Abstract
Copaiba oil, extracted from Copaifera multijuga Hayne, Fabaceae, is widely used for medicinal purposes, especially to treat inflammatory processes. However, there is no report regarding its effect on reproductive performance after used in repeated doses orally. The present study evaluated the effects of the oral administration of Copaiba oil (at doses of 200, 500 or 2500 mg/kg) or water (control) for eight weeks in male Wistar rats. Treated males mated untreated females, and parameters as fertility rates, absolute and relative mass of accessory sexual organs and histology and development of the offspring were evaluated. Chemical analysis revealed the presence of 22 components accounting for 99.11% of the Copaiba oil. The main compounds identified were sesquisterpenes. The reproductive toxicology results indicate that there was no difference between the treated groups compared with the control group in any of the parameters, suggesting that the oral treatment with C. multijuga oil for eight weeks does not affect reproductive performance of male Wistar rats.
Copaifera multijuga oil; Copaiba oil; Reproductive performance; Reproductive toxicity; Chronic treatment; Behavioral parameters
Introduction
Copaifera multijuga Hayne, Fabaceae, is one of the most used medicinal plants in Brazil (Veiga-Júnior and Pinto, 2002Veiga-Júnior, V.F., Patitucci, M.L., Pinto, A.C., 1997. Controle de autenticidade de óleos de copaíba comerciais por cromatografia gasosa de alta resolução. Quim. Nova 20, 612-615.). The oleoresin extracted from its trunk, the Copaiba oil, can be found in almost all fairs, and herbal and natural products popular markets nationwide (Maciel et al., 2002Maciel, M.A.M., Pinto, A.C., Veiga-Júnior, V.F., 2002. Plantas medicinais: A necessidade de estudos multidisciplinares. Quim. Nova 25, 429-438.).
Phytochemical studies showed that C. multijuga oil contains fatty acids (palmitic and linoleic acids) and mixtures of sesquiterpenes (Craveiro et al., 1978Craveiro, A.A., Maia, J.G.S., Varejão, M.J.C., Filho, W.W., Mourão, A.P., Alencar, J.W., 1978. Estudo químico de óleos essenciais, oleaginosas e láticas da Amazônia I. Composição e oxidação do óleo de uma espécie de Copaifera. Acta Amaz. 8, 705-706.). The sesquiterpene caryophyllene have been shown to be the main component, and it is the most used biomarker to authenticate this plant (Soares et al., 2003Soares, J.G. Vareão, M.J.C., Wolter-Filho, W., Mourão, A.P., Craveiro, A.A.R., Alencar, J.C., 2003. Estudo químico de óleos essenciais, oleaginosas e láticas da Amazônia I. Composição e oxidação do óleo de uma espécie de Copaifera. Acta Amaz. 9, 65-59.).
The popular use of Copaiba oil as an anti-inflammatory and healing agent is reported since the sixteenth century and occurs in all regions of Brazil. The oil or its ointments are administered orally or topically (Maciel et al., 2002). Pharmacologically, Copaiba oil has been shown to elicit anti-inflammatory (Carvalho et al., 2005Carvalho, J.C.T., Cascon, V., Possebon, L.S., Morimoto, M.S.S., Cardoso, L.G.V., Kaplan, M.A.C., Gilbert, B., 2005. Topical antiinflammatory and analgesic activities of Copaifera duckei Dwyer. Phytother. Res. 19, 946-950.; Veiga-Júnior et al., 2007), antitumor (Lima et al., 2003Lima, S.R.M., Veiga-Júnior, V.F., Christo, H.B., Pinto, A.C., Fernandes, P.D., 2003. In vivo and in vitro studies on the anticancer activity of Copaifera multijuga Hayne and its fractions. Phytother. Res. 17, 1048-1053.), antimicrobial (Tincusi et al., 2002Tincusi, B.M., Jiménez, I.A., Bazzocchi, I.L., Moujir, L.M., Mamani, Z.A., Barroso, J.P., Ravelo, A.G., Hernández, B.V., 2002. Antimicrobial terpenoids from the oleoresin of the Peruvian medicinal plant Copaifera paupera. Planta Med. 68, 808-812.; Santos et al., 2008Santos, A.O., Ueda-Nakamura, T., Dias-Filho, B.P., Veiga-Júnior, V.F., Pinto, A.C., Nakamura, C.V., 2008. Antimicrobial activity of Brazilian copaiba oils obtained from different species of the Copaifera genus. Mem. I. Oswaldo Cruz. 103, 277-281.) and antinociceptive activities (Gomes et al., 2007Gomes, N.M., Rezende, C.M., Fontes, S.P., Matheus, M.E., Fernandes, P.D., 2007. Antinociceptive activity of Amazonian Copaiba oils. J. Ethnopharmacol. 109, 486-492.).
