Abstract
The antispasmodic effects of acqueous extracts (AE) and tinctures (T) of Aloysia polystachya (Griseb.) Moldenke and Aloysia gratissima (Gillies & Hook.) Tronc., Verbenaceae, were studied on rat isolated ileum and duodenum. These plants are used for gastrointestinal disorders and as eupeptic in South America. Both AE non-competitively inhibited the dose-response curves (DRC) of ACh and the DRC of Ca2+ in high-[K+]o, as well as the T. The T of A. polystachya and A. gratissima respectively inhibited the ACh-DRC at the IC50 of 3.15±0.57 and 6.46±2.28 mg leaves/mL. The Ca2+- antagonist activity of both T occurred with IC50 respectively similar to those of the ACh-DRC, and was potentiated by the depolarization produced by 10 mM TEA, a blocker of K+- channels. The spasmolytic effect of T does not involve DA release and binding to D2, since it was not reduced by 10 µ M metoclopramide. Also, T induced dose-dependent relaxation on the tonic contracture produced by high -[K+]o and ACh. By TLC there were detected in the leaves the presence of carvone, and flavonoids such as quercetin and hesperidin. By HPLC there were not found vitexin nor isovitexin, identified in A. citriodora. The monoterpene (-)- carvone non-competitively inhibited the ACh-DRC (pD'2 of 4.0±0.1) and the DRC of Ca2+ (pD'2 of 3.86±0.19), suggesting that the Ca2+- influx blockade is the mechanism of its antispasmodic effect. Results suggest that the antispasmodic effect of A. polystachya and A. gratissima are mostly explained by the non-competitive blockade of Ca+2 influx. It could be associated to the presence of flavonoids, and in the tinctures to some spasmolytic components of the essential oil such as carvone.
Aloysia antispasmodic Ca-antagonist (-)- carvone rat intestine Verbenaceae
Antispasmodic effects of Aloysia polystachya and A. gratissima tinctures and extracts are due to non-competitive inhibition of intestinal contractility induced by acethylcholine and calcium
Alicia E. Consolini* * Correspondence: Alicia E. Consolini, Departamento de Ciencias Biológicas, Facultad de Ciencias Exactas, Universidad Nacional de La Plata, 47 y 115 (1900), La Plata, Argentina dinamia@biol.unlp.edu.ar, Tel.: 54 221 423 5333 int.42 ; Andrea Berardi; María A. Rosella; María Volonté
Área Ciencias Farmacéuticas, Departamento de Ciencias Biológicas, Facultad de Ciencias Exactas, Universidad Nacional de La Plata, Argentina
ABSTRACT
The antispasmodic effects of acqueous extracts (AE) and tinctures (T) of Aloysia polystachya (Griseb.) Moldenke and Aloysia gratissima (Gillies & Hook.) Tronc., Verbenaceae, were studied on rat isolated ileum and duodenum. These plants are used for gastrointestinal disorders and as eupeptic in South America. Both AE non-competitively inhibited the dose-response curves (DRC) of ACh and the DRC of Ca2+ in high-[K+]o, as well as the T. The T of A. polystachya and A. gratissima respectively inhibited the ACh-DRC at the IC50 of 3.15±0.57 and 6.46±2.28 mg leaves/mL. The Ca2+- antagonist activity of both T occurred with IC50 respectively similar to those of the ACh-DRC, and was potentiated by the depolarization produced by 10 mM TEA, a blocker of K+- channels. The spasmolytic effect of T does not involve DA release and binding to D2, since it was not reduced by 10 µ M metoclopramide. Also, T induced dose-dependent relaxation on the tonic contracture produced by high -[K+]o and ACh. By TLC there were detected in the leaves the presence of carvone, and flavonoids such as quercetin and hesperidin. By HPLC there were not found vitexin nor isovitexin, identified in A. citriodora. The monoterpene (-)- carvone non-competitively inhibited the ACh-DRC (pD'2 of 4.0±0.1) and the DRC of Ca2+ (pD'2 of 3.86±0.19), suggesting that the Ca2+- influx blockade is the mechanism of its antispasmodic effect. Results suggest that the antispasmodic effect of A. polystachya and A. gratissima are mostly explained by the non-competitive blockade of Ca+2 influx. It could be associated to the presence of flavonoids, and in the tinctures to some spasmolytic components of the essential oil such as carvone.
