ABSTRACT
Esenbeckia febrifuga (A.St.-Hil.) A. Juss. ex Mart., Rutaceae, is known by several popular names including quina-do-mato. This name is a reference to the use of its bark as febrifuge and in the past was employed as a substitute of Cinchona sp. for treatment of malaria symptoms. This confusion may have been reinforced by the fact that the bark of these plants are similar in appearance and have a bitter taste. In view thereof this study presents the description morphological and anatomical and the histochemistry of the stem bark and contributes to the pharmacobotanical study of plant drugs identified as Brazilian quinas, in sequence to two others studies. Compared with the Cinchona species, the prismatic shape of calcium oxalate crystals and the fibers with adornate end walls proved to be the main characteristics for differentiation.
Keywords:
Bark anatomy; Pharmacobotanical study; Quina-do-mato; Brazilian quina
Introduction
Esenbeckia febrifuga (A.St.-Hil.) A. Juss. ex Mart. (basionym Evodia febrifuga A. St. Hil.) of the family Rutaceae, is a semi-deciduous tree growing to 5–11 m tall (outside of forest canopy it does not grow above 6 m). The trunk is very branched and tortuous with a diameter of 20–40 cm (Fig. 1A) with 3-foliolate leaves and capsule fruits (Fig. 1B) with 10.5–13 mm diameters, muricate, glabrous with loculicidal or septicidal dehiscence. This species is known in Brazil as “quina-do-mato”, “laranjeira-do-mato” and “três-folhas-vermelhas”. Its geographic distribution occurs in tropical rain forests of Brazilian states of the Northeast, Central-West, Southeast and South (Flora do Brasil 2020Flora do Brasil 2020 under construction. Jardim Botânico do Rio de Janeiro. Available at: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB583 (accessed 02.11.17).
http://floradobrasil.jbrj.gov.br/reflora...
). In the Argentina this species is known as “ivirá-oví-guazú” and occur in the Misiones Rainforest (Chebez and Mazariche, 2010Chebez, J.C., Mazariche, M., 2010. Nuestros árboles. De norte a sur, conociendo los árboles de la Argentina. Albatroz, Buenos Aires.) and in the Paraguay its vernacular name is “guatambú-mi” or “ivirá-ñeti-(m)í (Grandtner and Chevrette, 2013Grandtner, M.M., Chevrette, J., 2013. Dictionary of Trees, vol. 2: South America, Academic Press, San Diego, USA.). The bark of E. febrifuga is extremely bitter and astringent and considered as febrifuge in traditional medicine (Saint-Hilaire, 1824Saint-Hilaire, A., [1824]2014. Plantas usuais dos Brasileiros. Fino Traço, Belo Horizonte.; Lindley, 1838Lindley, J., 1838. Flora Medica: A Botanical Account of All the More Important Plants Used in Medicine in Different Parts of the World. Longman, Orme, Brown, Green, and Longmans, London, pp. 210.; Albuquerque, 1968Albuquerque, B.W.P., 1968. Rutaceae do Estado da Guanabara. An. Acad. Bras. Cienc. 40, 499-530.; Corrêa, 1969Corrêa, M.P., 1969. Dicionário de plantas úteis do Brasil e das exóticas cultivadas, vol. 4. Imprensa Nacional, Rio de Janeiro.; Chebez and Mazariche, 2010Chebez, J.C., Mazariche, M., 2010. Nuestros árboles. De norte a sur, conociendo los árboles de la Argentina. Albatroz, Buenos Aires.; Cosenza et al., 2013Cosenza, G.P.P., Somavila, N.S., Fagg, C.W., Brandão, M.G.L., 2013. Bitter plants used as substitute of Cinchona spp. (quina) in Brazilian traditional medicine. J. Ethnopharmacol. 149, 790-796.; Grandtner and Chevrette, 2013Grandtner, M.M., Chevrette, J., 2013. Dictionary of Trees, vol. 2: South America, Academic Press, San Diego, USA.) and was used in the past as a substitute of the true quina, Cinchona spp., Rubiaceae (Lindley, 1830Lindley, J., 1830. An introduction to the Natural System of Botany or, a Systematic View of the Organization, Natural Affinities, and Geographical Distribution, of the Whole Vegetable Kingdom. Longman, Orme, Brown, Green, and Longmans, London.; Peckolt, 1916Peckolt, W., 1916. Monographia das falsas quinas brasileiras, Rio de janeiro.). The bark resembles Angostura bark, and was imported into Europe about 1813 as “Brazilian China bark.” (Oberlin et Schlagdenhauffen, 1874Oberlin et Schlagdenhauffen, M.M., 1874. Étude pharmacographique et chimique d’un nouveau succedanea de l’écorce d’angusture. J. Pharm. Chim. Série 4, Tome 20, 105.; Kaastra, 1982Kaastra, R.C., 1982. Pilocarpine (Rutaceae). Flora Neotropica 33, 1-197.). Infusion of bark was cited as antiparasitic agent in the fight against malaria (Brandão et al., 1985Brandão, M.G.L., Botelho, M.G.A., Krettli, A.U., 1985. Quimioterapia experimental antimalárica com produtos naturais: uma abordagem mais racional? Ciênc. Cultur. 37, 1152-1163.; Dolabela et al., 2008Dolabela, M.F., Oliveira, S.G., Nascimento, J.M., Peres, J.M., Wagner, H., Póvoa, M.M., Oliveira, A.B., 2008. In vitro antiplasmodial activity of extract and constituents from Esenbeckia febrifuga, a planta traditionally used to treat malaria in the Brazilian Amazon. Phytomedicine 15, 367-372.). Previous studies have confirmed the antiplasmodial activity of the aqueous extract of E. febrifuga bark (1 g/kg), with a 43% reduction in the multiplication of Plasmodium berghei in infected mice (Carvalho et al., 1991Carvalho, L.H., Brandão, M.G.L., Santos Filho, D., Lopes, J.L.C., Krettli, A.U., 1991. Antimalarial activity of crude extracts from Brazilian plants studied in vivo in P. berghei-infected mice and in vitro against P. falciparum in culture. Braz. J. Med. Biol. Res. 24, 1113-1123.).