However, the use of medicinal plants and their components may exert adverse effects (Reboredo et al., 2007Reboredo, M.M., Lucinda, L.M.F., Rocha, C.B., Queiroz, G.T., Faria, V.C., Vieira, V.A., 2007. Avaliação da toxicidade do extrato aquoso de Caesalpinia ferrea em órgãos vitais, no sistema reprodutor e na produção de espermatozóides de ratos Wistar submetidos a tratamento subagudo. Bol. Cent. Biol. Reprod. Juiz de Fora 26, 11-17.), thus characterizing a serious problem of public health. These adverse effects, as well as the possible synergistic action with other drugs, are quite common. Toxicological tests assess the effects of the treatment on many organs (Barnes and Dourson, 1998Barnes, D.G., Dourson, M., 1988. Reference dose (RfD): Description and use in health risk assessments. Reg. Toxicol. Pharmacol. 8, 471-486.), as well as the influence of the treatment on reproduction (Clark, 1993Clark, D.O., 1993. Pharmacokinetic studies in developmental toxicology: practical consideractions and approaches. Toxicol. Method. 3, 223-251.). These parameters are used to characterize dose-response relationships, and help choose the doses used in studies of chronic exposure to certain substances (Barnes and Dourson, 1998).
The male reproductive system is very sensitive to the action of harmful factors, and the exposure to certain agents can cause changes in these organs, impairing the reproductive competence of the individual (Reboredo et al., 2007). The action of a toxic agent can interfere with sexual maturation, the production and transport of gametes, the spermatogenic cycle, the sexual behavior and/or fertility (Kimmel et al., 1995Kimmel, G.L., Clegg, E.D., Crisp, T.M., 1995. Reproductive toxicity testing: A risk assessment perspective. In: Witorsch RJ (Ed.). Reprod. Toxicol. New York: Raven Press. p. 75-98.).
Taking into consideration the popular use and great commercialization of Copaiba oil, and the lack of information regarding its reproductive toxicity, this study aims to evaluate the effects of the treatment with C. multijuga oil on the male reproductive system of rats subjected to oral treatment for eight weeks, as well as on the postnatal development of their offspring.
Materials and methods
Plant material
The plant specimens of Copaifera multijuga, Hayne, Fabaceae, were collected from their natural habitat, in the Amazonian region (Nhamundá-AM), Brazil (S 02°11'09" W 56°42'46") in April, 2009. A voucher specimen of the plant was identified by botanist Ramos, J.F. and deposited at the Instituto Nacional de Pesquisas da Amazônia herbarium, under the number 233154/2009. The C. multijuga Hayne oil was provided by Pronatus, Manaus-AM, Brazil. The density of this oil (0.9945 g/ml) was determined to calculate the volume administered to the animals.
