Abstract
OBJECTIVE: To evaluate viral vaccine antibody levels in children with acute lymphoblastic leukemia after chemotherapy and after vaccine booster doses. METHODS: Antibody levels against hepatitis B, rubella, measles and mumps vaccine antigens were evaluated in 33 children after completing chemotherapy (before and after vaccine booster doses) and the results were compared to the data of 33 healthy children matched for gender, age and social class. RESULTS: After chemotherapy, 75.9%, 67.9%, 59.3% and 51.7% of the patients showed low antibody titers that would be unlikely to protect against exposure to measles, rubella, hepatitis B and mumps, respectively. After receiving a vaccine booster dose for these antigens the patients had high antibody levels consistent with potential protection against measles, mumps and hepatitis B, but not against rubella. CONCLUSION: Extra doses of measles-mumps-rubella plus hepatitis B vaccines are recommended in acute lymphoblastic leukemia patients submitted to treatment after hematologic recovery. After this, viral vaccine antibody levels should be verified to define the individual's protective status.
Leukemia, lymphoid; Antineoplastic combined chemotherapy protocols; Immunization; Viral vaccines; Child
ORIGINAL ARTICLE
Antibody responses to Hepatitis B and measles-mumps-rubella vaccines in children who received chemotherapy for acute lymphoblastic leukemia
Simone Santana Viana; Gustavo Santos Araujo; Gustavo Baptista de Almeida Faro; Lana Luíza da Cruz-Silva; Carlos André Araújo-Melo; Rosana Cipolotti
Universidade Federal de Sergipe - UFS, Aracaju, SE, Brazil
Corresponding author Corresponding author: Rosana Cipolotti Av. Beira Mar, 2016 apto. 402, Bairro 13 de Julho 4902505 Aracaju, SE, Brazil rosanaci@yahoo.com
ABSTRACT
OBJECTIVE: To evaluate viral vaccine antibody levels in children with acute lymphoblastic leukemia after chemotherapy and after vaccine booster doses.
METHODS: Antibody levels against hepatitis B, rubella, measles and mumps vaccine antigens were evaluated in 33 children after completing chemotherapy (before and after vaccine booster doses) and the results were compared to the data of 33 healthy children matched for gender, age and social class.
RESULTS: After chemotherapy, 75.9%, 67.9%, 59.3% and 51.7% of the patients showed low antibody titers that would be unlikely to protect against exposure to measles, rubella, hepatitis B and mumps, respectively. After receiving a vaccine booster dose for these antigens the patients had high antibody levels consistent with potential protection against measles, mumps and hepatitis B, but not against rubella.
CONCLUSION: Extra doses of measles-mumps-rubella plus hepatitis B vaccines are recommended in acute lymphoblastic leukemia patients submitted to treatment after hematologic recovery. After this, viral vaccine antibody levels should be verified to define the individual's protective status.
Keywords: Leukemia, lymphoid; Antineoplastic combined chemotherapy protocols; Immunization; Viral vaccines; Child
Introduction
Acute Lymphoblastic Leukemia (ALL) is the most common malignant diseasein children; a high risk of occurrence has been reported in many locations. The moreintensive treatment and risk stratification adopted over the last decade have resulted inan improvement in the survival rate, which, in many cases, reaches 90%. Both the illnessand treatment affect the immune system. Immune competence decreases not only due tochemotherapy-induced neutropenia, but also due to the reduction of serum antibody titersgained from previous immunizations. Moreover, the efficacy of the immunization strategiesemployed is questionable(1-3). New data are required to be able to draw up evidence-basedrecommendations that will ensure adequate protection against infectious diseases in suchhigh-risk children(4).
The role of the revaccination of children who receive chemotherapy for malignant illnesses is still controversial(5,6). There are very few data from controlled trials regarding the residual immunization status against vaccine antigens after cessation of chemotherapy in children with ALL and about the capacity of these patients to respond to vaccine booster doses; thus consensual guidelines have not been developed so far. Different strategies have been adopted in different countries(6) and so the best vaccine scheme has not been conclusively demonstrated as of yet(7). Some recommendations, in general derived from studies with low levels of evidence, are not always followed by clinical oncologists(8).
