ABSTRACT
The current study explored hepatoprotective effect of Aegle marmelos (L.) Corrêa, Rutaceae, leaves extract. Potentiation of A. marmelos hepatoprotective effect with piperine co-administration was also explored. Wistar rats were randomly divided into seven groups: (i) normal control, (ii) paracetamol group, (iii) silymarin group, (iv) extract-25 group (25 mg/kg body), (v) extract-50 group: (50 mg/kg), (vi) extract-100 group (100 mg/kg) and (vii) extract-25 + piperine group. Hepatotoxicity was induced by administering paracetamol orally in a dose of 400 mg/kg for seven days. The drugs were administered 30 min prior to paracetamol administration and continued for seven days. Animals were ‘sacrificed’ at the end of treatment and serum was collected for evaluating alkaline phosphatase, bilirubin, lactate dehydrogenase, alanine aminotransferase, aspartate aminotransferase IL-10 and TNF-α levels. Liver homogenates were used for determination of oxidative stress (malondialdehyde, reduced glutathione, superoxide dismutase, catalase, glutathione reductase, GSH-S-transferase, glutathione peroxidase and glucose-6-phosphate dehydrogenase). Serum biochemical markers were significantly higher in paracetamol group as compared to normal control group. Significant increase in oxidative stress parameters and inflammatory mediators was also observed. Treatment with A. marmelos curtailed the toxic effects of paracetamol in a dose dependent fashion. 100 mg/kg dose of A. marmelos was found to be most hepatoprotective. The results of extract-100 group were comparable to silymarin group. Low dose of A. marmelos i.e., 25 mg/kg was combined with piperine to evaluate potentiation of hepatoprotective effects of A. marmelos. Piperine co-administration potentiated the hepatoprotective effects, because the combination group results were comparable to high dose A. marmelos group. A. marmelos exerts hepatoprotective activity through its antioxidant and anti-inflammatory properties which was enhanced by piperine.
Keywords:
TNF-α; Oxidative stress; Aspartate aminotransferase; Alanine aminotransferase; Bilirubin
Introduction
Liver plays a major role in various stages of biochemical and physiological activities such as energy and nutrient supply, homeostasis, immunity, detoxification as well as metabolism and storage of nutrients (Singh et al., 2016Singh, H., Sidhu, S., Chopra, K., Khan, M.U., 2016. Hepatoprotective effect of trans-chalcone on experimentally induced hepatic injury in rats: inhibition of hepatic inflammation and fibrosis. Can. J. Physiol. Pharmacol. 94, 879-887.). Industrial toxins, drugs, free radicals, food additives and alcohol are the risk factors for developing liver diseases (Abirami et al., 2015Abirami, A., Nagarani, G., Siddhuraju, P., 2015. Hepatoprotective effect of leaf extracts from Citrus hystrix and C. maxima against paracetamol induced liver injury in rats. Food Sci. Hum. Wellness 4, 35-41.). Drugs causing hepatotoxicity include PCM, NSAID, statins, isoniazid, and various anti-microbial agents (Verma and Neil, 2009Verma, S., Neil, K., 2009. Diagnosis, management and prevention of drug-induced liver injury. Gut 58, 1555-1564.). Treatment of drug induced hepatotoxicity is mainly supportive and discontinuation of offending drug is the first step. Certain hepatotoxic drug specific treatments are also available; for example, liver injury due to valproate is treated with carnitine. Likewise, PCM induced liver injury is treated with N-acetylcysteine (Leise et al., 2014Leise, M.D., Poterucha, J.J., Talwalkar, J.A., 2014. Drug-induced liver injury. Mayo Clin. Proc. 89, 95-106.). Search for newer efficacious hepatoprotective drug fewer side effects is desirable (Mahmood et al., 2014Mahmood, N.D., Mamat, S.S., Kamisan, F.H., Yahya, F., Kamarolzaman, M.F.F., Nasir, N., Mohtarrudin, N., Tohid, S.F., Zakaria, Z.A., 2014. Amelioration of paracetamol-induced hepatotoxicity in rat by the administration of methanol extract of Muntingia calabura L. leaves. Biomed. Res. Int., http://dx.doi.org/10.1155/2014/695678.
http://dx.doi.org/10.1155/2014/695678...
). Recent research has focused on evaluation of hepatoprotective natural products.
Aegle marmelos (L.) Corrêa, Rutaceae, popularly known as Bael in India, is a tough subtropical tree found all over the sub Himalayan forest. The fruits, roots, leaves, bark and seeds of the tree are reported to have medicinal value (Baliga et al., 2011Baliga, M.S., Bhat, H.P., Josepha, N., Fazala, F., 2011. Phytochemistry and medicinal uses of the bael fruit (Aegle marmelos Correa): a concise review. Food Res. Int. 44, 1768-1775.). A. marmelos leaves contain large number of phytochemicals such as eugenol, lupeol, cineol, citronellal, cuminaldehyde, skimmianine, citral, aegeline and marmesinine (Maity et al., 2009Maity, P., Hansda, D., Bandyopadhyay, U., Mishra, D.K., 2009. Biological activities of crude extracts and chemical constituents of Bael, Aegle marmelos (L.) Corr. Indian J. Exp. Biol. 47, 849-861.). A. marmelos leaves have been traditionally used in the treatment of fever, cardiac dysfunction, hepatitis, asthma, diabetes, dyspepsia, seminal weakness, inflammation and febrifuge (Sharma et al., 2011Sharma, G.N., Dubey, S.K., Sharma, P., Sati, N., 2011. Medicinal values of bael (Aegle marmelos) (L.) Corr.: a review. Int. J. Curr. Pharm. Rev. Res. 1, 12-22.). Earlier reports have shown antioxidant and nitric oxide scavenging properties of A. marmelos fruit pulp aqueous extract (Kamalakkannan and Stanely, 2003Kamalakkannan, N., Stanely, M.P.P., 2003. Effect of Aegle marmelos correa fruit extract on tissue antioxidants in streptozotocin diabetic rats. Indian J. Exp. Biol. 41, 285-288.; Jagetia and Baliga, 2004Jagetia, G.C., Baliga, M.S., 2004. The evaluation of nitric oxide scavenging activity of certain Indian medicinal plants in vitro: a preliminary study. J. Med. Food 7, 343-348.). Preclinical studies of A. marmelos whole plant extract and fruit pulp extract showing hepatoprotective effect are also reported. The observed hepatoprotective effects were attributed to enhancement of antioxidants levels and preventing hyper-proliferation in liver (Khan and Sultana, 2009Khan, T.H., Sultana, S., 2009. Antioxidant and hepatoprotective potential of Aegle marmelos Correa against CCl4-induced oxidative stress and early tumor events. J. Enzyme Inhib. Med. Chem. 24, 320-327.; Rajasekaran et al., 2009Rajasekaran, C., Kalaivani, T., Ramya, S., Jayakumararaj, R., 2009. Studies on hepatoprotective activity of ethanolic extracts of fruit pulp of Aegle marmelos (L.) Corr. J. Pharm. Res. 2, 1419-1423.). Previous studies with A. marmelos mainly explored pharmacological properties of the fruit. Hepatoprotective effect of A. marmelos leaves is not much explored.
