Abstract
Chemical study of three medicinal plants: from leaves of Piper renitens (Miq.) Yunck, Piperaceae, and Siparuna guianensis Aubl., Siparunaceae, and from flowers of Alternanthera brasiliana (L.) Kuntze, Amaranthaceae, resulted in isolation of nine compounds: three steroids, β-sitosterol, stigmasterol from P. renitens and sitosterol-3-O-β-D-glucopyranoside from A. brasiliana, the diterpene kaurane ent-kauran-16α,17-diol from P. renitens, two derivatives kaempferol-methylether, kumatakenine (kaempferol-3,7-dimethylether) and kaempferol-3,7,3'-trimethylether from S. guianensis and three flavones, crysoeriol (5,7,4'-trihydroxy-3'-methoxyflavone), tricin (5,7,4'-trihydroxy-3',5'-dimethoxyflavone) and 7-O-β-D-glucopyranoside-5,4'-dihydroxy-3'-methoxyflavone from A. brasiliana. Compounds structures were determinate using 1D and 2D ¹H NMR and 13C spectral data, mass and IR spectra, comparing with literature data.
Piper renitens; Siparuna guianensis; Alternanthera brasiliana; ent-kaunare diterpene; kaempferol-methylether; flavonoids
Chemical constituents from three medicinal plants: Piper renitens, Siparuna guianensis and Alternanthera brasiliana
Valdir A. FacundoI,* * Correspondence: Valdir A. Facundo Departamento de Química, Universidade Federal de Rondônia BR 364, km 9,5, 78.900-500 Porto Velho-RO, Brazil vfacundo@unir.br Tel. +55 69 2182 2100 ; Mariangela S. AzevedoI; Rosely V. RodriguesI; Leandro F. do NascimentoI; Júlio S. L. T. MilitãoI; Gil V. J. da SilvaII; Raimundo Braz-FilhoIII
IDepartamento de Química, Universidade Federal de Rondônia, Campus UNIR, Brazil
IIDepartamento de Química, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Brazil
IIIPesquisador visitante emérito-FAPERJ-UENF-UFRRJ
ABSTRACT
Chemical study of three medicinal plants: from leaves of Piper renitens (Miq.) Yunck, Piperaceae, and Siparuna guianensis Aubl., Siparunaceae, and from flowers of Alternanthera brasiliana (L.) Kuntze, Amaranthaceae, resulted in isolation of nine compounds: three steroids, β-sitosterol, stigmasterol from P. renitens and sitosterol-3-O-β-D-glucopyranoside from A. brasiliana, the diterpene kaurane ent-kauran-16α,17-diol from P. renitens, two derivatives kaempferol-methylether, kumatakenine (kaempferol-3,7-dimethylether) and kaempferol-3,7,3'-trimethylether from S. guianensis and three flavones, crysoeriol (5,7,4'-trihydroxy-3'-methoxyflavone), tricin (5,7,4'-trihydroxy-3',5'-dimethoxyflavone) and 7-O-β-D-glucopyranoside-5,4'-dihydroxy-3'-methoxyflavone from A. brasiliana. Compounds structures were determinate using 1D and 2D 1H NMR and 13C spectral data, mass and IR spectra, comparing with literature data.
Keywords: Piper renitens, Siparuna guianensis, Alternanthera brasiliana, ent-kaunare diterpene, kaempferol-methylether, flavonoids
Introduction
Plants utilization in treatment, cure and prevention of any kind of disease is a traditional practice in the entire world. The necessity of knowledge from chemical constituents of plants becomes important as many pharmaceutical products that exist today were developed from substances of that origin (Newman et al., 2003).
Piper genus is included as one of the most important in Piperaceae family and is well distributed in tropical and sub-tropical regions of the planet. Chemical and pharmaceutical studies of this genus are related to the isolation of various secondary metabolites classes (amides, flavonoids, lignans, terpenes, propenylphenols, alkaloids and cyclopetenediones derivatives) and to its use in treatment of some diseases (Parmar et al., 1997; Facundo et al., 2003). In Mexico and Brazil, leaves from P. amalago are used to treat stomachache and some infections. Leaves and stems from P. marginatum and P. tuberculatum are used against snakebite and as sedatives (Chaves et al., 2006; Araújo-Junior et al., 1999). P. renitens (Miq.) Yunck is known by the Amazonian rainforest natives as "bararupyangihua" and tea from leaves is used mainly to cure fever, infant diarrhea and skin inflammations (Ribeiro et al., 1999). Chemical volatile constituents from their leaves were related this specie (Soleane et al., 2007).