Keywords: Aloysia antispasmodic Ca-antagonist (-)- carvone rat intestine Verbenaceae
Introduction
Many plants are used in folk medicine to treat gastrointestinal disorders, such as spasm or indigestion. Several plants of the genus Aloysia, Verbenaceae, are used as eupeptic in South America. In a previous work it was described for the first time the antispasmodic effect of Aloysia citriodora Ortega ex Pers., which is known as "cedrón" or "hierba luisa". Its pharmacological mechanism of action was related to activation of the guanylil-cyclase and the potassium channels (Ragone et al., 2007). In this work, the antispasmodic properties of two other species of Aloysia were validated and the pharmacological basis of the popular use was evaluated, as well as the correlation with its chemical composition. These two species, Aloysia polystachya (Griseb.) Moldenke known as "burrito" or "té-burro" and Aloysia gratissima (Gillies & Hook.) Tronc. known as "palo amarillo", are also used as eupeptic in South America.The leaves are prepared as an aromatic infusion commonly used as dietary supplement or eupeptic tea; and also as decoction or alcoholic tincture for abdominal pain or against nausea and dizziness (Soraru & Bandoni, 1978; Alonso & Desmarchelier, 2005). In spite of its wide use and the local experiences for cultivation (Burdyn et al., 2006) there were not studies about its gastrointestinal properties. Nevertheless, there were described anxiolytic and antidepressant effects of A. polystachya (Mora et al., 2005; Hellion-Ibarrola et al., 2008). Also, it has been described the presence of (-)-carvone in the essential oil of A. polystachya (Werdin-González et al., 2010), which was related to antispasmodic effect of Mentha genus (de Sousa et al., 2008; Goncalves et al., 2008) as well as other terpenic compounds.
At a taxonomic level the genus Aloysia is related to the genus Lippia (Pascual et al., 2001), which is widely used in the North hemisphere and exhibits an antispasmodic activity. In this way, in this work we studied whether the aqueous extract and tinctures of A. polystachya and A. gratissima are antispasmodic on acethylcholine-induced contractions on the isolated rat intestine, and which are the mechanisms and relation to composition. Isolated rat duodenum and ileum were a useful model to study the gastrointestinal effects of the extract and drugs. They have muscarinic receptors and let to study the antispasmodic effects over the smooth muscle contractured by depolarization or cholinergic stimulation (Karamenderes & Apaydin, 2003; Emendorfer et al., 2005).
Materials and Methods
Plant material Aloysia polystachya (Griseb.) Moldenke and Aloysia gratissima (Gillies & Hook.) Tronc., Verbenaceae, were provided by a local herboristery, and the plants were authenticated by Prof. Dra. Etile Spegazzini with the voucher numbers 1153 (22-05-06) and 1155 (18-06-07) for A. polystachya and 1154 (23-06-06) and 1156 (19-07-07) for A. gratissima (LPE). Their samples are kept in the Herbarium Museum of Botany and Pharmacognosy (LPE), Facultad de Ciencias Exactas, Universidad Nacional de La Plata, Argentina.
Pharmacological studies
Extracts preparation
Aqueous extracts (AE) were prepared by boiling 20 g of dried leaves in 100 mL distilled water for 20 min, according to the ethomedicinal use. After filtration, the respective decoction was lyophilized, affording a 10% w/w yield. The lyophilized extract was diluted in distilled water and Tyrode solution for in vitro tests the day of each experiment. With this procedure, the essential oil is not included in the lyophilized sample. The ethanolic tinctures (T) were obtained by maceration during 8-10 h at 20% in ethanol 70º.
Animals
The research was conducted in accordance with the internationally accepted principles for the laboratory animal use and care as was established by US guidelines (NIH publication # 85-23 revised in 1985).
Biological preparation and contractile measurements
Sprague-Dawley rats (200-250 g) were subjected to a 24 h fasting with free access to water before experimentation. The animals were anaesthetized by inhalation with diethyl ether and then quickly sacrificed by the opening of torax and abdomen, in order to avoid a relaxant effect of any other anaesthetic on the intestinal tissue.
Duodenums and ileums (about 2 cm long) were prepared and mounted in organ baths of 20 mLcontaining Tyrode solution at 37 ºC constantly oxygenated with air (pH 8.2) as in other works (Emendorfer et al., 2005). The preparations were equilibrated for at least 45 min at 1 g of pre-load. Tissues were connected to either, an isotonic force transducer Letica TRO 015 (PanLab, Spain) or an isometric one Power Lab MLT0201 (Power Lab, AD Instruments). Both types of response were statistically compared in each protocol, without significant differences between results. Because of that, both types of data were mixed. The signals were acquired in a computer respectively by a National Instruments PC-516 NI-Daq SW with Virtual Bench logger and by Chart 4 of Power Lab program.
Solutions and drugs
The solutions used had the following composition:
Tyrode (Tyr): 150 mM NaCl, 2.7 mM KCl, 2 mM MgCl2, 12 mM NaHCO3, 0.4 mM PO4H2Na, 1.8 mM Ca Cl2 , bubbled with air (pH 8.2).
Tyrode-0Ca: by eliminating Ca Cl2.
Tyrode-0Ca-40 mM K+: by adding 0.6 ml KCl 10% to 20 mL Tyrode 0Ca.
