ABSTRACT
OBJECTIVE:
This study evaluated in vitro differentiation of mesenchymal stromal cells isolated from bone marrow, in tenocytes after treatment with bovine tendon extract.
METHODS:
Bovine tendons were used for preparation of the extract and were stored at -80 °C. Mesenchymal stromal cells from the bone marrow of three donors were used for cytotoxicity tests by means of MTT and cell differentiation by means of qPCR.
RESULTS:
The data showed that mesenchymal stromal cells from bone marrow treated for up to 21 days in the presence of bovine tendon extract diluted at diminishing concentrations (1:10, 1:50 and 1:250) promoted activation of biglycan, collagen type I and fibromodulin expression.
CONCLUSION:
Our results show that bovine tendon extract is capable of promoting differentiation of bone marrow stromal cells in tenocytes.
Keywords:
Tendon; Mesenchymal stromal cells from bone marrow; Tenocytes
RESUMO
OBJETIVO:
O estudo avalia a diferenciação in vitro das células mesenquimais isoladas do estroma da medula óssea em tenócitos após tratamento com extrato de tendão bovino.
MÉTODOS:
Tendões bovinos foram usados para confecção do extrato e estocados a -80 °C. Células mesenquimais do estroma da medula óssea (BMSCs) de três doadores foram usadas para os testes de citotoxicidade por MTT e diferenciação celular por qPCR.
RESULTADOS:
Os dados mostram que células mesenquimais do estroma da medula óssea tratadas por até 21 dias em presença do extrato de tendão bovino diluído em concentrações crescentes (1:10, 1:50 e 1:250) promovem a ativação da expressão de biglican, colágeno tipo I e fibromodulina.
CONCLUSÃO:
Nossos resultados mostram que o extrato de tendão bovino é capaz de promover a diferenciação das BMSCs em tenócitos.
Palavras-chave:
Tendão; Células mesenquimais do estroma da medula óssea; Tenócitos
Introduction
Tendons are a specialized type of tissue composed of tenoblasts and tenocytes, which are embedded in an extracellular matrix mostly composed of type I collagen. Tenocytes only have limited potential for proliferation and thus confer low regenerative capacity on tendons.11. Luo Q, Song G, Song Y, Xu B, Qin J, Shi Y. Indirect co- culture with tenocytes promotes proliferation and mRNA expression of tendon/ligament related genes in rat bone marrow mesenchymal stem cells. Cytotechnology. 2009;61(1-2):1-10.and22. Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13(10):1219-27.
Tendon injuries constitute a serious problem within orthopedic practice and generate high costs for the public healthcare system, as well as having an impact on the quality of life of the patients affected. Although regeneration is the aim of the clinical treatments used, the methods currently available continue to be ineffective. Thus, tendon dysfunctions lead to definitive physical incapacity.33. Lin Y. From collagen to tenocyte - how the equine superficial tendon responds to physiologic challenges and physical therapy. Universidade Utrecht, Faculdade Diergerneeskunde; 2005. Available from: http://dspace.library.uu.nl/bitstream/handle/1874/7411/index.htm;jsessionid=F9F0939F8C85434883E51A7B4AB1F0D6?sequence=8.
http://dspace.library.uu.nl/bitstream/ha...
,44. Andrade LR. Biomateriais utilizados em bioengenharia ortopédica. Rev Estud Biol. 2006;28(63):17-23.and55. Wang T, Xu Z, Jiang W, Ma A. Cell-to-cell contact induces mesenchymal stem cell to differentiate into cardiomyocyte and smooth muscle cell. Int J Cardiol. 2006;109(1):74-81.