Piperine is an alkaloid present in fruits of Piper nigrum, Piperaceae. Various pharmacological studies have showed diverse pharmacological properties of piperine (Lee et al., 1984Lee, E.B., Shin, K.H., Woo, W.S., 1984. Pharmacological study on piperine. Arch. Pharm. Res. 7, 127-132.). Piperine has been shown to possess anti-epileptic, analgesic and anti-inflammatory properties and usefulness in various gastrointestinal disorders (Mehmood and Gilani, 2010Mehmood, M.H., Gilani, A.H., 2010. Pharmacological basis for the medicinal use of black pepper and piperine in gastrointestinal disorders. J. Med. Food 13, 1086-1096.). Piperine is recognized as one of the most potent bioavailability enhancers. Studies have demonstrated that piperine can enhance bioavailability (30–200%) of wide range of drugs and nutrients (Atal and Bedi, 2010Atal, N., Bedi, K.L., 2010. Bioenhancers: revolutionary concept to market. J. Ayurveda Integr. Med. 1, 96-99.). Hepatoprotective activity of piperine at a dose of 25 mg/kg has also been reported (Koul and Kapila, 1993Koul, I.B., Kapila, A., 1993. Evaluation of the liver protective potential of piperine, an active principle of black and long peppers. Planta Med. 59, 413-417.; Sabina et al., 2010Sabina, E.P., Souriyan, A.D.H., Jackline, D., Rasool, M.K., 2010. Piperine, an active ingredient of black pepper attenuates acetaminophen-induced hepatotoxicity in mice. Asian Pac. J. Trop. Med. 3, 971-976.).
Hepatotoxicity induced by paracetamol (PCM) overdose is a commonly used experimental model to assess hepatoprotective activity of new pharmacological agents (Hussain et al., 2014Hussain, L., Ikram, J., Rehman, K., Tariq, M., Ibrahim, M., Akash, M.S.H., 2014. Hepatoprotective effects of Malva sylvestris L. against paracetamol-induced hepatotoxicity. Turk. J. Biol. 38, 396-402.; Abirami et al., 2015Abirami, A., Nagarani, G., Siddhuraju, P., 2015. Hepatoprotective effect of leaf extracts from Citrus hystrix and C. maxima against paracetamol induced liver injury in rats. Food Sci. Hum. Wellness 4, 35-41.). PCM is regarded as a safe drug at the therapeutic dose but at higher doses, PCM can cause centrilobular necrosis that eventually leads to liver failure (Lee et al., 1984Lee, E.B., Shin, K.H., Woo, W.S., 1984. Pharmacological study on piperine. Arch. Pharm. Res. 7, 127-132.). The major advantage of PCM model is that it is a clinically relevant model and is a dose dependent hepatotoxicant (Jaeschke et al., 2011Jaeschke, H., McGill, M.R., Williams, C.D., Ramachandran, A., 2011. Current issues with acetaminophen hepatotoxicity – a clinically relevant model to test the efficacy of natural products. Life Sci. 88, 737-745.). The major portion of PCM dose is conjugated with glucuronic acid or sulfate and the rest is converted into reactive metabolite N-acetyl-p-benzoquinoneimine (NAPQI) through cytochrome P450 enzymes (Nelson, 1990Nelson, S.D., 1990. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin. Liver Dis. 10, 267-278.). At therapeutic dose, NAPQI is conjugated with reduced glutathione to form mercapturic acid which is excreted in urine (Mitchell et al., 1973Mitchell, J.R., Jollow, D.J., Potter, W.Z., Gillette, J.R., Brodie, B.B., 1973. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J. Pharmacol. Exp. Ther. 187, 211-217.). However, in case of overdose, excess NAPQI depletes GSH content and binds covalently to hepatic cellular proteins resulting in mitochondrial dysfunction and mitochondrial oxidative stress that eventually induces necrosis and apoptosis of hepatocytes (Bhattacharyya et al., 2013Bhattacharyya, S., Pence, L., Beger, R., Chaudhuri, S., McCullough, S., Yan, K., Simpson, P., Hennings, L., Hinson, J., James, L., 2013. Acylcarnitine profiles in acetaminophen toxicity in the mouse: comparison to toxicity, metabolism and hepatocyte regeneration. Metabolites 3, 606-622.; Abirami et al., 2015Abirami, A., Nagarani, G., Siddhuraju, P., 2015. Hepatoprotective effect of leaf extracts from Citrus hystrix and C. maxima against paracetamol induced liver injury in rats. Food Sci. Hum. Wellness 4, 35-41.).
With this background, the current study was planned to explore hepatoprotective effect of A. marmelos leaves extract in PCM model alone and in combination with low dose (non-hepatoprotective) of piperine.
Material and methods
Aegle marmelos extract preparation
The leaves of Aegle marmelos (L.) Corrêa, Rutaceae, were collected from areas in and around Chandigarh, India during the month of January. The plant material was authenticated by Dr. Sujata Bhattacharya, Assistant Professor, School of Biological and Environmental Sciences, Shoolini University, Solan. Voucher specimens of the plant (SUBMS/89) were deposited in the School of Biological and Environmental Sciences, Shoolini University, Solan. The dried coarsely powdered leaves (500 g) were first extracted with petroleum ether followed by 70% ethanol by hot extraction process. The solvent was removed under reduced pressure after completion of extraction process and the extract was stored in vacuum desiccator till further use.
HPLC analysis of Aegle marmelos extract
The A. marmelos extract was chromatographically analyzed and the rutin content was determined using RP-HPLC (Zu et al., 2006Zu, Y., Fu, Y., Zhao, C., 2006. Simultaneous determination of catechin, rutin, quercetin, kampferol and isorhamnetin in the extracts of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. J. Pharm. Biomed. Anal. 41, 714-719.). The HPLC system of Agilent technologies composed of bin pumps combined with Agilent technologies ALS with photodiode array detector using, with column from Agilent eclipse XBD ® C 18 bonded with 5 µm (4.6 mm × 150 mm). The method was validated and system suitability parameter was calculated by taking percent RSD of the five standards injections using the same concentration of rutin by HPLC method. Limits of detection and quantification were calculated by a method based on standard deviation (σ) and slope (S) of calibration plot using the formula LOD = 3.3σ/S and LOQ = 10σ/S. The finest resolution and sensitivity of the method was obtained for rutin at 257 mm.