The drugs employed in biological tests were: Acetylcholine bromide (ACh, Sigma, USA), tetraethylammonium chloride (TEA, Sigma, USA) and metoclopramide (Novartis) and the monoterpene (-)-carvone (Sigma, USA). For phytochemical assays there were used the flavonoids quercetin (Sigma, USA), vitexin (Extrasynthese, France), isovitexin (Extrasynthese, France) and hesperidin (Sigma, USA), and also (-)-carvone (Sigma, USA). The flavonoids were initially dissolved in dimethylsulphoxide (DMSO) as well as (-) carvone, and then diluted with water to obtain the lower concentrations. A DRC control was done with DMSO at 1% or Ethanol 1% in the Tyrode medium, which were respectively not different from the DRC without vehicle.
Protocols
Dose-response curves to acetylcholine
Dose-response curves (DRC) to acetylcholine (ACh) were done for the rats duodenums and ileums after a stabilization of 45 min. ACh concentrations were cumulatively added to the bath (to reach from 0.01 to 10 µg/mL) in the absence and the presence of a unique concentration of either, the respective AE, the T, or a reference drug as it was shown in the respective figure. They were added 5 to 10 min before the DRC and remained during it in the bath, and control curves were done with the vehicle of the tinctures, ethanol of 70º. When several concentrations of AE or T were assed, a growing order of concentrations was used for making the DRC in each organ.
Dose-response curves to CaCl2
After an stabilization during 45 min in Tyrode, the external Ca+2 was eliminated with Tyrode-0Ca and then, the muscle was depolarized with Tyrode0Ca-40 mM K+. The DRC of Ca2+ were obtained by cumulatively adding CaCl2 to reach concentrations from 0.0195 to 17.5 mmol/L, in the absence and presence of growing concentrations of the respective AE or T. Each concentration of AE or T was added 5 min before the depolarization with high-K+ in Tyrode-0Ca and remained in the bath during it.
Dose relaxation curves of the extracts
Tissues were contracted by depolarization with ClK 40 mM in the Tyrode until obtaining a tonic response. At this point, growing concentrations of AE (shown in the respective figure) were cumulatively added to construct a dose-relaxation curve.
In another group of muscles, the tonic contracture was obtained by adding 10 µg/mL acetylcholine (ACh). Then, the relaxation curves of AE or T were obtained in the absence and in the presence of 40 mmol/L tetraethylammonium (TEA, a non-selective K+- channels blocker).
Pharmacological and statistical analysis
From the DRC there were calculated the pD2 of the agonist (as -log EC50, in molar), and the affinity (pD'2) of the pure non-competitive antagonists as pD'2= -log [B'] + log [(EA-EAB'max)/(EA-EAB')-1], where B' is the concentration of the non-competitive antagonist, in molar, and E, E' and Eare the maximal effects of the agonist respectively in the absence (A) and in the presence of either the used concentration of antagonist (B') and the maximal concentration of it (B'max) (Van der Brink, 1977; Kenakin, 1984). In order to evaluate whether carvone act as competitive antagonist of ACh or Ca, the Schild method was applied for the 50% effect of DRC and the linear correlation of log (concentrations ratio-1) vs. log[carvona] (in Molar) was statisticaly analyzed (Kenakin 1984; Wyllie & Chen, 2007). For the extracts, the IC50 was calculated by extrapolation to 50% the individual relaxation curves obtained by plotting the maximal effect of the agonist from the respective DRC curves vs [extract] (expressed as mg lyophilized by mL). All results are expressed as media±ESM. Regression of DRC and statistics were done by using Graph Pad Prism 4.0 program, and by applying one-way ANOVA test for multiple comparisons followed by Tukey's a posteriori tests. Paired t-test for comparison of paired results were also done. In all tests it was considered a significance of p<0.05.
Phytochemical studies
HPLC analysis
Since the presence of vitexin and isovitexin had been demonstrated in Aloysia citriodora leaves, it was evaluated whether these flavonoids were present in the AE of A. polystachya and A. gratissima. It was applied the previously developed method of high performance liquid chromatography (HPLC) contrasted with standards of both flavonoids (Ragone et al., 2007). It was used a 322-H2 series pump, a 155/156 UV/Vis detector and a work station equipped with the UniPoint LC, 3.3 version software (Gilson SAS, Villiers-Le-Bel, France). A Rheodyne 7125 manual sample injector (Rheodyne, CA, USA) with a fixed volume of 20 µL was also used. Cromatographic conditions were: LiChrocart RP-18 (250 mm x 4 mm internal diameter, 5 µm particle size) column (Merck, Darmstadt, Germany) as stationary phase under isocratic elution mode, at room temperature (25 ºC). The mobile phase was composed by 2-propanol:tetrahydrofurane:water (5:15:85) at pH 4.33, flow rate was set at 1.0 mL/min and detected at 336 nm. The samples were prepared from the lyophilized AE of both plants, in the mobile phase at 700 µg/mL of concentration, which was filtered and injected by triplicate in the HPLC system. The following compounds were used as standard: vitexin and isovitexin.
