Abstracts
OBJECTIVES: Changes in the hypothalamic-pituitary-adrenocortical (HPA) system are characteristic of depression. Because the effects of glucocorticoids are mediated by intracellular receptors including, most notably, the glucocorticoid receptor (GR), several studies have examined the number and/or function of GRs in depressed patients. METHODS: Review scientific evidences have consistently demonstrated that GR function is impaired in major depression, resulting in reduced GR-mediated negative feedback on the HPA axis and increased production and secretion of CRF in various brain regions postulated to be involved in the causality of depression. RESULTS: This article summarizes the literature on GR in depression and on the impact of antidepressants on the GR in clinical and preclinical studies, and supports the concept that impaired GR signalling is a key mechanism in the pathogenesis of depression, in the absence of clear evidence of decreased GR expression. The data also indicate that antidepressants have direct effects on the GR, leading to enhanced GR function and increased GR expression. Although the effects of antidepressants on glucocorticoid hormones and their receptors are relevant for the therapeutic action of these drugs, the molecular mechanisms underlying these effects are unclear. We propose that antidepressants in humans could inhibit steroid transporters localised on the blood-brain barrier and in neurones, like the multidrug resistance p-glycoprotein, and thus increase the access of cortisol to the brain and the glucocorticoid-mediated negative feedback on the HPA axis. CONCLUSION: Enhanced cortisol action in the brain might prove to be a successful approach to maximise therapeutic antidepressant effects. Hypotheses regarding the mechanism of these receptor changes involve non-steroid compounds that regulate GR function via second messenger pathways. Research in this field will lead to new insights into the pathophysiology and treatment of affective disorders.
Stress; Depression; Hypothalamus; Hidrocortisone; Antidepressive agents; Neuroendocrinology; Cytokines; Psychoneuroimmunology
OBJETIVO: As mudanças no eixo hipotálamo-pituitária-adrenal (HPA) são características da depressão. Devido aos efeitos dos glicocorticóides serem mediados por receptores intracelulares, como os receptores de glicocorticóides (RGs), inúmeros estudos examinaram o número e/ou função dos RGs em pacientes com depressão. MÉTODOS: Os autores fazem uma revisão das evidências científicas dos estudos que têm consistentemente demonstrado que a função dos RGs está prejudicada na depressão maior, em conseqüência da redução da resposta do eixo HPA ao feedback negativo mediado pelos RGs e a um aumento na produção e secreção de HLC em várias regiões cerebrais, sugerindo que esses mecanismos estão envolvidos na etiologia da depressão e no tratamento antidepressivo. RESULTADOS: Esta revisão faz um resumo da literatura atual sobre RG na depressão e sobre o impacto dos antidepressivos nos RGs em estudos clínicos e pré-clínicos, e dá suporte ao conceito de que a sinalização deficiente dos RGs é parte fundamental na fisiopatogenia da depressão, na ausência de evidências claras de redução na expressão dos RGs. Embora os efeitos dos antidepressivos nos hormônios glicocorticóides e seus receptores sejam relevantes para a ação terapêutica dessas drogas, os mecanismos moleculares subjacentes a esses efeitos ainda não estão esclarecidos. Estudos indicam que os antidepressivos têm efeitos diretos nos RGs, levando a uma melhora da função e a um aumento da expressão dos RGs. Nós propomos que, em humanos, os antidepressivos podem inibir os transportadores de esteróides localizados na barreira hemato-liquórica e nos neurônios, como o complexo de resistência a múltiplas drogas glicoproteína-p ("multidrug resistance p-glycoprotein"), e podem aumentar o acesso do cortisol ao cérebro e o feedback negativo mediado por glicocorticoides no eixo HPA. CONCLUSÃO: O aumento da ação do cortisol no cérebro pode ser uma abordagem eficaz para maximizar os efeitos terapêuticos dos antidepressivos. Hipóteses referentes aos mecanismos destes receptores envolvem compostos não esteróides que regulam a função dos RGs via segundos mensageiros. A pesquisa nesta área trará novos entendimentos à fisiopatologia e ao tratamento dos transtornos afetivos, em especial na depressão.
Estresse; Depressão; Hipotálamo; Hidrocortisona; Antidepressivos; Neuroendocrinologia; Citocinas; Pseudoneuroimunologia
REVIEW
The Hypothalamic Pituitary Adrenal axis, Glucocorticoid receptor function and relevance to depression
Mario F Juruena; Anthony J Cleare; Carmine M Pariante
Division of Psychological Medicine, Section of Neurobiology of Mood Disorders, Institute of Psychiatry, University of London, UK, and Affective Disorders Unit, South London Maudsley Trust, London, UK
Correspondence Correspondence to Mario Francisco Juruena 103 Denmark Hill SE5 8AZ London - UK Phone: 44 (20) 7848 5305 Fax: 44 (20) 7848 5408 E-mail: m.juruena@iop.kcl.ac.uk
ABSTRACT
OBJECTIVES: Changes in the hypothalamic-pituitary-adrenocortical (HPA) system are characteristic of depression. Because the effects of glucocorticoids are mediated by intracellular receptors including, most notably, the glucocorticoid receptor (GR), several studies have examined the number and/or function of GRs in depressed patients.
METHODS: Review scientific evidences have consistently demonstrated that GR function is impaired in major depression, resulting in reduced GR-mediated negative feedback on the HPA axis and increased production and secretion of CRF in various brain regions postulated to be involved in the causality of depression.
RESULTS: This article summarizes the literature on GR in depression and on the impact of antidepressants on the GR in clinical and preclinical studies, and supports the concept that impaired GR signalling is a key mechanism in the pathogenesis of depression, in the absence of clear evidence of decreased GR expression. The data also indicate that antidepressants have direct effects on the GR, leading to enhanced GR function and increased GR expression. Although the effects of antidepressants on glucocorticoid hormones and their receptors are relevant for the therapeutic action of these drugs, the molecular mechanisms underlying these effects are unclear. We propose that antidepressants in humans could inhibit steroid transporters localised on the blood-brain barrier and in neurones, like the multidrug resistance p-glycoprotein, and thus increase the access of cortisol to the brain and the glucocorticoid-mediated negative feedback on the HPA axis.
CONCLUSION: Enhanced cortisol action in the brain might prove to be a successful approach to maximise therapeutic antidepressant effects. Hypotheses regarding the mechanism of these receptor changes involve non-steroid compounds that regulate GR function via second messenger pathways. Research in this field will lead to new insights into the pathophysiology and treatment of affective disorders.
