Abstracts
The boiled juice of Stachytarpheta cayennensis, Verbenaceae, whole plant material is used ethnomedicinally in different countries of Latin-America to treat symptoms of malaria. In the ethyl acetate extract, five phenylethanoid glycosides could be identified. Three of these phenylethanoid glycosides, leucosceptoside A, martynoside and jionoside D were detected for the first time in the genus Stachytarpheta.
Stachytarpheta cayennensis; Verbenaceae; Phenylethanoid glycosides; Malaria; Traditional medicine
O suco fervido de Stachytarpheta cayennensis, Verbenaceae, do material vegetal inteiro é utilizado etnomedicinalmente em diferentes países da América Latina para tratar os sintomas da malária. No extrato acetato de etila, cinco glicosídeos feniletanóides puderam ser identificados. Três destes glicosídeos feniletanóides, leucosceptosídeo A, martinosídeo e jionosídeo D foram detectados pela primeira vez no gênero Stachytarpheta.
Stachytarpheta cayennensis; Verbenaceae; Glicosídeos feniletanóides; Malária; Medicina tradicional
ARTIGO
Phenylethanoid glycosides from Stachytarpheta cayennensis (Rich.) Vahl, Verbenaceae, a traditional antimalarial medicinal plant
Glicosídeos feniletanóides de Stachytarpheta cayennensis (Rich.) Vahl, Verbenaceae, uma planta medicinal tradicional anti-malárica
Sonja FroelichI; Mahabir P. GuptaII; Karsten SiemsIII; Kristina Jenett-SiemsI,* * E-mail: kjsiems@zedat.fu-berlin.de, Tel. +49-30-838 53720, Fax +49-30-838 51461
IInstitut für Pharmazie (Pharmazeutische Biologie), Freie Universität Berlin, Königin-Luise Str.2+4, D-14195 Berlin, Germany
IICentro de Investigaciones Farmacognosticas de la Flora Panameña (CIFLORAN), Universidad de Panama, Panamá, Republica de Panama
IIIAnalyticon Discovery GmbH, Hermannswerder Haus 17, D-14473 Potsdam, Germany
ABSTRACT
The boiled juice of Stachytarpheta cayennensis, Verbenaceae, whole plant material is used ethnomedicinally in different countries of Latin-America to treat symptoms of malaria. In the ethyl acetate extract, five phenylethanoid glycosides could be identified. Three of these phenylethanoid glycosides, leucosceptoside A, martynoside and jionoside D were detected for the first time in the genus Stachytarpheta.
Keywords: Stachytarpheta cayennensis, Verbenaceae, Phenylethanoid glycosides, Malaria, Traditional medicine.
RESUMO
O suco fervido de Stachytarpheta cayennensis, Verbenaceae, do material vegetal inteiro é utilizado etnomedicinalmente em diferentes países da América Latina para tratar os sintomas da malária. No extrato acetato de etila, cinco glicosídeos feniletanóides puderam ser identificados. Três destes glicosídeos feniletanóides, leucosceptosídeo A, martinosídeo e jionosídeo D foram detectados pela primeira vez no gênero Stachytarpheta.
Unitermos: Stachytarpheta cayennensis, Verbenaceae, Glicosídeos feniletanóides, Malária, Medicina tradicional.
INTRODUCTION
Stachytarpheta cayennensis (Rich.) Vahl (syn.: Stachytarpheta guatemalensis Moldenke), Verbenaceae, is an herb or small shrub commonly found in forest, thickets, and swamps and on savannas from Mexico to Panama. The plant is known as "camaq olal", "San Diego", "verbena", and "vervena". The boiled juice is used by natives as a remedy for malaria and dysentery (Moldenke, 1973). In Guatemala, a decoction of the leaves is drunk to relieve specific symptoms or conditions that often accompany malaria, such as weakness and fever (Comersford, 1996). In Brazil, an infusion of the entire plant is used to treat malaria (Milliken, 1997) and against respiratory diseases, it is drunkas tea until the symptoms disappear (Agra et al., 2008). In Nicaragua, a decoction of the dried leaf material is indicated to cure fever (Coe and Anderson, 1996). Additionally, the plant is used as an analgesic, anti-inflammatory and diuretic (Alice et al., 1991), as well as a remedy for liver disorders (Milliken, 1997).
