ABSTRACT
The use of high-dose chemotherapy with autologous support of hematopoietic progenitor cells is an effective strategy to treat various hematologic neoplasms, such as non-Hodgkin lymphomas and multiple myeloma. Mobilized peripheral blood progenitor cells are the main source of support for autologous transplants, and collection of an adequate number of hematopoietic progenitor cells is a critical step in the autologous transplant procedure. Traditional strategies, based on the use of growth factors with or without chemotherapy, have limitations even when remobilizations are performed. Granulocyte colony-stimulating factor is the most widely used agent for progenitor cell mobilization. The association of plerixafor, a C-X-C Chemokine receptor type 4 (CXCR4) inhibitor, to granulocyte colony stimulating factor generates rapid mobilization of hematopoietic progenitor cells. A literature review was performed of randomized studies comparing different mobilization schemes in the treatment of multiple myeloma and lymphomas to analyze their limitations and effectiveness in hematopoietic progenitor cell mobilization for autologous transplant. This analysis showed that the addition of plerixafor to granulocyte colony stimulating factor is well tolerated and results in a greater proportion of patients with non-Hodgkin lymphomas or multiple myeloma reaching optimal CD34+ cell collections with a smaller number of apheresis compared the use of granulocyte colony stimulating factor alone.
Keywords:
Hematopoietic progenitor cell mobilization; Autologous transplant; Plerixafor; Multiple myeloma; Non-Hodgkin lymphoma
High-dose chemotherapy with autologous hematopoietic stem cell transplantation is an effective strategy to treat various hematologic neoplasms, such as chemosensitive relapsed Hodgkin's lymphomas,11 Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin's disease: results of a BNLI randomised trial. Lancet. 1993;341(8852):1051-4.,22 Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359(9323):2065-71. non-Hodgkin lymphomas (NHL)33 Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333(23):1540-5.,44 Schouten HC, Qian W, Kvaloy S, Porcellini A, Hagberg H, Johnsen HE, et al. High-dose therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin's lymphoma: results from the randomized European CUP trial. J Clin Oncol. 2003;21(21):3918-27. and multiple myeloma (MM).55 Koreth J, Cutler CS, Djulbegovic B, Behl R, Schlossman RL, Munshi NC, et al. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: a systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transplant. 2007;13(2):183-96. Several clinical guidelines and consensus recommend the procedure as standard treatment in these conditions.66 Ljungman P, Bregni M, Brune M, Cornelissen J, de Witte T, Dini G, et al. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe 2009. Bone Marrow Transplant. 2010;45(2):219-34.
7 Hoppe RT, Advani RH, Ai WZ, Ambinder RF, Aoun P, Bello CM, et al. Hodgkin lymphoma, version 2.2012 featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10(5):589-97.
8 Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advani RH, Andreadis CB, et al. Non-Hodgkin's Lymphomas, Version 4.2014. J Natl Compr Canc Netw. 2014;12(9):1282-303.
9 Anderson KC, Alsina M, Bensinger W, Biermann JS, Cohen AD, Devine S, et al. Multiple myeloma, version 1.2013. J Natl Compr Canc Netw. 2013;11(1):11-7.
10 Hahn T, Wingard JR, Anderson KC, Bensinger WI, Berenson JR, Brozeit G, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of multiple myeloma: an evidence-based review. Biol Blood Marrow Transplant. 2003;9(1):4-37.-1111 Oliansky DM, Gordon LI, King J, Laport G, Leonard JP, McLaughlin P, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of follicular lymphoma: an evidence-based review. Biol Blood Marrow Transplant. 2010;16(4):443-68. According to the Center for International Blood and Marrow Transplant Research (CIBMTR), 12,047 autologous hematopoietic stem cell transplantations (AHSCT) were carried out in the United States in 2011, with MM and NHL being the main indications.1212 CIBMTR, Center for International Blood and Marrow Transplant Research. Summary Slides 2014 (PowerPoint); 2014. Available from: http://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx [cited 28.06.15].
http://www.cibmtr.org/ReferenceCenter/Sl...
In Brazil, data from the Brazilian Transplant Registry show that 1144 AHSCT were performed in 2013, slightly higher than the year before.1313 Registro Brasileiro de Transplantes: Dimensionamento dos transplantes no Brasil e em cada estado (2006–2013). São Paulo; 2013. Available from: http://www.abto.org.br/abtov03/Upload/file/RBT/2013/rbt2013-parcial(1).pdf [cited 28.06.15].
http://www.abto.org.br/abtov03/Upload/fi...
The CIBMTR shows that peripheral blood progenitor cells are the main source used to support autologous transplants.1212 CIBMTR, Center for International Blood and Marrow Transplant Research. Summary Slides 2014 (PowerPoint); 2014. Available from: http://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx [cited 28.06.15].
http://www.cibmtr.org/ReferenceCenter/Sl...
In addition to the possible chemoresistance of the cancer, mobilization of hematopoietic progenitor cells (HPC) is another potentially limiting step for AHSCT, with high failure rates (between 5% and 40%) associated with historically used mobilization strategies.1414 Sheppard D, Bredeson C, Allan D, Tay J. Systematic review of randomized controlled trials of hematopoietic stem cell mobilization strategies for autologous transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2012;18(8):1191-203.
A consensus published by the American Society for Blood and Marrow Transplantation (ASBMT) recommends collecting a minimum dose of 2 × 106 CD34+ cells/kg to perform AHSCT, but the decision to accept collections of between 1 × 106 and 2 × 106 CD34+ cells/kg can be individualized according to the circumstances of each patient. On the other hand, larger target numbers are needed if multiple transplants are planned.1515 Giralt S, Costa L, Schriber J, Dipersio J, Maziarz R, McCarty J, et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20(3):295-308.
Although the minimum dose of progenitor cells to be collected is well defined, the ideal target or the desirable maximum dose is less clear. Some data show that the use of ≥5 × 106 CD34+ cells/kg leads to quicker and more predictable grafting, achieving platelet transfusions independence significantly earlier with potential reductions in transplant costs.1616 Siena S, Schiavo R, Pedrazzoli P, Carlo-Stella C. Therapeutic relevance of CD34 cell dose in blood cell transplantation for cancer therapy. J Clin Oncol. 2000;18(6):1360-77.,1717 Limat S, Woronoff-Lemsi MC, Deconinck E, Racadot E, Jacquet M, Herve P, et al. Cost-effectiveness of CD34+ dose in peripheral blood progenitor cell transplantation for non-Hodgkin's lymphoma patients: a single centre study. Bone Marrow Transplant. 2000;25(9):997-1002. Thus, adequate progenitor cell mobilization is a key step when planning an AHSCT.
Biology related to mobilization of hematopoietic progenitor cells and therapeutic targets
Although mature hematopoietic cells are physiologically released from the bone marrow to the peripheral blood, immature cells are found in the circulation at a very low frequency. About 0.05% or less of the total circulating leukocytes are HPC and express the CD34+ surface marker.1818 Mohty M, Ho AD. In and out of the niche: perspectives in mobilization of hematopoietic stem cells. Exp Hematol. 2011;39(7):723-9. HPC adhere to the bone marrow microenvironment by a variety of adhesive interactions.1919 Bensinger W, DiPersio JF, McCarty JM. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 2009;43(3):181-95. Furthermore, they express a wide range of surface receptors, such as adhesion molecules associated with angiopoietin-1 lymphocytes, very late antigen 4 (VLA4), and Mac-1, C-X-C chemokine receptors type 4 (CXCR4) and type 2 (CXCR2), the surface glycoproteins CD44 and CD62L, and tyrosine kinase receptor c-kit.1919 Bensinger W, DiPersio JF, McCarty JM. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 2009;43(3):181-95. The bone marrow stroma contains stromal cell-derived factor 1 (SDF-1), CXC chemokine GRO-β, vascular cell adhesion molecule (VCAM-1), KIT-ligand, P-selectin glycoprotein ligand and hyaluronic acid, all of which are ligands for the stem cell adhesion molecules.2020 Bonig H, Papayannopoulou T. Mobilization of hematopoietic stem/progenitor cells: general principles and molecular mechanisms. Methods Mol Biol. 2012;904:1-14. Preclinical data show that inhibition of these receptor-ligand interactions results in increased mobilization of progenitor cells.1919 Bensinger W, DiPersio JF, McCarty JM. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 2009;43(3):181-95.
20 Bonig H, Papayannopoulou T. Mobilization of hematopoietic stem/progenitor cells: general principles and molecular mechanisms. Methods Mol Biol. 2012;904:1-14.-2121 To LB, Haylock DN, Simmons PJ, Juttner CA. The biology and clinical uses of blood stem cells. Blood. 1997;89(7):2233-58.