Siparuna (formerly Monimiaceae) contains about 72 species of shrubs, straggling shrubs, and trees (Renner et al. 1997). S. guianensis Aubl., Siparunaceae, is a small tree found in various parts of Brazilian forests, popularly known as "capituí", "negramena", "erva-santa", "limão-bravo" and others (Zoghbi et al., 1998). Several species of this genus are used in traditional medicine to handling stomach disorders and skin diseases (Leitão et al., 1999). Literature reports the use of S. guianensis leaves in popular medicine to cure various diseases, including, sinusitis, fever, rheumatism, migraine, body aches and as anti-inflammatory (Valentini et al., 2010; Rodrigues, 2006). In state of Rondônia, Brazil (an oriental Amazonian state), there are some reports of using leaves tea in treatment of malaria and against rheumatic pains. Chemical studies had revealed presence of volatile compounds, alkaloids and flavonoids (Braz-Filho et al., 1976; Chiu et al., 1982; Machado et al., 1994).
Alternanthera brasiliana (L.) Kuntze is a herbaceous plant from the Amaranthaceae family, popularly known as "penicilina", "terramicina" and "perpetua-do-mato", tea from leaves is widely used to treat several pathologies, including coughing, diarrhea and for its anti-inflammatory and analgesic properties (Delaporte et al., 2005; Araújo & Onofre, 2011). In state of Rondônia, Brazil, tea from leaf is used for its anti-inflammatory properties. Chemical study of the leaves of A. brasiliana had revealed the presence of six di- and triglycosyl kaempferol derivatives and quercetin (Brochado et al., 2003).
This work describes the isolation and structural characterization of the nine compounds from three medicinal plants popularly used as anti-inflammatory: β-sitosterol 1 and stigmasterol 2 from leaves of P. renitens, sitosterol-3-O-β-D-glucopyranoside 9 from flowers of A. brasiliana, ent-kauran-16α,17-diol 3 from leaves of P. renitens, kumatakenine (kaempferol-3,7-dimethylether) 4 and kaempferol-3,7,4'-trimethylether 5, from leaves of S. guianensis and crysoeriol (5,7,4'-trihydroxy-3'-methoxyflavone) 6, tricin (5,7,4'-trihydroxy-3',5'-dimethoxyflavone) 7 and 7-O-β-D-glucopyranoside-5,4'-dihydroxy-3'-methoxyflavone 8, from flowers of A. brasiliana. The structures were established by spectroscopic techniques, mainly EIMS and 1D and 2D NMR. This is the first report of the occurrence of these compounds in these plants.
Material and methods
Melting points were obtained on a Mettler FP82HT apparatus and are uncorrected. IR spectra were recorded using a Perkin Elmer 1000 FT-IR spectrophotometer. The mass spectra were obtained on a Hewlett-Packard 5971 mass spectrometer by electron impact ionization (70 eV). 1H and 13C NMR where recorded on a Bruker Avance DRX-500 (500 MHz for 1H and 125 MHz for 13C); Silica gel 60 (Merck, kiesegel 60 F254, 0.20 mm) were used for analytical TLC. Silica gel 60 (Merck, 230-240 mesh) was used for column chromatography. All compounds were visualized on TLC by spraying with AlCl3/EtOH and vanillin/perchloric acid/EtOH followed by heating.