S. cayennensis belongs to a widely distributed and complex family of about 76 genera and 3435 specific and subspecific taxa, found all over the world except the driest and hottest parts of the Sahara Desert and in the Arctic and Antarctic regions. The family Verbenaceae is closely related to the Lamiaceae from which it is distinguished with difficulty (Moldenke, 1973).
A previous study determined the antiplasmodial activity of hydrophilic and lipophilic extracts from S. cayennensis against the chloroquine-sensitive strain poW and the multiresistant clone Dd2 of Plasmodium falciparum. The petrol ether/ethyl acetate extract showed only low activity at 50 µg/ml (Jenett-Siems et al., 1999). In addition, the hydroalcoholic extract of S. cayennensis inhibits the growing of Leishmania promastigotes forms in vitro accounting for the folk use of this vegetal in skin ulcers caused by Leishmania (Moreira et al., 2007).
In this study, the species was investigated phytochemically which led to the isolation of five phenylethanoid glycosides.
MATERIAL AND METHODS
Spectroscopic methods
1H NMR spectra were recorded on a Bruker AVANCE DPX 400-spectrometer (400 MHz) using TMS as internal standard. Samples were dissolved in methanol-d4.
FAB-MS were obtained on a Varian MAT CH5DF instrument with DMSO/Glycerin, Xenon; CHCl3/slm-Nitrobenzylalkohol, Xenon or MeOH/ Glycerin, Xenon as solvents.
Plant material
S. cayennensis was collected in an area around Panama City by PD Dr. Jenett-Siems. It was identified by Prof. Dr. M. D. Correa A., Panama. A voucher specimen CIFLORAN 2756 was deposited in the herbarium of the University of Panama.
Extraction and isolation of compounds
Air dried whole plant material (430 g) was extracted with methanol (3x2.0 L). The extract was concentrated under reduced pressure yielding 42 g of the crude methanol extract (CME). The dry CME was acidified with a 2 % (m/v) aqueous solution of tartaric acid. The aqueous layer was extracted with petrol ether (3x0.40 L), CH2Cl2 (3x0.40 L) and EtOAc (3x0.40 L). Of the evaporated EtOAc-extract (3.16 g), 0.5 g was dissolved in 1.5 mL methanol. Purification by preparative TLC (HCO2H-H2O-EtOAc 9:9:82) yielded iso-acteoside (1) (Rf 0.56, 4.6 mg) and acteoside (2) (Rf 0.63, 5.2 mg).
The remaining EtOAc-extract (2.6 g) was subjected to column chromatography on LiChroprep® RP-18 material [Merck, 40-63 µm, 28.5 g] and fractionated into 14 fractions (H2O-MeOH 80:20 to MeOH). Fraction C2 (H2O-MeOH 60:40) allowed the isolation of compounds leucosceptoside A (3) (Rf 0.57, 5.9 mg), and a mixture of martynoside (4) and jionoside D (5) (Rf 0.61, 27.2 mg) by preparative TLC (HCO2H-H2O-EtOAc 9:9:82).