Esenbeckia febrifuga. (A) Overview of whole plant. (B) Detail of twig with leaves and fruits. (C–F) Stem bark. (C) Curved and quill aspect of the dry samples. (D) Outer surface. (E) Inner surface. (F) Maceration. Scales: B (2 cm), C (1 cm), D–F (2 mm).
We have previously studied the stem barks of two Brazilian quinas (Somavilla et al., 2017Somavilla, N.S., Cosenza, G.P., Fagg, C.W., Brandão, M.G.L., 2017. Morpho-anatomy and chemical profile of native species used as substitute of quina (Cinchona spp.) in Brazilian traditional medicine Part II: Remijia ferruginea. Rev. Bras. Farmacogn. 27, 153-157.) and they showed important characters that can be useful for differentiation. The objective of this study was to characterize the stem bark of E. febrifuga, contributing for its better knowledge and pharmacobotanical study.
Materials and methods
Plant material
Stem bark samples of Esenbeckia febrifuga (A.St.-Hil.) A. Juss. ex Mart., Rutaceae, were collected at the Campus Pampulha – Universidade Federal de Minas Gerais, Belo Horizonte, state of Minas Gerais, Brazil and registered as DAT-278 in the DATAPLAMT (http://www.dataplamt.org.br). A voucher specimen Fagg CW 2196 was deposited in the University of Brasilia herbarium (UB).
Morphological, anatomical and histochemical analysis
External and internal aspects such as coloring and texture of the samples were described. For anatomical characterization part of these samples were fixed in solution of formaldehyde-acetic acid-ethanol 50 (1:1:18, Johansen, 1940Johansen, D.A., 1940. Plant Microtechnique. McGraw-Hill Book Company, New York.), rinsed in ethanol 50% and then stored in ethanol 70%. After fixation, these samples were sectioned in microtome type Ranvier and stained with astra blue and fuchsin dyes (Kraus and Arduim, 1997Kraus, J.E., Arduim, M., 1997. Manual básico de métodos em morfologia vegetal. EDUR, Rio de Janeiro.) and mounted on slides with verniz vitral incolor 500® (Paiva et al., 2006Paiva, J.G.A., Fank-de-Carvalho, S.M., Magalhães, M.P., Graciano-Ribeiro, D., 2006. Verniz vitral incolor 500®: uma alternativa de meio de montagem economicamente viável. Acta Bot. Bras. 20, 257-264.). Fresh samples, after being cut with the Ranvier microtome, were submitted to histochemical tests: ferric chloride (Johansen, 1940Johansen, D.A., 1940. Plant Microtechnique. McGraw-Hill Book Company, New York.) and potassium dichromate (Gabe, 1968Gabe, M., 1968. Techniques histologiques. Masson & Cie, Paris.) to detect phenolic compounds, vanillin hydrochloric acid for tannins (Gardner, 1975Gardner, R.O., 1975. Vanillin-hydrochloric acid as a histochemical test for tannin. Biotech. Histochem. 50, 315-317.), acid phloroglucin for lignin (Johansen, 1940Johansen, D.A., 1940. Plant Microtechnique. McGraw-Hill Book Company, New York.), solution of lugol for starch (Johansen, 1940Johansen, D.A., 1940. Plant Microtechnique. McGraw-Hill Book Company, New York.), Sudan III (Sass, 1951Sass, J.E., 1951. Botanical Microtechnique. The Iowa State College Press, Ames.) and Sudan IV (Pearse, 1972Pearse, A.G.E., 1972. Histochemistry: Theoretical and Applied, vol. 2., 3rd ed. The Williams & Wilkins Company, Baltimore.) for lipids, Dittmar and Wagner reagents for alkaloids (Furr and Mahlberg, 1981Furr, M., Mahlberg, P.G., 1981. Histochemical analyses of laticifers and glandular trichomes in Cannabis sativa. J. Nat. Prod. 44, 153-159.); hydrochloric acid and acetic acid were used for crystals tests (Kraus and Arduim, 1997Kraus, J.E., Arduim, M., 1997. Manual básico de métodos em morfologia vegetal. EDUR, Rio de Janeiro.). Part of the samples was macerated for tissue components analysis. For that, the samples were placed in Franklin solution and maintained in an oven (60 ºC) for 72 h (Franklin, 1945Franklin, G.L., 1945. Preparation of thin sections of synthetic resins and wood-resin composites, and a new macerating method for wood. Nature 155, http://dx.doi.org/10.1038/155051a0.