A pilot study conducted in a country with a low-risk profile suggested a single booster dose should be applied six months after concluding chemotherapy(9), but other authorsrecommend re-starting the vaccination protocol from the beginning three months after the end of treatment, followed by subsequent booster doses(10). This study aimed to evaluate the antibody levels associated to potential protection against viral vaccine antigens to the hepatitis B and measles-mumps-rubella (MMR) vaccines in children treated for ALL after cessation of chemotherapy and after the application of a booster dose.
Methods
A case-control study was conducted from June 2007 to June 2009 in the onlygovernment pediatric oncology clinic in Aracaju, the capital and main city of the state ofSergipe, located in the northeastern region of Brazil. This study only enrolled participantswho had completed the basic vaccination program according to the Brazilian vaccinationprogram, which includes three doses for hepatitis B (at birth, and in the first and sixth months) and two doses of the triple viral vaccine against mumps, measles and rubella, given at the end of the first and fourth yearsof life. The immunization data of all participants was verified on their vaccination cards, a document that is compulsory for allchildren in Brazil. This project was approved by the ResearchEthics Committee of the Universidade Federal de Sergipe (UFS-#0049.0.107.000-07).
Case Group
Thirty-three over 15-month-old children treated for ALLwere enrolled in this study after cessation of chemotherapy according to the current Brazilian protocol (Grupo Brasileiro de Tratamento de Leucemia da Infância - GBTLI - 1999(11) which is similar to the main protocols applied in Europe and North America(12)), after remission confirmed by a bone marrow exam, at least four weeks from treatment withdrawal and after complete neutrophil recovery. Patients did not receive any additional vaccine doses after diagnosis of the disease. A convenience sample of thirty-three patients was eligible for the study.
Baseline serologic antibody titers were collected after aminimum period of four weeks from the end of the treatment. Then,booster doses of the MMR and hepatitis B vaccines were applied andafter another period of four weeks the serologic tests were repeated.
Control Group
Thirty-three healthy children among the siblings of patients matched to cases by age and gender were selected. The same exams were performed at the moment of inclusion in the study.
Serology
All antibody levels were measured using the ELISA method.
The following cut-off points according to the manufacturer's instructions on commercial kits (Enzygnost®, Dade-Behring)were considered:
hepatitis B: 10 IU/mL
rubella: 15 IU/mL
measles: 1.10 IU/mL
mumps: 1.10 IU/mL
For analysis, 'protected' individuals were those with antibody titers equal to or above these cut-off points and 'unprotected' those with titers below these values.
Statistical analysis
Fisher's exact test, Mann-Whitney test and the paired t-test were used to compare groups (cases versus controls) and tocompare variables within the case group before and after vaccine booster doses. The two-tailed type I significance level was set for a p-value < 0.05.
Results
A total of sixty-six children were recruited to the study(33 cases and 33 controls) with a mean age of 10.31 yearsold and 69.7% (n = 46) were male. There were no significantdifferences between the characteristic of the two groups.Seventeen (51.5%) of the Case Group were stratified as standard risk according to clinical and immunophenotypiccriteria. The mean age at diagnosis was 5.75 years (± 3.23years) and at the time of the first serologic tests the mean agewas 10.31 years (± 3.59 years). The mean time that elapsedbetween the end of treatment and the first sample collectionwas 15.41 months (± 17.2 months) and between first and thesecond tests, 5.34 months (± 5.83 months).
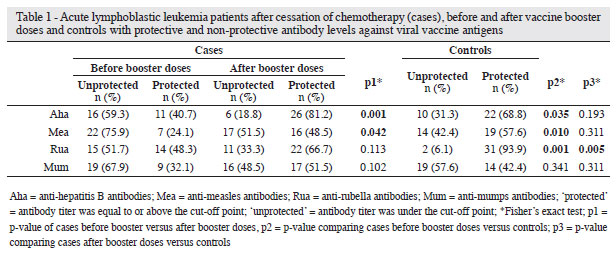
When the individuals who had finished treatment but had not received the booster doses were compared with theControl Group, there was a smaller number of potentiallyprotected individuals against all diseases, but the differencewas statistically significant for rubella (p-value > 0.001),measles (p-value = 0.010) and hepatitis B (p-value = 0.035) asshown in Table 1. This difference virtually disappeared afterthe patients had received booster doses, except for the rubellaantigen (p-value = 0.006).