Iso-acteoside [1]: (-)-FAB-MS m/z 623 [M-H]-, 1H NMR (methanol-d4, 400 MHz): δ 1.24 (3H, d, J = 6.0 Hz, H-6''); 2.77 (2H, t, J = 7.0 Hz, H-7); 3.30 (1H, m, H-2', obscured by methanol); 3.38 (1H, m, H-4''), 3.39 (1H, m, H-4'); 3.52 (1H, m, H-3'); 3.53 (1H, m, H-5'); 3.69 (1H, dd, J = 3.2 Hz, J = 9.5 Hz, H-3''); 3.70 (1H, m, H-8a); 3.93 (1H, dd, J = 2.0 Hz, J = 3.3 Hz, H-2''); 3.97 (1H, m, H-8b); 3.98 (1H, m, H-5''); 4.32 (1H, d, J = 8.0 Hz, H-1'); 4.34 (1H, dd, J = 6.0 Hz, J = 12.0 Hz, H-6'a); 4.49 (1H, dd, J = 2.0 Hz, J = 12.0 Hz, H-6'b); (1H, d, J = 1.5 Hz, H-1''); 6.28 (1H, d, J = 16.0 Hz, H-8'''); 6.53 (1H, dd, J = 2.0 Hz, J = 8.0 Hz, H- 6); 6.62 (1H, d, J = 8.0 Hz, H-5); 6.66 (1H, d, J = 2.0 Hz, H-2); (1H, d, J = 8.0 Hz, H-5'''); 6.89 (1H, dd, J = 2.0 Hz, J = 8.0 Hz, H-6'''); 7.02 (1H, d, J = 2.0 Hz, H-2'''); (1H, d, J = 16.0 Hz, H-7''').
Acteoside [2]: (-)-FAB-MS m/z 623 [M-H]-, 1H NMR (methanol-d4, 400 MHz): δ 1.09 (3H, d, J = 6.1 Hz, H-6''); 2.79 (2H, t, J = 8.0 Hz, H-7); 3.28 (1H, t, J = 9.5 Hz, H-4''); 3.39 (1H, t, J = 9.0 Hz, H-2'); 3.53-3.61 (5H, m, H-6'a, H-5'', H-5', H-3'', H-6'b); 3.72 (1H, m, H-8a); 3.81 (1H, t, J = 9.0 Hz, H-3'); 3.91 (1H, d, J = 2.0 Hz, J = 3.0 Hz, H-2''); 4.05 (1H, m, H-8b); 4.37 (1H, d, J = 8.0 Hz, H-1'); 4.95 (1H, H-4', obscured by water); (1H, d, J = 1.5 Hz, H-1''); 6.27 (1H, d, J = 15.9 Hz, H-8'''); 6.56 (1H, dd, J = 2.0 Hz, J = 8.0 Hz, H-6); 6.67 (1H, d, J = 8.0 Hz, H-5); 6.69 (1H, d, J = 2.0 Hz, H-2); (1H, d, J = 8.0 Hz, H-5'''); 6.95 (1H, dd, J = 2.0 Hz, J = 8.0 Hz, H-6'''); 7.05 (1H, d, J = 2.0 Hz, H-2'''); (1H, d, J = 15.9 Hz, H-7''').
Leucosceptoside A [3]: (+)-FAB-MS m/z 661 [M+Na]+, 1H NMR (methanol-d4, 400 MHz): δ 1.10 (3H, d, J = 6.2 Hz, H-6''); 2.79 ( 2H, t, J = 6.2 Hz, H-7); 3.20-4.03 (9H, m, H-2', H-3', H-5', H-6'a, H-6'b, H-2'', H-3'',H-4'', H-5''); 3.79 (1H, m, H-8a); 3.88 (3H, s, OCH3-3'''); 4.05 (1H, m, H-8b); 4.37 (1H, d, J = 7.9 Hz, H-1'); 4.93 (1H, t, J = 9.1 Hz, H-4'); 5.19 (1H, d, J = 1.5 Hz, H-1''); 6.37 (1H, d, J = 15.8 Hz, H-8'''); 6.56 (1H, dd, J = 1.9, J = 8.1 Hz, H-6); 6.67 (1H, d, J = 7.9 Hz, H-5); 6.69 (1H, d, J = 1.9 Hz, H-2); 6.81 ( 1H, d, J = 8.2 Hz, H-5'''); 7.08 (1H, dd, J = 1.7 Hz, J = 8.2 Hz, H-6'''); 7.19 (1H, d, J = 1.7 Hz, H-2'''); 7.66 (1H, d, J = 15.8 Hz, H-7''').
