) and 15-30 min (late phase).
Anti arthritic activity
Freund's complete adjuvant-induced arthritis
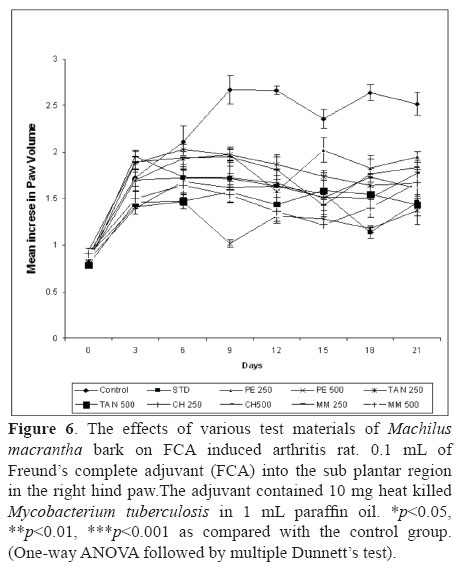
Arthritis was induced by injection of 0.1 mL of Freund's complete adjuvant (FCA, Sigma, USA) into the sub plantar region in the right hind paw (Newbould, 1963; Barbier et al., 1986) of rat. The adjuvant contained 10 mg heat killed Mycobacterium tuberculosis in 1 mL paraffin oil. Group I served as control. Group II served as standard dexamethasone (5 mg/kg, p.o.). One hour prior to the induction of arthritis different groups of animals were administered orally test samples at the dose 250 and 500 mg/kg, once a day, for fourteen days. Rats developed signs of polyarthritis in 8-10 days following the adjuvant injection. Volume of the injected paws was measured using a plethysmograph (Ugo Basile 7140, Italy) before and every three days after the induction of arthritis till day 21. On day 21st, at the end of the experimental period, the animals were sacrificed by cervical decapitation and blood was collected and separated plasma for assaying the hematological and biochemical parameters (Gokhale et al., 2002; Chattopadhyay et al., 2004). Arthritis was also assessed by body and spleen weight of rats.
Hematological parameters
Blood samples were collected from the retro-orbital plexus for laboratory tests on day 21 after FCA injection. The hematological parameters like Red Blood Cell (RBC), White Blood Cell (WBC), and Erythrocyte Sedimentation Rate (ESR, mm/h) was determined.
RA and CRP Test
Rheumatoid arthritis (RA) testing was carried out by utilizing the agglutination reaction between immunoglobulin and rheumatoid factor. C-reactive protein (CRP) factor based on the immunologic reaction between CRP as an antigen and latex particles have been coated with monospecific anti human CRP and sensitized to detect levels. Both the test was measured in serum using a commercial kit.
Determination of tissue marker enzymes
The marker enzymes glutamate oxalo acetate transaminase/aspartate aminotransferase (GOT/AST) and glutamate pyruvate transaminase/alanine aminotransferase (GPT/ALT) and alkaline phosphatase (ALP) were analyzed in serum. A lysosomal enzyme such as acid phosphatase (ACP) was estimated in plasma. All the tissue enzymes were spectrophotometrically estimated by using commercial diagnostic kit (Reitman & Frankel, 1957; Kind & King, 1954). Spleen were dissected out, washed and transferred to an ice-cold saline solution. The organs were weighed. Evaluation of membrane stabilizing property (in vitro)
Leukocyte migration assay in mice
The test was carried out using the technique of Hong et al. (2007). After oral administration of test samples of M. macrantha, animals received an intraperitonial injection of 1% CMC-Na solution in normal saline. Four hours later, mice were sacrificed and the peritoneal cavities were washed with 5 mL of the normal saline. Twenty micro liter of peritoneal fluid was mixed with 0.38 mL of Turk's solution (0.01% crystal violet in 3% acetic acid) and the number of leukocytes was counted under a light microscope. Dexamethasone was administrated to mice as a positive control in this experiment.