There was an overall increase, albeit not statistically significant, in antibody titers against rubella, measles, mumps, and hepatitis B in the Case Group after vaccine booster doses (Table 1). After the application of booster doses in the Case Group, there was a increase in the rate of individuals with potential protection against rubella, mumps, measles and hepatitis B, which was statistically significant for measles (p-value = 0.042) and hepatitis B (p-value = 0.001 - Table 2).
The time between the end of treatment and the first evaluation of antibodies was not different for individuals with or without protective antibody titers for hepatitis B and mumps. However, 75.9% and 51.7% of patients remained susceptible for measles and rubella for more than 18 months, respectively (measles: 18.45 months, p-value = 0.045; rubella: 20.73 months, p-value = 0.025). The time between the first and the second antigen tests did not influenced the level of protection against rubella, measles, mumps and hepatitis B (Table 3).
After the end of the treatment, 92.9% of the patients stratified and treated as high-risk were susceptible to measles (p-value = 0.049). There was no significant difference in antibody titers between the risk groups after booster doses. Protective levels for rubella, mumps and hepatitis B were not different between genders and in relation to mean age at diagnosis (data not shown).
Discussion
The peak incidence of ALL at two to five years of age(13) corresponds to the age range in which Brazilian children shouldhave already received the basic vaccinations responsible forthe formation of immunological memory. When undergoingchemotherapy, children with ALL are prone to lose the antibodyprotection previously acquired by vaccination. The reasons forthis loss, although not fully understood, seem to be related to theintensity of treatment, individual sensitivity to chemotherapyand time interval required for the recovery of B lymphocytes(14).
Previous studies indicate that the number of individuals susceptible to measles at the end of leukemia treatment ranged from 25 to 71%(7,8,14,15) and that their antibody titers against viral vaccine antigens were significantly lower compared to the healthy population(6). In another study, measles serology was available for 77 children; 45 (58%) were seronegative(16). In this study, 75.9% of patients were potentially susceptible to measles at the end of treatment, a proportion significantly lower than in the Control Group. After the booster dose, the proportions in the Case and Control Groups were similar. This is comparable to the results of a previous study(7) that suggested that the immune memory was preserved and could be stimulated with a vaccine booster dose. This response was found for hepatitis B, both in the present study and in a previous one(14), but differs from the results obtained by Fioredda et al.(17). In 2005 this group found protective levels of antibodies against hepatitis B in 84% and 80% of patients, respectively six and twelve months after the end of chemotherapy. In a recent study it was observed that 67% of patients who had received routine immunization, continued protected despite immunosuppressant therapy(18). It seems that the age of olderpatients (as in the present and in a previous study(14)) may hamper the immune reconstitution by the stimulation of memory cells with the vaccine booster dose, thereby justifying the results(17). In another study(19), the proportion of patients who were potentially unprotected against hepatitis B at the end of treatment did not change significantly after the booster vaccination; the proportion remained higher than in healthy individuals(20) different of what was observed in this study.
Data about rubella and mumps are different: it was observed that for both vaccines the proportion of patients at treatment whose serum antibodies were not protective was not significantly modified by the booster vaccination. However, with respect to mumps, the proportion of individuals potentially unprotected was similar and high for both cases (before and after the booster vaccination) and controls. There was a higher proportion of individuals susceptible to mumps in the Control Group, which is similar to what is reported in the literature(7). Moreover, a high proportion of individuals with potential protection against rubella was found in the Case Group before the booster dose. Other studies have identified lower proportions of patients susceptible to rubella at the end of treatment (11% to 28%)(12,13,21).