Total flavonoids content
Total content of flavonoids was evaluated from the aerial parts of both, A. polystachya and A. gratissima, by adaptation of the Kostennikova method modified by Méndez (Gutiérrez-Gaitén et al., 2000). Briefly, 1 g of aerial parts were extracted at reflux during 2 h with 20 mL sol. 10% H2SO4 and 20 mL of ethanol 50º. After cooling, filtering, and washing with 30 mL ethanol 50º, it was evaporated on a water boiling bath up to the half of volume, and cooling on an ice bath. The obtained precipitated was filtered and washing with cool water (10 ºC, four times of 10 mL). Both, the precipitate and the remaining solid obtained during cooling, were dissolved again with ethanol 96º previously heated at 50 ºC, transferred and completed volume to 100 mL with ethanol 96º. A volume of 5 mL of this solution was diluted to 100 mL (1:20) to read the absorbance at a wavelength of 285 nm (A285) on a Thermo spectrophotometer, Helios-beta model (Thermo Fisher Scientific, Waltham, MA, USA). As a standard it was used a 40 µg/mL solution of hesperidin (Sigma) in ethanol 50º, and this one was measured as a blank. Then, the standard and hydroalcoholic extractions of both species of Aloysia were submitted to the measure of an UV-spectra in the range of 200-400 nm in order to stablish the wavelength of maximum absorption. From those results, it was calculated the concentration of flavonoids in the sample expressed as µg/mL hesperidin (Cm) as Cm=Ch.Am/Ah, where Ch is the hesperidin concentration (in µg/mL), Am is the absorbance of the sample at 285 nm, and Ah is the absorbance of hesperidin at 285 nm. Then, it was calculated the % of total flavonoids expressed as hesperidin (g flavonoids by 100 g of aerial parts of the plant).
TLC analysis
The presence of the ketonic monoterpene (-)-carvone in both species of Aloysia was evaluated by thin-layer chromatography (TLC), on silica gel F 254 Merck 0.25 mm and three systems of mobile phase: (a) toluene/ethylacetate (7:3), (b) toluene/ethylacetate (96:4), and (c) toluene/ethylacetate (9:1). The samples were 10% hexanic extractive solutions of the aerial parts of A. pachystachya and A. gratissima, and a standard of (-)-carvone (Sigma) at 1% in dichloromethane.
The presence of flavonoids was evaluated by TLC on silica gel F254 Merck 0.25 mm and several mobile phases, as follows: (d) Cl2CH2/MeOH (95:5), (e) Cl2CH2/MeOH (99:1), (f) EtOAc/MeOH/ H2O (100:17:10), and (g) EtOAc/MetH/AcH/ H2O (100:11:11:26) (superior phase). As a standard it was used a 1% methanol solution of quercetin (Sigma). The detection of bands of flavonoids was performed under UV light (254 and 366 nm) with and without ammonia clouds, and with natural product spray (1% methanol solution of 2-amino ethyl-diphenil-boric acid) and it was observed under visible and UV light. The 10% methanol extractive solutions of aerial parts of both species of Aloysia and the lyophilized of 20% AE were run.
Results
Pharmacological studies
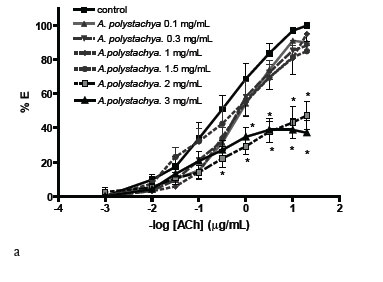
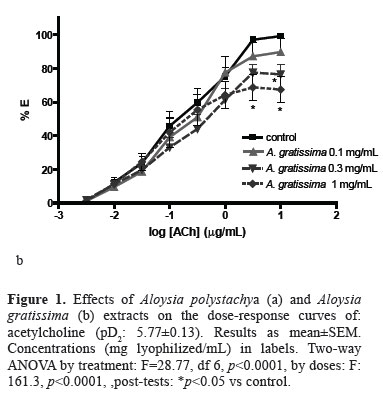
The extract of both, Aloysia polystachya (AEA.p) and A. gratissima (AE-A.g) reduced the maximal effect of the DRC on a dose-dependent way (Figure 1A), suggesting a non-competitive antagonism over the cholinergic contraction. The extrapolated IC50 of AE-A.p was 1.8±0.27 mg lyophilized/mL, but for AE-A.g it was only possible to find an IC25 of 0.276±0.077 mg lyophilized/mL because it inhibited the DRC in a lower degree (Figure 1B). The tinctures of both plants also non-competitively inhibited the DRC of Ach in a higher degree than the AE (Figure 2), with IC50 of 3.15±0.57 mg drug/mL for T-A.p and 6.46±2.28 mg drug/mL for T-A.g.
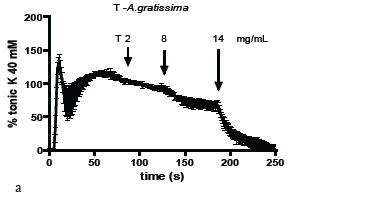
On the tonic contracture induced by Tyrode-K 40 mM the EA-A.p did not produce relaxation, but EA-A.g relaxed in a concentration-dependent way (Figure 3A). A similar behavior of the EA was obtained when the tonic contracture was induced by ACh, in which only the EA-A.g relaxed in a concentration-dependent way and it was unaffected by the unspecific blocking of all types of K+- channels with 40 mM TEA (Figure 3B). Nevertheless, both T-A.p. and T-A.g. relaxed in a concentration-dependent way the ACh-induced tonic contracture (Figure 3C). The effect of T-A.p. was not reduced but potentiated by the specific blocking of Ca2+ -dependent K+- channels with 1 mM TEA.