Keywords: Stress; Depression; Hypothalamus; Hidrocortisone; Antidepressive agents; Neuroendocrinology; Cytokines; Psychoneuroimmunology
Introduction
Hormones play a critical role in the development and expression of a wide range of behaviours. One aspect of the influence of hormones on behaviour is their potential contribution to the pathophysiology of psychiatric disorders and the mechanism of action of psychotropic drugs, particularly in major depression. Of all endocrine axes, the hypothalamic-pituitary-adrenal (HPA) axis has been the most widely evaluated.1,2 The HPA axis plays a fundamental role in the response to external and internal stimuli including psychological stressors. Abnormalities in the function of the HPA axis have been described in people experiencing psychiatric disorders. Moreover, is well known the fundamental role of stress in precipitating episodes of psychiatric disorders in predisposed individuals.1 These abnormalities seem related to changes in the ability of circulating glucocorticoids to exert their negative feedback on the secretion of HPA hormones through binding to the mineralocorticoid receptor (MR) and the glucocorticoid receptor (GR) in HPA tissues.2,3,6 In fact, previous studies have described both an impaired HPA negative feedback, leading to hypercortisolemia, as in melancholic depression.2,6 In addition to melancholic depression, a spectrum of other conditions may be associated with increased and prolonged activation of the HPA axis, including anorexia nervosa with or without malnutrition, obsessivecompulsive disorder, panic anxiety, chronic active alcoholism, alcohol and narcotic withdrawal, excessive exercising, poorly controlled diabetes mellitus, childhood sexual abuse and hyperthyroidism.7 Another group of states is characterized by hypoactivation of the stress system, rather than sustained activation, in which chronically reduced secretion of CRH may result in pathological hypoarousal and an enhanced HPA negative feedback. Patients with post-traumatic stress disorder, atypical, seasonal depression and the chronic fatigue syndrome fall in this category (see Table 1). Similarly, patients with fibromyalgia have decreased urinary free cortisol excretion and frequently complain of fatigue. Hypothyroid patients also have clear evidence of CRH hyposecretion.8-11 Finally, the hypothesis that antidepressants exert their clinical effects through direct modulation of the glucocorticoid hormones and their receptor is one of the most striking and innovative models of the mechanism of action of this class of drugs.12-13
We will review the evidences supporting that: 1) HPA axis hyperactivity plays an important role in the pathogenesis of major depression; 2) this hyperactivity is mainly due to an impaired feedback inhibition by circulating glucocorticoid hormones on the HPA axis; 3) this impaired feedback inhibition is related to a decreased function of the glucocorticoid receptors (GR), which mediate the effects of glucocorticoid hormones, including the negative feedback on the HPA axis; and 4) antidepressants act by reversing these changes in the GR function and thus normalising HPA axis hyperactivity in patients with major depression.
The regulation of the HPA axis
HPA axis activity is governed by the secretion of the adrenocorticotrophic hormone-releasing factor (CRF) and vasopressin (AVP) from the hypothalamus, which in turn activate the secretion of adrenocorticotrophic hormone (ACTH) from the pituitary, which finally stimulates the secretion of the glucocorticoids from the adrenal cortex.2 Glucocorticoids then interact with their receptors in multiple target tissues including the HPA axis, where they are responsible for feedback inhibition of the secretion of ACTH from the pituitary and CRF from the hypothalamus. Although glucocorticoids regulate the function of almost every tissue in the body, the best known physiological effect of these hormones is the regulation of energy metabolism. The anti-inflammatory and immunosuppressive effects of glucocorticoids are evident at pharmacological doses, while physiologically these hormones have an important regulatory role in the immune system.5
A number of factors regulate HPA axis activity. Evidence for a direct catecholaminergic, serotonergic and dopaminergic innervation of the CRF neurones in the hypothalamus has been described, and these and other neurotransmitters have been shown to influence CRF release. For example serotonin has been found to exert a stimulatory influence on CRF, through 5-HT1A , 5-HT1B , 5-HT1C and 5-HT2 receptors subtypes. Norepinephrine has a more variable effect, being stimulatory at low doses (via alpha1 receptors) and inhibitory at high doses (via beta receptors).14
Abnormalities of HPA axis in depression
Hyperactivity of the HPA axis in major depression is one of the most consistent findings in psychiatry. A significant percentage of patients with major depression have been shown to exhibit increased concentrations of cortisol (the endogenous glucocorticoid in humans) in plasma, urine and cerebrospinal fluid (CSF); an exaggerated cortisol response to adrenocorticotropic hormone (ACTH); and an enlargement of both the pituitary and adrenal glands.2-3,6,14-15 Adrenal hypertrophy in depressed patients has been demonstrated, and this finding likely explains why the cortisol response to CRF is similar in depressed and control subjects, because the enlarged adrenal gland is capable of compensating for the blunted ACTH response to CRF commonly observed in depressed patients.2 Increased pituitary volume in these patients has also been described, and it has also been considered a marker of HPA axis activation.16 In a recent study the first episode of a psychosis is associated with a larger pituitary volume, and suggested that this is due to activation of the HPA axis. The smaller pituitary volume in subjects with established psychosis could also be the consequence of repeated episodes of HPA axis hyperactivity.17
In general, HPA axis changes appear in chronic depressive and more severely episodes. Moreover, HPA axis changes appear to be state dependent, tending to resolve upon resolution of depressive syndrome.12
Findings derived from multiple lines of research have provided evidence that, during depression, dysfunction of limbic structures, including the hypothalamus and the hippocampus, results in hypersecretion of CRF and AVP, which in turn induces pituitary-adrenal activation. Moreover, the CSF concentrations of CRF are increased in drug-free depressed patients, and the decreased number of CRF receptors in the frontal cortex of suicide victims18 was found. A number of studies have provided evidence that CRF may play a role in the behavioural signs and symptoms of the depression (decreased libido, decreased appetite, psychomotor alterations and disturbed sleep).
Although the mechanism by which extra-hypothalamic CRF is elevated in depression has not been resolved, the increased levels of CRF in the hypothalamus are thought to be related to altered feedback inhibition by endogenous glucocorticoids. Data supporting the notion that glucocorticoids-mediated feedback inhibition is impaired in major depression comes from several studies3 demonstrating no suppression of cortisol secretion. These studies were complemented by many neuroendocrine function tests examining the suppression of ACTH and corticosteroids by the synthetic glucocorticoid dexamethasone (DEX; dexamethasone suppression test, DST). The DST showed that a high proportion of patients with various affective disorders have elevated cortisol levels,19 thus escaping the suppressive effect of DEX. After CRF was discovered and characterized by Vale and coworkers20 initial studies employing ovine or human CRF in depressives showed that the ACTH response after injection of this neuropeptide was decreased, suggesting desensitized pituitary CRF receptors due to homologous downregulation by hypersecreted CRF.21-22 The most sensitive neuroendocrine function test to detect HPA dysregulation combines the DST and the CRF stimulation test (DEX/CRF test):23-25 indeed, Heuser et al25 concluded from their studies that the sensitivity of this test is above 80%, depending on age and gender. Whereas While CRF-elicited ACTH response is blunted in depressives, DEX pretreatment produces the opposite effect and paradoxically enhances ACTH release following CRF. Similarly, CRF-induced cortisol release is much higher in DEX-pretreated patients than following a challenge with CRF alone. The interpretation of the above findings is as follows: DEX due to its low binding to corticosteroid binding globulin and its decreased access to the brain,26 acts primarily at the pituitary to suppress ACTH. The subsequent decrease of cortisol and the failure of DEX to compensate for the decreased cortisol levels in the nervous tissue create a situation that is sensed by central regulatory elements of the HPA system as a partial and transient adrenalectomy. In response to this situation, the secretion of central neuropeptides that are capable of activating ACTH secretion - mainly CRF and vasopressin - is increased. When vasopressin is infused at a low rate into DEX pretreated controls, concurrent infusion with CRF induces an ACTH and cortisol response which is similar to the hormone secretory profile of depressives receiving the combined DEX CRF-test but without simultaneous vasopressin treatment.27
There are however some limitations to this test. In particular, DEX pharmacodynamics and pharmacokinetics differ markedly from those of cortisol. DEX only binds to the GR (not the MR) in vivo, does not bind to the corticosteroid binding globulin (CBG), and has a much longer half-life compared to cortisol.4,28 In response to these concerns, we have recently developed an HPA suppressive test using prednisolone, which is similar to endogenous glucocorticoids.29 Prednisolone is a synthetic glucocorticoid that is similar to cortisol in its pharmacodynamics (binds to both the MR and the GR, and to the CBG) and pharmacokinetics (its half-life is similar to that of cortisol).28 We propose that prednisolone at the 5 mg dose (which gives partial HPA suppression), together with the assessment of salivary cortisol, can be used to investigate both impaired and enhanced glucocorticoid-mediated negative feedback in large samples of patients with psychiatric disorders.