ALL severity was not related to immunization status after treatment in previous studies(17,13,21) which is different to our findings. Here, we found a lower immunological competency in individuals with more severe risk categorization. These findings may reflect the intensity of different chemotherapy regimens or even biological differences of disease, reflected in the risk stratification. Recent studies demonstrated that the increased intensity of treatment was correlated with increased immune dysfunction. Children treated on high-intensity regimens had poor response to vaccination associated with the loss of humoral memory. Patients on low-intensity regimens had a similar response to vaccination and lymphocyte levels as controls(22).
Comparing the time interval between the end of treatment and the collection of the first blood sample to determine antibodies (before booster vaccination), a previous study(19) reported that,among individuals without protection against hepatitis B, the time gap was significantly higher than among those with potentialprotection, suggesting an influence of time after treatment on thereconstruction of immunity. The current study did not confirmthese findings as there was no statistically significant difference in the proportion of patients with potential protection against hepatitisB and mumps over time. Furthermore, most patients remainedsusceptible to measles and rubella even eighteen-months after theend of treatment, similar to results of other authors(15).
In this study, the age at diagnosis did not influence the proportion of individuals with protective titers of any of the antigens studied. These results are consistent with Volc et al.(15) although other studies suggest that children without protection were younger at the time of diagnosis(19,21).
High proportions of individuals susceptible to measles, rubella, mumps and hepatitis B at the end of chemotherapy for ALL were found. After a vaccine booster dose, the antibody titers recovered for measles and hepatitis B but remained without potential protection for rubella. For mumps the results did not differ from those found among healthy individuals. This may be because rubella vaccination in Brazil includes booster doses for healthy individuals at later ages as a way of preventing congenital forms of this disease, and because it is a live virus vaccine and herd immunity allows high levels of protection between healthy individuals (controls) but not for those who receive chemotherapy. Thus, possibly, additional booster doses may be required. With respect to mumps, the lack of difference occurred for other reasons: a high proportion of subjects with non-protective titers was observed not only among the cases before the booster dosebut also after the booster and among controls. Possibly, a similar strategy to that of rubella vaccination should be recommended for mumps, even for healthy individuals.
Regarding the time between the end of treatment and the vaccination, a minimum interval of four weeks, associated to hematological recovery, was used and there was no difference in the mean of this range on comparing the groups with and without protective titers for any of the four vaccine antigens.
At the end of chemotherapy for ALL, the administration of MMR and hepatitis B vaccine booster doses is recommendable after hematologic recovery, followed by an evaluation of vaccine antibody titers for subsequent individualized interventions.
Acknowledgements
The authors thank Prof. Charles Anthony Hart (inmemoriam), who contributed very much to this project, to PIBIC (Scientific Initiation Program - CNPq), and to FAPITEC-SE (Research and Innovation Aid Foundation of Sergipe) for research funding.
Submitted: 2/5/2012
Accepted: 4/26/2012
Conflict-of-interest disclosure: The authors declare no competing financial interest
- 1. Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166-78.
- 2. Pui CH, Robison LL, Look AT. Acute lymphoblastic leukemia. Lancet. 2008;371(9617):1030-43.
- 3. Calaminus G, Hense B, Laws HJ, Groeger M, Mackenzie CR, Göbel U. Diphtheria (D) and tetanus (T) antibody values in children with acute lymphoblastic leukaemia (ALL) after treatment according to Co-ALL 05/92. Klin Padiatr. 2007;219(6):355-60.
- 4. Esposito S, Cecinati V, Brescia L, Principi N. Vaccinations in children with cancer. Vaccine. 2010;28(19):3278-84.
- 5. Ridgway D, Wolff LJ, Deforest A. Immunization response varies with intensity of acute lymphoblastic leukemia therapy. Am J Dis Child. 1991;145(8):887-91.
- 6. Fioredda F, Cavillo M, Banow L, Plebani A, Timitilli A, Castagnola E. Immunization after the elective end of antineoplastic chemotherapy in children. Pediatr Blood Cancer. 2009;52(2):165-8.
- 7. Ercan TE, Soycan LY, Apak H, Celkan T, Ozkan A, Akdenizli E, et al. Antibody titers and immune response to diphtheria-tetanus-pertussis and measles-mumps-rubella vaccination in children treated for acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2005;27(5):273-7.