Animals
Adult male and female Wistar rats (Rattus norvegicus), weighing 310-340 g, and 200-240 g, respectively, were obtained from the Department of Physiology and Pharmacology from Federal University of Pernambuco, Brazil. Animals were kept under controlled temperature (22 ± 2°C) and 12:12 light-dark cycle. Commercial food (Labina(r)) and water were available ad libitum. All the experimental protocols were approved by the Animal Experimentation Ethics Committee of the Federal University of Pernambuco, under license 027253; in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Chemical analysis of the Copaifera multijuga fingerprint
The analysis of C. multijuga was performed on a gas chromatograph (Agilent 7890A) coupled to a mass spectrometer (Agilent 5975C MSD) equipped with a 5% Phenyl Methyl Siloxane column (30 m × 250 µm × 0.25 µm, Agilent HP - 5MS). The carrier gas used was helium at a constant flow of 1 ml/min. The initial temperature of the oven was 40°C (maintained for 1 min) and it was increased to 105°C at a rate of 50°C/min, then increased to 150°C at a rate of 1.5°C/min, and finally raised to 280°C at a rate of 30°C/min. The column was maintained at the final temperature for 10 min to ensure the elution of all components. The injector was maintained at 270°C and used in the 1:50 Split. The ionization source was preserved at 230°C and the ionization potential was 70 eV. The retention indices (RI) were determined for the oil components by co-injection of the sample with a homologous series of linear hydrocarbons (C8 to C30) and subsequent application of Van den Dool and Kratz equation (1963)Van den Dool, H., Kratz, P.D., 1963. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 11, 463-471.. The constituents were initially identified by comparing the calculated RI to those published in the literature (Adams, 2007Adams, R.P., 2007. Identification of essential oil components by gas chromatography/mass spectrometry. 4th ed. Carol Stream: Allured Publ.). Their identities were confirmed by comparing the mass spectra obtained with those found in NIST, Adams, MassFinder4, GC/MS libraries and other literature.
Chronic treatment and reproductive performance
Adult naïve male rats were randomly divided into four groups (n = 5/group) and then treated by oral route with C. multijuga (at the doses of 200, 500 or 2500 mg/kg) or water (control) for eight consecutive weeks. During treatment, the body weight was recorded weekly, and food consumption and water intake of the animals were recorded every two days. Animals were observed twice daily for signs of toxicity, such as piloerection, hair loss, diarrhea, changes in locomotor activity, and mortality throughout the experimental period. Animals were kept in individual cages and mated with untreated and fertile females (n = 10/group) in natural estrus (sexually receptive) for five days (male: female ratio, 1:2). The next morning, after the presence of a vaginal plug and sperm in the vaginal smear, were defined as the first gestational day. Pregnant rats were allowed to complete their pregnancy to term. During the pregnancy, rats were closely observed twice a day for survival, changes in appearance, behavior, and signs of vaginal bleeding. The day of parturition was designated as day 1 of postnatal life. On day 4 of postnatal life, litters were culled to seven neonates. In the cases where fewer than seven pups were delivered, additional neonates were fostered from other litters. After birth the following reproductive parameters were analyzed: duration of pregnancy (day), number of live and dead fetuses, the offspring/dam ratio, fertility index (number of pregnant rats/number of mated females × 100), viability index (number of live pups on day 4 of postnatal life/number of live offspring born × 100), and lactation index (number of live pups on day 21 of postnatal life/number of live pups on day 4 of postnatal life × 100). Body weight (g) and length (cm) of the newborn rats were determined on postnatal days 1, 4, 7 and 21. The offspring were closely observed for any external malformation (Silva et al., 2009Silva, E.J.R., Costa-Silva, J.H., Baratella-Evêncio, L., Fraga, M.C.A., Coelho, M.O.C., Wanderley, A.G., 2009. Reproductive assessment of hydroalcoholic extract of Calendula officinalis L. in Wistar rats. Phytother. Res. 23, 1392-1398.).
Behavioral parameters of offspring
After birth, one third of the offspring from each group was analyzed for the following behavioral parameters: postural reflex, day of eye opening, day of adult walking and spontaneous ambulation. The pups' postural reflexes were evaluated on the first and seventh days of life. The pups were put on a plane surface, in supine position, and the righting reflex was measured in seconds. The day of eye opening was determined by the observation of the partial displacement of the palpebral fissure of at least one eye. It was considered day of adult walking when pups ambulated without dragging the hind feet and without touching the belly on the floor. The spontaneous ambulation was determined on postnatal day 20 using a square measuring 30 × 30 cm and divided into nine equal spaces. The pup was put on the central part and for two minutes the number of invaded squares (when the pup put at least three feet on the square) was recorded (Costa-Silva et al., 2006Costa-Silva, J.H., Lyra, M.M.A., Lima, C.R., Arruda, V.M., Araújo, A.V., Ribeiro, A.R., Arruda, A.C., Fraga, M.C.C.A., Lafayette, S.S.L., Wanderley, A.G., 2006. Reproductive toxicological study of Carapa guianensis Aublet (Andiroba) in female Wistar rats. Acta Farm. Bonaer. 25, 425-428.).