Experimental protocol
Wistar albino rats weighing 180–200 g were used in the study. The animals were housed in temperature controlled (25 ± 1 °C) environment and provided free access to pellet food and drinking water. Animals were acclimatized to laboratory conditions one week prior to start of experiments. Institute Animal Ethics Committee (1201/a/08/CPCSEA) approved the study protocol. Rats were randomly divided into seven groups of six animals each, (i) normal control group (2 ml/kg distilled water), (ii) PCM group (400 mg/kg PCM), (iii) silymarin group (positive control, PCM 400 mg/kg + silymarin 200 mg/kg, (iv) A. marmelos extract-25 group (PCM 400 mg/kg + extract-25 mg/kg), (v) extract-50 group (PCM 400 mg/kg + extract-50 mg/kg), (vi) extract-100 group (PCM 400 mg/kg + extract-100 mg/kg) and (vii) extract-25 + piperine group (PCM 400 mg/kg + extract-25 mg/kg + piperine 20 mg/kg). PCM was administered orally in a dose of 400 mg/kg for seven days for the induction of hepatotoxicity (Ravindran et al., 2013Ravindran, C.A., Murugaiyah, V., Khiang, P.K., Xavior, R., 2013. Hepatoprotective activity of leaf of methanol extract of Laurus nobilis L. against paracetamol induced hepatotoxicity in rats. Asian J. Pharm. Clin. Res. 6, 153-157.). The high dose of A. marmelos (100 mg/kg) used in the present study was based on the LD50 values (Veerappan et al., 2007Veerappan, A., Miyazaki, S., Kadarkaraisamy, M., Ranganathan, D., 2007. Acute and subacute toxicity studies of Aegle marmelos Corr., an Indian medicinal plant. Phytomedicine 14, 209-215.). Further, a low dose of 25 mg/kg and intermediate dose of 50 mg/kg were selected. Silymarin was used at a dose of 200 mg/kg (Mahmood et al., 2014Mahmood, N.D., Mamat, S.S., Kamisan, F.H., Yahya, F., Kamarolzaman, M.F.F., Nasir, N., Mohtarrudin, N., Tohid, S.F., Zakaria, Z.A., 2014. Amelioration of paracetamol-induced hepatotoxicity in rat by the administration of methanol extract of Muntingia calabura L. leaves. Biomed. Res. Int., http://dx.doi.org/10.1155/2014/695678.
http://dx.doi.org/10.1155/2014/695678...
) and piperine at 20 mg/kg (Bano et al., 1991Bano, G., Raina, R.K., Zutshi, U., Bedi, K.L., Johri, R.K., Sharma, S.C., 1991. Effect of piperine on bioavailability and pharmacokinetics of propranolol and theophylline in healthy volunteers. Eur. J. Clin. Pharmacol. 41, 615-617.; Hiwale et al., 2002Hiwale, A.R., Dhuley, J.N., Naik, S.R., 2002. Effect of co-administration of piperine on pharmacokinetics of beta-lactam antibiotics in rats. Indian J. Exp. Biol. 40, 277-281.). Piperine is known to exert hepatoprotective effect at a dose of 25 mg/kg. Lower dose is inactive and in the current study, lower dose of both piperine and A. marmelos was combined to explore the augmentation of hepatoprotective effect with piperine co-administration. The A. marmelos extract and piperine were suspended separately in carboxy methyl cellulose and administered through oral gavage separately. All the drugs were administered 30 min before PCM administration orally once daily for seven days.
On the day of sacrifice 2 h after PCM administration rats were injected with thiopentone (50 mg/kg i.p.), and blood was withdrawn by cardiac puncture. The serum was separated by centrifugation at 4000 × g for 15 min at 4 °C. The liver was rapidly removed and washed in ice-cold saline solution. A part of liver was homogenized in phosphate buffer saline (0.1 M PBS, pH 7.4). The homogenates were centrifuged at 4000 × g for 20 min at 4 °C, and supernatant was stored at −80 °C for biochemical estimation. The second part of liver was used for determination of IL-10 and TNF-α level by ELISA and the third was stored in 10% neutral buffered formalin for histopathological study.
Hepatic damage serum biomarkers
Serum biomarkers, alkaline phosphatase (ALP), bilirubin, lactate dehydrogenase (LDH), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured by an auto-analyzer using the Accurex kits (Accurex Biomedical Pvt. Ltd, India).
Oxidative stress parameters
The liver homogenates were used for evaluation of oxidative stress parameters. Malondialdehyde (MDA) level in the liver was determined according to the method of Ohkawa et al. (1979)Ohkawa, H., Ohishi, N., Yagi, K., 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351-358.. Results are expressed as nM MDA/mg of protein. Reduced glutathione level was estimated by the method of Ellman (1959)Ellman, G.L., 1959. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82, 70-77.. The results are expressed as µg/mg of protein. Superoxide dismutase (SOD) activity was estimated according to the method of Robak and Gryglewski (1988)Robak Jm, Gryglewski, R.J., 1988. Flavonoids are scavengers of superoxide anions.Biochem. Pharmacol. 37, 837–841.. The results are expressed as U/mg of protein. Catalase (CAT) activity was measured by the method of Aebi (1974)Aebi, H., 1974. Catalase. In: Bergmeyer, H.U. (Ed.), Methods of Enzymatic Analysis. Academic Press, New York, pp. 673–677.. The results are expressed as µM of hydrogen peroxide decomposed/mg of protein. Glutathione reductase (GR) activity was measured by the method of Carlberg and Mannervik (1975)Carlberg, I., Mannervik, B., 1975. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 250, 5475-5480.. The rate of NADPH oxidation is directly proportional to the GR activity in the sample. GR activity is expressed as nM of NADPH oxidized/min/mg of protein. GSH-S-transferase (GST) activity was measured spectrophotometrically by the method of Habig et al. (1974)Habig, W.H., Pabst, M.J., Jakoby, W.B., 1974. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249, 7130-7139.. GST enzyme activity was calculated as nM of CDNB–GSH conjugate formed/min/mg of protein. Glutathione peroxidase (GPx) activity was calculated as described by Athar and Iqbal (1998)Athar, M., Iqbal, M., 1998. Ferric nitrilotriacetate promotes N-diethylnitrosamine-induced renal tumorigenesis in the rat: implications for the involvement of oxidative stress. Carcinogenesis 19, 1133-1139.. The activity was recorded at 340 nm and expressed as nM of NADPH oxidized/min/mg of protein. Glucose-6-phosphate dehydrogenase (G6PD) activity was determined by the method of Zaheer et al. (1965)Zaheer, N., Tewari, K.K., Krishnan, P.S., 1965. Exposure and solubilization of hepatic mitochondrial shunt dehydrogenases. Arch. Biochem. Biophys. 109, 646-648.. The changes in absorbance were recorded at 340 nm and enzyme activity was calculated as nM of NADPH formed/min/mg of protein. The total protein was estimated by Lowry et al. (1951)Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J., 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265-275. method.
Determination of IL-10 and TNF-α level
IL-10 and TNF-α levels were estimated by ELISA. The concentration of the cytokines in 100 µl sample volume was determined according to the manufacturer's protocol. IL-10 and TNF-α concentrations are expressed as pg/ml.
Histopathological examination
Liver of rats from different groups was fixed in 10% neutral buffered formalin. After fixation, liver samples were dehydrated in alcohol, cleared in xylene, and embedded in paraffin wax 56 °C in hot air oven for 24 h. Paraffin embedded tissue blocks were prepared for sectioning at 5 mm thickness by a microtome. The obtained tissue sections were collected on glass slides, deparaffinized, and stained by hematoxylin and eosin (H&E) stain for histopathological examination through the light microscope.