Growth factors [granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF)] are the most widely used agents for progenitor cell mobilization; they have two main mechanisms of action. The first is the production of proteases by hyperplastic myelomonocytic series, which induces the cleavage of SDF-1 by preventing its binding to CXCR4. The most studied protease is matrix metallopeptidase 9 (MMP-9), although dipeptidase CD26 seems to have a greater role in this process.2222 Alvarez P, Carrillo E, Velez C, Hita-Contreras F, Martinez-Amat A, Rodriguez-Serrano F, et al. Regulatory systems in bone marrow for hematopoietic stem/progenitor cells mobilization and homing. Biomed Res Int. 2013;2013:312656.,2323 Angelopoulou MK, Tsirkinidis P, Boutsikas G, Vassilakopoulos TP, Tsirigotis P. New insights in the mobilization of hematopoietic stem cells in lymphoma and multiple myeloma patients. Biomed Res Int. 2014;2014:835138. The second main mechanism is also proteolysis induced and is responsible for degradation of VCAM-1, osteopontin and fibronectin, leading to reduced adhesion of progenitor cells through its VLA-4 receptor in bone marrow stroma.2323 Angelopoulou MK, Tsirkinidis P, Boutsikas G, Vassilakopoulos TP, Tsirigotis P. New insights in the mobilization of hematopoietic stem cells in lymphoma and multiple myeloma patients. Biomed Res Int. 2014;2014:835138.
The addition of a chemotherapeutic agent to a cytokine in the mobilization regimen has effects which are not fully elucidated.1919 Bensinger W, DiPersio JF, McCarty JM. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 2009;43(3):181-95. It is speculated that the addition of cyclophosphamide to growth factors has a synergistic effect on the release of granulocytic proteases in the bone marrow as its administration in isolation leads to cleavage of SDF-1, CXCR4 and c-kit adhesion molecules.1919 Bensinger W, DiPersio JF, McCarty JM. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 2009;43(3):181-95. Furthermore, the toxicity of chemotherapeutic agents on bone marrow stroma can release HPC as a result of damage to the functional ability of stromal cells in supporting them.1919 Bensinger W, DiPersio JF, McCarty JM. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 2009;43(3):181-95.
Plerixafor (AMD3100) is a reversibly bicyclam inhibitor of CXCR4 that breaks the binding between SDF-1 and CXCR4 receptors, blocking the chemotactic signaling with stromal cells.2323 Angelopoulou MK, Tsirkinidis P, Boutsikas G, Vassilakopoulos TP, Tsirigotis P. New insights in the mobilization of hematopoietic stem cells in lymphoma and multiple myeloma patients. Biomed Res Int. 2014;2014:835138. Among the hypotheses for its mobilization mechanism is the loss of sensitivity of progenitor cells to SDF-1 caused by the inhibition of CXCR4. Consequently, these cells are attracted to the circulation through signaling probably related to sphingosine-1-phosphate (S1P), a sphingolipid implicated in the chemotaxis control of progenitor cells from bone marrow, blood and other tissues.2323 Angelopoulou MK, Tsirkinidis P, Boutsikas G, Vassilakopoulos TP, Tsirigotis P. New insights in the mobilization of hematopoietic stem cells in lymphoma and multiple myeloma patients. Biomed Res Int. 2014;2014:835138. Studies also suggest that plerixafor keeps the progenitor cells in the circulation by binding to CXCR4, leading to a loss of chemoattraction to SDF-1, decreasing HPC homing, which also contributes to mobilization.2424 Bonig H, Papayannopoulou T. Hematopoietic stem cell mobilization: updated conceptual renditions. Leukemia. 2013;27(1):24-31.
Mobilization of hematopoietic progenitor cells for multiple myeloma and lymphoma: results of the historically most used strategies show limitations
Traditionally, the most widely used mobilization strategies have been the use of growth factors alone (G-CSF/GM-CSF) or in combination with chemotherapeutic agents. Among the available growth factors, the most commonly used is recombinant G-CSF filgrastim, while others, such as, G-CSF pegfilgrastim, G-CSF lenograstim and GM-CSF molgramostim, are used less frequently.1414 Sheppard D, Bredeson C, Allan D, Tay J. Systematic review of randomized controlled trials of hematopoietic stem cell mobilization strategies for autologous transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2012;18(8):1191-203.
G-CSF alone as first-line mobilization is an attractive option owing to the predictable mobilization kinetics, which in turn allows predictable apheresis scheduling and staffing while decreasing costs of growth factors and the collection procedure compared with cyclophosphamide (CY).2525 Chao NJ, Grima DT, Carrum G, Holmberg L, Fung HC, Brown S, et al. Chemo-mobilization provides superior mobilization and collection in autologous stem cell transplants but with less predictability and at a higher cost. Blood (ASH Annual Meeting Abstracts). 2011;118:4048.
26 Desikan KR, Barlogie B, Jagannath S, Vesole DH, Siegel D, Fassas A, et al. Comparable engraftment kinetics following peripheral-blood stem-cell infusion mobilized with granulocyte colony-stimulating factor with or without cyclophosphamide in multiple myeloma. J Clin Oncol. 1998;16(4):1547-53.-2727 Hoggatt J, Pelus LM. Hematopoietic stem cell mobilization with agents other than G-CSF. Methods Mol Biol. 2012;904:49-67.
GM-CSF has been shown to be inferior to G-CSF in terms of number of stem cells collected and in post-transplantation outcomes related to hematopoietic recovery, transfusion and antibiotic support, febrile episodes and hospitalizations.2828 Weaver CH, Schulman KA, Wilson-Relyea B, Birch R, West W, Buckner CD. Randomized trial of filgrastim, sargramostim, or sequential sargramostim and filgrastim after myelosuppressive chemotherapy for the harvesting of peripheral-blood stem cells. J Clin Oncol. 2000;18(1):43-53.,2929 Arora M, Burns LJ, Barker JN, Miller JS, Defor TE, Olujohungbe AB, et al. Randomized comparison of granulocyte colony-stimulating factor versus granulocytemacrophage colony-stimulating factor plus intensive chemotherapy for peripheral blood stem cell mobilization and autologous transplantation in multiple myeloma. Biol Blood Marrow Transplant. 2004;10(6):395-404. It is most often used in remobilization strategies, alone or in combination with other cytokines or chemotherapy.2828 Weaver CH, Schulman KA, Wilson-Relyea B, Birch R, West W, Buckner CD. Randomized trial of filgrastim, sargramostim, or sequential sargramostim and filgrastim after myelosuppressive chemotherapy for the harvesting of peripheral-blood stem cells. J Clin Oncol. 2000;18(1):43-53.,2929 Arora M, Burns LJ, Barker JN, Miller JS, Defor TE, Olujohungbe AB, et al. Randomized comparison of granulocyte colony-stimulating factor versus granulocytemacrophage colony-stimulating factor plus intensive chemotherapy for peripheral blood stem cell mobilization and autologous transplantation in multiple myeloma. Biol Blood Marrow Transplant. 2004;10(6):395-404. Data on the use of pegfilgrastim in steady-state mobilization are both limited and mixed, but one study demonstrated predictable mobilization kinetics and similar collection yields and apheresis days compared with a separate G-CSF cohort.3030 Hosing C, Qazilbash MH, Kebriaei P, Giralt S, Davis MS, Popat U, et al. Fixed-dose single agent pegfilgrastim for peripheral blood progenitor cell mobilisation in patients with multiple myeloma. Br J Haematol. 2006;133(5):533-7.
CY may be incorporated into the initial induction or salvage therapy cycles, or may be administered as a standalone cycle separately from standard therapy. The most common stand-alone regimens include cyclophosphamide at a range of doses between 2 and 7 g/m2. CY is associated with higher cell yields, lower or similar failure rates,2525 Chao NJ, Grima DT, Carrum G, Holmberg L, Fung HC, Brown S, et al. Chemo-mobilization provides superior mobilization and collection in autologous stem cell transplants but with less predictability and at a higher cost. Blood (ASH Annual Meeting Abstracts). 2011;118:4048.
26 Desikan KR, Barlogie B, Jagannath S, Vesole DH, Siegel D, Fassas A, et al. Comparable engraftment kinetics following peripheral-blood stem-cell infusion mobilized with granulocyte colony-stimulating factor with or without cyclophosphamide in multiple myeloma. J Clin Oncol. 1998;16(4):1547-53.-2727 Hoggatt J, Pelus LM. Hematopoietic stem cell mobilization with agents other than G-CSF. Methods Mol Biol. 2012;904:49-67.,3131 Mohty M, Hubel K, Kroger N, Aljurf M, Apperley J, Basak GW, et al. Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: a position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2014;49(7):865-72.