Martynoside [4]: (+)-FAB-MS m/z 675 [M+Na]+, 1H NMR (methanol-d4, 400 MHz): δ 1.10 (3H, d, J = 6.2 Hz, H-6''); 2.82 (2H, t, J = 7.5 Hz, H-7); 3.37-4.03 (7H, m, H-2', H-3', H-5', H-6'a, H-6'b, H-2'', H-5''); 3.75 (1H, m, H-8a); 3.81 (3H, s, OCH3-4); 3.89 (3H, s, OCH3-3'''); 4.07 (1H, m, H-8b); 4.37 (1H, d, J = 8.0 Hz, H-1'); 4.92 (1H, t, J = 9.4 Hz, H-4'); 5.19 (1H, d, J = 1.7 Hz, H-1''); 6.36 (1H, d, J = 15.9 Hz, H-8'''); 6.68 (1H, dd, J = 2.0 Hz, J = 8.1 Hz, H-6); 6.73 (1H, d, J = 1.9 Hz, H-2); 6.80 (1H, d, J = 8.2 Hz, H-5); 6.82 (1H, d, J = 8.1 Hz, H-5'''); 7.08 (1H, dd, J = 1.8 Hz, J = 8.2 Hz, H-6'''); 7.19 (1H, d, J = 1.8 Hz, H-2'''); 7.66 (1H, d, J = 15.9 Hz, H-7''').
Jionoside D [5]: (+)-FAB-MS, m/z 661 [M+Na]+, 1H NMR (methanol-d4, 400 MHz): δ 1.15 (3H, d, J = 6.2 Hz, H-6''); 2.82 (2H, t, J = 7.5 Hz, H-7); 3.36-4.03 (9H, m, H-2', H-3', H-5', H-6'a, H-6'b, H-2'', H-3'', H-4'', H-5''); 3.76 (1H, m, H-8a); 3.81 (3H, s, OCH3-4); 4.05 (1H, m, H-8b); 4.37 (1H, d, J = 8.0 Hz, H-1'); 4.92 (1H, t, J = 9.4 Hz, H-4'); 5.20 (1H, d, J = 1,7 Hz, H-1''); 6.27 (1H, d, J = 15.9 Hz, H-8'''); 6.68 (1H, dd, J = 2,0 Hz, J = 8.1 Hz, H-6); 6.73 (1H, d, J = 2.0 Hz, H-2); 6.76 (1H, d, J = 8.2 Hz, H-5'''); 6.82 (1H, d, J = 8.1 Hz, H-5); 6.94 (1H, dd, J = 2.0 Hz, J = 8.0 Hz, H-6'''); 7.06 (1H, d, J = 1.7 Hz, H-2'''); 7.59 (1H, d, J = 15.6 Hz, H-7''').
RESULTS AND DISCUSSION
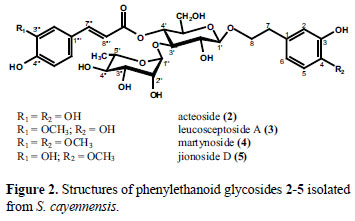
The ethyl acetate extract was separated by using a combination of column chromatography and preparative TLC which resulted in the isolation of five phenylethanoid glycosides (Figure 1 and 2). Upon preparative TLC acteoside (2) and its isomer isoacteoside (1) were obtained as amorphous compounds.