Interestingly, recent studies have found that cortisol itself has a limited access to the human brain.30 Therefore it is unclear at this stage whether or not DEX is able to compensate in the brain when the levels of plasma cortisol are low, since it is not clear in the first place how much of the circulating cortisol is able to enter the brain in normal circumstances (see below).
The HPA hyperactivity in major depression is believed to be secondary to hypersecretion of CRF. CRF has behavioral effects in animals that are similar to those seen in depressed patients including alterations in activity, appetite, and sleep.14 Moreover, depressed patients exhibit increased concentrations of CRF in the CSF, increased CRF mRNA and protein in the paraventricular nucleus (PVN) of the hypothalamus (postmortem samples), and a blun-ted ACTH response to a CRF challenge.2,6 The findings of blun-ted adrenocorticotropic hormone (ACTH) response to human CRF22 and to ovine CRF, 26the elevated levels of CRF in the CSF,31 the decreased number of CRF binding sites in the frontal cortex of patients with depression who have committed suicide,32 the increased number of CRF-expressing neurons in the hypothalamic paraventricular nucleus of patients with depression,33 and the finding that CRF concentrations in the spinal fluid decrease during long-term treatment with fluoxetine or amitriptyline34 support the idea that CRF is the key neuropeptide responsible for HPA alterations in depression.
The questions of whether psychological signs and symptoms of depression are also related to hypersecretion of CRF and vasopressin and how antidepressants work to remedy both the neuroendocrine and the behavioral aspect of this dysregulation have been the subject of intense preclinical research. Several research groups have formulated a hypothesis relating aberrant stress hormone dysregulation to causality of depression and have proposed that antidepressants may act through normalisation of these HPA changes.3,5,15 While nonsuppression to DEX in the DST and the DEX-CRH test likely represents impaired feedback inhibition at the level of the pituitary,4,35 impaired responsiveness to hydrocortisone challenge in depressed patients suggests these feedback alterations also occur in the brain.36
Studies conducted both in animals and in humans suggest that stress in early phases of development can induce persistent changes in the ability of the HPA axis to respond to stress in the adult life, and that mechanism can lead to a raised susceptibility to depression.37 Interestingly, persistent HPA hyperactivity has been associated with higher rates of relapse.38-40 Studies conducted in patients receiving a range of antidepressants have shown that those who fail to show a normalisation of post-DEX cortisol level tend to have a worse outcome in terms of re-hospitalisation, suicide and recurrence of depression.38 Two recent reports have described a prospective study on the relationship between results at the DEX/CRF test and clinical outcome. Specifically, Zobel et al39-40 have described a cohort of patients receiving the DEX/CRF test on two separate occasions: within one week after the admission (or after starting the first antidepressant treatment) and few days before the discharge. The patients were then followed-up for 6 months after discharge. The study found that those patients who had an increase in the cortisol levels after the DEX/CRF test between admission and discharge tended to relapse during the follow-up period, while those who showed a decrease in the post DEX/CRF cortisol levels tended to remain clinically stable in the follow-up period. Therefore, these studies suggest that evaluation of the HPA axis during the antidepressant treatment may be helpful in identifying those who are at higher risk of relapse (see Table 3).2,6,14-15,25
As Because a wide variety of stressors reliably activate the hypothalamic-pituitary-adrenal (HPA) axis, and because glucocorticoids are the end product of HPA axis activation, these hormones have been most commonly seen as the agents provocateurs, or even in extreme cases as the physical embodiment, of stress-induced pathology. Indeed, it has been suggested that prolonged overproduction of glucocorticoids, whether as a result of ongoing stress or a genetic predisposition to HPA axis hyperactivity, damages brain structures (especially the hippocampus) essential for HPA axis restraint.41 Such damage, in turn, has been hypothesized to lead to a feed-forward circuit in which ongoing stressors drive glucocorticoid overproduction indefinitely (the "glucocorticoid cascade hypothesis"). Because of the capacity of high concentrations of glucocorticoids to disrupt cellular functioning in ways that can lead to a host of ills, this glucocorticoid overproduction is believed to contribute directly to many of the adverse behavioral and physiological sequelae associated with chronic stress.41-42
Despite the popularity of the glucocorticoid cascade hypothesis, however, increasing data provide evidence that, in addition to glucocorticoid excess, insufficient glucocorticoid signaling may play a significant role in the development and expression of pathology in stress-related disorders.
Although not occurring together, both hypocortisolism and reduced responsiveness to glucocorticoids (as determined by dexamethasone challenge tests) were reliably found. Stress-related neuropsychiatric disorders were also associated with immune system activation/inflammation, high SNSCNS tone, and CRF hypersecretion, which are all consistent with insufficient glucocorticoid-mediated regulation of stress hyperresponsiveness. Finally, antidepressants, a mainstay in the treatment of stress-related disorders, were regularly associated with evidence of enhanced glucocorticoid signaling.
We define insufficient glucocorticoid signaling as any state in which the signaling capacity of glucocorticoids is inadequate to restrain relevant stress-responsive systems, either as a result of decreased hormone bioavailability (e.g., hypocortisolism) or as a result of attenuated glucocorticoid responsiveness (e.g., secondary to reduced glucocorticoid receptor sensitivity). Thus defined, insufficient glucocorticoid signaling implies no specific mechanism or absolute deficiency but focuses instead on the end point of glucocorticoid activity. The fundamental question is whether the glucocorticoid message is getting through in a manner adequate to the environment (external and internal) in which an organism finds itself. Therefore, even in the case of glucocorticoid hypersecretion, glucocorticoid insufficiency can exist, if reduced glucocorticoid sensitivity in relevant target tissues outweighs excess circulating hormone.43
Recent neuroendocrine data provide evidence of insufficient glucocorticoid signaling in stress-related neuropsychiatric disorders. Impaired feedback regulation of relevant stress responses, especially immune activation/inflammation, may, in turn, contribute to stress-related pathology, including alterations in behavior, insulin sensitivity, bone metabolism, and acquired immune responses. From an evolutionary perspective, reduced glucocorticoid signaling, whether achieved at the level of the hormone or its receptor, may foster immune readiness and increase arousal. Emphasis on insufficient glucocorticoid signaling in stress-related pathology encourages development of therapeutic strategies to enhance glucocorticoid signaling pathways.44
The glucocorticoid receptor
Steroid hormones (e.g., glucocorticoids, estrogen, testosterone, and mineralocorticoids), are small, lipid-soluble ligands that diffuse across cell membranes. Unlike the receptors for peptide hormones, which are located in the cell membrane, the receptors for these ligands are localized in the cytoplasm. In response to ligand binding, steroid hormone receptors translocate to the nucleus, where they regulate the expression of certain genes by binding to specific hormone response elements (HREs) in their regulatory regions. The MR has a high affinity for endogenous corticosteroids and is believed to play a role in the regulation of circadian fluctuations in these hormones (especially the regulation of ACTH secretion during the diurnal trough in cortisol secretion).