http://dx.doi.org/10.1038/155051a0...
modified by Kraus and Arduim, 1997Kraus, J.E., Arduim, M., 1997. Manual básico de métodos em morfologia vegetal. EDUR, Rio de Janeiro.). After this process, the macerated material was washed with distilled water to completely remove the Franklin solution and stained with ethanolic safranin 1%. The slides obtained from these preparations were analyzed by microscopy Axio Lab A1 (Zeiss) with a camera AxioCamERc 5S attached, and the acquisition, measurement and description of the photomicrography were obtained by ZEN 2012 (Zeiss) program. The botanical description followed the recommendations of Junikka (1994)Junikka, L., 1994. Survey of English macroscopy bark terminology. IAWA J. 15, 3-45. and Richter et al. (1996)Richter, H.G., Mazzoni-Viveiros, S.C., Alves, E.S., Luchi, A.E., Costa, C.G., 1996. Padronização de critérios para a descrição anatômica da casca: lista de características e glossário de termos. IF-Sér. Reg. S. Paulo 16, 1-25.. Mean and standard deviation of the length and width measurement of the phloem fiber cells were performed by Microsoft Excel® 2010 in 100 of these cells.
Results and discussion
Stem bark of E. febrifuga is very thin, ranging from 0.5 to 1.5 mm of thickness. When dried it is curved and single quill (Fig. 1C) and the outer surface is dark gray and lighter gray lichens may occur on its surface. The texture is rough with longitudinal wrinkles and transversal fissures and lenticels (Fig. 1D). Inner surface is yellowish and longitudinally striated (Fig. 1E). This description is similar to that Oberlin et Schlagdenhauffen (1878)Oberlin et Schlagdenhauffen, M.M., 1878. Étude histologique et chimique de differéntes ècorces de la famille des Diosmées. J. Pharm. Chim. Série 4, Tome 28, 225-272., however the coloring of the inner surface is described as reddish by those authors. The powder is yellowish with dark gray dots (Fig. 1F).
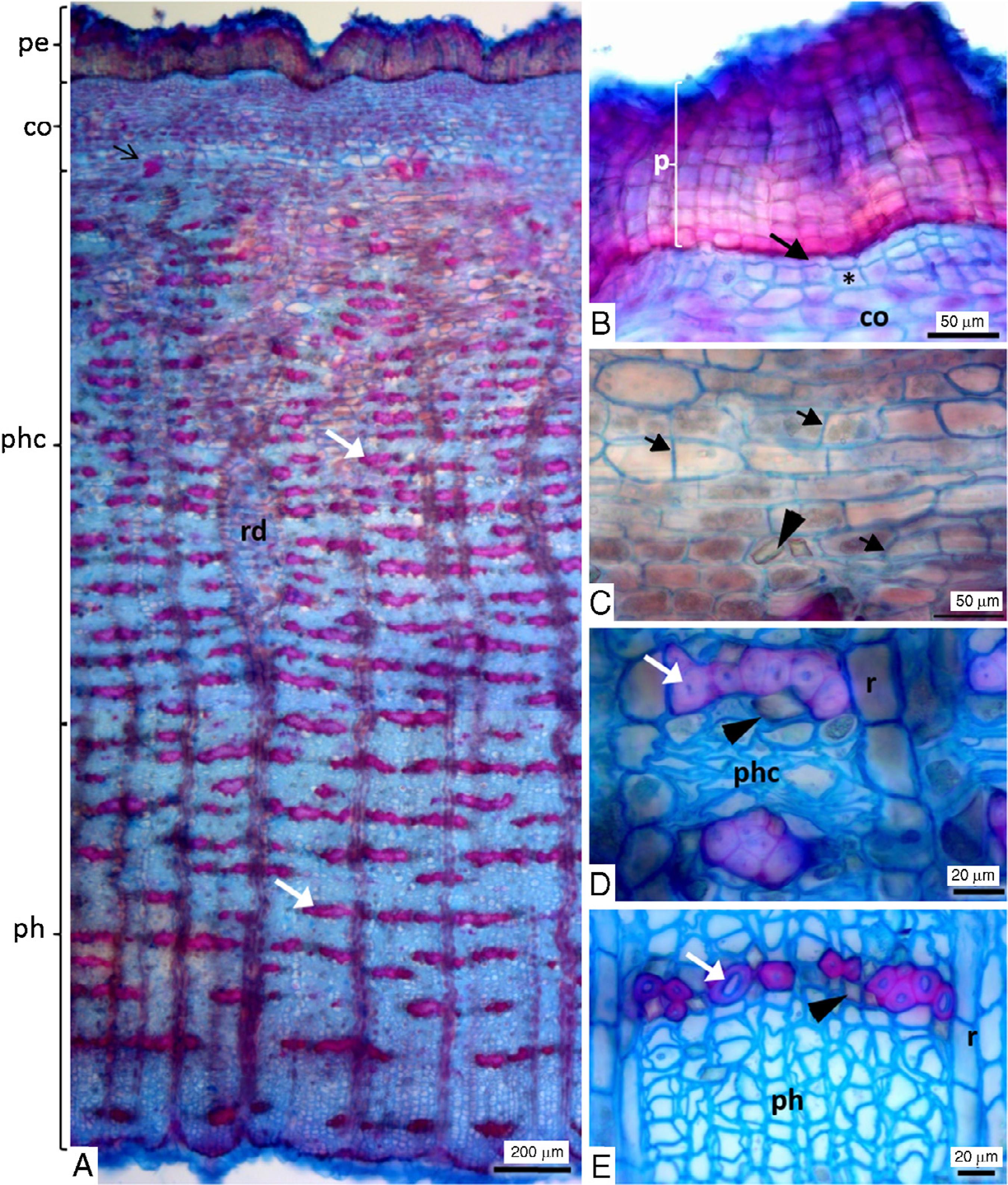