Leaves from Siparuna guianensis Aubl., Siparunaceae, and Piper renitens (Miq.) Yunck, Piperaceae, were collected in Mirante da Serra County, Rondonia State, Brazil on April, 2010, and May, 2009, respectively. The plant material was classified systematically by the botanist Dr. J. Gomes from Instituto Nacional de Pesquisa da Amazônia herbarium where voucher specimens were deposited: 216220 for S. guianensis and 213523 for P. renitens. Leaves from P. renitens (1.1 kg) and S. guianensis (1.8 kg) were dried, triturated and submitted to ethanol extraction at room temperature in portions of 2 and 3 L, respectively, for three times. After solvent distillation at reduced pressure, 26.0 g and 41.0 g, respectively, of extracts were obtained. A portion of 22.0 g of the ethanol extract from P. renitens was adsorbed in silica gel (100.3 g) and eluted under reduced pressure in a chromatographic column, using successively hexane, chloroform, ethyl acetate and methanol as solvents.
Chloroform fraction was treated with MeOH:H2O (90:10) and kept under refrigeration overnight to induce chlorophyll separation. This material was dried and re-chromatographed on a silica gel column and eluting with mixtures of hexane and chloroform of increasing polarity, obtaining a mixture of β-sitosterol 1 and of stigmasterol 2 [32.3 mg (hexane/chloroform 40:60), 13C NMR (CDCl3) data, see Jácome et al., 2004] and ent-kauran-16α,17-diol 3 [22.1 mg (hexane/chloroform 50:50), 187-188 ºC, 13C NMR (CDCl3) data, see Nascimento & Lopes, 2003].
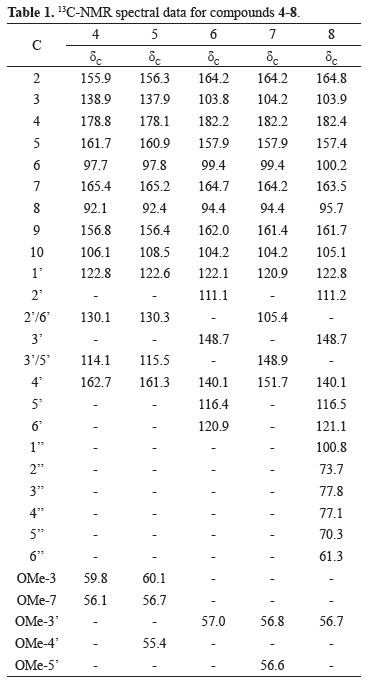
Similarly, a portion of 35.5 g of the ethanol extract from S. guianensis were adsorbed by silica gel (90.0 g) and eluted under reduced pressure in a chromatographic column, using successively hexane, chloroform, ethyl acetate and methanol as solvents. Chromatography on a silica gel column of the chloroform fraction (9.3 g) eluting with mixtures of hexane and chloroform of increasing polarity yielded 23 sub-fractions. The subfractions 32 to 54 were combined and re-chromatographed on a silica gel column and eluted with hexane and ethyl acetate mixture with increasing polarity yielded kumatakenin (kaempferol-3,7-dimethylether) 4 [31.8 mg (hexane/ethyl acetate 60:40), 243-245 ºC, 13C NMR (CDCl3) data, see table 1, Silva et al., 2009] and kaempferol-3,7,4'-trimethylether 5 [21.5 mg (hexane/ethyl acetate 55:45), 146-148 ºC, 13C NMR (CDCl3) data, see table 1, Paula et al., 2006].
Flowers from Alternanthera brasiliana (L.) Kuntze, Amaranthaceae, were collected in Porto Velho, Rondônia State, Brazil on September 2008. The plant material was classified systematically by the botanist Dra. Ana Cristina R. de Souza from Dr. Ary Tupinamba Pena Pinheiro Herbarium in Faculdade Sao Lucas (Porto Velho, Rondonia State, Brazil) where may be found a voucher specie identified by 445801 descriptor.