Statistical analysis
Results are expressed as mean ± SEM. The statistical analysis was performed by Graph Pad Prism 5.0 Software version for Windows (San Diego, CA, USA). The data were analyzed statistically by using one way analysis of variance (ANOVA) followed by post hoc Tukey's test. p < 0.05 was considered to be statistically significant.
Results
HPLC analysis
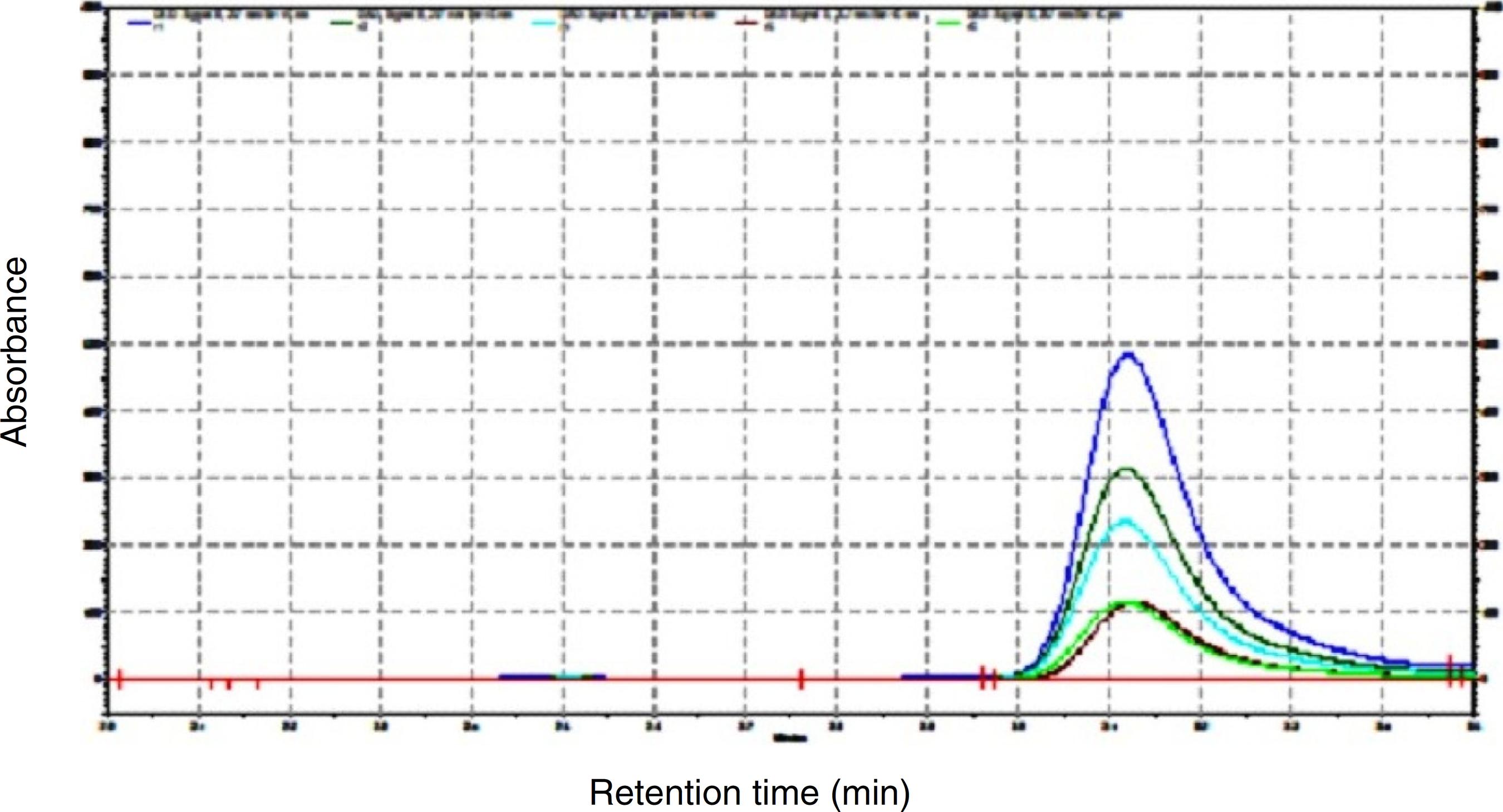
A HPLC analysis for the estimation of rutin in the crude extract was performed. The mobile phase consisted of methanol, acetonitrile and water of HPLC grade in the ratio of 40:15:45 with 1% acetic acid (v/v) in RP-HPLC at flow rate of 1 ml/min. The calibration curve of rutin was prepared by using the different concentrations (100, 200, 300, 400 and 500 µg/ml) (Fig. 1). Typical chromatogram with optimized condition gave sharp and symmetric peak with specific retention time of 3.107 ± 0.0145 min. The linearity range obtained was 5–25 ppm. For the regression equation, the slope was 36081 and the intercept was 351181. The regression coefficient was 0.995. The percentage accuracy obtained was SD = 1.215415, percentage RSD was 1.2 and the mean was 100.6967. The LOD and LOQ values obtained were 0.572 and 1.734, respectively.
Chromatograms of pure rutin at different concentrations (100 µg/ml, 200 µg/ml, 300 µg/ml, 400 µg/ml and 500 µg/ml).
Effect of Aegle marmelos treatment on serum biochemical parameters
The estimation of serum hepatic enzymes is a useful marker for determining extent and type of hepatocellular damage. Administration of PCM caused significant liver damage as revealed by elevated serum hepatic enzymes (AST, ALT, ALP and LDH) and reduced total proteins increased level of total bilirubin. PCM group showed significant increase in AST (p < 0.001), ALT (p < 0.001), ALP (p < 0.001) and LDH (p < 0.001) levels as compared to normal control group. Treatment with silymarin and A. marmelos at a dose of 50 mg/kg and 100 mg/kg significantly reduced the elevated serum enzymes as compared to PCM group. The enzyme levels in extract-25 group were not significantly different from the PCM group, thereby revealing that 25 mg/kg dose of A. marmelos was ineffective. But, co-administration of A. marmelos dose of 25 mg/kg and piperine at a dose of 20 mg/kg (sub hepatoprotective dose) significantly (p < 0.001) lowered the AST, ALT, ALP and LDH levels as compared to PCM group (Table 1) asserting that the addition of piperine amplified the activity of low dose of A. marmelos (25 mg/kg). The same inference was obtained when silymarin treatment group was compared with the A. marmelos treatment groups. The enzyme levels were significantly higher in extract-25 group as compared to silymarin group [AST (p < 0.001), ALT (p < 0.001), ALP (p < 0.001) and LDH (p < 0.01)] thereby confirming that the low dose of A. marmelos (25 mg/kg) alone was ineffective. On the other hand, no significant difference was observed between piperine + extract-25 mg/kg group and silymarin group (Table 1). The comparison between various A. marmelos treatment groups showed dose dependent reductions in the enzyme levels. Treatment extract-100 group reduced the serum enzymes compared to extract-50 group but the reduction was non-significant. Both, extract-50 and extract-100 groups had significantly lower enzymes levels as compared to extract-25 group. The enzymes levels in extract-25 + piperine group were comparable to extract-50 and extract-100 group with no significant difference (Table 1).