Glucocorticoids mediate their actions, including feedback regulation of HPA axis, through two distinct intracellular corticosteroid receptor subtypes referred to as mineralocorticoid receptor (MR) and GR (4; 45). In contrast to the MR, the GR has a high affinity for DEX and a lower affinity for endogenous corticosteroids. The GR is therefore believed to be more important in the regulation of the response to stress when endogenous levels of glucocorticoids are high. Spencer et al46 and de Kloet et al4 have clarified that GR activation is necessary for the HPA feedback regulation when levels of glucocorticoids are high (response to stress, circadian peak), but that MR also plays an important role by modulating GR-dependent regulation.
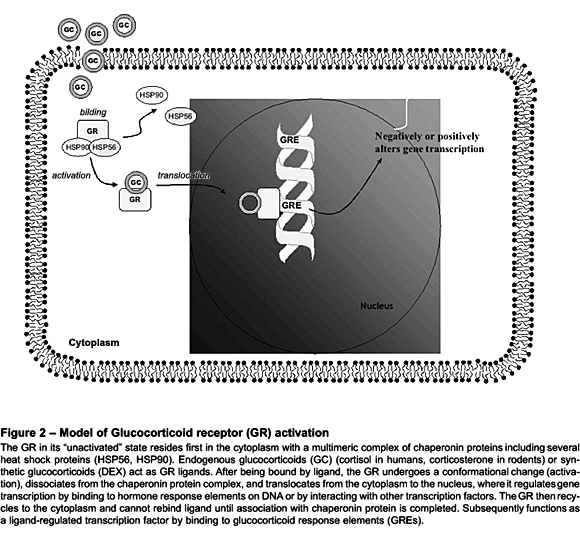
As we have previously mentioned, according to the '"nucleocytoplasmic traffic'" model of GR action (see Figure 2), the GR in its '"unactivated'" form resides primarily in the cytoplasm in association with a multimeric complex of chaperone proteins including several heat shock proteins (HSPs).47 After being bound by steroid, the GR undergoes a conformational change ('"activation'"), dissociates from the chaperone protein complex, and translocates from the cytoplasm to the nucleus, where it either binds to glucocorticoid response elements (GREs) on DNA or interacts with other transcription factors.48 GREs can confer either positive or negative regulation on the genes to which they are linked. The activated GR cannot rebind ligand since association with the chaperone protein complex is required for maintaining the receptor in a conformational state receptive to hormone.47 GRs have a low affinity but high capacity for cortisol and are very responsive to changes in cortisol concentrations. While MRs are thought to be involved in the tonic inhibitory activity within the HPA axis, GRs appear to '"switch off'" cortisol production at times of stress.49
Various research groups have suggested that the overactivity of the HPA axis in depression may be due to an abnormality of the GR at the limbichippocampal level.3,5,15,50 This abnormality results in a defect in or resistance to glucocorticoid. In fact, several findings in depression are consistent with an abnormality of the GR. Most notably, patients with depression fail to show most of the physical symptoms of corticosteroid excess, despite the frequent presence of hypercortisolism,51 suggesting that peripheral GRs may be abnormal or insensitive in depression. Consistent with the fact that GR is more important in the regulation of HPA when endogenous levels of glucocorticoids are high,4 and that patients with major depression exhibit impaired HPA negative feedback in the context of elevated circulating levels of cortisol,2 a number of studies have described reduced GR function in depressed patients (GR resistance) and that antidepressants act by reversing these putative GR changes.5
Glucocorticoid receptors in depression
A number of studies have assessed GR in patients with major depression. In general, these studies have measured GR number directly or have examined the in vitro or in vivo influence of glucocorticoids on functions known to be regulated by the GR. Limited information exists regarding the number and function of GR in the central nervous system.
In general, studies have found a lack of changes in total GR expression but have also found a decreased GR in the cellular cytosolic fraction.5 These studies suggest that the GR changes seen in depression are likely secondary to nuclear compartmentalization of the GR (activation, hence the translocation to the nucleus and therefore the reduced GR level in the cytoplasm). Of course it remains speculative to what extent one can draw inferences of central corticosteroid receptor function from studies of peripheral GRs, like lymphocyte GRs, that may not accurately reflect GRs in the pituitary and brain.52 Recently, a post-mortem brain study has found a reduced frontal and hippocampal GR gene expression and a reduced frontal GR and MR gene expression not only in patients with major depression but also with patients with schizophrenia and bipolar disorder.53 These studies provide suggestive evidence that HPA axis may be abnormal in some patients with bipolar disorder and schizophrenia and support the view that HPA axis dysregulation may play a role in different psychiatric disorders.54 However, another brain post-mortem study by Lopez et al55 has found no differences in GR mRNA (but lower MR mRNA) in the hippocampus of six suicide victims with a history of depression compared to a group of six controls.
Chronic stress has also been associated with increased HPA axis function and altered GR function. This work has evaluated the impact of glucocorticoids on peripheral cell functions (immune function) known to be inhibited by GR activation, and, in particular, the well-known capacity of DEX to inhibit the ability of peripheral blood mononuclear cells to proliferate in response to polyclonal mitogens. For instance, it has been suggested that chronically elevated cortisol levels may produce a state of steroid resistance enabling lymphocytes to respond with less intensity to GCs. Recent work produced by our group56 revealed that chronic stress (i.e. caring for dementia patients) in humans was associated with significant elevations in cortisol levels and reduced lymphocyte sensitivity to GCs in vitro. These data suggest that chronic elevations in cortisol may underlie GR resistance in humans.
Studies exploring differences in GR function between depressives and controls have found far more consistent results. These studies have consistently found that lymphocytes from DEX nonsuppressor subjects were more resistant to the inhibitory effect of DEX administered in vitro.3,5 Moreover, the few studies that have investigated changes in GR sensitivity in vitro and in vivo in the same patients have found a remarkable consistency of response. In addition, Kok et al57 have shown that cortisol stimulates production of immunoglobulins (IgG and IgM) in vitro in healthy controls, while only IgG production was increased in depressed patients.
In most of these studies, lymphocytes from DST nonsuppressor depressed patients are more resistant to the inhibitory effect of DEX administered in vitro compared to DST suppressor depressed patients; moreover, there seems to be an inverse correlation between plasma cortisol concentration and the DEX-induced inhibition of the proliferative response, suggesting a link between hypercortisolemia and resistance to in vitro GR-mediated responses. After clinical recovery, hypercortisolemia tends to resolve and the sensitivity of lymphocytes to DEX returns to control levels. However, research from our laboratory has recently reported that acquired GR resistance can be demonstrated in treatment-resistant depressed (TRD) patients in the absence of elevated basal salivary cortisol levels. It was observed that glucocorticoid-induced suppression of T-cell proliferation and cytokine production in vitro is generally less marked in treatment-resistant depression (TRD) compared with healthy controls.58 In other study, it was observed that the impact of DEX administration in vivo on lymphocyte redistribution is greater for the control group than that seen for TRD patients.59 Overall, measures made of in vitro or in vivo lymphocyte function demonstrate that cells from TRD patients might be less sensitive to steroid. It is tempting, however, to speculate that drug resistance in this sample of patients may be related to steroid resistance. Although commonly grouped together, hypercortisolism and glucocorticoid resistance do not necessarily occur together and may represent distinct states of HPA axis dysfunction or at least different points along an evolution of HPA axis pathology.60-61
Interestingly, the findings of these studies have been recently confirmed by a study in vivo, showing that depressed subjects have a reduced vasoconstrictor response to topical application of beclomethasone when compared to healthy matched controls.62 This finding is suggestive of a defect in the sensitivity of peripheral GRs as the vasoconstrictor response to beclomethasone is mediated by these receptors and provides further support for the hypothesis of an abnormality of the GR in depression.50 Again, using the DST as a measure of HPA axis activation, no difference in dermal sensitivity to beclomethasone was found between patients with normal versus abnormal DST results. These findings suggest that peripheral GR function is abnormal in depression but that the reduced vasoconstrictor response to beclomethasone is not necessarily a secondary effect of hypercortisolaemia or HPA axis overactivity.62 Consistent with the presence of GR resistance in major depression, Maguire et al63 found that despite having higher plasma cortisol concentrations compared to controls, melancholic depressed patients exhibited no increase in plasma sialyltransferase levels. Sialytransferases are a family of enzymes that participate in oligosaccharide chain metabolism and are known to be stimulated by glucocorticoids via the GR. No changes in GR binding were found between groups. These findings suggest that impaired GR function, and not number, underlines the decreased sensitivity of plasma sialyltransferase levels to cortisol in depressed patients.