Heat induced haemolysis
Preparation of erythrocyte suspension: fresh whole rat blood (10 mL) was collected and transferred to heparinized centrifuge tubes. The tubes were centrifuged at 3000 x g for 5 min, and washed three times with equal volume of normal saline. The volume of the blood was measured and reconstituted as a 40% v/v suspension with isotonic buffer solution (10 mM sodium phosphate buffer pH 7.4). The isotonic buffer solution (5 mL) containing 200-400 µg/mL of test sample were put in centrifuge tubes. Control tubes contained 5 mL of the vehicle. Aspirin was used as reference standard. Rat erythrocyte suspension (0.02 mL) was added to each tube and gently mixed. The tubes were incubated at 54 °C for 20 min in a regulated water bath. At the end of the incubation, the reaction mixture was centrifuged at 1300 x g for 3 min and the absorbance (OD) of the supernatant measured spectrophotometrically at 540 nm using micro plate titer (Eliza). (Shinde et al., 1999; Dharmasiri et al., 2003). Percent inhibition=A 540 Control - A 540 Sample x 100/A 540 Control.
Inhibition of protein denaturation
The reaction mixture (0.5 mL) consisted of 0.45 mL bovine serum albumin (5% aqueous solution) and 0.05 mL of test extract. pH was adjusted at 6.3 using a small amount of 1N HCl. The samples were incubated at 37 °C for 20 min and then heated at 57 °C for 3 min, after cooling; 2.5 mL phosphate buffer saline (pH 6.3) was added to each tube. Turbidity was measured spectrophotometrically at 660 nm. For control tests 0.05 mL distilled water was used instead of extracts while product control tests lacked bovine serum albumin (Chaterjee & Das, 1996). The percentage inhibition of protein denaturation was calculated as: Percentage inhibition=100 - (O.D of test - O.D. of product control)/O.D. of control X100.
Statistical analysis
Data are reported as mean±SD and were analyzed statistically by analysis of variance (ANOVA) followed by Dunnett's test. Results with p<0.05 were considered significant.
Results
Extraction and phytochemical analysis of extract and fractions of M. macrantha
The yield of PE, CH, TAN and MM was found to be 2-2.5%, 3.8-4.2%, 15-17% and 11-13% w/w respectively. The preliminary phytochemical tests revealed the presence of steroids, alkaloids, tannins, carbohydrates and mucilage in bark of M. macrantha.
GC-MS analysis of M. macrantha
The gas chromatography analysis of unsaponifiable matter of pet. ether extract of M. macrantha resulted in the identification of steroids (cholesta-4,6-dien-3-ol, diisoeugenol, campesterol, stigmasterol, β-sitosterol) as displayed in Figure 1.
Chromatographic separation and identification of compound
Chromatographic separation and isolation of MMS 1 and MMS 2 gave yield 68 and 15 mg, respectively. Comparison of the spectral data by 1HNMR, 13C NMR, and mass fragmentation pattern by GC-MS of compounds established and allowed to proposing the structure of β-sitosterol and stigmasterol respectively. The major compounds in PEMM were analyzed with HPLC (Figure 2).
Total phenolics and proanthocyanidin content
The amount of total phenolics and proanthocyanidin was found as 50.20±2.36 mg of gallic acid equivalents and 10.25 mg of epicatechin equivalents/g in the TAN fraction of M. macrantha respectively.
Acute toxicity test
The oral toxicity study (LD50) of all samples of M. macrantha estimated more than 5 g/kg.
Anti inflammatory activity
The treatment with crude extract and fractions of M. macrantha namely PE, CH, TAN and MM (250 and 500 mg/kg), as well as diclofenac sodium (13.5 mg/kg) significantly (p<0.001) inhibited the carrageenan-induced rat paw edema formation (Figure 3), which was measured at the third hour of the experiment (peak of edema formation). The results were statistically significant in comparison to the control. There was a significant reduction (p<0.05) in histamine induced rat paw edema in all groups (Figure 4). The maximal paw thickness was observed at 1 h of after sub plantar injection in vehicle treated control group. The animals treated with higher doses of test sample of PE, CH, TAN and MM (500 mg/kg, p.o.) produced statistically significant (p<0.01 and p<0.001) inhibition of the edema induced by histamine at 2nd and 3rd h, when compared to the vehicle treated control groups.