Effect of Aegle marmelos on PCM-induced alterations in serum ALT, AST, ALP, LDH activities, total bilirubin and total protein content.
Administration of PCM significantly reduced the total protein concentration (p < 0.001) and increased the total bilirubin level (p < 0.001) as compared to normal control group (Table 1). Treatment with silymarin and extracts of A. marmelos at a dose of 100 mg/kg and 25 mg/kg + piperine restored the total proteins and reduced total bilirubin to the normal control level. The comparison between PCM group and extract-25 group showed increase in proteins and decrease in bilirubin with extract-25 group was non-significant. The extract-50 and extract-100 groups had significantly higher total proteins and lower total bilirubin as compared to PCM group. The low dose of A. marmelos i.e., 25 mg/kg alone was ineffective; however, when this dose was co-administered with low dose of piperine the combination significantly increased total proteins and decreased total bilirubin as compared to PCM group (Table 1). The total bilirubin in silymarin group, extract-100 group and extract + piperine group were not significantly different. This indicates equal efficacy of extract-100 group, extract-25 + piperine group and silymarin treatment in reducing total bilirubin. The total bilirubin in extract-25 (p < 0.001) and extract-50 (p < 0.01) groups was significantly higher as compared to silymarin group. The comparison of results with respect to the total protein levels was non-significant between silymarin group and extract-50, extract-100 and extract-25 + piperine group. In addition, the comparison within the various doses of A. marmelos revealed dose dependent effects. Extract-100 group had significantly higher total protein (p < 0.05) and total bilirubin (p < 0.01) as compared to extract-25 group (Table 1).
Effect of Aegle marmelos treatment on lipid peroxidation, GSH level, SOD and catalase enzymes activity
Administration of PCM induced oxidative stress as indicated by significant increase in MDA level (p < 0.001) and decrease in reduced glutathione activity (p < 0.001), as compared to normal control group. MDA level was significantly reduced by the administration of A. marmelos 50 mg/kg (p < 0.01), 100 mg/kg (p < 0.001) and the combination of low dose of A. marmelos 25 mg/kg and piperine 20 mg/kg (p < 0.05), as compared to PCM-injected group (Fig. 2A). Similarly, PCM-induced reduction on reduced glutathione activity was alleviated by the treatment with A. marmelos 50 mg/kg (p < 0.05), 100 mg/kg (p < 0.01) and the combination of low dose of A. marmelos 25 mg/kg and piperine 20 mg/kg (p < 0.05) (Fig. 2B). Treatment with silymarin200 mg/kg significantly ameliorated the PCM-induced alterations in MDA (p < 0.001) and reduced glutathione (p < 0.01) activity in the liver.
Effect of Aegle marmelos treatment on PCM-induced alterations in hepatic (A) lipid peroxidation, (B) GSH level, (C) SOD and (D) catalase enzymes activity (data presented as mean ± SEM (n = 6). ### p < 0.001 compared with normal control group; ***p < 0.001, **p < 0.01, *p < 0.05 compared with PCM-treated group).
The endogenous antioxidant enzymes i.e. SOD and catalase activities were significantly lowered by the administration of PCM. However, SOD activity was significantly increased by the treatment of A. marmelos 50 mg/kg (p < 0.01), 100 mg/kg (p < 0.001) and the combination of low dose of A. marmelos 25 mg/kg and piperine 20 mg/kg (p < 0.05) as compared to PCM group (Fig. 2C). Similarly, alterations in the catalase activity level had been significantly reversed by the treatment with A. marmelos 50 mg/kg (p < 0.01), 100 mg/kg (p < 0.001) and the combination of low dose of A. marmelos 25 mg/kg and piperine 20 mg/kg (p < 0.01) (Fig. 2D). Silymarin treatment significantly (p < 0.001) ameliorated the PCM-induced alterations in SOD and catalase enzymes activity.
Effect of Aegle marmelos treatment on glutathione reductase, GSH-S-transferase, glutathione peroxidase and glucose-6-phosphate dehydrogenase activity
Fig. 3 shows that PCM group caused a significant (p < 0.001) decrease in glutathione reductase, transferase and peroxidase and G6PD activities as compared with the normal control group. These alterations had been significantly reversed by treatment with different doses of A. marmelos. PCM-induced reduction in glutathione reductase activity was reversed by the treatment of A. marmelos 50 mg/kg (p < 0.01), 100 mg/kg (p < 0.001) and the combination of low dose of A. marmelos 25 mg/kg and piperine 20 mg/kg (p < 0.01) (Fig. 3A). Glutathione transferase activity was increased by A. marmelos 50 mg/kg (p < 0.01), 100 mg/kg (p < 0.001) and the combination of low dose of A. marmelos 25 mg/kg and piperine 20 mg/kg (p < 0.001) treatment in PCM-injected animals (Fig. 3B). Fig. 3C depicts the elevation of glutathione peroxidase activity by the treatment of A. marmelos 50 mg/kg (p < 0.05), 100 mg/kg (p < 0.001) and the combination of low dose of A. marmelos 25 mg/kg and piperine 20 mg/kg (p < 0.05). G6PD activity was increased by the treatment with A. marmelos 50 mg/kg (p < 0.01), 100 mg/kg (p < 0.001) and the combination of low dose of A. marmelos 25 mg/kg and piperine 20 mg/kg (p < 0.01) as compared to PCM-injected group (Fig. 3D). Silymarin 200 mg/kg significantly ameliorated the PCM-induced alterations in glutathione reductase, transferase and peroxidase and G6PD activities.
Effect of Aegle marmelos treatment on PCM-induced alterations in (A) glutathione reductase (GR), (B) glutathione transferase (GST), and (C) glutathione peroxidase (GPx) and (D) glucose-6-phosphate dehydrogenase (G6PD) activity levels (data presented as mean ± SEM (n = 6). ### p < 0.001 compared with normal control group; ***p < 0.001, **p < 0.01, *p < 0.05 compared with PCM-treated group).
Effect of Aegle marmelos treatment on TNF-α and IL-10
Pro-inflammatory cytokine (TNF-α) was significantly increased (p < 0.001) in the serum of PCM administered group as compared to normal control group. However, concomitant treatment with A. marmelos 50 mg/kg (p < 0.01), 100 mg/kg (p < 0.001) and the combination of low dose of A. marmelos 25 mg/kg and piperine 20 mg/kg (p < 0.01) significantly prevented the elevation of serum TNF-α level (Fig. 4A). On the other hand, IL-10, an anti-inflammatory cytokine level was found higher after 7 days of PCM administration. Treatment with A. marmelos 100 mg/kg in PCM administered group significantly increased (p < 0.05) the IL-10 level (Fig. 4B). Silymarin 200 mg/kg did not show any significant effect on the serum IL-10 level but silymarin treatment significantly lowered down the PCM-induced elevation of serum TNF-α level.
Effect of Aegle marmelos treatment on PCM-induced alterations in (A) pro-inflammatory (TNF-α) and (B) anti-inflammatory (IL-10) cytokines (data presented as mean ± SEM (n = 6). ### p < 0.001 compared with normal control group; ***p < 0.001, **p < 0.01, *p < 0.05 compared with PCM-treated group).