32 Alegre A, Tomás JF, Martínez-Chamorro C, Gil-Fernández JJ, Fernández-Villalta MJ, Arranz R, et al. Comparison of peripheral blood progenitor cell mobilization in patients with multiple myeloma: high-dose cyclophosphamide plus GM-CSF vs G-CSF alone. Bone Marrow Transplant. 1997;20(3):211-7.
33 Desikan KR, Tricot G, Munshi NC, Anaissie E, Spoon D, Fassas A, et al. Preceding chemotherapy, tumour load and age influence engraftment in multiple myeloma patients mobilized with granulocyte colony-stimulating factor alone. Br J Haematol. 2001;112(1):242-7.-3434 Dingli D, Nowakowski GS, Dispenzieri A, Lacy MQ, Hayman S, Litzow MR, et al. Cyclophosphamide mobilization does not improve outcome in patients receiving stem cell transplantation for multiple myeloma. Clin Lymphoma Myeloma. 2006;6(5):384-8. and improved engraftment kinetics,3535 Narayanasami U, Kanteti R, Morelli J, Klekar A, Al-Olama A, Keating C, et al. Randomized trial of filgrastim versus chemotherapy and filgrastim mobilization of hematopoietic progenitor cells for rescue in autologous transplantation. Blood. 2001;98(7):2059-64.
36 Dazzi C, Cariello A, Rosti G, Argnani M, Sebastiani L, Ferrari E, et al. Is there any difference in PBPC mobilization between cyclophosphamide plus G-CSF and G-CSF alone in patients with non-Hodgkin's lymphoma?. Leuk Lymphoma. 2000;39(3/4):301-10.
37 Glaspy JA. Economic considerations in the use of peripheral blood progenitor cells to support high-dose chemotherapy. Bone Marrow Transplant. 1999;23Suppl. 2:S21-7.
38 Hiwase DK, Bollard G, Hiwase S, Bailey M, Muirhead J, Schwarer AP. Intermediate-dose CY and GCSF more efficiently mobilize adequate numbers of PBSC for tandem autologous PBSC transplantation compared with low-dose CY in patients with multiple myeloma. Cytotherapy. 2007;9(6):539-47.
39 Hamadani M, Kochuparambil ST, Osman S, Cumpston A, Leadmon S, Bunner P, et al. Intermediate-dose versus low-dose cyclophosphamide and granulocyte colony stimulating factor for peripheral blood stem cell mobilization in patients with multiple myeloma treated with novel induction therapies. Biol Blood Marrow Transplant. 2012;18(7):1128-35.-4040 Sizemore CA, Laporte J, Holland HK, Mccollum J, Westerman J, Morris LE, et al. A comparison of toxicity and mobilization efficacy following two different doses of cyclophosphamide for mobilization of hematopoietic stem cells in non-Hodgkin's lymphoma patients. Biol Blood Marrow Transplant. 2010;16 Suppl. 2:S206. but also may result in more toxicity, febrile neutropenia, transfusions, hospitalizations and higher costs.1414 Sheppard D, Bredeson C, Allan D, Tay J. Systematic review of randomized controlled trials of hematopoietic stem cell mobilization strategies for autologous transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2012;18(8):1191-203.,3434 Dingli D, Nowakowski GS, Dispenzieri A, Lacy MQ, Hayman S, Litzow MR, et al. Cyclophosphamide mobilization does not improve outcome in patients receiving stem cell transplantation for multiple myeloma. Clin Lymphoma Myeloma. 2006;6(5):384-8.,4141 Pusic I, Jiang SY, Landua S, Uy GL, Rettig MP, Cashen AF, et al. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant. 2008;14(9):1045-56. The published literature on the various CY approaches is vast. In general, studies demonstrate that CY will mobilize more stem cells than G-CSF alone and help in the role of mobilizing traditionally difficult patients, such as those with lymphoma.4242 Damon LE, Johnson JL, Niedzwiecki D, Cheson BD, Hurd DD, Bartlett NL, et al. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J Clin Oncol. 2009;27(36):6101-8.,4343 Geisler CH, Kolstad A, Laurell A, Andersen NS, Pedersen LB, Jerkeman M, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo epurged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112(7):2687-93.
The primary goal of mobilization is to collect a sufficient number of progenitor cells for the patient to undergo AHSCT. The optimal mobilization, however, requires collection of a target number of cells and also strategies to minimize the time and number of apheresis, reducing the cost of the procedure and avoiding complications related to mobilization, such as hospitalization due to febrile neutropenia.1515 Giralt S, Costa L, Schriber J, Dipersio J, Maziarz R, McCarty J, et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20(3):295-308.
A systematic review of randomized clinical trials evaluated the results of 28 studies comparing different mobilization schemes. Eighteen included only patients with MM and/or lymphomas1414 Sheppard D, Bredeson C, Allan D, Tay J. Systematic review of randomized controlled trials of hematopoietic stem cell mobilization strategies for autologous transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2012;18(8):1191-203. and only four of these were multicenter. Table 1 shows the characteristics of interventions and results observed in studies included in this systematic review that recruited ≥10 patients in each arm and compared different regimens of growth factors with and without chemotherapy (Table 1).
Randomized clinical trials evaluating different mobilization strategies including growth factors with/without chemotherapy.1414 Sheppard D, Bredeson C, Allan D, Tay J. Systematic review of randomized controlled trials of hematopoietic stem cell mobilization strategies for autologous transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2012;18(8):1191-203.
These studies show that, in general, strategies with higher doses of G-CSF alone or chemotherapy with the addition of growth factors (G-CSF and/or GM-CSF) result in increased number of CD34+ cells collected. Furthermore, some strategies reduced the number of apheresis needed to achieve target collection.1414 Sheppard D, Bredeson C, Allan D, Tay J. Systematic review of randomized controlled trials of hematopoietic stem cell mobilization strategies for autologous transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2012;18(8):1191-203.
Although this systematic review has limitations such as restricted sample size of studies, their moderate quality and variability in the population, it demonstrates the limitations of techniques historically used in HPC mobilization. A major retrospective analysis, involving 2177 patients undergoing attempted mobilization in three Italian centers between 1999 and 2007, showed a rate of poor mobilizers (defined as a collection of <2 × 106 CD34+ cells/kg) of only 15% corroborating data from randomized trials.4444 Perseghin P, Terruzzi E, Dassi M, Baldini V, Parma M, Coluccia P, et al. Management of poor peripheral blood stem cell mobilization: incidence, predictive factors, alternative strategies and outcome. A retrospective analysis on 2177 patients from three major Italian institutions. Transfus Apher Sci. 2009;41(1):33-7.
Insufficient initial mobilization leads to new mobilization procedures (remobilization), which can negatively influence disease progression and significantly increase the use of resources.4141 Pusic I, Jiang SY, Landua S, Uy GL, Rettig MP, Cashen AF, et al. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant. 2008;14(9):1045-56. Several studies evaluated the success of remobilization with traditional measures based on the use of growth factors. A retrospective analysis assessed the results of remobilization of regimens containing G-CSF and/or GM-CSF, alone or combined with chemotherapy in 251 patients with lymphoma or MM.4141 Pusic I, Jiang SY, Landua S, Uy GL, Rettig MP, Cashen AF, et al. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant. 2008;14(9):1045-56. After remobilization, only 18.4% of patients who used G-CSF or GM-CSF and 26.5% of those who received a growth factor associated with chemotherapy achieved collections of ≥2 × 106 CD34+ cells/kg. When cells collected in the first mobilization were pooled, the failure rates were 28.1% in the group remobilized with growth factor alone and 47.1% in the group remobilized with growth factor associated with chemotherapy (Table 2).4141 Pusic I, Jiang SY, Landua S, Uy GL, Rettig MP, Cashen AF, et al. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant. 2008;14(9):1045-56.
Published data suggest that, although remobilization with growth factors can rescue patients who failed the first mobilization, the proportion of patients not meeting the minimum collection of HPC required to perform AHSCT is high (around 30%), confirming the need to evaluate more effective mobilization strategies.
Challenges for the identification and prospective characterization of poor mobilizers after strategies based on the use of growth factors
The possibility of predicting mobilization failure based on patient's characteristics in the early mobilization process is highly debatable.1515 Giralt S, Costa L, Schriber J, Dipersio J, Maziarz R, McCarty J, et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20(3):295-308. Each study proposes different definitions of poor mobilizers, limiting the uniformity of the characteristics that prospectively identify these patients.1818 Mohty M, Ho AD. In and out of the niche: perspectives in mobilization of hematopoietic stem cells. Exp Hematol. 2011;39(7):723-9.