To assay whether the effects of both T on the DRC of ACh were associated to K+- channels activation, the DRC of ACh were done in the absence and the presence of 10 mM TEA. Figure 4 shows that TEA did not inhibited the non-competitive antagonism of T-A.p. and T-A.g. on DRC-ACh, but potentiated it. In order to evaluate whether that potentiation was due to the release of a relaxant neurotransmitter such as dopamine (DA) evoked by the depolarization due to TEA, the DRC of ACh was done in the presence of 10 mM TEA, 6 mg/mL T-A.p and 10 mM metoclopramide to block the effect of dopamine in D2 receptors. The comparison of Figure 4A with Figure 4C shows that this combined treatment did not reduce but increased the non-competitive blockade produced by T-A.p. and TEA.
In order to elucidate whether the non-competitive antagonism of both T on the ACh-DRC were associated to inhibition of Ca+2 influx to the smooth muscle, DRC of Ca+2 were done on high-K+- medium to activate the channels. Figure 5 shows that both, T-A.p and T-A.g produced a non-competitive inhibition of Ca+2 influx. The extrapolated IC50 were respectively 1.8±0.08 mg/mL for A. polystachya and 9.3±1.4 mg/mL for A. gratissima.
Effects of (-)carvone
Since it was described the presence of (-)-carvone in the essential oil of A. polystachya (Werdin-González et al. 2010), it could contribute to the effects of tinctures. Since its mechanism has not been studied before, in this work we evaluated its effects on the DRC of ACh and Ca2+ . Figure 6 shows that (-)-carvone developed a non-competitive blockade of both DRC, since it decreased Emax. Also, the Schild method confirmed that (-)-carvone is not a competitive antagonist of ACh (slope different than 1) or Ca2+ (there is not significative linear correlation). From the DRC it was calculated the affinity of (-)-carvone on its own cellular site, which was estimated by the pD'2. It resulted 4.002±0.117 (n=20) from the DRC of ACh and 3.86±0.19 (n=18) from the DRC of Ca2+ .
Contrarily to what was described for Aloysia citriodora (Ragone et al., 2007) the HPLC chromatograms showed no presence of vitexin or isovitexin in the AE of Aloysia polystachya and Aloysia gratissima (Figure 7). Retention times of the standard drugs were Rt 11.51 min for vitexin and 16.85 min for isovitexin, with a good separation profile comparable to that previously described.
Total content of flavonoids
Figure 8 shows that hesperidin exhibited a maximum absorption at 285 nm with a lower peak absorption at 230 nm. The extractives from A.polystachya and A.gratissima exhibited also two peaks at 285 nm and 330 nm, by which it was chosen the wavelength of 285 nm for measuring. Table 1 shows the results of absorbance as well as the calculated % of flavonoids total content.
TLC results
Table 2 shows the results obtained with TLC for detecting the presence of flavonoids in the systems of higher resolution. The methanol extracts of both plants yielded until 10 common bands with Rf between 0.50 and 1.00. The extract of A. gratissima has another extra band at Rf 0.40 with strong blue clear fluorescence. According to Wagner & Bladt (1995) this is typical of extracts with flavonoids containing phenol-carboxylic acids, such as caffeic and chlorogenic acids. After boric acid, they appear until four bands yellow-orange (Rf 0.50; 0.80; 0.85 and 0.90) characteristics of flavonols and flavones and their glycosides, the stronger at Rf 0.50. The band at Rf 0.90 is similar to that of the standard, the flavonol quercetin. On this system, the aglycons flavonoids run with the front of mobile phase (Rf 0.80 a 1.00) while the glycosides run in the middle. The AE of A. polystachya developed three bands of very low intensity (Rf 0.45; 0.60 and 0.80) which correspond with those of the methanolic extract. The AE of A. gratissima only showed two bands (Rf 0.45 and 0.80).
Table 3 shows the results of TLC done with the hexane extractives of both Aloysia spp. The ketonic monoterpene (-)-carvone was used as a standard. The cromatographic profiles are very similar for both species. At 254nm there were found 6 bands with Rf between 0 and 0.60. One of them (Rf 0.40) has the same Rf and aspect than that of the standard (-)-carvone. At 366 nm it appears a band with blue clear fluorescence (Rf 0.25), which does not react with sulphuric anisaldehyde. With this compound were seen the same seven bands, from which it is stronger the band of Rf 0.30 in violet. In system (c) there were found similar profile of bands for both Aloysia spp., except a low-intensity band at 0.45 in A.polystachya. In both, a band at Rf 0.30 with blue clear fluorescence appears at 366, which does not react with sulphuric anisaldehyde. Also, there is a band at Rf 0.50 which became red violet after sulphuric anisaldehyde, with Rf and aspect similar to that of (-)-carvone. Then, TLC results suggest that both species of Aloysia have similar content in monoterpenic compounds, such as carvone.