The negative FAB-MS of acteoside (2) showed a pseudomolecular ion [M-H]-at m/z 623. The 1H NMR spectrum of (2) consisted of a set of signals at δ6.67 ppm (1H, d, J = 8.0 Hz, H-5), 6.69 ppm (1H, d, J = 2.0 Hz, H-2), and 6.56 ppm (1H, dd, J = 2.0 Hz, J = 8.0 Hz, H-6) and another set at δ 6.77 ppm (1H, d, J = 8.0 Hz, H-5'''), 6.95 ppm (1H, dd, J = 2.0 Hz, J = 8.0 Hz, H-6''') and 7.05 ppm (1H, d, J = 2.0 Hz, H-2'''). These resonances are characteristic for the presence of two 1,3,4-trisubstituted aromatic systems. One of these aromatic systems is part of a caffeoyl-moiety due to the signals for two olefinic protons at δ 6.27 ppm (1H, d, J = 15.9 Hz, H-8''') and δ 7.59 ppm (1H, d, J = 15.9 Hz, H-7'''), indicating a trans geometry. Proton signals at δ 2.79 ppm (2H, d, J = 8.0 Hz, H-7), 3.72 ppm (1H, m, H-8a) and δ 4.05 ppm (1H, m, H-8b) revealed that the second aromatic system is part of a phenylethanoid moiety. The 1H NMR spectrum also displayed a number of signals between 3.00 and 4.00 ppm, suggesting the existence of sugar in the molecule. Accordingly, the signal at δ 1.09 ppm (3H, d, J = 6.1 Hz, H-6'') was ascribed to the methyl group of a rhamnose moiety and furthermore a signal at δ 5.18 ppm (1H, d, J = 1.5 Hz, H-1'') with a small coupling constant could be assigned to the anomeric proton of an α-L-rhamnose. A second anomeric proton at δ 4.37 ppm (1H, d, J = 8.0 Hz, H-1') with a coupling constant of 8.0 Hz, pointed to a β-hexopyranose. Comparing the 1H-NMR data with previously published data (Sasaki et al. 1978; Miyase et al., 1982; Andary et al., 1989) led to the identification of acteoside (2). In 1978 acteoside was isolated for the first time out of fresh leaves and stems of Martynia louisiana Mill., Pedaliaceae (Sasaki et al., 1978). Additionally, acteoside was afforded from fresh roots of Leucosceptrum japonicum (Miq.) Kitamura & Murata, Lamiaceae (Miyase et al., 1982), from roots of Plantago crassifolia Forssk., Plantaginaceae (Andary et al., 1989), and from numerous other plant species.
The negative FAB-MS of iso-acteoside (1) showed a pseudomolecular ion [M-H]-at m/z 623. In the 1H-NMR spectrum of (1), characteristic signals arising from six aromatic protons at δ 6.62 ppm (1H, d, J = 8.0 Hz, H-5), 6.66 ppm (1H, d, J = 2.0 Hz, H-2) and 6.53 ppm (1H, dd, J = 2.0 Hz, J = 8.0 Hz, H-6), as well as at 6.76 ppm (1H, d, J = 8.0 Hz, H-5'''), 6.89 ppm (1H, dd, J = 2.0 Hz, J = 8.0 Hz, H-6''') and 7.02 ppm (1H, d, J = 2.0 Hz, H-2''') showed the presence of two 1,3,4-trisubstituted benzenes. One of these aromatic systems is part of a caffeoyl-moiety due to the signals for two olefinic protons at δ 6.28 ppm (1H, d, J = 16.0 Hz, H-8''') and δ 7.55 ppm (1H, d, J = 16.0 Hz, H-7'''), representing a trans-geometry. Proton signals at δ 2.77 ppm (2H, t, J = 7.0 Hz, H-7), 3.70 ppm (1H, m, H-8a) and δ 3.97 ppm (1H, m, H-8b) could be assigned to a phenylethanoid moiety. Furthermore, two doublet signals that appeared at δ 4.32 ppm (J = 8.0 Hz) and δ 5.17 ppm (J = 1.5 Hz) were attributed to the anomeric protons of a β-glucose and an α-rhamnose unit, respectively. The downfield shift of H-6'a+b compared to (2) led to the identification of (1) as iso-acteoside (Miyase et al., 1982). In earlier studies iso-acteoside was isolated e.g. out of fresh roots of Leucosceptrum japonicum (Miq.) Kitamura & Murata, Lamiaceae (Miyase et al., 1982).