Some identifiable characteristics before mobilization have often been associated with a higher risk of failure of procedures in patients with lymphoproliferative diseases.2929 Arora M, Burns LJ, Barker JN, Miller JS, Defor TE, Olujohungbe AB, et al. Randomized comparison of granulocyte colony-stimulating factor versus granulocytemacrophage colony-stimulating factor plus intensive chemotherapy for peripheral blood stem cell mobilization and autologous transplantation in multiple myeloma. Biol Blood Marrow Transplant. 2004;10(6):395-404. Although some of these factors are unanimously pointed out in the literature as predictive of poor mobilization, such as the intensity of prior exposure to chemotherapy or radiation, the use of chemotherapeutic agents and failure in a previous attempt of mobilization others, such as patient age, have a more controversial relationship with poor mobilization.4747 Jantunen E, Kvalheim G. Mobilization strategies in hard-to-mobilize patients with lymphoid malignancies. Eur J Haematol. 2010;85(6):463-71.
The Italian working group, Gruppo Italiano Trapianto di Midollo Osseo (GITMO), recently proposed a definition for ‘poor mobilizers’ for patients with lymphoma or myeloma.4848 Olivieri A, Marchetti M, Lemoli R, Tarella C, Iacone A, Lanza F, et al. Proposed definition of ‘poor mobilizer’ in lymphoma and multiple myeloma: an analytic hierarchy process by ad hoc working group Gruppo ItalianoTrapianto di Midollo Osseo. Bone Marrow Transplant. 2012;47(3):342-51. This definition came from a consensus of experts and considers the factors described in Table 3.
Definitions for patients with lymphoma or myeloma who have prediction or evidence of poor mobilization.
The concept of proved poor mobilizer based on the circulating CD34+ cell count after 4-6 days of mobilization is very relevant given the availability of preemptive strategies with potential for reducing risk of complications related to the necessity of a greater number of apheresis or higher need for remobilizations and possible reduced cost.
Use of plerixafor to mobilize hematopoietic progenitor cells: randomized clinical trials showing its effectiveness
Plerixafor has proved to be an agent that generates rapid mobilization of HPC in murine models and subsequently in healthy human volunteers.4949 Stiff P, Micallef I, McCarthy P, Magalhaes-Silverman M, Weisdorf D, Territo M, et al. Treatment with plerixafor in non-Hodgkin's lymphoma and multiple myeloma patients to increase the number of peripheral blood stem cells when given a mobilizing regimen of G-CSF: implications for the heavily pretreated patient. Biol Blood Marrow Transplant. 2009;15(2):249-56. The phase I and II studies attested the mobilizing effect and safety of the G-CSF plus plerixafor combination in patients with NHL and MM. The rapid and significant increase in white blood and CD34+ cell counts in peripheral blood, included patients whose failure to mobilize with G-CSF alone was proven.4949 Stiff P, Micallef I, McCarthy P, Magalhaes-Silverman M, Weisdorf D, Territo M, et al. Treatment with plerixafor in non-Hodgkin's lymphoma and multiple myeloma patients to increase the number of peripheral blood stem cells when given a mobilizing regimen of G-CSF: implications for the heavily pretreated patient. Biol Blood Marrow Transplant. 2009;15(2):249-56.
50 Devine SM, Flomenberg N, Vesole DH, Liesveld J, Weisdorf D, Badel K, et al. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin's lymphoma. J Clin Oncol. 2004;22(6):1095-102.-5151 Flomenberg N, Devine SM, Dipersio JF, Liesveld JL, McCarty JM, Rowley SD, et al. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106(5):1867-74. The combination guaranteed collections of ≥2 × 106 CD34+ cells/kg in 95.9% of patients with 77.6% achieving a collection of ≥5 × 106 CD34+ cells/kg (22.4% of patients achieved this dose after only one apheresis).
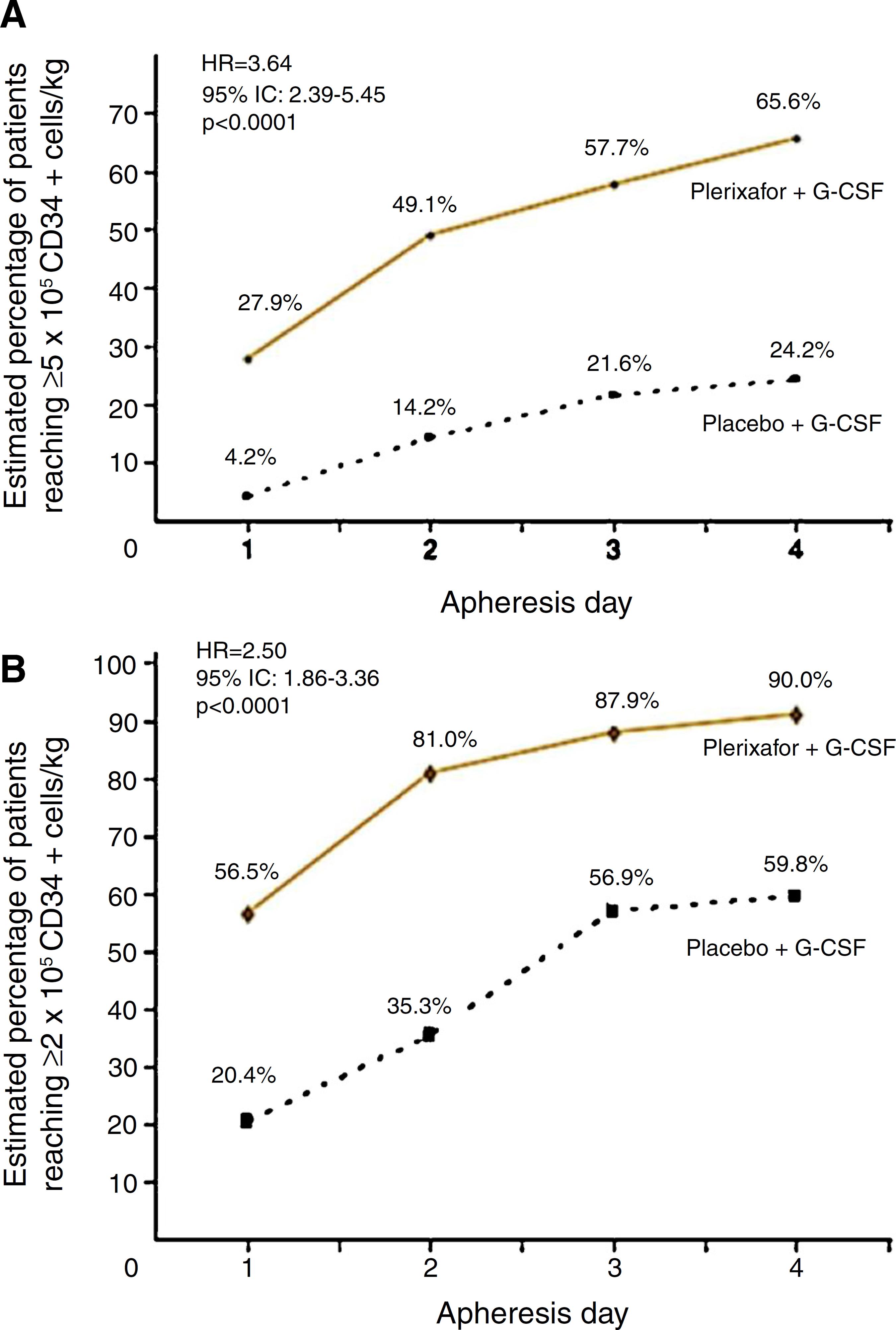
Two randomized, comparative, double-blind, placebo controlled and prospective multicenter studies evaluated the use of G-CSF plus plerixafor versus G-CSF alone in the initial mobilization strategy of patients. These studies recruited 298 patients with NHL5252 DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol. 2009;27(28):4767-73. and 302 patients with MM.5353 DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720-6. NHL patients treated with the combined therapy obtained 65.6% of successful collections of ≥5 × 106 CD34+ cells/kg versus 24.2% in the G-CSF arm. Collection of ≥2 × 106 CD34+ cells/kg was achieved by 90% with the combination against 59.8% in the G-CSF group within four apheresis days (p-value < 0.001) (Figure 1). The median number of CD34+ cells collected was also higher in combination group (5.69 × 106 cells/kg versus 1.98 × 106 cells/kg).5252 DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol. 2009;27(28):4767-73. Incidence of adverse events, mostly mild to moderate, was similar between groups with only 5.3% of serious events in the combination group and 6.9% in the G-CSF group during the mobilization period.5252 DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol. 2009;27(28):4767-73. The most common non-serious adverse events were diarrhea (38%), injection site erythema (29.3%), nausea (17.3%), headache (11.3%) and bone pain (10.7%), all of which were easily manageable.5252 DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol. 2009;27(28):4767-73.