Histopathology
Histological analysis revealed that PCM caused marked hepatotoxicity as evident by shrinkage of central veins, hepatocellular hypertrophy and necrosis. Fig. 5 shows normal architecture of hepatocytes and liver parenchyma, distinct hepatic cords and central vein in normal control group. Treatment with A. marmelos (100 mg/kg) and the combination with piperine reduced severity of hepatic damage as compared to PCM group. Moreover, vascular distortion and lymphocyte infiltration were also reduced in extract-100 and piperine group, which further confirms its hepatoprotective effect.
Discussion
The present study investigated hepatoprotective effects of A. marmelos treatment. Further, it was evaluated if piperine co-administration potentiated hepatoprotective effects of A. marmelos.
Aegle marmelos treatment produced dose dependent hepatoprotection. The results of A. marmelos group were comparable to that of silymarin group (positive control) further confirming the beneficial effects of A. marmelos. The results of piperine group (A. marmelos + piperine) as compared to extract-25 group showed significant improvement indicating augmentation of Aegle marmelos effects by piperine. Rutin was identified as the major constituent of the A. marmelos leaves extract. A number of pharmacological properties are attributed to rutin. Rutin has also shown hepatoprotective potential (Ganeshpurkar and Saluja, 2017Ganeshpurkar, A., Saluja, A.K., 2017. The pharmacological potential of rutin. Saudi Pharm. J. 25, 149-164.).
Elevation in serum levels of ALT is considered a reliable indicator of hepatotoxicity. ALT rarely gives false positive results (Ozer et al., 2008Ozer, J., Ratner, M., Shawc, M., 2008. The current state of serum biomarkers of hepatotoxicity. Toxicology 245, 194-205.). The enzymes are normally present in cytoplasm; however, due to the liver injury caused by PCM there is an alteration in hepatic cells membrane permeability thereby resulting in elevation of their levels in blood serum (Gupta and Misra, 2006Gupta, A.K., Misra, N., 2006. Hepatoprotective activity of aqueous ethanolic extract of Chamomile capitula in paracetamol intoxicated albino rats. Am. J. Pharmacol. Toxicol. 1, 17-20.; Abirami et al., 2015Abirami, A., Nagarani, G., Siddhuraju, P., 2015. Hepatoprotective effect of leaf extracts from Citrus hystrix and C. maxima against paracetamol induced liver injury in rats. Food Sci. Hum. Wellness 4, 35-41.). ALT, AST and ALP enzymes are increased during hepatic injury due to the impaired transport function of hepatocytes. High level of AST in the serum indicates cellular injury and disturbance in transport function of cell membrane in the liver (Gupta and Misra, 2006Gupta, A.K., Misra, N., 2006. Hepatoprotective activity of aqueous ethanolic extract of Chamomile capitula in paracetamol intoxicated albino rats. Am. J. Pharmacol. Toxicol. 1, 17-20.). Serum ALT, AST, ALP and LDH were increased after PCM administration indicating hepatocellular injury. Treatment with A. marmelos dose dependently reduced elevated serum enzymes by maintaining integrity of hepatocellualar membrane. Bilirubin is a product formed from the breakdown of red blood cells within the reticuloendothelial system. Bilirubin is a marker of liver function; elevated levels indicate hepatic injury (Abirami et al., 2015Abirami, A., Nagarani, G., Siddhuraju, P., 2015. Hepatoprotective effect of leaf extracts from Citrus hystrix and C. maxima against paracetamol induced liver injury in rats. Food Sci. Hum. Wellness 4, 35-41.). PCM increased serum bilirubin indicating abnormal liver functioning. Treatment with A. marmelos dose dependently reduced bilirubin levels. Oxidative stress plays an important role in the development of PCM induced hepatotoxicity (Radosavljević et al., 2010Radosavljević, T., Mladenović, D., Vucević, D., Vukićević, R.J., 2010. [The role of oxidative/nitrosative stress in pathogenesis of paracetamol-induced toxic hepatitis]. Med. Pregl. 63, 827-832.). A. marmelos treatment significantly improved the activities of SOD, CAT and GPx which are important antioxidant enzymes. SOD converts superoxide radicals into hydrogen peroxide. Sequentially, catalase converts toxic hydrogen peroxide into water and oxygen. GPx plays a key role in maintaining the balance in redox status under oxidative stress and thereby protects lipids and proteins from oxidative stress (Abirami et al., 2015Abirami, A., Nagarani, G., Siddhuraju, P., 2015. Hepatoprotective effect of leaf extracts from Citrus hystrix and C. maxima against paracetamol induced liver injury in rats. Food Sci. Hum. Wellness 4, 35-41.). GSH plays an important role in detoxifying N-acetyl-p-benzoquinone imine (NAPQI). Under limited therapeutic dose, NAPQI is readily detoxified by forming conjugate with GSH. But at higher dose of PCM, excessively formed NAPQI depletes GSH content and thereafter binds to hepatic cellular proteins causing mitochondrial dysfunction (Radosavljević et al., 2010Radosavljević, T., Mladenović, D., Vucević, D., Vukićević, R.J., 2010. [The role of oxidative/nitrosative stress in pathogenesis of paracetamol-induced toxic hepatitis]. Med. Pregl. 63, 827-832.). GSH levels reduced by toxic dose of PCM were significantly increased by A. marmelos treatment. MDA content in livers of PCM group was increased, indicating that PCM evoked oxidative stress. The reduction SOD and CAT level is attributed to the excessive consumption of SOD and CAT enzymes in ROS detoxification (Radosavljević et al., 2010Radosavljević, T., Mladenović, D., Vucević, D., Vukićević, R.J., 2010. [The role of oxidative/nitrosative stress in pathogenesis of paracetamol-induced toxic hepatitis]. Med. Pregl. 63, 827-832.). The antioxidant properties of A. marmelos observed are probably due to high phenolic components of A. marmelos (Kamalakkannan and Prince, 2003aKamalakkannan, N., Prince, P.S.M., 2003. Effect of Aegle marmelos Correa (Bael) fruit extract on tissue antioxidants in streptozotocin diabetic rats. Indian J. Exp. Biol. 41, 1285-1288.,bKamalakkannan, N., Prince, P.S.M., 2003. Hypoglycaemic effect of water extracts of Aegle marmelos fruits in streptozotocin diabetic rats. J. Ethnopharmacol. 87, 207-210.).
Numerous experimental reports demonstrate that piperine acts as a bioenhancer when used in combination with other pharmacological agents (Bano et al., 1991Bano, G., Raina, R.K., Zutshi, U., Bedi, K.L., Johri, R.K., Sharma, S.C., 1991. Effect of piperine on bioavailability and pharmacokinetics of propranolol and theophylline in healthy volunteers. Eur. J. Clin. Pharmacol. 41, 615-617.; Hiwale et al., 2002Hiwale, A.R., Dhuley, J.N., Naik, S.R., 2002. Effect of co-administration of piperine on pharmacokinetics of beta-lactam antibiotics in rats. Indian J. Exp. Biol. 40, 277-281.; Patil et al., 2011Patil, U.M., Singh, A., Chakraborty, A.K., 2011. Role of piperine as a bioavailability enhancer. Int. J. Recent Adv. Pharm. Res. 1, 16-23.). Piperine has also shown hepatoprotective effect in experimental studies (Patil et al., 2011Patil, U.M., Singh, A., Chakraborty, A.K., 2011. Role of piperine as a bioavailability enhancer. Int. J. Recent Adv. Pharm. Res. 1, 16-23.). Although in the present study A. marmelos at dose of 25 mg/kg did not significantly inhibit hepatotoxicity, the combination of this dose with piperine significantly inhibited hepatotoxicity. These results suggested that piperine potentiates hepatoprotective effect of A. marmelos.