Flowers (0.8 kg) from A. brasiliana, were dried, triturated and submitted to ethanol extraction at room temperature in portions of 1.5 L, in three times. After solvent distillation at reduced pressure, 21.3 g of extract were obtained. All the extract were absorbed by silica gel and eluted in a chromatography column with chloroform and ethyl acetate as binary mixtures with increasing polarity yielded 104 sub-fractions. The subfraction 9 (1.2 g chloroform/ethyl acetate 90:10) yielded a solid residue which after recrystallization from acetone yielded crysoeriol (5,7,4'-trihydroxy-3'-methoxyflavone) 6 [21.9 mg, 324-326 ºC, 13C NMR (DMSO-d6) data, see table 1, Awaad et al., 2006]. The subfractions 17 to 34 (2.2 g chloroform/ethyl acetate 85:15) were combined, the resulting fraction was rechromatographed on a silica gel column and eluted with chloroform and ethyl acetate mixture with increasing polarity yielded tricin (5,7,4'-trihydroxy-3',5'-dimethoxyflavone) 7 [23.1 mg (chloroform/ethyl acetate 82:18), 278-280 ºC, 13C NMR (DMSO-d6) data, see table 1, Jiao et al., 2007] and 7-O-β-D-glucopyranoside-5,4'-dihydroxy-3'-methoxyflavone 8 [17.4 mg (chloroform/ethyl acetate 40:60), 176-178 ºC, 13C NMR (DMSO-d6) data, see table 1, Markham & Moore, 1980]. The subfraction 56 (chloroform/ethyl acetate 90:10) yielded a solid residue which after recrystallization from methanol yielded sitosterol-3-O-β-D-glucopyranoside 9 [34.2 mg, 232-235 ºC, 13C NMR (DMSO-d6) data, see table 1, Macari et al., 1990].
Results and Discussion
The structure of the steroids β-sitosterol 1 and stigmasterol 2, isolated from the leaves of P. renitens, and sitosterol-3-O-β-D-glucopyranoside 9, isolated from flowers of A. brasiliana were assigned based on the analysis of the 1D and 2D 1H and 13C NMR spectra and by comparison with the literature data (Jácome et al., 2004; Macari et al., 1990).
Compound 3 was obtained from the leaves of P. renitens, their EIMS spectrum showed the molecular ion peak at m/z 306, compatible with the molecular formula C20H34O2. The IR spectrum displayed absorption bands indicating the presence of OH (3430 cm-1) group. The 13C NMR spectrum of compound 3 showed twenty signals, which were attributed to three CH3 (δC 17.7, 21.5, 33.5), ten CH2 (δC 66.4, 53.4, 42.0, 42.1, 40.3, 37.3, 26.4, 20.67, 18.6, 18.3), three CH (δC 56.7, 56.2, 45.5) and four C (δC 81.9, 44.7, 39.4, 33.2). The 1H NMR spectrum showed two doublet at δH 3.78 (J=11.0 Hz) e 3.67 (J=11.0 Hz), revealed the presence of a carbinolic methylene (CH2O-17) and three singlets assigned to methyl groups at δH 1.03, 0.85 and 0.81. The peak at δC 81.9 and literature data comparison with 16α,17-ent-kauranediol, was decisive to locate the hydroxymethyl and hydroxyl groups at C-16 and consider the same structure for 3 (Nascimento & Lopes, 2003).
Compounds 4 and 5 were isolated from the leaves of S. guianensis and were identified as O-methylated derivatives of kaempferol. These compounds show positive test for flavonoids using AlCl3/EtOH in TLC plate. The IR spectrum displayed O-H stretching bands at 3350 cm-1 for 4 and 3375 cm-1 for 5, and conjugated carbonyl group absorptions at 1675 cm-1 and 1667 cm-1, respectively. The 1H NMR spectra of compounds 4 and 5 showed two meta-coupled doublets for A-ring, at δH 6.55 (d, J=2.2 Hz, H-6), 6.13 (d, J=2.2 Hz, H-8) for 4 and 6.35 (d, J=2.0 Hz, H-6), 6.44 (d, J=2.0 Hz, H-8) for 5 and two orto-coupled doublets, for B-ring, at δH 8.02 (d, J=8.8 Hz, H-2'/6') and 6.99 (d, J=8.8 Hz, H-3'/5') for 4 and 8.08 (d, J=7.9 Hz, H-2'/6'), 7.02 (d, J=7.8 Hz, H-3'/5') for 5, commonly observed in the kaempferol nucleus (Pizzolatti et al., 2003). Also, one signal for a chelated hydroxyl (OH-5) was observed at δH 12.76 for 4 and 12.53 for 5. The 13C NMR spectra of compounds 4 and 5 showed signals for methoxyl groups at δC 59.8 and 56.1 for 4 and at δC 60.1, 56.7 and 55.4 for 5. The downfield shift of the methoxyl groups at δC 59.8 for 4 and 60.1 for 5, suggests a crowded position such as C-3 (Silva et al., 2009); this assignment is supported by the absence of the characteristic absorption at δH 6.80 of a vinylic hydrogen in flavones (Fossen & Andersen, 2006). A correlation peak in HMBC between C-7 (δC 165.4) and a methoxy group (δH 3.77) completed the elucidation of the structure of compound 4. Therefore, compound 4 was established as kumatakenin (kempferol-3,7-dimethylether) (Silva et al., 2009).