Mesenchymal cells isolated from bone marrow stromal cells (BMSCs) are known to be a promising therapeutic option within the field of cell therapy and bioengineering of musculoskeletal tissues.55. Wang T, Xu Z, Jiang W, Ma A. Cell-to-cell contact induces mesenchymal stem cell to differentiate into cardiomyocyte and smooth muscle cell. Int J Cardiol. 2006;109(1):74-81.,66. Chen WH, Lai MT, Wu AT, Wu CC, Gelovani JG, Lin CT, et al. In vitro stage-specific chondrogenesis of mesenchymal stem cells committed to chondrocytes. Arthritis Rheum. 2009;60(2):450-9.,77. Csaki C, Matis U, Mobasheri A, Shakibaei M. Co- culture of canine mesenchymal stem cells with primary bone- derived osteoblasts promotes osteogenic differentiation. Histochem Cell Biol. 2009;131(2):251-66.and88. Alberton P, Popov C, Prägert M, Kohler J, Shukunami C, Schieker M, et al. Conversion of human bone marrow- derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem Cells Dev. 2012;21(6):846-58. Their use in association with synthetic biomaterials has been proposed as an option for modern treatments aiming to toward tendon reconstruction, using an allograft, autograft or xenograft.99. Juncosa-Melvin N, Boivin GP, Gooch C, Galloway MT, West JR, Dunn MG, et al. The effect of autologous mesenchymal stem cells on the biomechanics and histology of gel- collagen sponge constructs used for rabbit patellar tendon repair. Tissue Eng. 2006;12(2):369-79.and1010. Sahoo S, Toh SL, Goh JC. A bFGF- releasing silk/PLGA-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells. Biomaterials. 2010;31(11):2990-8. Use of autologous BMSCs biosynthetic grafts has the aims of improving the results from conservative surgery and reducing the time taken for the pre-injury biomechanical properties to be restored.1111. Bashir J, Sherman A, Lee H, Kaplan L, Hare JM. Mesenchymal stem cell therapies in the treatment of musculoskeletal diseases. PM R J. 2014;6(1):61-9. Furthermore, the low immunogenicity of BMSCs makes it possible to use them allogeneically and minimizes the need for immunosuppression of the receptor.1212. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402-8.
Despite the significant therapeutic potential of BMSCs, little is yet known about the mechanisms and signaling pathways involved in determining that BMSCs will differentiate toward a tenogenic route, or in relation to progression of their differentiation. Considering that BMSCs seem to respond to stimuli that are present in extracts from healthy mature tissues and have specific phenotypic characteristics,1313. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739-49.and1414. Lee IC, Wang JH, Lee YT, Young TH. The differentiation of mesenchymal stem cells by mechanical stress or/and co- culture system. Biochem Biophys Res Commun. 2007;352(1):147-52. we developed the hypothesis that tendon extracts might induce differentiation of BMSCs into tenocytes. Thus, the present study had the objective of evaluating the influence of treatment of human BMSCs with different concentrations of bovine tendon extract, on in vitro differentiation toward a tenocytic route.
Material and methods
Isolation and expansion of mesenchymal cells from the stroma of human bone marrow
BMSCs were isolated from surgical waste that originated from hip arthroplasty procedures on five patients (two men and three women) aged 45-60 years, who did not present any comorbidities. Informed consent was obtained from all of these individuals after approval of the study protocol by the institutional ethics committee. After the samples had been collected in the surgical center, they were stored in sterile flasks containing Iscove's modified Dulbecco's medium (IMDM; Sigma-Aldrich, St. Louis, MO, USA), supplemented with 20% bovine fetal serum (BFS; Gibco, Grand Island, NY, USA), at 4 °C for not more than 18 h. To isolate the total cellular fraction, the bone marrow was resuspended in phosphate-buffered saline (PBS) solution and was mechanically dissociated from any bone fragments. The cell suspensions thus obtained were collected in 50 mL tubes and were centrifuged at 836 × g and 4 °C for 5 min. The cells were then resuspended in 50 mL of IMDM supplemented with 20% BFS and were counted using a Neubauer chamber. Following this, 6 × 105 mononuclear cells were distributed in culturing flasks of volume 75 cm2, in 10 mL of IMDM with 20% BFS, and were maintained at 37 °C under 5% CO2. Three days later, the non-adherent fraction was removed by means of lavage with PBS and the culturing medium was changed. After a further 14 days, the cells were removed using a solution of 0.125% trypsin and 0.78 mM EDTA and were expanded.