Discussion
This work gives support to the popular use of Aloysia polystachya ("burrito") and A. gratissima ("palo amarillo") as antispasmodic, since both directly relaxed the intestinal smooth muscle and non-competitively inhibited the DRC of ACh. There were assessed two phytotherapic preparations, the tinctures obtained by maceration (T) and the aqueous extract obtained by decoction (AE). The T induced a higher antispasmodic effect than the AE, as it was evidenced by the higher reduction in the effects of ACh (see the Emax of the DRC). These differences suggest either that the ethanolic maceration could extract more active principles or that these ones are thermo-sensitives. The non-competitive inhibition of the ACh effect suggests that one or more active principles would be interacting with a cellular site different from the muscarinic receptor, as it is classically interpreted from the DRC (Van der Brink, 1977; Kenakin, 1984). Several antispasmodic plants demonstrated to be non-competitive antagonists of ACh on duodenal or ileal smooth muscles (Sánchez de Rojas et al., 1995; Karamenderes & Apaydin, 2003; Camara et al., 2003; Emendorfer et al., 2005). This behaviour was also found in flavonoids, such as quercetin (Di Carlo et al., 1999). The presence of flavonoids as quercetin was evidenced in the AE by TLC, which could explain part of the effects of both Aloysia spp. It was reported previously that quercetin relaxed the high-K+contracture on rat intestine (Ragone et al., 2007) and guinea-pig ileum (Di Carlo et al., 1999). Also, the total content of flavonoids was estimated by a method which compares the UV-absorption spectrum of the extracts with that of hesperidin. This compound could be present among other flavonoids, according to the characterization done by TLC. The flavonoids total content of A. pachystachya was slightly higher than that of A. gratissima. This difference could be the origin of the pharmacological differences: A. pachystachya induced higher reduction of the ACh effect than A. gratissima in the extracts, and higher potency in the tinctures (IC50 of 3.14±0.57 vs 6.46±2.28 mg/mL, respectively). Nevertheless, the flavonoids vitexin and isovitexin were not found by HPLC in A. polystachya or A. gratissima. These flavonoids have been identified in the AE of Aloysia polystachya in which explain part of the antispasmodic effect (Ragone et al., 2007), and there were also described in other plants as the genus Passiflora (Muller et al., 2005). Then, the antispasmodic effects of the species of Aloysia could be related to several types of flavonoids which differ among them.
The first hypothesis underlying the non-competitive inhibition of ACh contractions was that the plants could activate K+- channels, as Aloysia citriodora does. A K+- channel opener hyperpolarizes and relaxes the smooth muscles exposed to agonists but not those exposed to high-K+ depolarization, and this is used as a tool to evidence this mechanism (Karaki et al., 1997). Then, results of the AE and T on the tonic contractures induced by high [K+] and ACh suggested that A. polystachya could have that mechanism. Nevertheless, the relaxation was not inhibited by TEA at 10-40 mM (concentrations at which it non-selectively blocks all types of K+- channels), or TEA at 1 mM (which selectively block the Ca-dependent K+channels KCa without affecting the voltage-dependent ones, Kv) (Zhao et al., 2009). Contrarily, the non-competitive inhibition of the T on the ACh dose-response curve was potentiated by TEA, suggesting that the depolarization induced by TEA could stimulate the release of a relaxant neurotransmitter such as dopamine (DA). Also, the depolarization could be contributing to activate and inactivate the Ca2+ channels and then, it could be the origin of the potentiated blockade of T on the smooth muscle. It would suggest that T may contain Ca-blockers among the active principles. The pre-treatment with the competitive antagonist of D2 receptor, metoclopramide, did not reduce the effect of T-A. polystachya. Then, the relaxant effect of T-A. polystachya with or without TEA was not due to release of dopamine acting on D2 receptors. The potentiation of metoclopramide on the non-competitive blockade of the T suggests also interaction with other constituents of the plant.