The location of the two methoxyl groups at C-7 and C-4' for compound 5, was supported by analysis of the HMBC spectrum, where a correlation between C-7 at δC 165.2 and a methoxyl group at δH 3.87 and a correlation between C-4' at δC 161.3 and a methoxyl group at δH 3.86 were observed. On this basis the compound 5 was unambiguously identified as kaempferol-3,7,3'-trimethylether (Paula et al., 2006).
Compounds 6-8 were isolated from the flowers of A. brasiliana and were identified as three flavones. These compounds show positive test for flavonoids using AlCl3/EtOH in TLC plate. The IR spectra of compounds 6-8 displayed absorption bands indicating hydroxyl and conjugated carbonyl groups at 3387 and 1673 cm-1, respectively (Silva et al., 2006). The HMBC spectra of compounds 6 and 8 exhibited correlations between the hydrogen signal at δH 6.91 (s, H-3) for 6, 6.90 (s, H-3) for 7 and 6.90 (s, H-3) for 8, and the three quaternary carbon resonances at δC 122.1 (C-1'), 111.1 (C-2) and 182.2 (C-4) for 6, 120.9 (C-1'), 105.4 (C-2) and 182.2 (C-4) for 7 and 122.8 (C-1'), 111.2 (C-2) and 182,4 (C-4) for 8, respectively, which allowed us to unequivocally assign this hydrogen at position 3 of the flavone nucleous (Fossen & Andersen, 2006). 1H NMR spectra of compounds 6-8 showed signals at δH 12.98 for 6, 12.91 for 7 and 12.93 for 8 assigned to the chelated hydroxyl group at C-5, two meta-coupled doublets at δH 6.21 (d, J=2.0 Hz, H-6) and 6.50 (d, J=2.0 Hz, H-8) for 6, 6.20 (d, J=1.9 Hz, H-6), 6.54 (d, J=1.9 Hz, H-8) for 7 and 6.46 (d, J=1.9 Hz, H-6) and 6.89 (d, J=1.9 Hz, H-8) for 8, suggesting an A-ring substituted at positions C-5 and C-7. 1H NMR spectra of compounds 6 and 8 showed one broad singlet and two doublets at δH 7.56 (bs, H-2'), 6.95 (d, J=8.9 Hz, H-5') and 7.55 (d, J=8.9 Hz, H-6') for 6 and 7.56 (bs, H-2'), 6.96 (d, J=8.8 Hz, H-5') and 7.58 (d, J=8.8 Hz, H-6') for 8, suggesting a B-ring substituted at positions C-3' and C-4'. The 1H NMR spectra of compounds 7 showed one signal at δH 7.56 (bs), compatible with a B-ring substituted at positions C-3', C-4' and C-5'. The 1H and 13C NMR spectra of compounds 6-8 showed signals at δH 3.91 (3H, s), for 6, 3.90 (6H, s) for 7 and 3.92 (3H, s) for 8 and δC 57.0 for 6, 56.6 and 56.8 for 7 and 56.7 for 8 assigned to methoxyl groups. For compound 8, additional signals were observed at δH 5.06 (1H, d, J=7.3 Hz, H-1''), 3.46 (1H, m, H-2''), 3.35 (1H, m, H-3''), 3.30 (1H, m, H-4''), 3.21 (1H, m, H-5''), 3.75(1H, dd, J=11.6 and J=2.2), 3.51(1H, dd, J=11.6 and J=2.2 Hz) and δC 100.8, 77.8, 77.1, 73.7, 70.3, and 61.3, which supported the presence of an O-β-D-glucose moiety. The HMBC spectra of compounds 6-8 exhibited unambiguous correlations between the hydrogens of OCH3-3' (δH 3.91) and C-3' (δC 148.6) for 6, OCH3-3'/5' (δH 3.90) and C-3'/5' (δC 148.8) for 7 and OCH3-3' (δH 3.91) and C-3' (δC 148.7) for 8. The HMBC spectrum of 8 exhibited also a correlation between the anomeric hydrogen at δH 5.06 (1H, d, J=7.3 Hz, H-1'') and C-7 (δC 163.5).