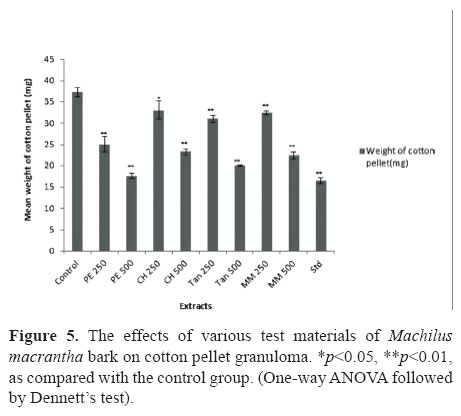
In acetic acid induced vascular permeability assay it was observed a significant activity (p<0.01) for the groups treated with PE, CH, TAN and MM at 500 mg/kg and the positive drug, indomethacin (20 mg/kg), produced dose-related inhibitory effect on peritoneal capillary permeability produced by acetic acid in mice by 66.76, 42.44, 68.44, 63.7 and 83.90%, respectively (Table1). In order to assess its efficacy against the proliferative phase of inflammation in which tissue degeneration and fibrosis occur the widely used cotton pellet granuloma test was employed. In this experiment it was observed a significant anti-proliferative effect (p<0.001) only for the groups treated with PE and TAN (500 mg/kg, p.o.) and diclofenac (13.5 mg/kg, p.o.), daily for six days. The reductions were 52.82, 46.29 and 55.95 %, respectively (Figure 5).
Analgesic activity
TAN and MM at a dose of 500 mg/kg, as well as aspirin inhibited the number of writhings significantly (Table 1). In M. macrantha extracts, produced inhibition on formalin-induced biphasic pain responses (neurogenic and inflammatory pain) in rat. The analgesic effect of PE and CH (250 and 500 mg/kg) fraction occurred predominately during the second phase; thus these Fraction significantly reduced formalin-induced pain at the both phase with the percentage inhibition.
Anti arthritic activity
Freund's complete adjuvant-induced arthritis in rats
The results presented in Figure 6 showed that extracts of M. macrantha injected orally was capable of reducing the severity of arthritic lesions and a statistically significant (p<0.05) inhibition of the paw edema as compared to the control group. The test extracts of M. macrantha namely PE, CH, TAN and MM at the doses of 250 and 500 mg/kg (p.o.) showed 37.28, 59.45, 39.58 44.59, 33.54, 42.84, 35.21 and 48.33% inhibition of rat paw edema, respectively, while the standard was found to inhibit this edema to an extent of 57.76% at 21 days. Swelling and redness developed over a 24 h period in the foot injected with adjuvant. This inflammatory reaction subsided slightly during the next 11 to 14 days and then increased at that time when disseminated arthritis appeared. In rats treated from the day of adjuvant injection, the paw swelling was completely suppressed and no secondary increase was seen. The drug treatment for 14 days from the day of adjuvant injection suppressed the secondary increase in swelling of the injected foot that occurred with the appearance of polyarthritis.
Hematology
Table 3 represents the hematological changes associated with arthritic condition. Levels of RBC were decreased in arthritic rats with concomitant increases in WBC, platelet count and ESR. These changes were reverted to near normal levels in test drugs treated animals. The adjuvant-reagent treated rat produced the significant increase in RA and CRP factors (RA: 10, CRP: 10) and treatment of rats with test drugs containing M. macrantha extracts exhibited potent inhibitory effects on RA and CRP factors.
Biochemical estimation
A marked increase in aminotransferase and alkaline phosphatase was observed in arthritic rat in serum but it was significantly reduced after the administration of test drugs, compared to the arthritic control rat. Levels of acid phosphatase were increased significantly in rat with adjuvant-induced arthritis while the administration of test drugs i.e. PE at 500 mg/kg to arthritic rat significantly reversed the changes to normal level. Elevated levels of serum ALP in AIA rats can be due to increase in the liver and bone fraction or due to an increase of both isoenzymes (Table 4).
Splenomegaly and body weight
An approximately 40% increase in spleen weight was observed in arthritic animals compared to normal (Figure 7), which was significantly suppressed in rats treated with PE at 500 mg/kg. Almost all test samples moderately able to inhibit the development of splenomegaly. A change in the body weight is a useful index to assess the course of the disease and the response to therapy of anti-inflammatory drugs under investigation (Figure 8).
Membrane stabilizing activity
PE, CH, TAN and MM of M. macrantha at 500 mg/kg markedly decreased leucocytes counts from 5.49±0.09 to 4.73±0.14, 4.16±0.08, 4.34±0.08 and 3.86±0.06 x 103 cells/mL, respectively, in CMC-Na induced leucocytes migration in the mice peritoneal cavity (Table 5). All the extracts of M. macrantha inhibited heat-induced haemolysis of RBCs to varying degrees. PE and TAN exhibited the most potent and conc. dependent inhibition of hemolytic activity. Denaturation of protein as a one the cause of inflammation. Effect of M. macrantha extracts on inhibition of protein denaturation at different concentrations (200 and 400 μg/mL) provided significant protection against denaturation of proteins Table 6.