Percentage of patients with non-Hodgkin lymphoma achieving collection targets of ≥5 × 106 CD34+ cells/kg (A) and of ≥2 × 106 CD34+ cells/kg (B) after mobilization with G-CSF plus placebo or G-CSF plus plerixafor.
MM patients treated with G-CSF plus plerixafor obtained ≥6 × 106 CD34+ cells/kg within two apheresis days, with 86.8% achieving this target versus 55.9% in the G-CSF group (p-value <0.0001). The combination was also more effective to collect ≥6 × 106 CD34+ cells/kg within four apheresis days (75.7% vs. 51.3%; p-value <0.001) and ≥2 × 106 CD34+ cells/kg days (95.3% vs. 88.3%; p-value = 0.031)3636 Dazzi C, Cariello A, Rosti G, Argnani M, Sebastiani L, Ferrari E, et al. Is there any difference in PBPC mobilization between cyclophosphamide plus G-CSF and G-CSF alone in patients with non-Hodgkin's lymphoma?. Leuk Lymphoma. 2000;39(3/4):301-10. (Figure 2). The median number of CD34+ cells collected was also higher in the combination group (12.97 vs. 7.31 × 106 cells/kg). The incidence of adverse events was similar between study groups. The most common non-serious adverse events occurred were injection site erythema (20.4%), diarrhea (18.4%), nausea (16.3%), bone pain (9.5%) and fatigue (8.2%), all of which were easily manageable.5353 DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720-6. The majority of these events occurred in the period after mobilization and were unrelated to the study drug.5353 DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720-6.
Percentage of patients with multiple myeloma achieving collection targets of ≥6 × 106 CD34+ cells/kg after mobilization with G-CSF plus placebo or G-CSF plus plerixafor.
These studies show that the combination of plerixafor with G-CSF is well tolerated and results in a greater proportion of NHL or MM patients achieving CD34+ cell collections considered optimal for transplant within a smaller number of apheresis days when compared with G-CSF alone.
Strategies for preemptive use of plerixafor
Many studies, including phase III studies, have shown that the use of plerixafor reduces risk of poor progenitor cell mobilization and risk of failure from up to 40% to less than 10%. However, the cost of plerixafor may be a limiting factor for its unrestricted use in the initial mobilization.5454 Shaughnessy P, Chao N, Shapiro J, Walters K, McCarty J, Abhyankar S, et al. Pharmacoeconomics of hematopoietic stem cell mobilization: an overview of current evidence and gaps in the literature. Biol Blood Marrow Transplant. 2013;19(9):1301-9.
An alternative strategy discusses the use of plerixafor as rescue treatment in patients failing prior mobilization. Micallef et al. analyzed 52 patients with NHL who failed the initial mobilization with G-CSF in a phase III study. The analysis showed that patients who received remobilization with G-CSF associated with plerixafor presented a median collection of 2.9 × 106 CD34+ cells/kg in remobilization after a median of three apheresis.5555 Micallef IN, Stiff PJ, DiPersio JF, Maziarz RT, McCarty JM, Bridger G, et al. Successful stem cell remobilization using plerixafor (mozobil) plus granulocyte colony-stimulating factor in patients with non-Hodgkin lymphoma: results from the plerixafor NHL phase 3 study rescue protocol. Biol Blood Marrow Transplant. 2009;15(12):1578-86. In this study, 63.5% of patients achieved a collection of ≥2 × 106 CD34+ cells/kg in up to four apheresis days in the second mobilization and 88.75% were able to undergo the transplant.
Some centers developed algorithms for the preemptive use of plerixafor in which patients initially receive customary regimens for mobilization. In this case, plerixafor is indicated, if necessary, to immediately rescue patients with verified poor mobilization before collection of progenitor cells.17>17 Limat S, Woronoff-Lemsi MC, Deconinck E, Racadot E, Jacquet M, Herve P, et al. Cost-effectiveness of CD34+ dose in peripheral blood progenitor cell transplantation for non-Hodgkin's lymphoma patients: a single centre study. Bone Marrow Transplant. 2000;25(9):997-1002.,5656 Micallef IN, Sinha S, Gastineau DA, Wolf R, Inwards DJ, Gertz MA, et al. Cost-effectiveness analysis of a risk-adapted algorithm of plerixafor use for autologous peripheral blood stem cell mobilization. Biol Blood Marrow Transplant. 2013;19(1):87-93.
One study evaluated 314 patients with myeloma, amyloidosis or lymphoma undergoing mobilization with G-CSF and tested two algorithms for plerixafor use. One used plerixafor only in patients with a CD34+ cell count in peripheral blood of <10/µL on Day 4 of mobilization or on any day of apheresis, with collections of <0.5 × 106 CD34+ cells/kg (plerixafor-1 algorithm - including 216 patients). The other used plerixafor if CD34+ cell count in peripheral blood reached <10/µL on Day 4 of mobilization (<20/µL if multiple transplants were planned) or if collection was <1.5 × 106 CD34+ cells/kg on Day 1 of apheresis or <0.5 × 106 CD34+ cells/kg in any subsequent apheresis day (plerixafor-2 algorithm - including 98 patients). The target was the collection of ≥4 × 106 CD34+ cells/kg (optimal collection) or ≥2 × 106 CD34+ cells/kg (minimum collection). The results were compared with those obtained in another cohort of 278 patients mobilized before the introduction of plerixafor, and with data from the same prospective database of transplant (Mayo Clinic), presenting similar characteristics.5757 Abhyankar S, DeJarnette S, Aljitawi O, Ganguly S, Merkel D, McGuirk J. A risk-based approach to optimize autologous hematopoietic stem cell (HSC) collection with the use of plerixafor. Bone Marrow Transplant. 2012;47(4):483-7. Some other studies evaluating different algorithms for the preemptive use of plerixafor showed lower collecting failure rates of 0-7%.5757 Abhyankar S, DeJarnette S, Aljitawi O, Ganguly S, Merkel D, McGuirk J. A risk-based approach to optimize autologous hematopoietic stem cell (HSC) collection with the use of plerixafor. Bone Marrow Transplant. 2012;47(4):483-7.,5858 Abusin GA, Abu-Arja RF, Gingrich RD, Silverman MD, Zamba GK, Schlueter AJ. An algorithm for utilizing peripheral blood CD34 count as a predictor of the need for plerixafor in autologous stem cell mobilization - cost-effectiveness analysis. J Clin Apher. 2013;28(4):293-300.
A review of pharmacoeconomic studies presented some algorithms for the preemptive use of plerixafor that resulted in reduced consumption of several health resources, including the possibility of reducing the total cost of mobilization procedures.5454 Shaughnessy P, Chao N, Shapiro J, Walters K, McCarty J, Abhyankar S, et al. Pharmacoeconomics of hematopoietic stem cell mobilization: an overview of current evidence and gaps in the literature. Biol Blood Marrow Transplant. 2013;19(9):1301-9.
Cost effective analyses
There are some analyses related to plerixafor cost-benefit in front line and in remobilization scenarios,5656 Micallef IN, Sinha S, Gastineau DA, Wolf R, Inwards DJ, Gertz MA, et al. Cost-effectiveness analysis of a risk-adapted algorithm of plerixafor use for autologous peripheral blood stem cell mobilization. Biol Blood Marrow Transplant. 2013;19(1):87-93.,5959 Micallef IN, Sinha S, Gastineau DA, Wolf R, Inwards DJ, Gertz MA, et al. A cost effective analysis of a risk-adapted algorithm for plerixafor use in autologous peripheral blood stem cell mobilization. Biol Blood Marrow Transplant. 2011;17 Suppl. 2:S159-60.
60 Li J, Hamilton E, Vaughn L, Graiser M, Renfroe H, Lechowicz MJ, et al. Effectiveness and cost analysis of justin-time salvage plerixafor administration in autologous transplant patients with poor stem cell mobilization kinetics. Transfusion. 2011;51(10):2175-82.