In transversal section, the bark is composed of periderm, parenchymatous cortex and phloem (Fig. 2A). Periderm has a variable number of layers of phellem (cork or suber), generally five to twenty layers, followed by one layer of phellogen and two to three layers of phelloderm (Fig. 2B). Cortical cells exhibit anticlinal divisions and tangential elongation (Fig. 2C) and this avoids the breakage of tissues with the increase in the stem circumference. Cortical cells have calcium oxalate prismatic crystals (Fig. 2C). Green color of the fresh bark is due to deposition of chloroplasts in the outer cortical cells and this feature was also found in the stem bark of another Rutaceae – Phellodendron lavallei Dode (Meskheli et al., 2010Meskheli, M., Mchedlidze, K., Gogitidze, T.S., 2010. Anatomical and morphological description of Phellodendron lavallei Dode stem bark. Georgian Med. News 182, 32-38.). Normally, in some Rutaceae species this photosynthetic cortex is replaced by a rhytidome in the older trunk (Kyriakis and Fasseas, 2010Kyriakis, G., Fasseas, C., 2010. A novel type of tube network within the stem bark of Olea europaea L.. Flora 205, 90-93.), however E. febrifuga maintains this tissue even with the secondary growth of the trunk. Secondary phloem is divided into collapsed phloem and phloem non-collapsed with clusters of fibers tangentially distributed (Fig. 2A, D and E). These clusters demarcate the limit of the phloem with the cortical parenchyma and Brocadet (1921)Brocadet, A.P., 1921. Plantes utiles du Brésil. Vigor Fréres, Paris. also emphasizes this feature in E. febrifuga when describing species of pseudo-quina. There is a deposit of prismatic crystals in the parenchyma cells adjacent to fiber clusters (Fig. 2E). These prismatic crystals were dissolved with hydrochloric acid and did not dissolve with acetic acid indicating calcium oxalate (Kraus and Arduim, 1997Kraus, J.E., Arduim, M., 1997. Manual básico de métodos em morfologia vegetal. EDUR, Rio de Janeiro., p. 136).
Stem bark of Esenbeckia febrifuga. (A) Transversal section shown periderm (pe), cortex (co), collapsed (phc) and non-collapsed (ph) phloem and dilated ray (rd). White arrow: clusters of fibers. (B) Detail of periderm shown phellem (p), phellogen (arrow) and phelloderm (asterisk). (C) Detail of cortical cells with anticlinal division (arrow) and prismatic crystals (arrowhead). (D and E) Collapsed secondary phloem (phc) and non-collapsed secondary phloem (ph), respectively, shown fibers (white arrow), ray cells (r) and prismatic crystals (arrowhead).