Although the above data provide strong evidence of glucocorticoid resistance in major depression, there is some data suggesting that glucocorticoid sensitivity in depressed patients remains intact in at least some body compartments. Specifically, depressed patients have been found to exhibit increased intra-abdominal fat deposition,64 which. Increased intra-abdominal fat deposition is seen in medical illnesses characterized by hypercortisolemia such as Cushing's syndrome and following chronic treatment with glucocorticoids. These findings suggest that intra-abdominal GRs may maintain their sensitivity to glucocorticoids, while other tissues/cell types are resistant. In support of this possibility, studies also have shown decreased bone mineral density in depressed patients,65,66 since elevated glucocorticoids have also been associated with bone loss.
Molecular mechanisms of GR resistance in depression
As previously discussed, the GR data do not provide a compelling case for GR downregulation secondary to hypercortisolism in major depression. Nevertheless, it is conceivable that hypercortisolism could overburden the recycling capacity of the GR with consequent diminished capability of the cell to respond to further stimulation. However, a second possibility is that GR function is altered in major depression via ligand-independent mechanisms.3,5 The concept of '"ligand-independent'" regulation of GR function derives from findings that steroid receptor function is regulated not only by steroid ligand binding, but also by signal transduction pathways driven by compounds unrelated to steroids.67 For example, research has demonstrated that GR function can be influenced by a myriad of non-steroid compounds including proinflammatory cytokines, such as interleukin-1,68-69 and participants in the cAMP cascade including protein kinase A (PKA).70 We will focus the remainder of this review on two mechanisms that have been investigated in our laboratory and that are also potential targets for antidepressant treatment: proinflammatory cytokines and steroid hormone transporters (see below). However, others factors not reviewed here could also be involved in acquired GR resistance in depression. Phosphorylation of the GR and/or other steroid receptor coactivators by cAMP-dependent protein kinase has a relevant role in the regulation of GR function. These findings are particularly intriguing in view of the fact that depressed patients had been found to exhibit reduced G protein function in mononuclear cells71 and reduced cAMP-dependent protein kinase activity in cultured fibroblasts.72 Therefore, it is possible that disruption in the cAMP/PKA pathway described in major depression is linked to GR resistance in this disorder and that antidepressants may overcome these receptor alterations via a direct effect on this pathway. Of note, it has been recently shown that a non-ligand binding b-isoform of the human GR (hGRb) may also be implicated in acquired steroid resistance. The hGRb heterodimerises with ligand-bound hGRa and translocates into the nucleus to act as a dominant negative inhibitor of the classic receptor. Therefore, a high expression of hGRb might participate in the development of acquired steroid resistance, whereas abnormally high expression of hGRa and low expression of hGRb might lead to glucocorticoid hypersensitivity state.73 It is possible that GRa/GRb ratio may be altered in DST nonsuppressors leading to acquired GR resistance. We cannot exclude the participation of changes in the GR transduction system (e.g. altered AP-1 and NF-kB expression, heat shock proteins) in promoting tissue sensitivity to glucocorticoids.74 In summary, various mechanisms may mediate "acquired steroid resistance" in depression (see Table 3), and we will explore some of these mechanisms in further details below.
Antidepressants and the glucocorticoid receptor
The hypothesis that antidepressants exert their clinical effects through direct modulation of the glucocorticoid receptor (GR) is one of the most striking and innovative models of the mechanism of action of this class of drugs.5,12,75-77 Specifically, studies in depressed patients, animals, and cellular models, have demonstrated that antidepressants increase GR expression, enhance GR function and promote GR nuclear translocation; this, in turn, is associated with enhanced GR-mediated negative feedback by endogenous glucocorticoids, and thus with reduced resting and stimulated HPA axis activity5 (see Table 4). These effects, in turn, can contribute to the therapeutic action of this class of drug (see Figure 3). However, the relationship between chemical structure, known pharmacological mechanisms and effects on the GR have yet to be clarified.
Work developed in our laboratory and elsewhere over the last few years has attempted to understand the mechanisms by which antidepressants regulate GR by examining this interaction in vitro. We have described in L929 cells (mouse fibroblasts) that incubation with the tricyclic antidepressant, desipramine, induces GR translocation from the cytoplasm to the nucleus in the absence of steroids.75,78 Moreover, we have found that coincubation of desipramine and DEX leads to enhanced GR-mediated gene transcription, while preincubation of desipramine followed by DEX leads to reduced GR-mediated gene transcription.75,78 This latter finding has been recently replicated by Budziszewska et al77 who also found that preincubation of L929 mouse fibroblast cells with various antidepressant (including desipramine) reduce GR-mediated gene transcription induced by a subsequent treatment with corticosterone or DEX.
Some of our most recent work suggests a possible role of membrane steroid transporters, like the multiple drug resistance p-glycoprotein (MDR PGP), in the regulation of GR function during antidepressant treatment and possibly - in major depression. Some GR ligands, like cortisol and DEX (but not corticosterone), are actively excreted from cells by the MDR PGP and other membrane transporters belonging to the ATP-binding cassette family of transporters.4,75,79 The MDR PGP has been extensively described to regulate intracellular concentrations of steroids, to secrete naturally occurring metabolites and toxic substances directly into the urinary or gastrointestinal tracts, and to confer treatment resistance to tumor cells by expelling anticancer agents.80-81 Moreover, the MDR PGP localized on the apical membrane of the endothelial cells of the blood-brain barrier has been described to limit the access of DEX and cortisol (but not corticosterone) to the human brain as well as human peripheral cells like lymphocytes.82-83 In vitro expression of the MDR PGP can induce GR resistance in a tymoma cell line,83 thus reproducing a condition similar to that described in lymphocytes of patients with major depression. Moreover, some antidepressants have been shown to inhibit the MDR p-glycoprotein in tumor cells84-86 and to be transported by the MDR p-glycoprotein.87 Based on this evidence, we hypothesized that one mechanism by which antidepressants regulate GR function in vitro (and theoretically, in vivo) is by regulating the function of MDR PGP, and therefore the intracellular access of glucocorticoids.