61 Vishnu P, Roy V, Paulsen A, Zubair AC. Efficacy and cost-benefit analysis of risk-adaptive use of plerixafor for autologous hematopoietic progenitor cell mobilization. Transfusion. 2012;52(1):55-62.-6262 Kymes SM, Pusic I, Lambert DL, Gregory M, Carson KR, DiPersio JF. Economic evaluation of plerixafor for stem cell mobilization. Am J Manag Care. 2012;18(1):33-41. but they were not performed in Brazilian institutions nor did they analyze real private or public costs. Another important question about these analyses is that the calculated costs did not include supportive care costs, including transfusions, antimicrobials, hospitalization, cryopreservation, nursing and medical costs, patient's personal costs, logistics, or even costs with previous failures, remobilization5656 Micallef IN, Sinha S, Gastineau DA, Wolf R, Inwards DJ, Gertz MA, et al. Cost-effectiveness analysis of a risk-adapted algorithm of plerixafor use for autologous peripheral blood stem cell mobilization. Biol Blood Marrow Transplant. 2013;19(1):87-93.,5959 Micallef IN, Sinha S, Gastineau DA, Wolf R, Inwards DJ, Gertz MA, et al. A cost effective analysis of a risk-adapted algorithm for plerixafor use in autologous peripheral blood stem cell mobilization. Biol Blood Marrow Transplant. 2011;17 Suppl. 2:S159-60.
60 Li J, Hamilton E, Vaughn L, Graiser M, Renfroe H, Lechowicz MJ, et al. Effectiveness and cost analysis of justin-time salvage plerixafor administration in autologous transplant patients with poor stem cell mobilization kinetics. Transfusion. 2011;51(10):2175-82.
61 Vishnu P, Roy V, Paulsen A, Zubair AC. Efficacy and cost-benefit analysis of risk-adaptive use of plerixafor for autologous hematopoietic progenitor cell mobilization. Transfusion. 2012;52(1):55-62.-6262 Kymes SM, Pusic I, Lambert DL, Gregory M, Carson KR, DiPersio JF. Economic evaluation of plerixafor for stem cell mobilization. Am J Manag Care. 2012;18(1):33-41. and, specifically, the worse outcome in lymphoma and myeloma patients failure to undergo AHSCT.4141 Pusic I, Jiang SY, Landua S, Uy GL, Rettig MP, Cashen AF, et al. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant. 2008;14(9):1045-56.,6363 Gertz MA, Wolf RC, Micallef IN, Gastineau DA. Clinical impact and resource utilization after stem cell mobilization failure in patients with multiple myeloma and lymphoma. Bone Marrow Transplant. 2010;45(9):1396-403.,6464 Pavone V, Gaudio F, Console G, Vitolo U, Iacopino P, Guarini A, et al. Poor mobilization is an independent prognostic factor in patients with malignant lymphomas treated by peripheral blood stem cell transplantation. Bone Marrow Transplant. 2006;37(8):719-24.,6565 Gordan LN, Sugrue MW, Lynch JW, Williams KD, Khan SA, Wingard JR, et al. Poor mobilization of peripheral blood stem cells is a risk factor for worse outcome in lymphoma patients undergoing autologous stem cell transplantation. Leuk Lymphoma. 2003;44(5):815-20.
In the study of Micallef et al.,5656 Micallef IN, Sinha S, Gastineau DA, Wolf R, Inwards DJ, Gertz MA, et al. Cost-effectiveness analysis of a risk-adapted algorithm of plerixafor use for autologous peripheral blood stem cell mobilization. Biol Blood Marrow Transplant. 2013;19(1):87-93. although the earlier identification of poor mobilizers and addition of plerixafor results in higher per-patient costs over baseline, it is associated with higher apheresis yields, fewer days of mobilization and collection, and lower failure rates. The median total costs of mobilization per patient, including any remobilization attempt, was US$12,500 in the baseline group, US$12,500 in the plerixafor-1 group, and US$20,000 in the plerixafor-2 group (Table 4). Although both the median and mean costs were higher in the plerixafor-2 group, the mobilization failure rate was 1%, with fewer days of apheresis, fewer days of plerixafor, and fewer total days of mobilization and collection compared to the plerixafor-1 algorithm.5656 Micallef IN, Sinha S, Gastineau DA, Wolf R, Inwards DJ, Gertz MA, et al. Cost-effectiveness analysis of a risk-adapted algorithm of plerixafor use for autologous peripheral blood stem cell mobilization. Biol Blood Marrow Transplant. 2013;19(1):87-93. There is an urgency to have more accurate pharmacoeconomic matrixes to calculate the other important and neglected costs mentioned above and to decide about the real cost-benefit in each institution.
Consensus recommendations suggest the use of plerixafor as a mobilization strategy particularly in patients with a higher target for collection of progenitor cells, as a preemptive approach based on the monitoring of the CD34+ cell count and in cases of remobilization.1515 Giralt S, Costa L, Schriber J, Dipersio J, Maziarz R, McCarty J, et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20(3):295-308. The European Group for Blood and Marrow Transplantation (EGBMT) recommends preemptive use of plerixafor in patients with CD34+ cell counts <10/µL before apheresis3131 Mohty M, Hubel K, Kroger N, Aljurf M, Apperley J, Basak GW, et al. Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: a position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2014;49(7):865-72. (Figure 3). Each center should develop and implement its own algorithms for applying different mobilization strategies, with the goal of optimizing collection yields and costs-benefits.
Conclusion
Collection of adequate numbers of HPC is a critical step in autologous transplantation procedures. Traditional strategies, based on the use of growth factors with or without chemotherapy, have some limitations even when remobilizations are performed.
The addition of plerixafor shows a consistent increase in collection rate success, reducing the number of apheresis and not increasing toxicity. Strategies of preemptive use of plerixafor have been considered a promising way to optimize and rationalize the use of this agent in patients who have high chance of failure with classic mobilization based on G-CSF with or without chemotherapy. This strategy would reduce failure in mobilization, especially in poor mobilizers, ensuring collection and transplantation as well as reducing time and costs of the mobilization procedure. However, external validity of these algorithms is limited, so it is recommended that each institution sets up a strategy appropriate to its standards for the preemptive use of plerixafor.
-
FundingPublication support provided by Sanofi.
REFERENCES
-
1Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin's disease: results of a BNLI randomised trial. Lancet. 1993;341(8852):1051-4.
-
2Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359(9323):2065-71.
-
3Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333(23):1540-5.
-
4Schouten HC, Qian W, Kvaloy S, Porcellini A, Hagberg H, Johnsen HE, et al. High-dose therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin's lymphoma: results from the randomized European CUP trial. J Clin Oncol. 2003;21(21):3918-27.
-
5Koreth J, Cutler CS, Djulbegovic B, Behl R, Schlossman RL, Munshi NC, et al. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: a systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transplant. 2007;13(2):183-96.
-
6Ljungman P, Bregni M, Brune M, Cornelissen J, de Witte T, Dini G, et al. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe 2009. Bone Marrow Transplant. 2010;45(2):219-34.
-
7Hoppe RT, Advani RH, Ai WZ, Ambinder RF, Aoun P, Bello CM, et al. Hodgkin lymphoma, version 2.2012 featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10(5):589-97.
-
8Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advani RH, Andreadis CB, et al. Non-Hodgkin's Lymphomas, Version 4.2014. J Natl Compr Canc Netw. 2014;12(9):1282-303.
-
9Anderson KC, Alsina M, Bensinger W, Biermann JS, Cohen AD, Devine S, et al. Multiple myeloma, version 1.2013. J Natl Compr Canc Netw. 2013;11(1):11-7.
-
10Hahn T, Wingard JR, Anderson KC, Bensinger WI, Berenson JR, Brozeit G, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of multiple myeloma: an evidence-based review. Biol Blood Marrow Transplant. 2003;9(1):4-37.
-
11Oliansky DM, Gordon LI, King J, Laport G, Leonard JP, McLaughlin P, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of follicular lymphoma: an evidence-based review. Biol Blood Marrow Transplant. 2010;16(4):443-68.
-
12CIBMTR, Center for International Blood and Marrow Transplant Research. Summary Slides 2014 (PowerPoint); 2014. Available from: http://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx [cited 28.06.15].
» http://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx -
13Registro Brasileiro de Transplantes: Dimensionamento dos transplantes no Brasil e em cada estado (2006–2013). São Paulo; 2013. Available from: http://www.abto.org.br/abtov03/Upload/file/RBT/2013/rbt2013-parcial(1).pdf [cited 28.06.15].
» http://www.abto.org.br/abtov03/Upload/file/RBT/2013/rbt2013-parcial(1).pdf -
14Sheppard D, Bredeson C, Allan D, Tay J. Systematic review of randomized controlled trials of hematopoietic stem cell mobilization strategies for autologous transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2012;18(8):1191-203.