In radial and tangential longitudinal section, the secondary phloem is not storied and displays sieve-tube elements with simple (Fig. 3A and B) and compound (Fig. 3C and D) sieve plates on inclined end walls and lateral sieve areas (Fig. 3C). Phloem rays are multiseriate and heterocellular with procumbent and upright cells (Fig. 3E and F). In the maceration the secondary phloem fibers (Fig. 3I–K) are elongated with length ranges from 383.28 to 831.19 µm (630.52 ± 102.97, mean ± standard deviation) and the width in the cell middle region ranges from 11.09 to 24.07 µm (16.41 ± 2.61). The fiber cell wall is not lamellar and usually has the adornate end wall (Fig. 3J–K). Phellem cells appear in the maceration as pieces of tissue with polygonal cells (Fig. 3K). The results of histochemical tests performed on the stem bark show lignin in the fibers and xylem elements, lipids in the cell wall of phellem and large amounts of oil droplets in the parenchyma (Fig. 3G) of the cortex and secondary phloem. Small starch grains appear in the secondary phloem but are very sporadically distributed. Phenolic compounds and tannin tests were negative. Tests for alkaloids was not conclusive because the large amounts of oil droplet in the fresh samples did not allow to verify the staining expected by the test in the cortex and phloem parenchyma, and there was no difference in the phellem and phelloderm cells between control test and that with reagents. Negative tests with reagents of alkaloids was highlighted by Dolabela (2007, p. 131)Dolabela, M.F., Thesis 2007. Atividade antiplasmódica e citotoxicidade de Esenbeckia febrifuga (A. St-Hil.) Juss. Ex Mart. (Rutaceae) e de espécies do gênero Aspidosperma (Apocynaceae). Universidade Federal de Minas Gerais, www.dominiopublico.gov.br/download/texto/cp104928.pdf.
www.dominiopublico.gov.br/download/texto...
and the use of Draggendorff reagent in chromatographic plates (CCDS) containing extracts of this species. The author proposed that such result is due to the fact that the bases are amides and, therefore, will be less likely to give a positive result with Draggendorff reagent.
Secondary phloem and phellem cells of the stem bark of Esenbeckia febrifuga. (A–F) Longitudinal section of secondary phloem. (A, C and E). Radial section. (B, D and F) Tangential section. (A and B) Simple sieve plate (thin arrow and ellipse, respectively). (C and D) Compound sieve plate (thin arrow and ellipse – showing sieve area (sa) and bars (b) of the compound sieve plate), lateral sieve area (arrowhead) and prismatic crystals (thick arrow) in parenchyma ray cells. (E and F) Heterocellular and multiseriate ray with procumbent (star) and upright (diamond) cells. (G) Transversal section shown oil droplets into the parenchyma cells (Sudan III test). (H–K) Maceration. (H) Phellem cells. (I and J) Fibers of secondary phloem. (K) Detail of fiber end cell.
Since it presents a curved aspect when dried, bark pieces of E. febrifuga could be confounded with Cinchona species, however it is possible to use characteristics to differentiate them. In relation to the bark of C. succirubra Pav. ex Klotzsch (synonym of C. pubescens Vahl), the reddish color in the inner surface and in the powder (Wichtl, 2004Wichtl, M., 2004. Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis, 3rd ed. CRC Press, Florida, pp. 134.) is the mean feature for distinction. However, other species such as C. calisaya Weed. (syn. C. ledgeriana (Howard) Bern. Moens ex Trimen) and C. officinalis L. (syn. C. peruviana Mutis) have yellowish or a pale color to the inner surface of the bark (Wallis, 1951Wallis, T.E., 1951. Textbook of Pharmacognosy, 2nd ed. J. and A. Churchill Ltd, London.), so the microscopy description is the most indicative. Although the anatomical descriptions are very similar between these species (Deutschmann et al., 1984Deutschmann, F., Hohmann, B., Sprecher, E., Stahl, E., 1984. Pharmazeutische Biologie 3. Drogenanalyse I: Morphologic und Anatomie. G. Fischer Verlag, New York.) and E. febrifuga, these Cinchona species show parenchymatous idioblasts filled with microprisms (crystal sand) of calcium oxalate in the cortex and the spindle-shaped phloem fibers, without adornate end walls and larger in diameter and length (Claus, 1961Claus, E.P., 1961. Pharmacognosy, 4th ed. Lea and Febiger, Philadelphia.; British and Pharmacopoeia, 2009British Pharmacopoeia, 2009. Her Majesty's Stationary Office, London.), which are the main