Morphological study
After successful mating, the rats were weighed and euthanized by sodium thiopental overdose (0.140 g/kg, i.p.). Necropsy was made for external macroscopic evaluation of testicles, epididymis, vas deferens and seminal vesicles. These organs were removed and weighed individually. Seminal vesicles and the vas deferens were weighed without fluid. The organ weight was expressed in terms of absolute (g) and relative weight (g/100 g of body weight). For microscopic analysis, these organs were fixed in totum in 10% buffered formalin for 48 h at room temperature; afterwards, each sample was washed with water and immersed in 70% ethanol for 3 to 4 days, and then embedded in paraffin. Paraffin sections of 5 µm were obtained and stained with hematoxylin/eosin (HE), and they were subsequently analyzed using the digital image acquisition system MicroDIP/Kacil(r)(Dimech et al., 2006Dimech, G.S., Gonçalves, E.S., Araújo, A.V., Arruda, V.M., Baratella-Evêncio, L., Wanderley, A.G., 2006. Avaliação do extrato hidroalcoólico de Mentha crispa sobre a performance reprodutiva em ratos Wistar. Rev. Bras. Farmacogn. 16, 152-157.).
Statistical analysis
Results were expressed as mean value ± standard error of mean (S.E.M). Variances in data for body and relative organ weights and reproductive parameters were checked for homogeneity by Bartlett's method. If the variances were homogeneous, the data were assessed by variance analysis (ANOVA) followed, as necessary, by Newman-Keuls test. When the data could not be assumed to follow a normal distribution, nonparametric Kruskall-Wallis test followed by the Dunn test was performed. The fertility, viability and lactation indexes were compared by Fisher's exact test. A probability level of less than 5% (p < 0.05) was considered statistically significant. All statistical analyses were performed by GraphPad Prism 5.0.
Results
Chemical analysis of the Copaifera multijuga fingerprint
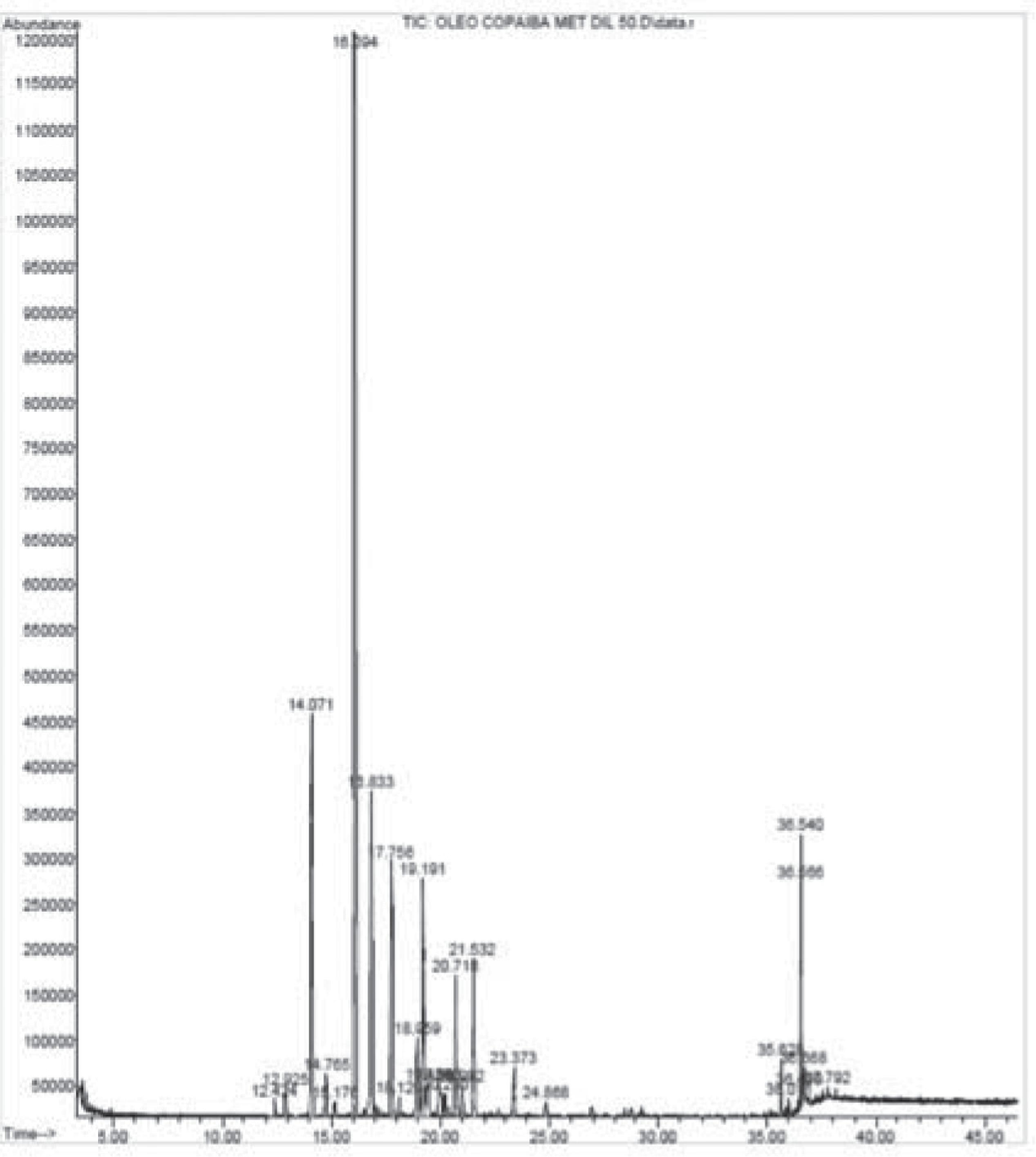
The chemical characterization of C. multijuga oil identified 22 components accounting for 99.11% of total composition (Table 1). The major compounds identified were sesquiterpenes caryophyllene (51.64%), copaene (8.04%), α-trans-bergamotene (7.57%) and germacrene D (6.22%). The Fig. 1 represents the chromatographic profile of the oil.
Chromatographic profile of Copaifera multijuga oil obtained by a gas chromatograph coupled to a mass spectrometer.