We recently explored this hypothesis by examining the effects of a range of antidepressants on GR function (GR-mediated gene transcription) in the presence of steroids that are differentially affected by the L929 membrane steroid transporter. Moreover, we assessed the ability of the inhibitor of the membrane steroid transporter, verapamil, to reverse the effects of antidepressants on GR function. Although it is still unclear whether the L929 cells membrane steroid transporter is identical to MDR p-glycoprotein,88,89 we and others have shown that they share the same substrate profile.75,89-90 Indeed, our findings are strongly suggestive that an antidepressant-induced inhibition (or downregulation) of the L929 membrane steroid transporter is relevant for the in vitro enhancement of GR function.75 In fact, we found that three different antidepressants (desipramine, clomipramine and paroxetine) all increase GR function in the presence of DEX and cortisol (that are expelled from the cells by the MDR PGP) but not with corticosterone (that is not expelled by this transporter). Moreover, clomipramine (the antidepressant that gives the strongest potentiation of GR-mediated gene transcription in the presence of Dex or cortisol) fails to have any effect in the presence of DEX, after blocking the steroid transporter with verapamil.
It is of note that our in vitro data are consistent with animal studies. For example, clomipramine at 10 mg/Kg/day for two days completely overcomes resistance to anticancer drugs of subcutaneous tumors in mice.85 Of note is that the dose used in this study is within the range (10-20 mg/Kg/day) used in most animal studies showing GR upregulation by tricyclic antidepressants.5 A recent paper by Uhr et al87 has described that amitriptyline, but not fluoxetine, is transported by the MDR-p-glycoprotein. Moreover, our data are also consistent with the study by Przegalinski et al91 showing that pretreatment of rat with nifedipine (another MDR p-glycoprotein inhibitor) prevents the hippocampal GR upregulation induced by chronic treatment with desipramine, amitriptyline or electroconvulsive shock. Furthermore, our hypothesis that modulation of the membrane steroid transporters is important for the effects of antidepressants on the GR is a potential explanation of how chemically and pharmacologically unrelated drugs may have similar effects on the GR. In fact, antidepressants, as other membrane steroid transporters inhibitors, seem to modulate MDR by interacting directly with the membrane phospholipids, an effect that is not receptor-mediated and is related to the drugs physiochemical properties, that is, lipophilicity and electric charge.79 Finally, our data are consistent with all in vivo evidence, in humans and animals, supporting the notion that antidepressant treatment increases GR function.5 Therefore, it seems plausible that the effects of antidepressants in vivo are mainly related to the effects on the membrane steroid transporters, leading to increased GR function, and we propose that membrane steroid transporters, especially those regulating access of glucocorticoids to the brain in vivo like the MDR p-glycoprotein, could be a fundamental target for antidepressant action.
In summary, the effects of glucocorticoids are mediated GR. Several studies have demonstrated that GR function is impaired in major depression, resulting in reduced GR-mediated negative feedback on the HPA axis and increased production and secretion of CRH in various brain regions postulated to be involved in the causality of depression. The concept that impaired GR signalling is a key mechanism in the pathogenesis of depression. The data indicate that antidepressants have direct effects on the GR, leading to enhanced GR function and increased GR expression.The mechanism of these receptor changes also involve non-steroid compounds, like cytokines and neurotransmitters. Moreover, evidence suggestsing that membrane steroid transporters like the MDR p-glycoprotein, could be fundamental target of antidepressant treatment. Research in this field is leading to new insights into the pathophysiology and treatment of affective disorders.
References
1. Checkley S. The neuroendocrinology of depression and chronic stress. Br Med Bull. 1996;52(3):597-617.
2. Nemeroff CB. The corticotropin-releasing factor (CRF hypothesis of depression: new findings and new directions. Mol Psychiatry. 1996;1(4):336.
3. Juruena MF, Cleare AJ, Bauer ME, Pariante CM. Molecular mechanism of GR sensitivity and relevance for affective disorders for special issue Acta Neuropsychiatrica. 2003;15(3):354-67.
4. de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19(3):269-301.
5. Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49(5):391-404.
6. Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestation of depression. Relation to the neurobiology of stress. N Engl J Med. 1988;319(7):;413-20. Erratum in: N Engl J Med. 1988;319921):1428.
7. Tsigos C, Chrousos GP. Hypothalamicpituitaryadrenal axis, neuroendocrine factors and stress. J Psychosom Res. 200253(4):865-71.
8. Kellner M, Yehuda R. Do panic disorder and posttraumatic stress disorder share a common psychoneuroendocrinology? Psychoneuroendocrinology. 1999;24(5):485-504.
9. Cleare AJ, Blair D, Chambers S, Wessely S. Urinary free cortisol in chronic fatigue syndrome. Am J Psychiatry. 2001;158(4):641-3.
10. Cleare AJ, Miell J, Heap E, Sookdeo S, Young L, Malhi GS, et al. Hypothalamo -pituitary-adrenal axis function in chronic fatigue syndrome, and the effects of low-dose hydrocortisone therapy. J Clin Endocrinol Metab. 2001;86(8):3545-54.
11. Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7(3):254-75.
12. Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23(5):477-501.
13. McQuade R, Young AH. Future therapeutic targets in mood disorders: the glucocorticoid receptor. Br J Psychiatry. 2000;177:390-5.
14. Owens MJ, Nemeroff CB. The ole of HLC in the pathophysiology of affective disorders. New York: John Wiley & Sons; 1993. (Laboratory and Clinical Studies, 172).
15. Holsboer F, Barden N. Antidepressants and hypothalamic-pituitaryadrenocortical regulation. Endocr Rev. 1996;17:187-205.
16. Axelson DA, Doraiswamy PM, Boyko OB, Rodrigo EP, McDonald WM, Ritchie JC, et al. In vivo assessment of pituitary volume with magnetic resonance imaging and systematic stereology: relationship to dexamethasone suppression test results in patients. Psychiatry Res. 1992;44(1):63-70.
17. Pariante CM, Vassilopoulou K, Velakoulis D, Phillips L, Soulsby B, Wood SJ, et al. Pituitary volume in psychosis. Br J Psychiatry. 2004;185:5-10.
18. Heuser IJ, Bissette G, Dettling M, Schweiger U, Gotthardt U, Schmider J, et al. Cerebrospinal fluid concentrations of corticotropin-releasing-hormone vasopressin, and somatostatin in depressed patients and healthy controls: response to amitriptyline treatment. Depress Anxiety. 1998;8(2):71-9.
19. Carroll J. Clinical applications of the dexamethasone suppression test for endogenous depression. Pharmacopsychiatria. 1982;15(1):19-24.
20. Vale W, Spiess I, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and betaendorphin. Science. 1981;213(4514):1394-7.
21. Gold PW, Loriaux DL, Roy A, Kling MA, Calabrese JR, Kellner CH, et al. Responses to corticotrophin-releasing hormone in the hyporcortisolism of depression and Cushing's disease. N Engl J Med.1986;314(21):1329-35.
22. Holsboer F, Gerken A, von Bardeleben U, Grimm W, Beyer H, Muller OA, Stalla GK. Human corticotropinreleasing hormone (HLC) in patients with depression, alcoholism and panic disorder. Biol Psychiatry. 1986;21(7):601-11.
23. von Bardeleben U, Holsboer F. Cortisol response to a combined dexamethasone- hHLC challenge in patients with depression. J Neuroendocrinol. 1989;1:485-8.
24. von Bardeleben U, Holsboer F. Effect of age upon the cortisol response to human corticotropin-releasing hormone in depressed patients pretreated with dexamethasone. Biol Psychiatry. 1991;29(10):1042-50.
25. Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: A refined laboratory test for psychiatric disorders. J Psychiatr Res. 1994;28(4):341-56.