TNF-α is a pro-inflammatory cytokine implicated in regulating hepatocyte proliferation, wound healing, and matrix remodeling. Previous experimental studies showed that TNF-α plays a key role in mediating liver injury (Chastre et al., 2012Chastre, A., Bélanger, M., Beauchesne, E., Nguyen, B.N., Desjardins, P., Butterworth, R.F., 2012. Inflammatory cascades driven by tumor necrosis factor-alpha play a major role in the progression of acute liver failure and its neurological complications. PLoS ONE 7, .
https://doi.org/10.1371/journal.pone.004...
; Tahir et al., 2013Tahir, M., Rehman, M.U., Lateef, A., Khan, R., Khan, A.Q., Qamar, W., Ali, F., O’Hamiza, O., Sultana, S., 2013. Diosmin protects against ethanol-induced hepatic injury via alleviation of inflammation and regulation of TNF-α and NF-κB activation. Alcohol 47, 131-139.). Thus, TNF-α is regarded as an important marker in determining protective effect of new drugs against hepatotoxicity. In our study, we found that PCM increased TNF-α level. IL-10 is an anti-inflammatory cytokine. A. marmelos treatment decreased TNF-α level and increased IL-10 level. These results suggested that A. marmelos exerts hepatoprotective actions through inhibition of pro-inflammatory cytokines and activation of anti-inflammatory cytokines. The anti-inflammatory effects of A. marmelos are in concordance with the previous reports (Benni et al., 2011Benni, J.M., Jayanthi, M.K., Suresha, R.N., 2011. Evaluation of the anti-inflammatory activity of Aegle marmelos (Bilwa) root. Indian J. Pharmacol. 43, 393-397.; Kumari et al., 2014Kumari, K.D., Weerakoon, T.C., Handunnetti, S.M., Samarasinghe, K., Suresh, T.S., 2014. Anti-inflammatory activity of dried flower extracts of Aegle marmelos in Wistar rats. J. Ethnopharmacol. 151, 1202-1208.).
Conclusion
In conclusion, the results of this study demonstrate that A. marmelos leaf extract has hepatoprotective properties. The hepatoprotective activity of A. marmelos leaf extract was due to its antioxidant and anti-inflammatory properties that are further supported by the presence of phenolic compounds, rutin in higher amounts in the extracts. A. marmelos treatment significantly enhanced the level of anti-inflammatory cytokines in PCM-treated group. The hepatoprotective effect of A. marmelos leaf extract was comparable to that of silymarin. The hepatoprotective activity of A. marmelos leaf extract increased when used in combination with piperine. The concomitant treatment of piperine with A. marmelos leaves extract exerts synergistic hepatoprotective effect possibly by increasing the absorption or inhibiting the metabolism of A. marmelos leaves phyto-constituents that lead to enhancement of bioavailability of A. marmelos leaves phyto-constituents. The present study is an exploratory study. However, our study results warrant further detailed investigations to determine the other possible hepatoprotective mechanism(s) involved and to isolate and identify the pharmacological bioactive compounds from A. marmelos.
Ethical disclosures
-
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).Confidentiality of data. The authors declare that no patient data appear in this article.Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Acknowledgements
The research facility provided by IKG Punjab Technical University, Kapurthala gratefully acknowledged. Further, the authors would like to acknowledge Dr Sujata Bhattacharya for plant authentication.
References
- Abirami, A., Nagarani, G., Siddhuraju, P., 2015. Hepatoprotective effect of leaf extracts from Citrus hystrix and C. maxima against paracetamol induced liver injury in rats. Food Sci. Hum. Wellness 4, 35-41.
- Aebi, H., 1974. Catalase. In: Bergmeyer, H.U. (Ed.), Methods of Enzymatic Analysis. Academic Press, New York, pp. 673–677.
- Atal, N., Bedi, K.L., 2010. Bioenhancers: revolutionary concept to market. J. Ayurveda Integr. Med. 1, 96-99.
- Athar, M., Iqbal, M., 1998. Ferric nitrilotriacetate promotes N-diethylnitrosamine-induced renal tumorigenesis in the rat: implications for the involvement of oxidative stress. Carcinogenesis 19, 1133-1139.
- Baliga, M.S., Bhat, H.P., Josepha, N., Fazala, F., 2011. Phytochemistry and medicinal uses of the bael fruit (Aegle marmelos Correa): a concise review. Food Res. Int. 44, 1768-1775.
- Bano, G., Raina, R.K., Zutshi, U., Bedi, K.L., Johri, R.K., Sharma, S.C., 1991. Effect of piperine on bioavailability and pharmacokinetics of propranolol and theophylline in healthy volunteers. Eur. J. Clin. Pharmacol. 41, 615-617.
- Benni, J.M., Jayanthi, M.K., Suresha, R.N., 2011. Evaluation of the anti-inflammatory activity of Aegle marmelos (Bilwa) root. Indian J. Pharmacol. 43, 393-397.
- Bhattacharyya, S., Pence, L., Beger, R., Chaudhuri, S., McCullough, S., Yan, K., Simpson, P., Hennings, L., Hinson, J., James, L., 2013. Acylcarnitine profiles in acetaminophen toxicity in the mouse: comparison to toxicity, metabolism and hepatocyte regeneration. Metabolites 3, 606-622.
- Carlberg, I., Mannervik, B., 1975. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 250, 5475-5480.
- Chastre, A., Bélanger, M., Beauchesne, E., Nguyen, B.N., Desjardins, P., Butterworth, R.F., 2012. Inflammatory cascades driven by tumor necrosis factor-alpha play a major role in the progression of acute liver failure and its neurological complications. PLoS ONE 7, .
» https://doi.org/10.1371/journal.pone.0049670 - Ellman, G.L., 1959. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82, 70-77.
- Ganeshpurkar, A., Saluja, A.K., 2017. The pharmacological potential of rutin. Saudi Pharm. J. 25, 149-164.
- Gupta, A.K., Misra, N., 2006. Hepatoprotective activity of aqueous ethanolic extract of Chamomile capitula in paracetamol intoxicated albino rats. Am. J. Pharmacol. Toxicol. 1, 17-20.
- Habig, W.H., Pabst, M.J., Jakoby, W.B., 1974. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249, 7130-7139.
- Hiwale, A.R., Dhuley, J.N., Naik, S.R., 2002. Effect of co-administration of piperine on pharmacokinetics of beta-lactam antibiotics in rats. Indian J. Exp. Biol. 40, 277-281.