Leucosceptoside A (3) differs structurally from acteoside (2) by the replacement of caffeic acid with ferulic acid. The 1H-NMR spectrum of (3) was similar to that of acteoside (2) and iso-acteoside (1) due to the characteristic signals arising from six aromatic protons at δ 6.67 ppm (1H, d, J = 7.9 Hz, H-5), δ 6.56 ppm (1H, dd, J = 1.9 Hz, J = 8.1 Hz, H-6) and δ 6.69 ppm (1H, d, J = 1.9 Hz, H-2) as well as signals at δ 6.81 ppm (1H, d, J = 8.2 Hz, H-5'''), δ 7.08 ppm (1H, dd, J = 1.7 Hz, J = 8.2 Hz, H-6''') and δ 7.19 ppm (1H, d, J = 1.7 Hz, H-2''') showing the presence of two 1,3,4-trisubstituted aromatic systems. One of these benzenes is part of a feruloyl-moiety due to the signals for two olefinic protons at δ 6.37 ppm (1H, d, J = 15.8 Hz, H-8''') and δ 7.66 ppm (1H, d, J = 15.8 Hz, H-7'''), which indicate the trans-geometry and the characteristic signal at δ 3.88 ppm (3H, s, OCH3) for the methoxygroup. Proton signals at δ 2.79 ppm (2H, t, J = 6.2 Hz, H-7), 3.79 ppm (1H, m, H-8a) and δ 4.05 ppm (1H, m, H-8b) revealed that the second aromatic system is part of a phenylethanoid moiety. The sugar resonances were again similar to that of (2), allowing the identification of (3) as leucosceptoside A (Miyase et al., 1982; Sasaki et al., 1989). In previous investigations, leucosceptoside A was isolated together with iso-acteoside and acteoside out of the under terrestrial parts of Leucosceptrum japonicum (Miq.) Kitamura & Murata, Lamiaceae (Miyase et al., 1982) and as well out of dried roots of Rehmannia glutinosa Libosch. var. purpurea Makino, Scrophulariaceae (Sasaki et al., 1989).
Martynoside (4) and jionoside D (5) were isolated as a 3:1 mixture. Both compounds have similar features but differ structurally in the number of methoxy groups. This is reflected in the positive FABMS showing pseudomolecular peaks [M+Na]+ at m/z 675 (4), respectively [M+Na]+ at m/z 661 (5). The 1H NMR spectrum of martynoside (4) showed a signal for a methoxy group at δ 3.89 ppm (3H, s, OCH3-3''') in the acid moiety and furthermore a signal at δ 3.81 ppm (3H, s, OCH3-4) in the phenylethanoid portion (Sasaki et al. 1978; Miyase et al. 1982; Calis et al. 1984; Teborg and Junior 1989) (Figure 2), whereas jionoside D possesses only one methoxy group at δ 3.81 ppm (3H, s, OCH3-4) (Sasaki et al. 1989). Martynoside (4) was isolated from Miyase and co-workers in 1982 from Leucosceptrum japonicum (Miq.) Kitamura & Murata, Lamiaceae (Miyase et al., 1982) and furthermore together with jionoside D (5) out of Rehmannia glutinosa Libosch. var. purpurea Makino, Scrophulariaceae (Sasaki et al., 1989).
Phenylethanoid glycosides are characteristic constituents of families belonging to the order Lamiales, as e.g. Lamiaceae and also the closely related Verbenaceae. Two of the isolated compounds, namely acteoside (2) and iso-acteoside (1) were already found in Stachytarpheta cayennensis before (Schapoval et al., 1998; Adebajo et al., 2007; Pereira et al., 2008), and acteoside also in Stachytarpheta glabra (Viccini et al., 2008), whereas the other phenylethanoid glycosides leucosceptoside A (3), martynoside (4) and jionoside D (5) were detected for the first time in the genus Stachytarpheta.
ACKNOWLEDGMENTS
The authors are grateful to Mrs. U. Ostwald (Institut für Organische Chemie, FU Berlin) for providing the FAB-MS spectra. Thanks are due to Mrs. G. Rehork and Mrs. B. Zeisig for measuring NMR spectra.
Received 16 July 2008; Accepted 15 September 2008
- Adebajo AC, Olawode EO, Omobuwajo OR, Adesanya SA, Begrow F, Elkhawad A, Akanmu MA, Edrada R, Proksch P, Schmidt TJ, Klaes M, Verspohl EJ 2007. Hypoglycaemic constituents of Stachytarpheta cayennensis leaf. Planta Med 73:241-250.