Preparation of bovine tendon extract
Five bovine calcaneal tendons were obtained. They were macerated mechanically and then were ground up using an electric blender of power 20 W, in the proportions of 1 g of tissue to 2 mL of IMDM, without BFS.1515. Dietz FR, Mukhopadhyay B, Becker G, Daniels K, Solursh M. Peripheral nerve extract effects on mesenchymal cells. Iowa Orthop J. 1996;16:46-57. The tissue extract was°C centrifuged at 836 × g and 8 °C for 5 min and was then stored at -80 °C for a maximum of two months.
Analysis of cell viability using the MTT method
BMSCs were cultured on 24-well plates, at a density of 2.5 × 104 cells/well and were treated with bovine tendon extract diluted in the proportions of 1:10, 1:50 or 1:250 (v/v) in IMDM supplemented with 10% BFS. Cell viability was assessed 24, 72, 120 and 168 hours after the treatment, in the presence of MTT (thiazolyl blue tetrazolium bromide; Sigma-Aldrich) at a concentration of 25 mg/mL. Equal concentrations of dimethyl sulfoxide (DMSO) were used as a negative control. Colorimetric evaluation was performed at a wavelength of 550 nm, using the SIRIO S SEAC reader (Burladingen, Germany).
Analysis of gene expression using qPCR
BMSCs were cultured under the different experimental conditions described above (1:10, 1:50 or 1:250, v/v), for 7, 14 or 21 days. The cells were then washed and the total RNA was extracted using the Trizol(r) method (Invitrogen Corp., Carlsbad, CA, USA) and was treated using DNase (Ambion(r) DNA-free(tm) DNase treatment; Life Technologies), in accordance with the manufacturer's instructions. The integrity and quantity of the RNA were evaluated by means of electrophoresis on denaturing gel and by means of spectrophotometry (Nanodrop(tm) 1000; Thermo Fisher Scientific, Inc.). Reverse transcription for synthesis of complementary DNA (cDNA) was performed in duplicate, from 1.0 µg of RNA, using the ImProm-II(tm) reverse transcription system (Promega), in accordance with the manufacturer's protocol. qPCR was performed using the Power Sybr Green Master MIX(r) detection system (Applied Biosystems, Molecular Probes, Inc.) in the Step One equipment (Applied Biosystems, Molecular Probes, Inc.). Primers from the constitutive genes (rDNA 28S and actin) were used as controls for the experiment. The expression of the genes for type I collagen, biglycan and fibromodulin was normalized in relation to the expression of the constitutive gene for ß-actin (Table 1). The 2-ΔΔCT1313. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739-49. method was used for analyzing the expression of the target genes of this study in relation to the constitutive gene. The values for relative expression that have been presented took the fixed control-group value of 1.0 as the reference (calibrator).
Results
With the aim of evaluating the toxicity of bovine tendon extract toward BMSCs, the MTT test was performed. The results showed that the bovine tendon extract did not alter the viability of the BMSCs at any of the concentrations tested (Fig. 1). Thus, we can infer that the bovine tendon extract did not have any cytotoxic effect on the mesenchymal stem cells.
Evaluation of the cytotoxicity of bovine tendon extract in mesenchymal cells of the bone marrow stroma. C represents control condition; 1:10, 1:50 and 1:250 represent the dilutions of the bovine tendon extract in IMDM; B represents the control condition for the technique in which there were no cells. Under all conditions, IMDM was used with supplementation with 1% BFS.
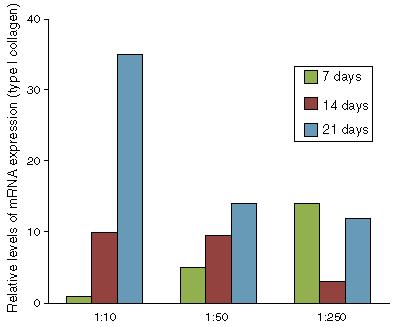
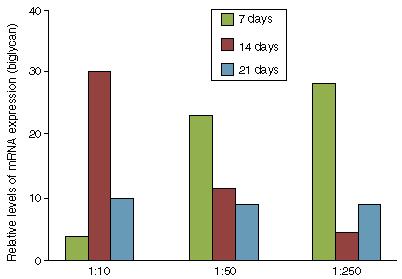
Given that there was no cytotoxic effect, the potential of the bovine tendon extract for differentiation was tested with the aim of evaluating whether the growth factors present in the extract would stimulate differentiation of the mesenchymal progenitor cells. We observed that in the BMSCs, the extract was capable of activating expression of the genes for type I collagen (Fig. 2), biglycan (Fig. 3) and fibromodulin (Fig. 4) over periods of 7, 14 and 21 days. These results showed that the bovine tendon extract was not cytotoxic and that it was capable of inducing expression of the genes implicated in tenocytic differentiation of mesenchymal stem cells. Furthermore, the tendon protein extract promoted induction in a dose-dependent manner and as a function of the duration of exposure to the extract.