26. Meijer OC, de Lange ECM, Breimer DD, de Boer AC, Workel JO, de Kloet ER. Penetration of dexamethasone into brain glucocorticoid targets is enhanced in mdrlAP-glycoprotein knockout mice. Endocrinology. 1998;139(4):1789-93.
27. von Bardeleben U, Holsboer F, Stalla GK, Muller OA. Combined administration of human corticotropin-releasing factor and lysine vasopressin induces cortisol escape from dexamethasone suppression in healthy subjects. Life Sci. 1985;37(17):1613-9.
28. Orth DN, Kovacs WJ. The adrenal cortex. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR, editors. Williams textbook of endocrinology, 9th ed. Philadelphia: W.B. Saunders 1998. p. 517-664.
29. Pariante CM, Papadopoulos AS, Poon L, Checkley SA, English J, Kerwin RW, et al. A novel prednisolone suppression test for the hypothalamic-pituitary-adrenal axis. Biol Psychiatry. 2002;51(11):922-30.
30. Karssen AM, Meijer OC, van der Sandt IC, Lucassen PJ, de Lange EC, de Boer AG, et al. Multidrug resistance P-glycoprotein hampers the access of cortisol but not of corticosterone to mouse and human brain. Endocrinology. 2001;142(6):2686-94.
31. Nemeroff CB, Widerlov E, Bissette C, Walleus H, Karlsson I, EkLund K, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226(4680):1342-4.
32. Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M. Reduced corticotropin- releasing factor (HLC) binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry. 1988;45(6):377-9.
33 Raadsheer FC, Hoogendijk WIG, Stam FC, Tilders FHJ, Swaab DF. Increased numbers of corticotropinreleasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436-44.
34. de Bellis MD, Gold PW, Geracioti TD Jr, Listwak SI, Kling MA. Association of fluoxetine treatment with reductions in CSF concentrations of corticotropinreleasing hormone and arginine vasopressin in patients with major depression. Am J Psychiatry. 1993;150(4):656-7.
35. Miller AH, Spencer RL, Pulera M, Kang S, McEwen BS, Stein M. Adrenal steroid receptor activation in rat brain and pituitary following dexamethasone: Implications for the dexamethasone suppression test. Biol Psychiatry. 1992;32(10):850-69.
36. Young EA, Haskett RF, Murphy.Weinberg V, Watson SI, Akil H. Loss of glucocorticoid fast feedback in depression. Arch Gen Psychiatry. 1991;48(8):693-9.
37. Glover V, O'Connor TG. Effects of antenatal stress and anxiety: Implications for development and psychiatry Br J Psychiatry. 2002;180:389-91.
38. Ribeiro SCM, Tandon R, Grunhaus L, Greden JF. The DST as a predictor of outcome in depression: a meta-analysis. Am J Psychiatry. 1993;150(11):1618-29.
39. Zobel AW, Yassouridis A, Frieboes RM, Holsboer F. Prediction of medium-term outcome by cortisol response to the combined dexamethasone-CRH test in patients with remitted depression. Am J Psychiatry. 1999;156(6):949-51.
40. Zobel AW, Nickel T, Sonntag A, Uhr M, Holsboer F, Ising M. Cortisol response in the combined dexamethasone/CRH test as predictor of relapse in patients with remitted depression a prospective study. J Psychiatr Res. 2001;35(2):83-94.
41. Sapolsky RM. Glucocorticoid toxicity in the hippocampus: reversal by supplementation with brain fuels. J Neurosci. 1986;6(8):2240-4.
42. McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30-47.
43. Sapolsky RM, Romero M, Munck AU. How do glucocorticoids influence stress responses? integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55-89.
44. Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160(9):1554-65.
45. McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886(1-2):172-89.
46. Spencer RL, Kim PJ, Kalman BA, Cole MA. Evidence for mineralocorticoid receptor facilitation of glucocorticoid receptor-dependent regulation of hypothalamic-pituitary-adrenal axis activity. Endocrinology. 1998;139(6):2718-26.
47. Pratt WB. The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor. J Biol Chem. 1993;268(29):21455-8.
48. Guiochon-Mantel A, Delabre K, Lescop P, Milgrom E. The Ernst Schering Poster Award. Intracellular traffic of steroid hormone receptors. J Steroid Biochem Mol Biol. 1996;56(1-6):3-9.
49. Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117(6):2505-11.
50. Modell S, Yassouridis A, Huber I, Holsboer F. Corticosteroid receptor function is decreased in depressed patients. Neuroendocrinology. 1997;65(3):216-22.
51. Murphy BEP. Treatment of major depression with steroid suppressive drugs. J Steroid Biochem Mol Biol. 1991;39(92):239-44.
52. Gametchu B. Glucocorticoid receptor-like antigen in lymphoma cell membranes: correlation to cell lysis. Science. 1987;236(4800):456-61.
53. Webster MJ, O'Grady J, Orthmann C, Weickert C. Decreased glucocorticoid receptor mRNA levels in individuals with depression, bipolar disorder and schizophrenia [abstract] Schizophr Res. 2000;41(1):111.[Presented 10th Biennial Winter Workshop on Schizophrenia. Davos, Switzerland, February 5-11, 2000].
54. Cotter D, Pariante CM. Stress on the progress of the developmental hypothesis of schizophrenia? Br J Psychiatry. 2002;181:363-5.
55. Lopez JF, Chalmers DT, Little KI, Watson SJ. E. Bennett Research Award. Regulation of serotonin 1A, glucocorticoid and mineralocorticoid receptor in rat and human hippocampus: Implications for the neurobiology of depression. Biol Psychiatry. 1998;43(8):547-73.
56. Bauer M, Vedhara K, Perks P, Wilcock G, Lightman S, Shanks N. Chronic stress in caregivers of dementia patients is associated with reduced lymphocyte sensitivity to glucocorticoids. J Neuroimmunol. 2000;103(1):84-92.
57. Kok F, Heijnen C, Bruijn J, Westenberg H, Van Ree J. Immunoglobulin production in vitro in major depression: a pilot study on the modulating action of endogenous cortisol. Biol Psychiatry. 1995;38(4):217-26.
58. Bauer ME, Papadopoulus A, Poon L, Perks P, Lightman SL, Checkley S, et al. Altered glucocorticoid immunoregulation in treatment resistant depression. Psychoneuroendocrinology. 2003 ;28(1):49-65.
59. Bauer M, Papadopoulos A, Poon L, Perks P, Lightman S, Checkley S, Shanks N. Dexamethasone-induced effects on lymphocyte distribution and expression of adhesion molecules in treatment resistant major depression. Psychiatry Res. 2002;113(1-2):1-15.
60. Asnis GM, Haibreich U, Ryan ND, Rabinowicz H, Puig-Antich J, Nelson B, et al. The relationship of the dexamethasone suppression test (1 mg and 2 mg) to basal plasma cortisol levels in endogenous depression. Psychoneuroendocrinology. 1987;12(4):295-301.
61. Miller AH, Sastry G, Speranza Jr AJ, Lawlor BA, Mohs RC, Ryan TM, et al. Lack of association between cortisol hypersecretion and nonsuppression on the DST in patients with Alzheimer's disease. Am J Psychiatry. 1994;151(2):267-70.
62. Cotter P, Mulligan O, Landau S, Papadopoulos A, Lightman S, Checkley S. Vasoconstrictor response to topical beclomethasone in major depression. Psychoneuroendocrinology. 2002;27(4):475-87.