Discussion and Conclusions
In the present study, the evaluation of anti-inflammatory, analgesic and anti arthritis activity was undertaken using different animal models to fully investigate the potential of M. macrantha to be used in the treatment of inflammatory disorders.
Carrageenan-induced edema is an experimental animal model for acute inflammation and it is believed to be biphasic. The edema and inflammation induced by carrageenan is shown to be mediated by histamine and 5-HT during first 1 h, after which increased vascular permeability is maintained by the release of kinins up to 2.30 h and from 2.30 to 6 h, the mediators appear to be prostaglandins, the release of which is closely associated with migration of leucocytes into the inflamed site (Brito & Antonio, 1998; Di Rosa et al., 1971). M. macrantha bark extracts significantly inhibited the formation of the carrageenan-induced rat paw edema in both phases. Histamine and serotonin are important inflammation mediators and they are potent vasodilator substances as well as increase the vascular permeability (Cuman et al., 2001; Linardi et al., 2002). M. macrantha extracts showed anti inflammatory action in late phase i.e. 3 h indicated inhibiting prostaglandin synthesis. It has been reported that the second phase of edema is sensitive to most clinically effective anti-inflammatory agents.
In vascular permeability assay, test drugs produced significant (p<0.01) dose-related inhibitory effect on peritoneal capillary permeability produced by acetic acid in mice. The positive drug, indomethacin (20 mg/kg), also inhibited (83.90%) peritoneal capillary permeability. Chemical-induced vascular permeability (such as seen with acetic acid) causes an immediate sustained reaction that is prolonged over 24 h and its inhibition suggests that the extract may effectively suppress the exudative phase of acute inflammation (Okoli et al., 2007). The dry weight of the pellet correlates with the amount of the granulomatous tissue (Olajide et al., 2000). Decrease in the granuloma weight indicates a suppression of the proliferative phase being effectively inhibited by the extracts. The effect of PE (500 mg, 52.82%) on dry weight of the cotton pellet was almost near to that of diclofenac (55.95%). This effect may be due to the cellular migration to injured sites and accumulation of collagen and mucopolysaccharide (Vanu et al., 2004).
Acetic acid induced abdominal constriction acts indirectly by inducing the release of endogenous mediators, such as PGE2 (prostaglandin E2) and PGF2α in peritoneal fluids as well as lipooxygenase products, which stimulate the nociceptive neurons sensitive to NSAID (Anindya et al., 2007). Therefore, the result of the acetic acid-induced writhing strongly suggests that the mechanism of M. macrantha extract may be linked partly to inhibition of lipooxygenase and/or cyclooxygenase in peripheral tissues, thereby reducing PGE2 synthesis and interfering with the mechanism of transduction in primary afferent nociceptor (Sulaiman et al., 2008). The significant inhibitory effect of fraction of M. macrantha on nociceptive response in the both phase of formalin test suggest that the anti-nociceptive might be due present of active analgesic principles acting both centrally and peripherally (Sulaiman et al., 2008).
AA is very similar to human RA both in pathological and serological changes, including the involvement of inflammatory mediators in the arthritic etiology (Qin et al., 2008; Fan et al., 2005). In this study, we found that all rats swelled and appeared red in the injected ankle on the 2nd day after FCA injection. On the 9th day, the injected rats appeared secondary affection of the left foot and forelimbs. After 21 days of treatment with extracts of M. macrantha the swelling dimensions of both first and secondary sides significantly decreased as compared to the model group (Vanu et al., 2004).The decrease in RBC levels in arthritic rats reflects the presence of anemia, is the most common extra cellular manifestation in RA and a moderate hypo chromic; normocytic anemia due to reduction in the RBC count. The increase in both WBC and platelet counts might be due to the stimulation of immune system against the invading pathogenic microorganism. This is evident by the infiltration of inflammatory mononuclear cells in the joints of AIA rats (Rajendran et al., 2008). RA factor is observed positively in 80% of arthritis control rat and is also increased in diffuse collagen disease. CRP factor is a diagnostic index of bacterial infection, chronic rheumatoid arthritis, supprurative arthritis, gout, malignant tumor and rheumatoid fever. In this AIA rat model, both ESR and CRP were found to be markedly associated with the development of the disease, and significantly elevated ESR and CRP levels were noted throughout the course of the experiment as compared to control rats (Cai et al., 2006).