differing features from E. febrifuga. Crystal shape is a good feature for comparison because a particular species will form only a certain crystal type or subset of crystal morphologies (Franceschi and Nakata, 2005Franceschi, V.R., Nakata, P.A., 2005. Calcium oxalate in plants: formation and function. Annu. Rev. Plant Biol. 56, 41-71.). Although E. febrifuga also had fibers without adorned end walls, the constant presence of fibers with adorned end walls in its macerate constitutes an important differential feature. The same anatomical information plus the color and morphological aspects of the bark can be used for avoid misidentification when compared to other Brazilian quinas: Polyouratea hexasperma (A. St.-Hil.) Tiegh (Somavilla et al., 2013Somavilla, N.S., Cosenza, G.P., Fagg, C.W., Brandão, M.G.L., 2013. Morpho-anatomy and chemical profile of native species used as substitutes of quina (Cinchona spp.) in Brazilian traditional medicine Part I: Polyouratea hexasperma. Rev. Bras. Farmacogn. 23, 592-599.), Remijia ferruginea (A. St. Hil.) DC. (Somavilla et al., 2017Somavilla, N.S., Cosenza, G.P., Fagg, C.W., Brandão, M.G.L., 2017. Morpho-anatomy and chemical profile of native species used as substitute of quina (Cinchona spp.) in Brazilian traditional medicine Part II: Remijia ferruginea. Rev. Bras. Farmacogn. 27, 153-157.) and Bathysa cuspidata (A. St.-Hil.) Hook.f. ex K. Schum. (Coelho et al., 2012Coelho, V.P.M., Leite, J.P.V., Nunes, L.G., Ventrella, M.C., 2012. Anatomy, histochemistry and phytochemical profile of leaf and stem bark of Bathysa cuspidata (Rubiaceae). Aust. J. Bot. 60, 49-60.).
A phytochemical study carried out by Dolabela et al. (2008)Dolabela, M.F., Oliveira, S.G., Nascimento, J.M., Peres, J.M., Wagner, H., Póvoa, M.M., Oliveira, A.B., 2008. In vitro antiplasmodial activity of extract and constituents from Esenbeckia febrifuga, a planta traditionally used to treat malaria in the Brazilian Amazon. Phytomedicine 15, 367-372. found two coumarins (bergapten and isopimpinellin), four furoquinoline alkaloids (flindersiamine, kokusaginine, skimmiamine and γ-fagarine), one acridone (1-hydroxy-3-methoxy-N-methylacridone) and a limonoid (rutaevine) in the E. febrifuga stem ethanol extract. They also evaluated the in vitro antiplasmodial activity of the ethanol extract and concluded that only the furoquinoline alkaloids are related to this activity, specially skimmiamine and γ-fagarine. These two alkaloids, obtained from another Rutaceae species, proved to be promising therapeutical agents due to their efficacy and safety against promastigote forms of Leishmania tropica (Östan et al., 2007Östan, P., Iam, H.S., Limoncu, M.E., Ertabaklar, H., Toz, S.Ö., Özbel, Y., Özbilgin, A., 2007. In vitro and in vivo activities of Haplophyllum myrtifolium against Leishmania tropica. New Microbiol. 30, 439-445.) and Leishmania braziliensis (Santos et al., 2011Santos, R.A.N., Batista, J., Rosa, S.I., Torquato, H.F., Bassi, C.L., Ribeiro, T.A., De Sousa, P.T., Bessera, A.M., Fontes, C.J., Da Silva, L.E., Piuvezam, M.R., 2011. Leishmanicidal effect of Spiranthera odoratíssima (Rutaceae) and its isolated alkaloid skimmianine occurs by a nitric oxide dependent mechanism. Parasitology 138, 1224-1233.), causative agents of cutaneous leishmaniasis. Moreover, Napolitano et al. (2004)Napolitano, H.B., Silva, M., Ellena, J., Rodrigues, B.D.G., Almeida, A.L.C., Vieira, P.C., Oliva, G., Thiemann, O.H., 2004. Aurapten, a coumarin with growth inhibition against Leishmania major promastigotes. Braz. J. Med. Biol. Res. 37, 1847-1852. isolated one coumarin (aurapten) in the leaves of E. febrifuga with significant inhibition of the in vitro growth of Leishmania major promastigotes, another agent of cutaneous leishmaniasis. These data reinforces the importance of this species as a source of active principles with activity against these parasitic diseases, which are co-occurring in geographical regions (Gelb and Hol, 2002Gelb, M.H., Hol, W.G.J., 2002. Drugs to combat tropical protozoan parasites. Science 297, 343-344.; Kvist et al., 2006Kvist, L.P., Christensen, S.B., Rasmussen, H.B., Mejia, K., Gonzalez, A., 2006. Identification and evaluation of Peruvian plants used to treat malaria and leishmaniasis. J. Ethnopharmacol. 106, 390-402.) and considered as the most prevalent tropical diseases caused by protozoan parasites (WHO, 2015WHO, 2015. World Health Statistics 2015. WHO Graphics, World Health Organiza-tion, Luxembourg.).