Click to enlarge

Comparison of these data and the literature leads to the following conclusion: (a) compound 6 is crysoeriol (5,7,4'-trihydroxy-3'-methoxyflavone) (Awaad et al., 2006), (b) compound 7 is tricin (5,7,4'-trihydroxy-3',5'-dimethoxyflavone) (Jiao et al., 2007) and (c) compound 8 is 7-O-β-D-glucopyranoside-5,4'-dihydroxy-3'-methoxyflavone (Markham & Moore, 1980).
Acknowledgements
Authors thank the CNPq for financial support, Dr. J. Gomes and Dra. Ana Cristina R. de Souza for identification of plant material and CENAUREMN (Universidade Federal do Ceará) for the NMR spectra.
Received 2 Sep 2011
Accepted 23 Jan 2012
- Araújo-Júnior JX, Chaves MCO, Cunha EVL, Gray AI 1999. Cepharanone B from Piper tuberculatum. Biochem Syst Ecol 27:325-327.
- Araújo D, Onofre SB 2011. Ação do extrato hidroalcoólico Alternanthera brasiliana (L.) O. Kunt., (Amaranthaceae) sobre a atividade de antimicrobianos utilizados na terapêutica. Rev Saúde e Biol 6:1-8.
- Awaad AS, Maitland DJ, Soliman GA 2006. Hepatoprotective activity of Schouwia thebica Webb. Bioorg Med Chem 16:4624-4628.
- Braz-Filho R, Gabriel SJ, Gomes CMR, Gottlieb OR, Bichara MGA, Maia JGS 1976. Oxoaporphine alkaloids from Fusea longifolia and Siparuna guianensis. Phytochemistry 15:1187-1188.
- Brochado CO, Almeida AP, Barreto BP, Costa LP, Ribeiro LS, Pereira RLC, Koatz VLG, Costa SS 2003. Flavonol robinobiosides and rutinosides from Alternanthera brasiliana (Amaranthaceae) and their effects on lymphocyte proliferation in vitro. J Braz Chem Soc 14:449-451.
- Chaves MCO, Oliveira AH, Santos BVO 2006. Aristolactams from Piper marginatum Jacq. (Piperaceae). Biochem Sys Ecol 34:75-77.
- Chiu SY, Dobberstein RH, Fong HHS, Farnsworth NR 1982. Oxoporphine alkaloids from Sipaaruna guianensis. J Nat Prod 45:229-230.
- Delaporte RH, Guzenl KP, Takemura1 OS, Mello JCP 2005. Estudo mineral das espécies vegetais Alternanthera brasiliana (L.) Kuntze e Bouchea fluminensis (Vell) Mold. Rev Bras Farmacogn 15:133-136.
- Facundo VA, Sá AL, Silva SAF, Morais SM, Matos CRR, Braz-Filho R 2003. Three new natural cyclopentenedione derivatives from Piper carniconnectivum. J Braz Chem Soc 15:140-145.
- Fossen T, Andersen OM 2006. Flavonoids Chemistry, Biochemistry and Applications: Spectroscopic Techniques Applied to Flavonoids; Anderson, O. M.; Markham, K. R., eds.; Taylor & Francis: New York, p. 37-142.
- Jácome RLRP, Oliveira AB, Rasian DS, Wagner H 2004. Estudo químico e perfil cromatográfico das cascas de Aspidosperma parvifolium A. DC. ("pau-pereira"). Quim Nova 27:897-900.
- Jiao J, Zhang Y, Liu C, Liu J, Wu X, Zhang Y 2007. Separation and purification of tricin from an antioxidant product derived from bamboo leaves. J Agric Food Chem 55:10086-10092.