The activities of aminotransferase and ALP were significantly increased in FCAIA rats due to increased lipid per oxidation, permeability of cell membrane or altered metabolism of these enzymes, since these are good indices of liver injury (Rajendran et al., 2008). The decreased enzyme levels on herbal drugs treatments emphasizes the decreased bone loss and organ protective role of M. macrantha in AIA rats, since treatment of NSAID which are hepatotoxic result in elevated levels of GOT and GPT in RA. The enhanced protective effect of drugs might be due to the combined effect of polyphenols, sterols and alkaloids.
The reduction in the release of lysosomal enzymes proves beneficial and indirectly confirms the protective effect of M. macrantha extracts ( Amresha et al., 2007). Ideally, an agent active in adjuvant disease should restore the spleen weights and morphology to normal as is the case with herbal drugs (Musculoskel, 2001). The loss of body weight observed in FCA induced arthritic rats may be due to the reduced absorption of glucose and leucine in the rat intestine (Somasundram et al., 1983). M. macrantha treated rats showed significant increase in body weight reveals the restoration of absorption capacity of the intestine in the arthritic animals (Yokota et al., 2006; Bahorun & Trotin, 1994; Gupta et al., 1980).
Phytochemical analysis of the M. macrantha bark extract and its fractions indicated that the large majority of its constituents were phytosterols, polyphenols, alkaloids and mucilage polysaccharides. Major component as phytosterols include β-sitosterol, stigmasterol and campesterol help to reduce the activity of the cells that cause inflammation, restoring the normal balance to the immune system and allowing the body to repair itself (Patrick, 2002). In addition to its phytosterols are active in immune modulation and have anti-inflammatory and antipyretic activity (Gupta et al., 1980), it inhibiting the production of inflammatory cells and chemical agents (cytokines) which cause tissue damage. A 70 kg 60 year old man has knee joint pain and radio logically diagnosed degenerative joint disorder. Intake of daily basis of supplement that contain 100 mg green tea extract and 300 mg avocado-soybean, unsaponifiable reduces their symptoms (US Patent US2006/0029685A1).
In the present study, acetone extracts, which is rich with proanthocyanidin (catechin) and hydrolysable tannins, markedly inhibited joint swelling, pain and down regulated the index of polyarthritis in rats with AA. Catechin may represent a new class of effective anti-inflammatory agents. Catechin inhibited the production of IL-1 and TNF-a. Therefore, it might ameliorate the secondary inflammatory reaction of AA by influencing the secretory function of activated synoviocytes. Catechin and hydrolysable tannins protects inflammatory joints from destruction, in part by blocking PGE2 production. The pharmacologic effects of tannin rich extracts strongly suggest its potential therapeutic role in the treatment of patients with autoimmune disease, especially RA (Li-Qin et al., 2007). Mixture of catechins, including epicatechin (EC) epigallocatechin (EGC), epicatechin gallate (ECG) and epigallocatechin gallate (EGCG) have potent antioxidant activity. Recent studies have shown that polyphenols significantly reduce the incidence of collagen-induced arthritis in mice (US Patent US2006/0029685A1). Pharmacological studies (Viana et al., 1997) demonstrated that this tannin-enriched fraction presents potent analgesic and anti-inflammatory effects, in several experimental models.
Generally, leukocytes migrate to sites of inflammation in response to chemotactic stimulus (Weismann et al., 1980; Wagner & Roth, 2000) and play a pivotal role in the pathogenesis of inflammatory disorders of both acute and chronic types. Thus, herbal sample containing phytosterols and proanthocyanidin may reduce the incidence of super oxide radicals' release by inhibiting leukocyte migration Okoli & Akah, 2004).The membrane stabilization test is based on the finding that potent extracts inhibit heat induced lysis of erythrocytes. The erythrocyte membrane may be considered as model of the lysosomal membrane, phytoconstituents that can prevent the rupture of latter and thereby prevent damage to the tissue caused by the release of hydrolytic enzymes contained within the lysosome may be expected to alleviate some symptoms of inflammation (Olajide et al., 2000). M. macrantha inhibited both heat- induced lysis and protein denaturation. These provide evidence for membrane stabilization as an additional mechanism of their anti-inflammatory effect. Denaturation of protein as a one the cause of inflammation (Mizushima et al.,1965). Production of auto antigens in inflammation disease may be due to in vivo denaturation of protein. Their effect on heat-induced denaturation human serum albumin (HSA) in comparison with several fatty acids which are known to be potent stabilizers of this protein (Saso et al., 2001).