Reproductive parameters
During the eight weeks of treatment with C. multijuga (200, 500 or 2500 mg/kg) by oral route, there were no deaths and the treatment did not produce changes in body mass gain, behavioral abnormalities or signs of toxicity in the male rats.
Table 2 shows the reproductive parameters obtained from the mating of untreated females and males treated with C. multijuga. There was no significant difference in mass gain and time of pregnancy indicators of the treated groups compared with the control group.
After the birth of the offspring, we did not encounter stillbirths or any fetal malformation, through macroscopic examination of the pups neither from treated groups nor control group, indicating no teratogenic effects externally visible. Moreover, no significant difference was observed in the offspring/dam relationship and in the percentage of dead fetuses.
Fertility, Viability and Lactation indices of the rats treated with C. multijuga were the same as those obtained in the control group. In addition, the exposure of male rats to Copaiba oil did not affect postnatal development of the offspring (Table 2).
Behavioral parameters of offspring
There was no significant difference in the average number of pups alive at birth between the control group and those fathered by rats treated with C. multijuga 200, 500 and 2500 mg/kg. Furthermore, no significant difference was observed in the behavioral parameters of pups (postural reflex, day of eye opening, day of adult walking and ambulation) among the groups (Table 3).
Morphological study
The treatment with C. multijuga did not change absolute and relative weights of testes, epididymis, seminal vesicle and vas deferens (Table 4).
Fig. 2 shows representative microphotographs of testicular sections from control and treated rats. Microscopic analysis revealed no abnormalities in the testes after treatment with C. multijuga at doses 500 and 2500 mg/kg. The tissue presented normal spermatogenesis, well-preserved sertoli cells and well-delineated peritubular cells in both control (A) and treated groups (B and C). The interstitium were also intact, without inflammatory cell infiltrates.
Cross-section of rat testis treated with Copaifera multijuga oil (Cm) for eight weeks. A, Control group (water); B, Cm 500 mg/kg and C) Cm 2500 mg/kg. Seminiferous tubule (ST), Germinal tissue (TG), Sperm (SP) and Leydig cells (L). Hematoxylin eosin (200 × magnification).