The following hypothesis was that the AE and especially the T have active principles which could act as antagonists of Ca+2influx. This hypothesis was supported by the relaxation of the tonic contracture induced by high-K+and ACh, and by the potentiation evoked by depolarization with TEA. The dose-response curves of CaCl2 were also non-competitively inhibited by the T of A. polystachya and A. gratissima. The IC50 on the Ca2+ -DRC were similar to (for A. gratissima) or lower than (for A. polystachya) the IC50 on the ACh-DRC. These comparisons suggest that the blockade of Ca2+ influx would be responsible for the non-competitive inhibition on theACh-induced contractions. It is known that the interference with the Ca2+ channels may result competitive for drugs as dihydropiridines, but some other typical drugs as diltiazem or verapamil interfere in a non-competitive way with the internal sites of the channel (Karaki et al., 1997). Alternatively, other cellular sites could be affected by these plants, such as inhibition on calmoduline or sensitivity of actomyosin myofilaments (Karaki et al., 1997). The AE of A. citriodora also inhibited the DRC of Ca2+ in a non-competitive way, but the effect was associated to the activation of guanylil-ciclase and K+channels (Ragone et al., 2007). The presence of flavonoids could also explain the Ca2+ -blockade, since these compounds induce relaxation of smooth muscle and blockade of calcium influx (Gharzouli & Holzer, 2004). The taxonomically related genus Lippia also contains flavonoids (Skaltsa & Shammas, 1988) and exhibits antispasmodic properties. Nevertheless, the specific flavonoids seems to differ among the different species of Aloysia, especially A. citriodora.
Furthermore, the presence of the ketonic monoterpene (-)-carvone was described in about an 85% in the essential oil of A. polystachya (Werdin-González et al., 2010). We found by TLC a similar band when there were assessed together the standard of this drug and the hexane extract of the aerial parts. The effect of (-)-carvone on smooth muscle was also assessed, finding that this compound non-competitively blocked the DRC of ACh and Ca2+ , both with the same pD'2 (about 4). This parameter estimates the affinity of a pure substance for its own receptor site, which differs from the agonist receptor. It is defined as the -log [antagonist] (in Molar) that reduces to the half the effect of the agonist (Kenakin et al., 1984). Also, the Schild analysis confirmed that (-)-carvone is not a competitive antagonist of ACh or Ca2+ , since there was obtained either absence of correlation (for ACh) or a slope different than one (for Ca2+ ) according to Kenakin et al. (1984) and Wyllie & Chen (2007). Neither the pD'2 nor the Schild graphs could be calculated for the extracts or tinctures because they are not pure substances, but the DRC showed non-competitive antagonism because of the fall in Emax (Van der Brink, 1977). In spite of the different parameters estimated (pD'2 vs IC50), either for the tinctures and for (-)-carvone assays, the parameters of inhibition on the DRC of ACh were similar to that of the DRC of Ca2+ . It suggests that in tinctures as well as in (-)-carvone the Ca2+ -influx blockade may be the origin of the interference with the ACh effect. Then, if considering that at least a fraction of the essential oil could be extracted in the tinctures, and that the pharmacological test is more sensitive than the TLC, it is possible to suggest that carvone could contribute to the effect of A. polystachya and A. gratissima tinctures. In summary, the results support the popular use of A. polystachya and A. gratissima as intestinal antispasmodics. Also, they are mostly explained by the non-competitive blockade of the Ca2+ influx. The effect was higher with the tinctures than with the acqueous extract, by which the maceration with ethanol of 70º must be extracting the active principles which cause the effect, such as some flavonoids and essential components as carvone. Since the flavonoids are known relaxants of the smooth muscle, their presence suggest that they would contribute to the antispasmodic effect of these plants. Here it is shown that also the monoterpene carvone could be in part responsible for the effect of the tinctures, since it non-competitively inhibits the effect of ACh and Ca2+ influx, at the same concentration range, as well as the tinctures.
Acknowledgments
We kindly thanks to Lic. Esperanza Ruiz for her technical assistance on the HPLC experiments, and to Dra Etile Spegazzini for the identification of the plants. This work was supported by the grants X-408 (2005-2008) and X-513 (2009-2012) from the Universidad Nacional de La Plata (UNLP), and was presented as a thesis of Andrea Berardi for obtaining the Magister en Plantas Medicinales from Facultad de Ciencias Exactas, UNLP.
Received 20 Dec 2010
Accepted 22 Apr 2011
- Alonso J, Desmarchelier C 2005. Plantas medicinales autóctonas de la Argentina. Literatura of Latin American. Buenos Aires: Ed. Buenos Aires. p. 88-92.
- Burdyn L, Luna C, Tarragó, J, Sanberro P, Dudit N, Gonzalez A, Mroginsky L 2006. Direct shoot regeneration from leaf and internode explants of Aloysia polystachya (Gris.) Mold. (Verbenaceae). In Vitro Cell Biol Plant 42: 235-239.
- Camara CC, Nascimento NR, Macedo-Filho CL, Almeida FB, Fonteles MC 2003. Antispasmodic effect of the essential oil of Plectranthus barbatus and some major constituents on the guinea-pig ileum. Planta Med 69: 1080-1085.
- De Sousa DP, Junior GA, Andrade LN, Calasans FR, Nunes XP, Barbosa-Filho JM, Batista JS. 2008. Structure and spasmolytic activity relationships of monoterpene analogues found in many aromatic plants. Z Naturforsch C 63: 808-812.
- Di Carlo G, Mascolo N, Izzo AA, Capasso, F. 1999. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci 65: 337-345.
- Emendorfer F, Emendorfer F, Bellato F, Noldin VF, Niero R, Cechinel-Filho V, Cardozo AM. 2005. Evaluation of the relaxant action of some Brazilian medicinal plants in isolated guinea-pig ileum and rat duodenum. J Pharm Pharmac Sci 8: 63-68.