In conclusion, these experimental results point out that the anti-inflammatory, antinociceptive and membrane stabilizing activity of the various extract and fraction of the bark of M. macrantha primarily due to the phytosterols (β-sitosterol and stigmasterol) and phenolic components (proanthocyanidin and gallic acid), alkaloids and mucilage polysaccharides in the plant, which seems to support the use of this plant in traditional medicine. In addition to this study reveals that M. macrantha is a good candidate for a rich source of potent natural compounds and phytochemical studies of the active principles based on the present results may help to discover the new drug for the development of new medicine. To the best of our knowledge, this is the first study evaluating the antinociceptive, anti-inflammatory and membrane stabilizing activity of M. macrantha growing western part of India.
Acknowledgements
The authors are grateful to the Director, Botanical survey of India, Pune for identification of plant specimen. The authors are also thankful to SICART, Vallbh Vidhyanagar, Anand, Gujarat, for carry out analytical work.
Received 1 Oct 2010
Accepted 30 Dec 2010
- Amresha G, Singh PN, Rao CH 2007.Antinociceptive and antiarthritic activity of Cissampelos pareira roots. J Ethnopharmacol 111: 531-536.
- Anindya BA, Sumanta M, Gupta JK, Ghosh T, Dash GK, Sudam S 2007. Analgesic, anti-inflammatory and antipyretic activities of the ethanolic extract and its fractions of Cleome rutidosperma. Fitoterapia 78: 515-520.
- Bahorun T, Trotin F 1994 .Antioxidant activities of Crataegus monogyna extracts. Planta Med 60: 323-326.
- Barbier A 1986. Studies on the chronic phase of adjuvant arthritis effect of SR 41319, a new diphosphonate. Ann Rheum Dis 45: 67-74.
- Baveja SK, Garg ML, Jonejs MP 1968.Investigation of Machilus macrantha Nees. Indian J Pharmacol 30: 11-13.
- Brito ARMS, Antonio MA 1998.Oral anti-inflammatory and antiulcerogenic activities of a hydro alcoholic extract and partitioned fractions of Turnera ulmifolia (Turneraceae). J Ethnopharmacol 61: 215-228.
- Cai X, Wong YF, Zhou H, Xie Y, Liu ZQ, Jiang Z, Bian ZX, XuHX Liu L 2006. The comparative study of Sprague-Dawley and Lewis rats in adjuvant-induced arthritis. N-S Arch Pharmacol 373: 140-147.
- Chatterjee S, Das SN 1996. Anti-Arthritic and Anti-inflammatory effect of a poly-herbal drug (Ease@): its mechanism of action. Indian J Pharmacol 28: 116-119.
- Chattopadhyay P, Besra SE, Gomes A, Das M, Sur P, Mitra S, Vedasiromoni JR 2004. Anti- inflammatory activity of tea (Camellia sinensis) root extract. Life Sci 74: 1839-1849.
- Chopra RN, Nayer SL 1992. Glossary of indian medicinal plants. New Delhi: Publication and Information Directorate, p. 162-163.
- Christel QD, Bernard G, Jacques V, Thierruy D, Claude B, Michel L 2000. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J Ethnopharmacol 72: 35-42.
- Cuman RKN, Bersani Amadio CA, Fortes ZB 2001. Influence of type 2 diabetes on the inflammatory response in rat. Inflam Res 50: 460-465.
- Dharmasiri MG, Jayakody JRAC, Galhena G, Liyanage SSP, Ratnasooriya WD 2003.Anti-inflammatory and analgesic activities of mature fresh leaves of Vitex negundo. J Ethnopharmacol 87: 199-206.
- Di Rosa M, Giroud JP, Willoughby DA 1971. Studies on the mediators of acute inflammatory response induced in rats in different sites of carrageenan and turpentine. J Pathology 104: 15-29.
- Fan AY, Lao L, Zhang RX, Zhoua AN, Wang LB, Moudgil KD, Lee DYW, Mac ZZ, Zhang WY, Bermana BM 2005. Effects of an acetone extract of Boswellia carterii Birdw. (Burseraceae) gum resin on adjuvant-induced arthritis in Lewis rats. J Ethnopharmacol 101: 104-109.