- Leitão GG, Simas NK, Soares SSV, Brito APP, Claros BMG, Brito TBM, Monache FD 1999. Chemistry and pharmacology of Monimiaceae: a special focus on Siparuna and Mollinedia. J Ethnopharmacol 65:87-102.
- Macari PAT, Emerenciano, VP, Ferreira ZGS 1990. Identificação dos triterpenos de Miconia albicans Triana através de análise por microcomputador. Quim Nova 13:260-262.
- Machado SMF, Ribeiro A, Facundo VA, Militão JSLT, Morais SM, Machado MIL 1994. Seasonal variation of (E)-nerolidol in Siparuna guianensis Aublet and 13C-NMR spectral assignments of (E)- and (Z)-nerolidol. J Essent Oil Res 13:130-132.
- Markham KR, Moore NA 1980. Comparative flavonoid glycoside biochemistry as a chemotaxonomic tool in the subdivision of the classical 'genus' Lycopodium. Biochem Syst Ecol 8:17-20.
- Nascimento IR, Lopes LMX 2003. Diterpene esters of aristolochic acids from Aristolochia pubescens. Phytochemistry 63:953-957.
- Newman DJ, Cragg GM, Snader KM 2003. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod 66:1022-1037.
- Parmar VS, Jain SC, Bisht KS, Jain R, Taneja P, Jha A, Tyagi OD, Prasad AK, Wengel J, Olsen CE, Boll PM 1997. Phytochemistry of the genus Piper. Phytochemistry 46:597-673.
- Paula VF, Cruz MP, Barbosa LC 2006. Constituintes químicos de Bombacopsis glabra (Bombacaceae). Quim Nova 29:213-215.
- Pizzolatti MG, Cunha Jr. A, Szpoganicz B, Sousa E, Braz-Filho, R, Schripsema J 2003. Flavonoides glicosilados das folhas e flores de Bauhinia forficata (Legminosae). Quim Nova 26:466-469.
- Renner SS, Schwarzbach AE, Lohmann L 1997. Phylogenetic position and floral function of Siparuna (Siparunaceae: Laurales). Int J Plant Sci 158 (6 Suppl.):S89-S98.
- Ribeiro JEL, Hopkins MJG, Vicentin A, Sothers CA, Costa MA, Brito JM, Souza MAD, Martins LHP, Lohmann LG, Assuncão PACL, Pereira EC, Mesquita MR, Procópio LC 1999. Flora da Reserva Ducke - Guia de identificação das plantas vasculares de uma floresta de terra-firme na Amazônia Central. INPA-DFID, Manaus, Amazonas, Brasil, p. 181-187.
- Rodrigues E 2006. Plants and Animals Utilized as Medicines in the Jaú National Park (JNP), Brazilian Amazon. Phytother Res 20:378-391.
- Soleane H, Azevedo MS, Facundo VA, Rover M, Santos OA, Slana GBC, Barreto AS 2007. Essential oil of Piper renitens (Miq.) Yunck leaves and stems (Piperaceae) from Brazilian amazonian forest. J Essent Oil Res 19:557-558.
- Silva DA, Silva TMS, Lins ACS, Costa DA, Cavalcante JMS, Matias WN, Sousa MFV 2006. Constituintes Químicos e Atividade Antioxidante de Sida galheiransi Ulbr. (Malvaceae). Quim Nova 29:1250-1254.
- Silva TMS, Carvalho MG, Braz-Filho R 2009. Estudo espectroscópico em elucidação estrutural de flavonóides de Solanum jabrense Agra & Nee e S. paludosa Moric. Quim Nova 32:1119-1128.
- Valentini CMA, Rodriguez-Ortiz CE, Coelho MFB 2010. Siparuna guianensis Aublet (negramina): uma revisão. Rev Bras Pl Med 12:96-104.
- Zoghbi MGB, Andrade EHA, Santos AS, Silva MHL, Maia JGS, Silva MHL, Haia JGS 1998. Essential oils of Siparuna guianensis Aubl. J Essent Oil Res 10:543-544.
Publication Dates
-
Publication in this collection
03 Apr 2012 -
Date of issue
Oct 2012
History
-
Received
02 Sept 2011 -
Accepted
23 Jan 2012