-
15Giralt S, Costa L, Schriber J, Dipersio J, Maziarz R, McCarty J, et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20(3):295-308.
-
16Siena S, Schiavo R, Pedrazzoli P, Carlo-Stella C. Therapeutic relevance of CD34 cell dose in blood cell transplantation for cancer therapy. J Clin Oncol. 2000;18(6):1360-77.
-
17Limat S, Woronoff-Lemsi MC, Deconinck E, Racadot E, Jacquet M, Herve P, et al. Cost-effectiveness of CD34+ dose in peripheral blood progenitor cell transplantation for non-Hodgkin's lymphoma patients: a single centre study. Bone Marrow Transplant. 2000;25(9):997-1002.
-
18Mohty M, Ho AD. In and out of the niche: perspectives in mobilization of hematopoietic stem cells. Exp Hematol. 2011;39(7):723-9.
-
19Bensinger W, DiPersio JF, McCarty JM. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 2009;43(3):181-95.
-
20Bonig H, Papayannopoulou T. Mobilization of hematopoietic stem/progenitor cells: general principles and molecular mechanisms. Methods Mol Biol. 2012;904:1-14.
-
21To LB, Haylock DN, Simmons PJ, Juttner CA. The biology and clinical uses of blood stem cells. Blood. 1997;89(7):2233-58.
-
22Alvarez P, Carrillo E, Velez C, Hita-Contreras F, Martinez-Amat A, Rodriguez-Serrano F, et al. Regulatory systems in bone marrow for hematopoietic stem/progenitor cells mobilization and homing. Biomed Res Int. 2013;2013:312656.
-
23Angelopoulou MK, Tsirkinidis P, Boutsikas G, Vassilakopoulos TP, Tsirigotis P. New insights in the mobilization of hematopoietic stem cells in lymphoma and multiple myeloma patients. Biomed Res Int. 2014;2014:835138.
-
24Bonig H, Papayannopoulou T. Hematopoietic stem cell mobilization: updated conceptual renditions. Leukemia. 2013;27(1):24-31.
-
25Chao NJ, Grima DT, Carrum G, Holmberg L, Fung HC, Brown S, et al. Chemo-mobilization provides superior mobilization and collection in autologous stem cell transplants but with less predictability and at a higher cost. Blood (ASH Annual Meeting Abstracts). 2011;118:4048.
-
26Desikan KR, Barlogie B, Jagannath S, Vesole DH, Siegel D, Fassas A, et al. Comparable engraftment kinetics following peripheral-blood stem-cell infusion mobilized with granulocyte colony-stimulating factor with or without cyclophosphamide in multiple myeloma. J Clin Oncol. 1998;16(4):1547-53.
-
27Hoggatt J, Pelus LM. Hematopoietic stem cell mobilization with agents other than G-CSF. Methods Mol Biol. 2012;904:49-67.
-
28Weaver CH, Schulman KA, Wilson-Relyea B, Birch R, West W, Buckner CD. Randomized trial of filgrastim, sargramostim, or sequential sargramostim and filgrastim after myelosuppressive chemotherapy for the harvesting of peripheral-blood stem cells. J Clin Oncol. 2000;18(1):43-53.
-
29Arora M, Burns LJ, Barker JN, Miller JS, Defor TE, Olujohungbe AB, et al. Randomized comparison of granulocyte colony-stimulating factor versus granulocytemacrophage colony-stimulating factor plus intensive chemotherapy for peripheral blood stem cell mobilization and autologous transplantation in multiple myeloma. Biol Blood Marrow Transplant. 2004;10(6):395-404.
-
30Hosing C, Qazilbash MH, Kebriaei P, Giralt S, Davis MS, Popat U, et al. Fixed-dose single agent pegfilgrastim for peripheral blood progenitor cell mobilisation in patients with multiple myeloma. Br J Haematol. 2006;133(5):533-7.
-
31Mohty M, Hubel K, Kroger N, Aljurf M, Apperley J, Basak GW, et al. Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: a position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2014;49(7):865-72.
-
32Alegre A, Tomás JF, Martínez-Chamorro C, Gil-Fernández JJ, Fernández-Villalta MJ, Arranz R, et al. Comparison of peripheral blood progenitor cell mobilization in patients with multiple myeloma: high-dose cyclophosphamide plus GM-CSF vs G-CSF alone. Bone Marrow Transplant. 1997;20(3):211-7.
-
33Desikan KR, Tricot G, Munshi NC, Anaissie E, Spoon D, Fassas A, et al. Preceding chemotherapy, tumour load and age influence engraftment in multiple myeloma patients mobilized with granulocyte colony-stimulating factor alone. Br J Haematol. 2001;112(1):242-7.
-
34Dingli D, Nowakowski GS, Dispenzieri A, Lacy MQ, Hayman S, Litzow MR, et al. Cyclophosphamide mobilization does not improve outcome in patients receiving stem cell transplantation for multiple myeloma. Clin Lymphoma Myeloma. 2006;6(5):384-8.
-
35Narayanasami U, Kanteti R, Morelli J, Klekar A, Al-Olama A, Keating C, et al. Randomized trial of filgrastim versus chemotherapy and filgrastim mobilization of hematopoietic progenitor cells for rescue in autologous transplantation. Blood. 2001;98(7):2059-64.
-
36Dazzi C, Cariello A, Rosti G, Argnani M, Sebastiani L, Ferrari E, et al. Is there any difference in PBPC mobilization between cyclophosphamide plus G-CSF and G-CSF alone in patients with non-Hodgkin's lymphoma?. Leuk Lymphoma. 2000;39(3/4):301-10.
-
37Glaspy JA. Economic considerations in the use of peripheral blood progenitor cells to support high-dose chemotherapy. Bone Marrow Transplant. 1999;23Suppl. 2:S21-7.
-
38Hiwase DK, Bollard G, Hiwase S, Bailey M, Muirhead J, Schwarer AP. Intermediate-dose CY and GCSF more efficiently mobilize adequate numbers of PBSC for tandem autologous PBSC transplantation compared with low-dose CY in patients with multiple myeloma. Cytotherapy. 2007;9(6):539-47.
-
39Hamadani M, Kochuparambil ST, Osman S, Cumpston A, Leadmon S, Bunner P, et al. Intermediate-dose versus low-dose cyclophosphamide and granulocyte colony stimulating factor for peripheral blood stem cell mobilization in patients with multiple myeloma treated with novel induction therapies. Biol Blood Marrow Transplant. 2012;18(7):1128-35.
-
40Sizemore CA, Laporte J, Holland HK, Mccollum J, Westerman J, Morris LE, et al. A comparison of toxicity and mobilization efficacy following two different doses of cyclophosphamide for mobilization of hematopoietic stem cells in non-Hodgkin's lymphoma patients. Biol Blood Marrow Transplant. 2010;16 Suppl. 2:S206.
-
41Pusic I, Jiang SY, Landua S, Uy GL, Rettig MP, Cashen AF, et al. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant. 2008;14(9):1045-56.
-
42Damon LE, Johnson JL, Niedzwiecki D, Cheson BD, Hurd DD, Bartlett NL, et al. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J Clin Oncol. 2009;27(36):6101-8.
-
43Geisler CH, Kolstad A, Laurell A, Andersen NS, Pedersen LB, Jerkeman M, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo epurged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112(7):2687-93.
-
44Perseghin P, Terruzzi E, Dassi M, Baldini V, Parma M, Coluccia P, et al. Management of poor peripheral blood stem cell mobilization: incidence, predictive factors, alternative strategies and outcome. A retrospective analysis on 2177 patients from three major Italian institutions. Transfus Apher Sci. 2009;41(1):33-7.
-
45Lefrere F, Levy V, Makke J, Audat F, Cavazzana-Calvo M, Miclea JM. Successful peripheral blood stem cell harvesting with granulocyte colony-stimulating factor alone after previous mobilization failure. Haematologica. 2004;89(12):1532-4.
-
46Boeve S, Strupeck J, Creech S, Stiff PJ. Analysis of remobilization success in patients undergoing autologous stem cell transplants who fail an initial mobilization: risk factors, cytokine use and cost. Bone Marrow Transplant. 2004;33(10):997-1003.
-
47Jantunen E, Kvalheim G. Mobilization strategies in hard-to-mobilize patients with lymphoid malignancies. Eur J Haematol. 2010;85(6):463-71.
-
48Olivieri A, Marchetti M, Lemoli R, Tarella C, Iacone A, Lanza F, et al. Proposed definition of ‘poor mobilizer’ in lymphoma and multiple myeloma: an analytic hierarchy process by ad hoc working group Gruppo ItalianoTrapianto di Midollo Osseo. Bone Marrow Transplant. 2012;47(3):342-51.