Acknowledgement
The authors are grateful to CNPq for financial support (563311/2010-0, 563563-2010-9) and fellowships (150523/2011-4 and 150453/2012).
References
- Albuquerque, B.W.P., 1968. Rutaceae do Estado da Guanabara. An. Acad. Bras. Cienc. 40, 499-530.
- Brandão, M.G.L., Botelho, M.G.A., Krettli, A.U., 1985. Quimioterapia experimental antimalárica com produtos naturais: uma abordagem mais racional? Ciênc. Cultur. 37, 1152-1163.
- British Pharmacopoeia, 2009. Her Majesty's Stationary Office, London.
- Brocadet, A.P., 1921. Plantes utiles du Brésil. Vigor Fréres, Paris.
- Carvalho, L.H., Brandão, M.G.L., Santos Filho, D., Lopes, J.L.C., Krettli, A.U., 1991. Antimalarial activity of crude extracts from Brazilian plants studied in vivo in P. berghei-infected mice and in vitro against P. falciparum in culture. Braz. J. Med. Biol. Res. 24, 1113-1123.
- Chebez, J.C., Mazariche, M., 2010. Nuestros árboles. De norte a sur, conociendo los árboles de la Argentina. Albatroz, Buenos Aires.
- Claus, E.P., 1961. Pharmacognosy, 4th ed. Lea and Febiger, Philadelphia.
- Coelho, V.P.M., Leite, J.P.V., Nunes, L.G., Ventrella, M.C., 2012. Anatomy, histochemistry and phytochemical profile of leaf and stem bark of Bathysa cuspidata (Rubiaceae). Aust. J. Bot. 60, 49-60.
- Corrêa, M.P., 1969. Dicionário de plantas úteis do Brasil e das exóticas cultivadas, vol. 4. Imprensa Nacional, Rio de Janeiro.
- Cosenza, G.P.P., Somavila, N.S., Fagg, C.W., Brandão, M.G.L., 2013. Bitter plants used as substitute of Cinchona spp. (quina) in Brazilian traditional medicine. J. Ethnopharmacol. 149, 790-796.
- Deutschmann, F., Hohmann, B., Sprecher, E., Stahl, E., 1984. Pharmazeutische Biologie 3. Drogenanalyse I: Morphologic und Anatomie. G. Fischer Verlag, New York.
- Dolabela, M.F., Thesis 2007. Atividade antiplasmódica e citotoxicidade de Esenbeckia febrifuga (A. St-Hil.) Juss. Ex Mart. (Rutaceae) e de espécies do gênero Aspidosperma (Apocynaceae). Universidade Federal de Minas Gerais, www.dominiopublico.gov.br/download/texto/cp104928.pdf
» www.dominiopublico.gov.br/download/texto/cp104928.pdf - Dolabela, M.F., Oliveira, S.G., Nascimento, J.M., Peres, J.M., Wagner, H., Póvoa, M.M., Oliveira, A.B., 2008. In vitro antiplasmodial activity of extract and constituents from Esenbeckia febrifuga, a planta traditionally used to treat malaria in the Brazilian Amazon. Phytomedicine 15, 367-372.
- Flora do Brasil 2020 under construction. Jardim Botânico do Rio de Janeiro. Available at: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB583 (accessed 02.11.17).
» http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB583 - Franceschi, V.R., Nakata, P.A., 2005. Calcium oxalate in plants: formation and function. Annu. Rev. Plant Biol. 56, 41-71.
- Franklin, G.L., 1945. Preparation of thin sections of synthetic resins and wood-resin composites, and a new macerating method for wood. Nature 155, http://dx.doi.org/10.1038/155051a0
» http://dx.doi.org/10.1038/155051a0 - Furr, M., Mahlberg, P.G., 1981. Histochemical analyses of laticifers and glandular trichomes in Cannabis sativa J. Nat. Prod. 44, 153-159.
- Gardner, R.O., 1975. Vanillin-hydrochloric acid as a histochemical test for tannin. Biotech. Histochem. 50, 315-317.
- Gabe, M., 1968. Techniques histologiques. Masson & Cie, Paris.
- Gelb, M.H., Hol, W.G.J., 2002. Drugs to combat tropical protozoan parasites. Science 297, 343-344.
- Grandtner, M.M., Chevrette, J., 2013. Dictionary of Trees, vol. 2: South America, Academic Press, San Diego, USA.
- Johansen, D.A., 1940. Plant Microtechnique. McGraw-Hill Book Company, New York.
- Junikka, L., 1994. Survey of English macroscopy bark terminology. IAWA J. 15, 3-45.
- Kaastra, R.C., 1982. Pilocarpine (Rutaceae). Flora Neotropica 33, 1-197.
- Kvist, L.P., Christensen, S.B., Rasmussen, H.B., Mejia, K., Gonzalez, A., 2006. Identification and evaluation of Peruvian plants used to treat malaria and leishmaniasis. J. Ethnopharmacol. 106, 390-402.