- Gokhale AB, Damre AS, Kulkarni KR, Saraf MN 2002. Preliminary evaluation of anti-inflammatory and anti-arthritic activity of S. lappa, A. speciosa and A. aspera Phytomedicine 9: 433-437.
- Gowda DC, Gowda JP, Anjaneyalu YV 1982. Structure of an arabinoxylan from the bark of Persea macrantha (Lauraceae). Carbohyd Res 108: 261-267.
- Gupta MB, Nath R, Srivastava N, Shanker K, Kishor K, Bhargava KP 1980. Anti inflammatory and antipyretic activities of β-sitosterol. Planta Med 39: 157-163.
- Habib MR, Nikkon F, Rahman M 2007. Isolation of stigmasterol and sitosterol from methanolic extract of root bark of Calotropis gigantean Linn. Pak J Biol Sci 10: 4174-4176.
- Hong YM, Jun PK, Jing RW, Bo YY 2007. Evaluation of the anti-inflammatory and analgesic activities of Liu-Shen-Wan and its individual fractions. J Ethnopharmacol 112: 108-114.
- Hunskaar S, Fasmer OB, Hole K 1985. Formalin test in mice: A useful technique for evaluating wild analgesics. J Neurosci Methods 14: 69-76.
- Kind P, King E 1954. Estimation of plasma phosphatase by determination of hydrolyzed phenol with amino-antipyrine. J Clin Pathol 7: 322-326.
- Kirtikar KR, Basu BD 1999. Indian medicinal plants 2 ed. vol 2. Dehradun: International Book Distributors, p. 2155-2156.
- Koster R, Anderson M, De Beer EJ 1959. Acetic acid for analgesic screening. Feder Proc 18: 412-417.
- Kulkarni YA, Gokhale SB, Veeranjaneyulu A, Surana SJ, Tatiya AU 2009. Effect of Persea macrantha against acute inflammation and adjuvant-induced arthritis in rat. Pharm Biol 47: 304-308.
- Linardi A, Costa SKP, DeSilva GR, Antunes E 2002. Involvement of kinins, mast cells and sensory neurons in the plasma exudation and paw edema induced by styphylococcal entrotoxin B in the mouse. Eur J Pharmacol 399: 235-242.
- Li-Qin T, Wei Wei, Xiao-Yu W 2007. Effects and mechanisms of catechin for adjuvant arthritis in rats. Adv Ther 3: 24.
- Mani V, Kumar KG, Parle M 2007. Antinociceptive and anti-inflammatory effects of Thespesia populnea bark extract. J Ethnopharmacol 109: 264-270.
- Mizushima Y 1965. Screening tests for anti-rheumatic drugs. Lancet 285: 169-170.
- Musculoskel J 2001. Animal models of rheumatoid arthritis. Neuron Interact 1: 377-385.
- Newbould BB 1963. Chemotherapy of arthritis induced in rats by mycobacterial adjuvant. Br J Pharmacol 21: 127-136.
- Okoli CO, Akah PA 2004. Mechanisms of the anti-inflammatory activity of the leaf extracts of Culcasia scandens P. Beauv (Araceae). Pharmacol Biochem Behav 79: 473-481.
- Okoli CO, Akah PA, Nwafor SV, Anisiobi AI, Ibegbunam IN, Erojikwe O 2007. Anti-inflammatory activity of hexane leaf extract of Aspilia africana C.D. Adams. J Ethnopharmacol 109: 219-225.
- Olajide OA, Awe SO, Makinde JM, Ekhelar AI, Olusola A, Morebise O, Okpako DT 2000. Studies on the anti-inflammatory, antipyretic and analgesic properties of the Alstonia boonei stem bark. J Ethnopharmacol 71: 179-186.
- Patrick JDB 2002. Sterols and sterolins: new drugs for the immune system? Therapeutic focus-reviews. DDT 7: 14.
- Price ML, Scoyoc SV, Butler LG 1978. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. J Agri Food Chem 26: 1214-1218.
- Qin G, Jinjun S, Liuqing D, Lijun Jiang, Huiqin Xu 2008. Therapeutic effects of daphnetin on adjuvant-induced arthritic rats. J Ethnopharmacol 120: 259-263.
- Rajendran M, Palanivelu S, Panchanadam, S 2008.Salubrious effect of Kalpaamruthaa, a modified indigenous preparation in adjuvant-induced arthritis in rats: A biochemical approach. Chemico-Bio l Inter 173: 148-158.