-
49Stiff P, Micallef I, McCarthy P, Magalhaes-Silverman M, Weisdorf D, Territo M, et al. Treatment with plerixafor in non-Hodgkin's lymphoma and multiple myeloma patients to increase the number of peripheral blood stem cells when given a mobilizing regimen of G-CSF: implications for the heavily pretreated patient. Biol Blood Marrow Transplant. 2009;15(2):249-56.
-
50Devine SM, Flomenberg N, Vesole DH, Liesveld J, Weisdorf D, Badel K, et al. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin's lymphoma. J Clin Oncol. 2004;22(6):1095-102.
-
51Flomenberg N, Devine SM, Dipersio JF, Liesveld JL, McCarty JM, Rowley SD, et al. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106(5):1867-74.
-
52DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol. 2009;27(28):4767-73.
-
53DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720-6.
-
54Shaughnessy P, Chao N, Shapiro J, Walters K, McCarty J, Abhyankar S, et al. Pharmacoeconomics of hematopoietic stem cell mobilization: an overview of current evidence and gaps in the literature. Biol Blood Marrow Transplant. 2013;19(9):1301-9.
-
55Micallef IN, Stiff PJ, DiPersio JF, Maziarz RT, McCarty JM, Bridger G, et al. Successful stem cell remobilization using plerixafor (mozobil) plus granulocyte colony-stimulating factor in patients with non-Hodgkin lymphoma: results from the plerixafor NHL phase 3 study rescue protocol. Biol Blood Marrow Transplant. 2009;15(12):1578-86.
-
56Micallef IN, Sinha S, Gastineau DA, Wolf R, Inwards DJ, Gertz MA, et al. Cost-effectiveness analysis of a risk-adapted algorithm of plerixafor use for autologous peripheral blood stem cell mobilization. Biol Blood Marrow Transplant. 2013;19(1):87-93.
-
57Abhyankar S, DeJarnette S, Aljitawi O, Ganguly S, Merkel D, McGuirk J. A risk-based approach to optimize autologous hematopoietic stem cell (HSC) collection with the use of plerixafor. Bone Marrow Transplant. 2012;47(4):483-7.
-
58Abusin GA, Abu-Arja RF, Gingrich RD, Silverman MD, Zamba GK, Schlueter AJ. An algorithm for utilizing peripheral blood CD34 count as a predictor of the need for plerixafor in autologous stem cell mobilization - cost-effectiveness analysis. J Clin Apher. 2013;28(4):293-300.
-
59Micallef IN, Sinha S, Gastineau DA, Wolf R, Inwards DJ, Gertz MA, et al. A cost effective analysis of a risk-adapted algorithm for plerixafor use in autologous peripheral blood stem cell mobilization. Biol Blood Marrow Transplant. 2011;17 Suppl. 2:S159-60.
-
60Li J, Hamilton E, Vaughn L, Graiser M, Renfroe H, Lechowicz MJ, et al. Effectiveness and cost analysis of justin-time salvage plerixafor administration in autologous transplant patients with poor stem cell mobilization kinetics. Transfusion. 2011;51(10):2175-82.
-
61Vishnu P, Roy V, Paulsen A, Zubair AC. Efficacy and cost-benefit analysis of risk-adaptive use of plerixafor for autologous hematopoietic progenitor cell mobilization. Transfusion. 2012;52(1):55-62.
-
62Kymes SM, Pusic I, Lambert DL, Gregory M, Carson KR, DiPersio JF. Economic evaluation of plerixafor for stem cell mobilization. Am J Manag Care. 2012;18(1):33-41.
-
63Gertz MA, Wolf RC, Micallef IN, Gastineau DA. Clinical impact and resource utilization after stem cell mobilization failure in patients with multiple myeloma and lymphoma. Bone Marrow Transplant. 2010;45(9):1396-403.
-
64Pavone V, Gaudio F, Console G, Vitolo U, Iacopino P, Guarini A, et al. Poor mobilization is an independent prognostic factor in patients with malignant lymphomas treated by peripheral blood stem cell transplantation. Bone Marrow Transplant. 2006;37(8):719-24.
-
65Gordan LN, Sugrue MW, Lynch JW, Williams KD, Khan SA, Wingard JR, et al. Poor mobilization of peripheral blood stem cells is a risk factor for worse outcome in lymphoma patients undergoing autologous stem cell transplantation. Leuk Lymphoma. 2003;44(5):815-20.
Publication Dates
-
Publication in this collection
Jan-Feb 2016
History
-
Received
17 Apr 2015 -
Accepted
17 July 2015


 Adapted from DiPersio et al.
Adapted from DiPersio et al.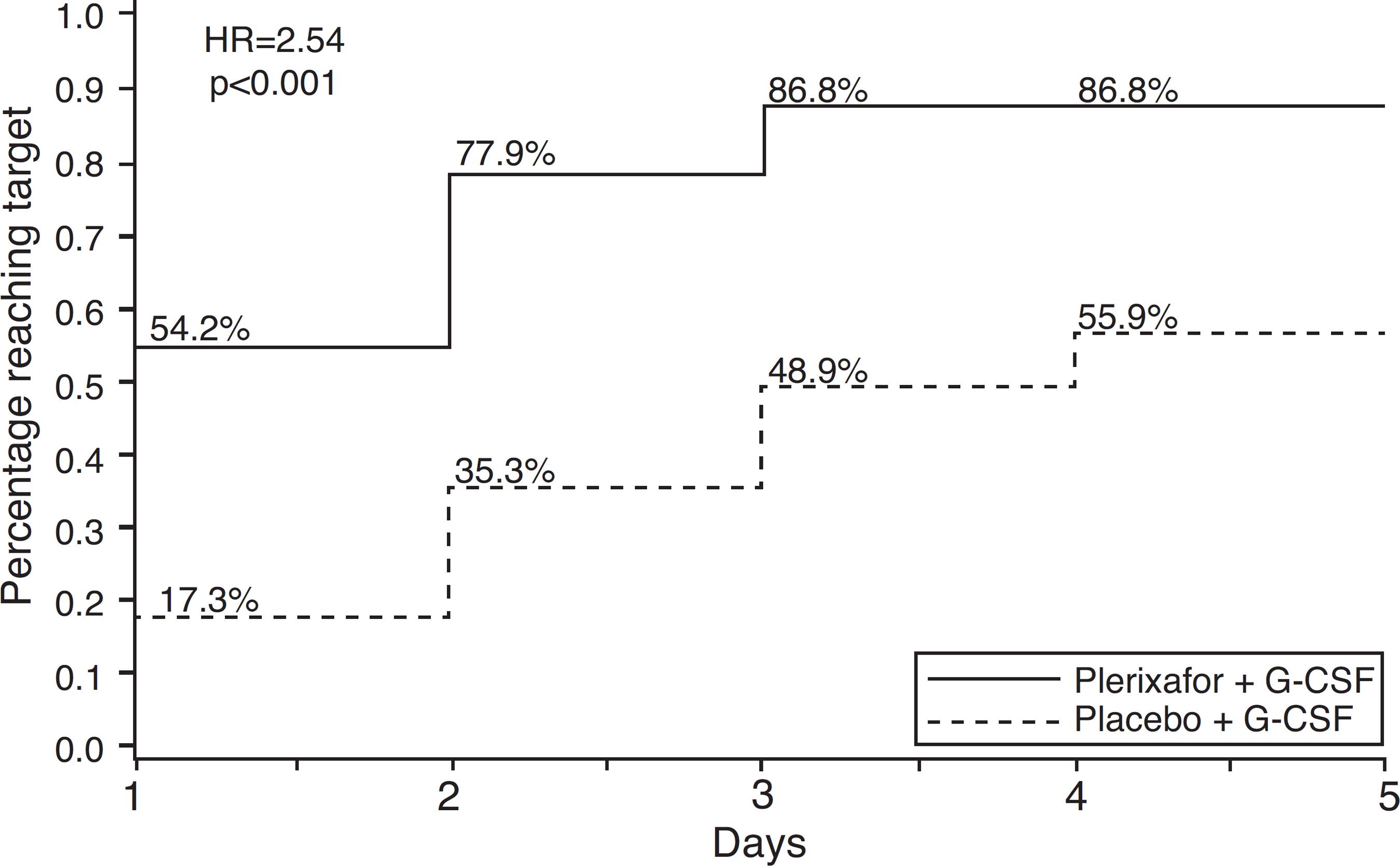 Adapted from DiPersio et al.
Adapted from DiPersio et al. Adapted from Mohty et al.
Adapted from Mohty et al.