Gene expression of type I collagen in mesenchymal cells of the bone marrow stroma, treated with increasing concentrations of bovine tendon extract (1:10, 1:50 and 1:250). Data normalized in relation to the expression of the constitutive gene ACTB.
Evaluation of biglycan expression through qPCR on mesenchymal stromal cells treated with increasing concentrations of bovine tendon extract (1:10, 1:50 and 1:250).
Evaluation of fibromodulin expression through qPCR on mesenchymal stromal cells treated with increasing concentrations of bovine tendon extract (1:10, 1:50 and 1:250).
Discussion
Our study showed that the bovine tendon extract had the potential to induce tenocytic differentiation of BMSCs and that this extract did not have cytotoxicity at any of the concentrations used. In our analyses, the results showed that there was a spatial and temporal window for success regarding differentiation of BMSCs into tenocytes. We emphasize that for the procedure to be efficient, the BMSCs (at 70% confluence) should be treated with tendon extract at 1:50 for seven days. We observed that the peak expression of biglycan and fibromodulin (which are markers for tenocytes)1616. Liu XN, Yin Q, Zhang H, Zhang H, Zhu SJ, Wei YJ, et al. Tissue extracts from infarcted myocardium of rats in promoting the differentiation of bone marrow stromal cells into cardiomyocyte-like cells. Biomed Environ Sci. 2008;21(2):110-7.,1717. Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128(19):3855-66.,1818. Aslan H, Kimelman- Bleich N, Pelled G, Gazit D. Molecular targets for tendon neoformation. J Clin Investig. 2008;118(2):439-44.,1919. Kuo CK, Tuan RS. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng A. 2008;14(10):1615-27.,2020. Zhu J, Li J, Wang B, Zhang WJ, Zhou G, Cao Y, et al. The regulation of phenotype of cultured tenocytes by microgrooved surface structure. Biomaterials. 2010;31(27):6952-8.and2121. Min BH, Han MS, Woo JI, Park HJ, Park SR. The origin of cells that repopulate patellar tendons used for reconstructing anterior cruciate ligaments in man. J Bone Joint Surg Br. 2003;85(5):753-7. was at this time. This indicated that the BMSCs became committed to a tenocytic lineage. The treatment protocol needs to proceed with increased concentration of the extract, which should be 1:10 for another 10 days. This allows the BMSCs to maintain type I collagen expression and provide an efficient extracellular matrix, so as also to maintain cell viability.
Our results suggest that in tendons, there are growth factors that stimulate differentiation of pluripotent cells into tendon cells. This opens up a possibility for the field of cell therapy, for treating tendinopathy. Finally, the potential was evaluated in an in vitro model and therefore there is a need for validation in an in vivo model, in order to confirm the results. Moreover, we raise the possibility that, in the future, the potential of tendon extracts originating from human tendons in cadaver donors might be evaluated.
Conclusions
The set of results showed that treatment of BMSCs with a protein extract from bovine tendon tissue promoted differentiation into tenocytes.
References
-
1Luo Q, Song G, Song Y, Xu B, Qin J, Shi Y. Indirect co- culture with tenocytes promotes proliferation and mRNA expression of tendon/ligament related genes in rat bone marrow mesenchymal stem cells. Cytotechnology. 2009;61(1-2):1-10.
-
2Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13(10):1219-27.