- Hussain, L., Ikram, J., Rehman, K., Tariq, M., Ibrahim, M., Akash, M.S.H., 2014. Hepatoprotective effects of Malva sylvestris L. against paracetamol-induced hepatotoxicity. Turk. J. Biol. 38, 396-402.
- Jaeschke, H., McGill, M.R., Williams, C.D., Ramachandran, A., 2011. Current issues with acetaminophen hepatotoxicity – a clinically relevant model to test the efficacy of natural products. Life Sci. 88, 737-745.
- Jagetia, G.C., Baliga, M.S., 2004. The evaluation of nitric oxide scavenging activity of certain Indian medicinal plants in vitro: a preliminary study. J. Med. Food 7, 343-348.
- Kamalakkannan, N., Prince, P.S.M., 2003. Effect of Aegle marmelos Correa (Bael) fruit extract on tissue antioxidants in streptozotocin diabetic rats. Indian J. Exp. Biol. 41, 1285-1288.
- Kamalakkannan, N., Prince, P.S.M., 2003. Hypoglycaemic effect of water extracts of Aegle marmelos fruits in streptozotocin diabetic rats. J. Ethnopharmacol. 87, 207-210.
- Kamalakkannan, N., Stanely, M.P.P., 2003. Effect of Aegle marmelos correa fruit extract on tissue antioxidants in streptozotocin diabetic rats. Indian J. Exp. Biol. 41, 285-288.
- Khan, T.H., Sultana, S., 2009. Antioxidant and hepatoprotective potential of Aegle marmelos Correa against CCl4-induced oxidative stress and early tumor events. J. Enzyme Inhib. Med. Chem. 24, 320-327.
- Koul, I.B., Kapila, A., 1993. Evaluation of the liver protective potential of piperine, an active principle of black and long peppers. Planta Med. 59, 413-417.
- Kumari, K.D., Weerakoon, T.C., Handunnetti, S.M., Samarasinghe, K., Suresh, T.S., 2014. Anti-inflammatory activity of dried flower extracts of Aegle marmelos in Wistar rats. J. Ethnopharmacol. 151, 1202-1208.
- Lee, E.B., Shin, K.H., Woo, W.S., 1984. Pharmacological study on piperine. Arch. Pharm. Res. 7, 127-132.
- Leise, M.D., Poterucha, J.J., Talwalkar, J.A., 2014. Drug-induced liver injury. Mayo Clin. Proc. 89, 95-106.
- Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J., 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265-275.
- Mahmood, N.D., Mamat, S.S., Kamisan, F.H., Yahya, F., Kamarolzaman, M.F.F., Nasir, N., Mohtarrudin, N., Tohid, S.F., Zakaria, Z.A., 2014. Amelioration of paracetamol-induced hepatotoxicity in rat by the administration of methanol extract of Muntingia calabura L. leaves. Biomed. Res. Int., http://dx.doi.org/10.1155/2014/695678
» http://dx.doi.org/10.1155/2014/695678 - Maity, P., Hansda, D., Bandyopadhyay, U., Mishra, D.K., 2009. Biological activities of crude extracts and chemical constituents of Bael, Aegle marmelos (L.) Corr. Indian J. Exp. Biol. 47, 849-861.
- Mehmood, M.H., Gilani, A.H., 2010. Pharmacological basis for the medicinal use of black pepper and piperine in gastrointestinal disorders. J. Med. Food 13, 1086-1096.
- Mitchell, J.R., Jollow, D.J., Potter, W.Z., Gillette, J.R., Brodie, B.B., 1973. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J. Pharmacol. Exp. Ther. 187, 211-217.
- Nelson, S.D., 1990. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin. Liver Dis. 10, 267-278.
- Ohkawa, H., Ohishi, N., Yagi, K., 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351-358.
- Ozer, J., Ratner, M., Shawc, M., 2008. The current state of serum biomarkers of hepatotoxicity. Toxicology 245, 194-205.
- Patil, U.M., Singh, A., Chakraborty, A.K., 2011. Role of piperine as a bioavailability enhancer. Int. J. Recent Adv. Pharm. Res. 1, 16-23.
- Radosavljević, T., Mladenović, D., Vucević, D., Vukićević, R.J., 2010. [The role of oxidative/nitrosative stress in pathogenesis of paracetamol-induced toxic hepatitis]. Med. Pregl. 63, 827-832.
- Rajasekaran, C., Kalaivani, T., Ramya, S., Jayakumararaj, R., 2009. Studies on hepatoprotective activity of ethanolic extracts of fruit pulp of Aegle marmelos (L.) Corr. J. Pharm. Res. 2, 1419-1423.
- Ravindran, C.A., Murugaiyah, V., Khiang, P.K., Xavior, R., 2013. Hepatoprotective activity of leaf of methanol extract of Laurus nobilis L. against paracetamol induced hepatotoxicity in rats. Asian J. Pharm. Clin. Res. 6, 153-157.
- Robak Jm, Gryglewski, R.J., 1988. Flavonoids are scavengers of superoxide anions.Biochem. Pharmacol. 37, 837–841.
- Sabina, E.P., Souriyan, A.D.H., Jackline, D., Rasool, M.K., 2010. Piperine, an active ingredient of black pepper attenuates acetaminophen-induced hepatotoxicity in mice. Asian Pac. J. Trop. Med. 3, 971-976.
- Sharma, G.N., Dubey, S.K., Sharma, P., Sati, N., 2011. Medicinal values of bael (Aegle marmelos) (L.) Corr.: a review. Int. J. Curr. Pharm. Rev. Res. 1, 12-22.
- Singh, H., Sidhu, S., Chopra, K., Khan, M.U., 2016. Hepatoprotective effect of trans-chalcone on experimentally induced hepatic injury in rats: inhibition of hepatic inflammation and fibrosis. Can. J. Physiol. Pharmacol. 94, 879-887.
- Tahir, M., Rehman, M.U., Lateef, A., Khan, R., Khan, A.Q., Qamar, W., Ali, F., O’Hamiza, O., Sultana, S., 2013. Diosmin protects against ethanol-induced hepatic injury via alleviation of inflammation and regulation of TNF-α and NF-κB activation. Alcohol 47, 131-139.
- Veerappan, A., Miyazaki, S., Kadarkaraisamy, M., Ranganathan, D., 2007. Acute and subacute toxicity studies of Aegle marmelos Corr., an Indian medicinal plant. Phytomedicine 14, 209-215.
- Verma, S., Neil, K., 2009. Diagnosis, management and prevention of drug-induced liver injury. Gut 58, 1555-1564.
- Zaheer, N., Tewari, K.K., Krishnan, P.S., 1965. Exposure and solubilization of hepatic mitochondrial shunt dehydrogenases. Arch. Biochem. Biophys. 109, 646-648.
- Zu, Y., Fu, Y., Zhao, C., 2006. Simultaneous determination of catechin, rutin, quercetin, kampferol and isorhamnetin in the extracts of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. J. Pharm. Biomed. Anal. 41, 714-719.
Publication Dates
-
Publication in this collection
Jan-Feb 2018
History
-
Received
1 Aug 2017 -
Accepted
23 Nov 2017