- Kyriakis, G., Fasseas, C., 2010. A novel type of tube network within the stem bark of Olea europaea L.. Flora 205, 90-93.
- Kraus, J.E., Arduim, M., 1997. Manual básico de métodos em morfologia vegetal. EDUR, Rio de Janeiro.
- Lindley, J., 1830. An introduction to the Natural System of Botany or, a Systematic View of the Organization, Natural Affinities, and Geographical Distribution, of the Whole Vegetable Kingdom. Longman, Orme, Brown, Green, and Longmans, London.
- Lindley, J., 1838. Flora Medica: A Botanical Account of All the More Important Plants Used in Medicine in Different Parts of the World. Longman, Orme, Brown, Green, and Longmans, London, pp. 210.
- Meskheli, M., Mchedlidze, K., Gogitidze, T.S., 2010. Anatomical and morphological description of Phellodendron lavallei Dode stem bark. Georgian Med. News 182, 32-38.
- Napolitano, H.B., Silva, M., Ellena, J., Rodrigues, B.D.G., Almeida, A.L.C., Vieira, P.C., Oliva, G., Thiemann, O.H., 2004. Aurapten, a coumarin with growth inhibition against Leishmania major promastigotes. Braz. J. Med. Biol. Res. 37, 1847-1852.
- Oberlin et Schlagdenhauffen, M.M., 1874. Étude pharmacographique et chimique d’un nouveau succedanea de l’écorce d’angusture. J. Pharm. Chim. Série 4, Tome 20, 105.
- Oberlin et Schlagdenhauffen, M.M., 1878. Étude histologique et chimique de differéntes ècorces de la famille des Diosmées. J. Pharm. Chim. Série 4, Tome 28, 225-272.
- Östan, P., Iam, H.S., Limoncu, M.E., Ertabaklar, H., Toz, S.Ö., Özbel, Y., Özbilgin, A., 2007. In vitro and in vivo activities of Haplophyllum myrtifolium against Leishmania tropica New Microbiol. 30, 439-445.
- Paiva, J.G.A., Fank-de-Carvalho, S.M., Magalhães, M.P., Graciano-Ribeiro, D., 2006. Verniz vitral incolor 500®: uma alternativa de meio de montagem economicamente viável. Acta Bot. Bras. 20, 257-264.
- Pearse, A.G.E., 1972. Histochemistry: Theoretical and Applied, vol. 2., 3rd ed. The Williams & Wilkins Company, Baltimore.
- Peckolt, W., 1916. Monographia das falsas quinas brasileiras, Rio de janeiro.
- Richter, H.G., Mazzoni-Viveiros, S.C., Alves, E.S., Luchi, A.E., Costa, C.G., 1996. Padronização de critérios para a descrição anatômica da casca: lista de características e glossário de termos. IF-Sér. Reg. S. Paulo 16, 1-25.
- Saint-Hilaire, A., [1824]2014. Plantas usuais dos Brasileiros. Fino Traço, Belo Horizonte.
- Santos, R.A.N., Batista, J., Rosa, S.I., Torquato, H.F., Bassi, C.L., Ribeiro, T.A., De Sousa, P.T., Bessera, A.M., Fontes, C.J., Da Silva, L.E., Piuvezam, M.R., 2011. Leishmanicidal effect of Spiranthera odoratíssima (Rutaceae) and its isolated alkaloid skimmianine occurs by a nitric oxide dependent mechanism. Parasitology 138, 1224-1233.
- Sass, J.E., 1951. Botanical Microtechnique. The Iowa State College Press, Ames.
- Somavilla, N.S., Cosenza, G.P., Fagg, C.W., Brandão, M.G.L., 2013. Morpho-anatomy and chemical profile of native species used as substitutes of quina (Cinchona spp.) in Brazilian traditional medicine Part I: Polyouratea hexasperma Rev. Bras. Farmacogn. 23, 592-599.
- Somavilla, N.S., Cosenza, G.P., Fagg, C.W., Brandão, M.G.L., 2017. Morpho-anatomy and chemical profile of native species used as substitute of quina (Cinchona spp.) in Brazilian traditional medicine Part II: Remijia ferruginea Rev. Bras. Farmacogn. 27, 153-157.
- Wallis, T.E., 1951. Textbook of Pharmacognosy, 2nd ed. J. and A. Churchill Ltd, London.
- Wichtl, M., 2004. Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis, 3rd ed. CRC Press, Florida, pp. 134.
- WHO, 2015. World Health Statistics 2015. WHO Graphics, World Health Organiza-tion, Luxembourg.
Publication Dates
-
Publication in this collection
Mar-Apr 2018
History
-
Received
23 Sept 2017 -
Accepted
6 Feb 2018 -
Published
9 Mar 2018