- 8. Crawford NW, Heath JA, Buttery JP. Immunization practices of paediatric oncologists: an Australasian survey. J Paediatr Child Health. 2007;43(9):593-6.
- 9. Patel SR, Otín M, Cohen BJ, Borrow R, Irving D, Sheldon J, et al. Revaccination of Children after Completion of Standard Chemotherapy for Acute Leukemia. Clin Infect Dis. 2007;44(5):635-42. Comment in: Clin Infect Dis. 2007;44(5):643-5.
- 10. Zengin E, Sarper N. Humoral immunity to diphtheria, tetanus, measles, and hemophilus influenzae type b in children with acute lymphoblastic leukemia and response to re-vaccination. Pediatr Blood Cancer. 2009;53(6):967-72. Comment in: Pediatr Blood Cancer. 2009;53(6):922-3.
-
11Portal Oncopediatria [Internet] [cited 2012 July 25]; available from www.oncopediatria.org.br/portal/artigos/profissionais/protocolos/lla99.jsp
» link - 12. Cazé MO, Bueno D,Santos ME. Estudo referencial de um protocolo quimioterápico para leucemia linfocítica aguda infantil. Rev HCPA. 2010;30(1):5-12.
- 13. Esparza SD, Sakamoto KM. Topics in pediatric leukemia- acute lymphoblastic leukemia. Med Gen Med. 2005;7(1):23.
- 14. Zignol M, Peracchi M, Tridello G, Pillon M, Fregonese F, D'Elia R, et al. Assessment of humoral to poliomyelitis, tetanus, hepatitis b, measles, rubella, and mumps in children after chemotherapy. Cancer 2004;101(3):635-41. Comment in: Cancer. 2005;103(8):1758-9; author reply 1760.
- 15. Volc SM, Almeida MT, Abadi MD, Cornacchioni AL, Odone-Filho V, Cristofani LM. Measles and rubella antibody status in children after treatment for acute lymphoblastic leukemia. J Pediatr (Rio J). 2006;82(6):481-4.
- 16. Aytac S, Yalcin SS, Cetin M, Yetgin S, Gumruk F, Tuncer M, et al. Measles, mumps, and rubella antibody status and response to immunization in children after therapy for acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2010;27(5):333-43
- 17. Fioredda F, Plebani A, Hanau G, Haupt R, Giacchino M, Barisone E, et al. Re-immunization schedule in leukaemic children after intensive chemotherapy: a possible strategy. Eur J Haematol. 2005;74(1):20-3. Comment in: Eur J Haematol. 2005;75(2):174; author reply 179-80; Eur J Haematol. 2005;75(2):175-6; author reply 179-80; Eur J Haematol. 2005;75(2):177-8; author reply 181-2.
- 18. Karaman S, Vural S, Yildirmak Y, Urganci N, Usta M. Assessment of hepatitis B immunization status after antineoplastic therapy in children with cancer. Ann Saudi Med. 2011;31(6):573-6.
- 19. Belloni C, Pistorio A, Tinelli C, Komakec J, Chirico G, Rovelli D, et al. Early immunization with hepatitis B vaccine: a five-year study. Vaccine. 2000;18(14):1307-11.
- 20. Brodtman DH, Rosenthal DW, Redner A, Lanzkowsky P, Bonagura VR. Immunodeficiency in children with acute lymphoblastic leukemia after completion of modern aggressive chemotherapeutic regimens. J Pediatr. 2005;146(5):654-61.
- 21. Nilsson A, De Milito A, Engström P, Nordin M, Narita M, Grillner L, et al. Current chemotherapy protocols for childhood acute lymphoblastic leukemia induce loss of humoral immunity to viral vaccination antigens. Pediatrics. 2002;109(6):e91.
- 22. Ek T, Josefson M, Abrahamsson J. Multivariate analysis of the relation between immune dysfunction and treatment intensity in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011;56(7):1078-87.
Corresponding author:
Publication Dates
-
Publication in this collection
06 Sept 2012 -
Date of issue
2012
History
-
Received
05 Feb 2012 -
Accepted
26 Apr 2012