Discussion
In this study, we tested the effects of the oral treatment with C. multijuga oil on reproductive parameters in male and female Wistar rats. Regarding the chemical constitution of the Copaifera multijuga Hayne, Fabaceae, oil, Craveiro et al. (1978) reported the presence of fatty acids, palmitic and linoleic acids, among others. Veiga-Júnior et al. (1997) identified terpene compounds, and these compounds are mixtures of sesquiterpenes (caryophyllene and β-bisabolene). Similar results were published by Dias et al. (2012)Dias, D.O., Colombo, M., Kelmanna, R.G., Souza, T.D., Bassani, V.L., Teixeira, H.F., Veiga-Júnior, V.F., Limberger, R.P., Koester, L.S., 2012. Optimization of headspace solid-phase microextraction for analysis of β-caryophyllene in a nanoemulsion dosage form prepared with copaiba (Copaifera multijuga Hayne) oil. Anal. Chim. Acta 721, 79-84., and in our study, we have also found similar components.
The great complexity of inputs of natural origin led the World Health Organization to consider and accept the chromatographic fingerprint as a more robust way to analyze the quality of natural products, focusing on the qualitative and systematic comparison of individual peaks in different samples. It facilitates the repeatability and credibility of pharmacological and toxicological properties of natural products (WHO, 1991).
The treatment of males before mating and conception may result in infertility, embryonic death and non-specific anomalies of the offspring, as well as a reduced birth weight, decreased litter size and delayed postnatal development. The characteristics of the fetus and its development capability and maturity are expressions of the fertilizing capacity of gametes, successful conception and subsequent intrauterine development. The reproductive toxic effect of plant-derived compounds may be also evaluated by changes in the weight of the male reproductive organs. The weight loss of the gonads, epididymis or sexual accessory glands may decrease the production of sperm and it is considered a standard criterion for the characterization of toxic agents (Reboredo et al., 2007). The spermatogenesis can be reduced by discontinuation of standard divisions of stem cells, degeneration and phagocytosis of cells of germinal tissue, delayed spermatogenesis and sloughing of germ cells, which may occur alone or in combination in the testicular parenchyma by hormonal deprivation or toxic effects exerted by chemicals (Moura et al., 2006Moura, C.S., Guerra, M.M.P., Silva-Júnior, V.A., Silva, C.G.C., Caju, F.M., Alves, L.C., 2006. Avaliação histomorfométrica do parênquima testicular de ratos adultos tratados com diferentes doses de ivermectina. Arq. Bras. Med. Vet. Zoot. 58, 799-808.). Moreover, the pregnancy index reflects the ability of the male to fertilize the female (Gupta et al., 2001Gupta, R.S., Yadav, V.P., Dixit, V.P., Dobhal, M.P., 2001. Antifertility studies of Colebrookia oppositifolia leaf extract in male rats with special reference to testicular cell population dynamics. Fitoterapia 72, 236-245.). In our study, there was no difference in all these parameters among the groups. Moreover, the histological analysis of the testes revealed that the pattern of organization of the seminiferous epithelium presented no obvious changes and that the overall morphology was maintained normal in the treated groups.
The perinatal and postnatal development potential depends on the subjects and their ability to meet varied environmental conditions (Hollenbach et al., 2010Hollenbach, C.B., Bortolini, C.E., Batista, J.M., Hollenbach, E.B., Schuch, T.L., Pacheco, M.H., Mello, F.B., Mello, J.R., 2010. Desenvolvimento pós-natal e potencial teratogênico da prole de ratos Wistar no estudo da toxicidade reprodutiva de duas preparações fitoterápicas contendo soja Glycine max (L.) Merr. Arq. Bras. Med. Vet. Zoot. 62, 845-852.). There is a need for longitudinal studies concerning the reproductive toxicology covering not only structural but also functional changes, such as the development of physical characteristics from the first days of life and even animal behavior (Hollenbach et al., 2010). According to Peters and Guerra (1995)Peters, V.M., Guerra, M.O., 1995. Effects of Dalbergia subcymosa Ducke decoction on rats and their offspring during pregnancy. J. Ethnopharmacol. 46, 161-165., a normal postnatal development is generally observed when the percentage of surviving newborns and their growth are similar to those obtained in control animals. In our study, these parameters were not altered by the treatment with C. multijuga. Additionally, the behavioral parameters were used to assess possible deleterious actions of C. multijuga on neurological and behavioral development of the offspring. The results are suggestive that the administration of C. multijuga in males for eight weeks did not alter the development of their offspring.