- Reitman S, Frankel S 1957. A calorimetric method for the determination of serum-oxalacetic and glutamic-pyruvic transaminase. Am J Clin Pathol 28: 56-63.
- Saso L, Valentin G, Casini ML, Grippa E, Gatto MT, Leone MG, Silvestrini B 2001. Inhibition of heat-induced denaturation of albumin by nonsteroidal anti-inflammatory drugs (NSAIDs): pharmacological implications. Arch Pharm Res 24: 150-158.
- Satyavati GV, Gupta AK 1987. Medicinal plants of India vol 2. New Delhi: Indian Council for Medical Research, p. 396.
- Saxena VK, Sosanna A 2005. Sitosterol-3-β-D-xylopyranoside from the flowers of Tridex procumbers Linn. J Chem Sci 117: 263-266.
- Shinde UA, Phadke AS, Nair AM, Mungantiwar, Dikshi VJ, Saraf MN 1999. Membrane stabilizing -a possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia 70: 251-257.
- Somasundram S, Sadique J, Subramaniam 1983. In vitro absorption of 14C leucine during inflammation and the effect of anti-inflammatory drugs in the jejunum of rats. Biochem Medica 29: 259-264.
- Sulaiman MR, Hussain MK, Zakariz ZA, Somchit MN, Moin S,Mohamad AS, Israf DA 2008. Evaluation of the antinociceptive activity of Ficus deltoidea aqueous extract. Fitoterapia 79: 557-561.
- Talapatra B, Ray T, Talapatra S 1978.Structure and partial syntheses of machicendiol, machicenonol and machicenin-three new nor-lignans from Machilus glucescens: Preferred confirmation of the side chain of machicendiol. Indian J Chem Soc LV: 1204-1208.
- Talapatra SK, Chakravarti R, Talapatra B 1979.Terpenoids and related compounds: Chemical constituents of Machilus parviflora Meissn (Lauraceae). Indian J Chem 17B: 298-299.
- Tatiya AU, Hatapakki BC 2003.Anti-inflammatory activity of bark of Machilus macrantha Nees (Lauraceae). Indian J Pharm Sci 3:240-242.
- Tebourbi O, Trabelsi C, Nasr CB, Sakly M 2006. Antioxidant activity of Extract of Rhus oxyacantha root cortex. Indian J Exp Biol 44: 246-249.
- Trease GE, Evans WC 1983. Textbook of Pharmacognosy London: Balliere-Tindal, p. 343-383.
- US Patent US2006/0029685A1 2006. Combination of unsaponifiable lipids combined with polyphenols and /or catechin for the protection, treatment and repair of cartilage in joints of human and animals. p. 1-5.
- Vanu RR, Palanivelu S, Panchanatham S 2004. Anti-Inflammatory effect of Semecarpus Anacardium Linn. Nut extract in acute and chronic inflammatory conditions. Biol Pharm Bull 27: 2028-2031.
- Viana GSB, Bandeira MAM, Moura LCM, Souza-Filho VP, Matos FJA, Ribeiro RA 1997. Analgesic and Anti-inflammatory effects of the tannin fraction from Myracrodruon urundeuva Fr. Phytother Res 11: 118-122.
- Wagner JG, Roth AR 2000. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev 52: 349-374.
- Weissmann G, Smolen JE, Korchak HM 1980. Release of inflammatory mediators from stimulated neutrophils. New Eng J Med 303: 24-7.
- Whittle BA 1964. The use of changes in capillary permeability in mice to distinguish between narcotic and non-narcotic analgesics. Br J Pharmacol 22: 246-253.
- Winter CA, Porter CC 1957. Effect of alteration in side chain upon anti-inflammatory and liver glycogen activities in hydrocortisone esters. J Am Pharm Assoc 46: 515-519.
- Winter CA, Risley EA, Nuss CW 1962. Carrageenan-induced edema in hind paws of the rat as an assay for anti-inflammatory drugs. P Soc Exp Biol Med 111: 554-547.
- Yokota J, Takuma D, Hamada A, Onogawa M, Yoshioka S, Kusunose M, Miyamura M, Kyotani S, Nishioka Y 2006. Scavenging of reactive oxygen species by Eriobotrya japonica seed extract. Biol Pharm Bull 29: 467-471.
Publication Dates
-
Publication in this collection
26 Aug 2011 -
Date of issue
Dec 2011
History
-
Received
01 Oct 2010 -
Accepted
30 Dec 2010