- Gharzouli K, Holzer P 2004. Inhibition of guinea pig intestinal by the flavonoids quercetin, naringin, apigenin and genistein. Pharmacol 70: 5-14.
- Goncalves JCR, Sousa Oliveira F, Benedito RB, de Sousa DP, Almeida RN, Araujo DAM 2008. Antinociceptive activity of (-)-carvone: evidence of association with decreased peripheral nerve excitability. Biol Pharm Bull 31: 1017-1020.
- Gutiérrez-Gaitén YI, Miranda Martínez M, Varona Torres N, Tania Rodríguez A 2000. Validación de dos métodos espectrofotométricos para la cuantificación de taninos y flavonoides (quercetina) en Psidium guajaba, L. Rev Cub Farm 34: 50-55.
- Hellion-Ibarrola MC, Ibarrola DA, Montalbetti Y, Kennedy ML, Heinichen O, Campuzano M, Ferro EA, Alvarenga N, Tortoriello J, De Lima TC, Mora S 2008. The antidepressant-like effects of Aloysia polystachya (Griseb.) Moldenke (Verbenaceae) in mice. Phytomedicine 15: 478-483.
- Hellion-Ibarrola MC, Ibarrola DA, Montalbetti Y, Kennedy ML, Heinichien O, Campuzano M, Tortoriello J, Fernández S, Wasowski C, Marder M, De Lima TC, Mora S 2006. The anxiolytic-like effects of Aloysia polystachya (Griseb.) Moldenke (Verbenaceae) in mice". J Ethnopharmacol 105: 400-408.
- Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K-I, Harada K-I, Miyamoto S, Nakazawa H, Won K-J, Sato K 1997. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev 49: 157-230.
- Karamenderes C, Apaydin S 2003. Antispasmodic effect of Achillea nobilis L. subsp. sipylea (O. Schwarz) Bassler on the rat isolated duodenum. J Ethnopharmacol 84: 175-179.
- Kenakin TP 1984. The classification of drugs and drug receptors in isolated tissues. Pharmacol Rev 36: 165-222.
- Mora S, Díaz-Véliz G, Millán R, Lungenstrass H, Quirós S, Coto-Morales T, Hellion-Ibarrola MC 2005. Anxiolytic and antidepressant-like effects of hydroalcoholic extract from Aloysia polystachya in rats. Pharmacol Biochem Behav 82: 373-378.
- Muller SD, Vasconcelos SB, Coelho M, Biavatti MW 2005. LC and UV determination of flavonoids from Passiflora alata medicinal extracts and leaves. J Pharm Biomed Analysis 37: 399-403.
- Pascual ME, Slowing K, Carretero E, Sánchez Mata D, Villar A 2001. Lippia: traditional uses, chemistry and pharmacology: a review. J Ethnopharmacol 76: 201-214.
- Ragone MI, Sella M, Conforti P, Volonté MG, Consolini AE 2007. The spasmolytic effect of Aloysia citriodora, Palau (South American cedrón) is partially due to its vitexin but not isovitexin on rat duodenums. J Ethopharmacol 113: 258-266.
- Sánchez de Rojas VR, Ortega T, Villar A 1995. Inhibitory effects of Cistus populifolius on contractile responses in the isolated rat duodenum. J Ethnopharmacol 46: 59-62.
- Skaltsa H, Shammas G 1988. Flavonoids from Lippia citriodora. Planta Med 54: 465.
- Soraru SB, Bandoni, AL 1978. Plantas de la medicina popular argentina Buenos Aires: Editorial Albatros, p. 107-109.
- Van der Brink FG 1977. General theory of drug-receptor interactions. Drug-receptor interaction models. Calculation of drug parameters. In Van Rossum, JM (ed.) Kinetics of Drug Action New York: Springer-Verlag, p. 169-254.
- Werdin-González JO, Gutiérrez MM, Murray AP, Ferrero AA 2010. Biological activity of essentials oils from Aloysia polystachya and Aloysia citriodora (Verbenaceae) against the soybean pest Nezara viridula (Hemiptera: Pentatomidae). Nat Prod Commun 5: 301-306.
- Wagner H, Bladt S 1995. Plant drug analysis. A thin layer chromatography atlas. 2 ed. Berlin: Springer-Verlag.
- Wyllie DJA, Chen PE 2007. Taking the time to study competitive antagonism. Br J Pharmacol 150: 541-551.
- Zaho P, Huang X, Wang Z, Qiu Z, Ham Y, Lu H, Kim Y, Xu W 2009. Dual effect of exogenous hydrogen sílfide on the spontaneous contraction of gastric smooth muscle in guinea-pig. Eur J Pharmacol 616: 223-228.
Publication Dates
-
Publication in this collection
12 Aug 2011 -
Date of issue
Oct 2011
History
-
Received
20 Dec 2010 -
Accepted
22 Apr 2011