Taken together, these results shows that the oral treatment with C. multijuga oil for eight weeks at the doses of 200, 500 or 2500 mg/ml induces no toxic effects in the male reproductive system, in the animals' fertility nor in their offspring development, which suggests no toxic effects on the reproductive performance of the animals.
Acknowledgements
The authors thank the Pronatus Laboratory for providing the Copaifera multijuga Hayne oil, to FINEP for financial support and Rejane de Souza Silva for technical assistance.
References
- Adams, R.P., 2007. Identification of essential oil components by gas chromatography/mass spectrometry. 4th ed. Carol Stream: Allured Publ.
- Barnes, D.G., Dourson, M., 1988. Reference dose (RfD): Description and use in health risk assessments. Reg. Toxicol. Pharmacol. 8, 471-486.
- Carvalho, J.C.T., Cascon, V., Possebon, L.S., Morimoto, M.S.S., Cardoso, L.G.V., Kaplan, M.A.C., Gilbert, B., 2005. Topical antiinflammatory and analgesic activities of Copaifera duckei Dwyer. Phytother. Res. 19, 946-950.
- Clark, D.O., 1993. Pharmacokinetic studies in developmental toxicology: practical consideractions and approaches. Toxicol. Method. 3, 223-251.
- Craveiro, A.A., Maia, J.G.S., Varejão, M.J.C., Filho, W.W., Mourão, A.P., Alencar, J.W., 1978. Estudo químico de óleos essenciais, oleaginosas e láticas da Amazônia I. Composição e oxidação do óleo de uma espécie de Copaifera. Acta Amaz. 8, 705-706.
- Costa-Silva, J.H., Lyra, M.M.A., Lima, C.R., Arruda, V.M., Araújo, A.V., Ribeiro, A.R., Arruda, A.C., Fraga, M.C.C.A., Lafayette, S.S.L., Wanderley, A.G., 2006. Reproductive toxicological study of Carapa guianensis Aublet (Andiroba) in female Wistar rats. Acta Farm. Bonaer. 25, 425-428.
- Dias, D.O., Colombo, M., Kelmanna, R.G., Souza, T.D., Bassani, V.L., Teixeira, H.F., Veiga-Júnior, V.F., Limberger, R.P., Koester, L.S., 2012. Optimization of headspace solid-phase microextraction for analysis of β-caryophyllene in a nanoemulsion dosage form prepared with copaiba (Copaifera multijuga Hayne) oil. Anal. Chim. Acta 721, 79-84.
- Dimech, G.S., Gonçalves, E.S., Araújo, A.V., Arruda, V.M., Baratella-Evêncio, L., Wanderley, A.G., 2006. Avaliação do extrato hidroalcoólico de Mentha crispa sobre a performance reprodutiva em ratos Wistar. Rev. Bras. Farmacogn. 16, 152-157.
- Gomes, N.M., Rezende, C.M., Fontes, S.P., Matheus, M.E., Fernandes, P.D., 2007. Antinociceptive activity of Amazonian Copaiba oils. J. Ethnopharmacol. 109, 486-492.
- Gupta, R.S., Yadav, V.P., Dixit, V.P., Dobhal, M.P., 2001. Antifertility studies of Colebrookia oppositifolia leaf extract in male rats with special reference to testicular cell population dynamics. Fitoterapia 72, 236-245.
- Hollenbach, C.B., Bortolini, C.E., Batista, J.M., Hollenbach, E.B., Schuch, T.L., Pacheco, M.H., Mello, F.B., Mello, J.R., 2010. Desenvolvimento pós-natal e potencial teratogênico da prole de ratos Wistar no estudo da toxicidade reprodutiva de duas preparações fitoterápicas contendo soja Glycine max (L.) Merr. Arq. Bras. Med. Vet. Zoot. 62, 845-852.