- Agra MF, Silva KN, Basílio IJLD, França PF, Barbosa-Filho JM 2008. Survey of medicinal plants used in the region Northeast of Brazil. Rev Bras Farmacogn 18:472-508.
- Alice CB, Vargas VMF, Silva GAAB, De Siqueira NCS, Schapoval EES, Gleve J, Henriques JAP, Henriques AT 1991. Screening of plants used in South Brazilian folk medicine. J Ethnopharmacol 35:165-171.
- Andary C, Ravn H, Wylde R, Heitz A, Motte-Florac E 1989. Crassifolioside, a caffeic acid glycoside ester from Plantago crassifolia. Phytochemistry 28:288-290.
- Calis I, Lahloub MF, Rogenmoser E, Sticher O 1984. Isomartynoside, a phenylpropanoid glycoside from Galeopsis pubescens. Phytochemistry 23:2313-2315.
- Coe FG, Anderson GJ 1996. Screening of medicinal plants used by the Garifuna of Eastern Nicaragua for bioactive compounds. Econ Bot 53:29-50.
- Comersford SC 1996. Medicinal plants of two Mayan healers from San Andres, Peten, Guatemala. Econ Bot 50:327-336.
- Jenett-Siems K, Mockenhaupt FP, Bienzle U, Gupta MP, Eich E 1999. In vitro antiplasmodial activity of Central American medicinal plants. Trop Med Int Health 4:611-615.
- Milliken W 1997. Traditional anti-malarial medicine in Roraima, Brazil. Econ Bot 51:212-237.
- Miyase T, Koizumi A, Ueno A, Tadataka N, Kuroyanagi M, Fukushima S, Akiyama Y, Takemoto T 1982. Studies on the acyl glycosides from Leucoseptum japonicum (Miq.) Kitamura et Murata. Chem Pharm Bull 30:2732-2737.
- Moldenke, HN 1973. Flora of Panama Part IX. Family 168. Verbenaceae. Ann Missouri Bot Gard 60:41-148.
- Moreira RCR, Costa GC, Lopes TC, Bezerra JL, Guerra RNM, Rebêlo JMM, Ribeiro MNS, Nascimento FRF, Costa JML 2007. Efeito leishmanicida in vitro de Stachytarpheta cayennensis (Rich.) Vahl (Verbenaceae). Rev Bras Farmacogn 17:59-63.
- Pereira AC, Carvalho HWP, Silva GH, Oliveira DF, Figueiredo HCP, Cavalheiro AJ, Carvalho DA 2008. Purification of an antibacterial compound from Lantana lilacina. Rev Bras Farmacogn 18:204-208.
- Sasaki H, Taguchi H, Endo T, Yosioka I, Higashiyama K, Otomasu H 1978. The glycosides of Martynia louisiana Mill. A new phenylpropanoid glycoside, Martynoside. Chem Pharm Bull 26:2111-2121.
- Sasaki H, Nishimura H, Chin M, Mitsuhashi H 1989. Hydroxycinnamic acid esters of phenylethylalcohol glycosides from Rehmannia glutinosa var. purpurea. Phytochemistry 28:875-879.
- Schapoval EES, Winter de Vargas MR, Chaves CG, Bridi R, Zuanazzi JA, Henriques AT 1998. Antiinflammatory and antinociceptive activities of extracts and isolated compounds from Stachytarpheta cayennensis. J Ethnopharmacol 60:53-59.
- Teborg D, Junior P 1989. Martynoside and the novel dimeric open-chain monoterpene glucoside digipenstroside from Penstemon digitalis. Planta Med 55:474-476.
- Viccini LF, Silva PS, de Almeida MV, Saraiva MF, Peixoto PHP, Salimena FRG, Diniz R, Rodrigues BL, Scowen I, Edwards HGM, Oliveira LFC 2008. Ipolamiide and fulvoipolamiide from Stachytarpheta glabra (Verbenaceae): A structural and spectroscopic characterization. J Mol Struct 875:27-31.
Publication Dates
-
Publication in this collection
18 Feb 2009 -
Date of issue
Dec 2008
History
-
Received
16 July 2008 -
Accepted
15 Sept 2008