-
3Lin Y. From collagen to tenocyte - how the equine superficial tendon responds to physiologic challenges and physical therapy. Universidade Utrecht, Faculdade Diergerneeskunde; 2005. Available from: http://dspace.library.uu.nl/bitstream/handle/1874/7411/index.htm;jsessionid=F9F0939F8C85434883E51A7B4AB1F0D6?sequence=8.
» http://dspace.library.uu.nl/bitstream/handle/1874/7411/index.htm;jsessionid=F9F0939F8C85434883E51A7B4AB1F0D6?sequence=8 -
4Andrade LR. Biomateriais utilizados em bioengenharia ortopédica. Rev Estud Biol. 2006;28(63):17-23.
-
5Wang T, Xu Z, Jiang W, Ma A. Cell-to-cell contact induces mesenchymal stem cell to differentiate into cardiomyocyte and smooth muscle cell. Int J Cardiol. 2006;109(1):74-81.
-
6Chen WH, Lai MT, Wu AT, Wu CC, Gelovani JG, Lin CT, et al. In vitro stage-specific chondrogenesis of mesenchymal stem cells committed to chondrocytes. Arthritis Rheum. 2009;60(2):450-9.
-
7Csaki C, Matis U, Mobasheri A, Shakibaei M. Co- culture of canine mesenchymal stem cells with primary bone- derived osteoblasts promotes osteogenic differentiation. Histochem Cell Biol. 2009;131(2):251-66.
-
8Alberton P, Popov C, Prägert M, Kohler J, Shukunami C, Schieker M, et al. Conversion of human bone marrow- derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem Cells Dev. 2012;21(6):846-58.
-
9Juncosa-Melvin N, Boivin GP, Gooch C, Galloway MT, West JR, Dunn MG, et al. The effect of autologous mesenchymal stem cells on the biomechanics and histology of gel- collagen sponge constructs used for rabbit patellar tendon repair. Tissue Eng. 2006;12(2):369-79.
-
10Sahoo S, Toh SL, Goh JC. A bFGF- releasing silk/PLGA-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells. Biomaterials. 2010;31(11):2990-8.
-
11Bashir J, Sherman A, Lee H, Kaplan L, Hare JM. Mesenchymal stem cell therapies in the treatment of musculoskeletal diseases. PM R J. 2014;6(1):61-9.
-
12Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402-8.
-
13Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739-49.
-
14Lee IC, Wang JH, Lee YT, Young TH. The differentiation of mesenchymal stem cells by mechanical stress or/and co- culture system. Biochem Biophys Res Commun. 2007;352(1):147-52.
-
15Dietz FR, Mukhopadhyay B, Becker G, Daniels K, Solursh M. Peripheral nerve extract effects on mesenchymal cells. Iowa Orthop J. 1996;16:46-57.
-
16Liu XN, Yin Q, Zhang H, Zhang H, Zhu SJ, Wei YJ, et al. Tissue extracts from infarcted myocardium of rats in promoting the differentiation of bone marrow stromal cells into cardiomyocyte-like cells. Biomed Environ Sci. 2008;21(2):110-7.
-
17Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128(19):3855-66.
-
18Aslan H, Kimelman- Bleich N, Pelled G, Gazit D. Molecular targets for tendon neoformation. J Clin Investig. 2008;118(2):439-44.
-
19Kuo CK, Tuan RS. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng A. 2008;14(10):1615-27.
-
20Zhu J, Li J, Wang B, Zhang WJ, Zhou G, Cao Y, et al. The regulation of phenotype of cultured tenocytes by microgrooved surface structure. Biomaterials. 2010;31(27):6952-8.
-
21Min BH, Han MS, Woo JI, Park HJ, Park SR. The origin of cells that repopulate patellar tendons used for reconstructing anterior cruciate ligaments in man. J Bone Joint Surg Br. 2003;85(5):753-7.
-
☆
Work developed at the Instituto Nacional de Traumatologia e Ortopedia (INTO), Rio de Janeiro, RJ, Brazil.
Publication Dates
-
Publication in this collection
jan-feb 2016
History
-
Received
20 Oct 2014 -
Accepted
03 Feb 2015