- Kimmel, G.L., Clegg, E.D., Crisp, T.M., 1995. Reproductive toxicity testing: A risk assessment perspective. In: Witorsch RJ (Ed.). Reprod. Toxicol. New York: Raven Press. p. 75-98.
- Lima, S.R.M., Veiga-Júnior, V.F., Christo, H.B., Pinto, A.C., Fernandes, P.D., 2003. In vivo and in vitro studies on the anticancer activity of Copaifera multijuga Hayne and its fractions. Phytother. Res. 17, 1048-1053.
- Maciel, M.A.M., Pinto, A.C., Veiga-Júnior, V.F., 2002. Plantas medicinais: A necessidade de estudos multidisciplinares. Quim. Nova 25, 429-438.
- Moura, C.S., Guerra, M.M.P., Silva-Júnior, V.A., Silva, C.G.C., Caju, F.M., Alves, L.C., 2006. Avaliação histomorfométrica do parênquima testicular de ratos adultos tratados com diferentes doses de ivermectina. Arq. Bras. Med. Vet. Zoot. 58, 799-808.
- Peters, V.M., Guerra, M.O., 1995. Effects of Dalbergia subcymosa Ducke decoction on rats and their offspring during pregnancy. J. Ethnopharmacol. 46, 161-165.
- Reboredo, M.M., Lucinda, L.M.F., Rocha, C.B., Queiroz, G.T., Faria, V.C., Vieira, V.A., 2007. Avaliação da toxicidade do extrato aquoso de Caesalpinia ferrea em órgãos vitais, no sistema reprodutor e na produção de espermatozóides de ratos Wistar submetidos a tratamento subagudo. Bol. Cent. Biol. Reprod. Juiz de Fora 26, 11-17.
- Santos, A.O., Ueda-Nakamura, T., Dias-Filho, B.P., Veiga-Júnior, V.F., Pinto, A.C., Nakamura, C.V., 2008. Antimicrobial activity of Brazilian copaiba oils obtained from different species of the Copaifera genus. Mem. I. Oswaldo Cruz. 103, 277-281.
- Silva, E.J.R., Costa-Silva, J.H., Baratella-Evêncio, L., Fraga, M.C.A., Coelho, M.O.C., Wanderley, A.G., 2009. Reproductive assessment of hydroalcoholic extract of Calendula officinalis L. in Wistar rats. Phytother. Res. 23, 1392-1398.
- Soares, J.G. Vareão, M.J.C., Wolter-Filho, W., Mourão, A.P., Craveiro, A.A.R., Alencar, J.C., 2003. Estudo químico de óleos essenciais, oleaginosas e láticas da Amazônia I. Composição e oxidação do óleo de uma espécie de Copaifera. Acta Amaz. 9, 65-59.
- Tincusi, B.M., Jiménez, I.A., Bazzocchi, I.L., Moujir, L.M., Mamani, Z.A., Barroso, J.P., Ravelo, A.G., Hernández, B.V., 2002. Antimicrobial terpenoids from the oleoresin of the Peruvian medicinal plant Copaifera paupera. Planta Med. 68, 808-812.
- Van den Dool, H., Kratz, P.D., 1963. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 11, 463-471.
- Veiga-Júnior, V.F., Patitucci, M.L., Pinto, A.C., 1997. Controle de autenticidade de óleos de copaíba comerciais por cromatografia gasosa de alta resolução. Quim. Nova 20, 612-615.
- Veiga-Júnior, V.F., Pinto, A.C., 2002. O Gênero Copaifera L. Quim. Nova 25, 273-286.
- Veiga-Júnior, V.F., Rosas, E.C., Carvalho, M.V., Henriques, M.G.M.O., Pinto, A.C., 2007. Chemical composition and antiinflammatory activity of copaiba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne. A comparative study. J. Ethnopharmacol. 112, 248-254.
- WHO, 1991. Guidelines for the Assessment of Herbal Medicines. World Health Organization. Geneva.
Publication Dates
-
Publication in this collection
May-Jun 2014
History
-
Received
07 Mar 2014 -
Accepted
05 May 2014