63. Maguire TM, Thakore J, Dinan TG, Hopwood S, Breen KC. Plasma sialyltransferase levels in psychiatric disorders as a possible indicator of HPA axis function. Biol Psychiatry. 1997;41(11):1131-6.
64. Thakore JH, Richards PJ, Reznek RH, Martin A, Dinan TG. Increased intra-abdominal fat deposition in patients with major depression illness as measured by computed tomography. Biol Psychiatry. 1997;41(11):1140-2.
65. Schweiger U, Deuschle M, Korner A, Lammers CH, Schmider J, Gotthard U, et al. Low lumbar bone mineral density in patients with major depression. Am J Psychiatry. 1994;151(11):1691-3.
66. Michelson D, Stratakis C, Hill L, Reynolds J, Galliven E, Chrousos G, Gold P. Bone mineral density in women with depression. N Engl J Med.1996;335(16):1176-81.
67. O'Malley BW, Schrader WT, Mani S, Smith C, Weigel NL, Conneely OM, et al. An alternative ligand independent pathway for activation of steroid receptors. Recent Prog Horm Res. 1995;50:333-47.
68. Miller AH, Pariante CM, Pearce BD. Effects of cytokines on glucocorticoid receptor expression and function: Glucocorticoid resistance and relevance to depression. Adv Exp Med Biol. 1999;461:107-16.
69 Pariante CM, Pearce BD, Pisell TL, Sanchez CI, Po C, Su C, Miller AH. The proinflammatory cytokine, interleukin-1 alpha, reduces glucocorticoid receptor translocation and function. Endocrinology. 1999;140(9):4359-66.
70. Rangarajan PN, Umesono K, Evans RM. Modulation of glucocorticoid receptor function by protein kinase A Mol Endocrinol. 1992;6(9):1451-7.
71. Avissar S, Nechamkin Y, Roitman G, Schreiber G. Reduced G protein functions and immunoreactive levels in mononuclear leukocyts of patients with depression. Am J Psychiatry. 1997;154(2):211-7.
72. Shelton RC, Mainer DH, Sulser F. cAMP-dependent protein kinase activity in major depression. Am J Psychiatry. 1996;153(8):1037-42.
73. Castro M, Elliot S, Kino T, Bamberger C, Karl M, Webster E, Chrousos G. The non-ligand binding beta-isoform of the human glucocorticoid receptor (hGC-beta): tissue levels, mechanism of action, and potential physiologic role. Mol Med. 1996;2(5):597-607.
74. Bronnegard M, Stierna P, Marcus C. Glucocorticoid resistant syndromes - molecular basis and clinical presentations. J Neuroendocrinol. 1996;8(6):405-15.
75. Pariante CM, Makoff A, Lovestone S, Feroli S, Heyden A, Miller AH, Kerwin RW. Antidepressants enhance glucocorticoid receptor function in vitro by modulating the membrane steroid transporters. Br J Pharmacol. 2001;13496):1335-43.
76. Barden N. Regulation of corticosteroid receptor gene expression in depression and antidepressant action. J Psychiatry Neurosci. 1999;24(1):25-39.
77. Budziszewska B, Jaworska-Feil L, Kajta M, Lason W. Antidepressant drugs inhibit glucocorticoid receptor mediated gene transcription-a possible mechanism. Br J Pharmacol. 2000;130(6):1385-93.
78. Pariante CM, Pearce BD, Pisell TL, Owens MJ, Miller AH. Steroid-independent translocation of the glucocorticoid receptor by the antidepressant desipramine. Mol Pharmacol. 1997;52(4):571-81.
79. Pariante CM, Pearce BD, Pisell TL, Su C, Miller AH. The steroid receptor antagonists, RU486 and RU40555, activate glucocorticoid receptor translocation and are not excreted by the steroid hormone transporter in L929 cells. J Endocrinol. 2001;169(2):309-20.
80. Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci. 2000;11(4):265-83.
81. Ueda K, Okamura N, Hirai M, Tanigawara Y, Saeki T, Kioka N, / et al. Human P-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. J Biol Chem. 1992;267(34):24248-52.
82. de Kloet ER, Meijer OC, Vreugdenhil E, Joels M.The Yin and Yang of nuclear receptors: symposium on nuclear receptors in brain, Oegstgeest, The Netherlands, 13-14 April 2000. Trends Endocrinol Metab. 2000;11(6):245-8.
83. Bourgeois S, Gruol DJ, Newby RF, Rajah FM. Expression of an mdr gene is associated with a new form of resistance to dexamethasone-induced apoptosis. Mol Endocrinol. 1993;7(5):840-51.
84. Varga A, Nugel H, Baehr R, Marx U, Hever A, Nacsa J, Ocsovsky I, Molnar J. Reversal of multidrug resistance by amitriptyline in vitro. Anticancer Res. 1996;16(1):209-12.
85. Merry S, Hamilton TG, Flanigan P, Freshney RI, Kaye SB. Circumvention of pleiotropic drug resistance in subcutaneous tumours in vivo with verapamil and clomipramine. Eur J Cancer. 1991; 27(1):31-4.
86. Szabó D, Szabó Jr G, Ocsovszki I, Aszalos A, Molnár J. Anti-psychotic drugs reverse multidrug resistance of tumor cell lines and human AML cell ex-vivo. Cancer Lett. 1999;1399(1):115-9.
87. Uhr M, Steckler T, Yassouridis A, Holsboer F. Penetration of amitriptyline, but not fluoxetine, into brain is enhanced in mice with blood-brain barrier deficiency due to MDR1a p-glycoprotein gene disruption. Neuropsychopharmacology. 2000;22(4):380-7.
88. Kralli A, Yamamoto KR. An FK506-sensitive transporter selectively decreases intracellular levels and potency of steroid hormones. J Biol Chem. 1996;271(29):17152-6.
89. Marsaud V, Mercier-Bodard C, Fortin D, Le Bihan S, Renoir JM. Dexamethasone and triamcinolone acetonide accumulation in mouse fibroblasts is differently modulated by the immunosuppressants cyclosporin A, FK506, rapamycin and their analogues, as well as by other p-glycoprotein ligands. J Steroid Biochem Molec Biol. 1998;66(1-2):11-25.
90. Medh RD, Lay RH, Schmidt TJ. Agonist-speciffic modulation of glucocorticoid receptor-mediated transcription by immunosuppressants. Mol Cell Endocrinol. 1998;138(1-2):11-23.
91. Przegalinski E, Budziszewska B, Siwanowicz J, Jaworska L. The effect of repeated combined treatment with nifedipine and antidepressant drugs or electroconvulsive shock on the hippocampal corticosteroid receptors in rats. Neuropharmacology. 1993;32(12):1397-400.
92. Pariante CM, Thomas SA, Lovestone S, Makoff A, Kerwin RW. Do antidepressants regulate how cortisol affects the brain? Psychoneuroendocrinology. 2004;29(4):423-47.
93. Pariante CM, Kim RB, Makoff A, Kerwin RW. Antidepressant fluoxetine enhances glucocorticoid receptor function in vitro by modulating membrane steroid transporters. Br J Pharmacol. 2003;139(6):1111-8.
94. Pariante CM, Hye A, Williamson R, Makoff A, Lovestone S, Kerwin RW. The antidepressant clomipramine regulates cortisol intracellular concentrations and glucocorticoid receptor expression in fibroblasts and rat primary neurones. Neuropsychopharmacology. 2003;28(9):1553-61.
95. Lowy M, Gormley G, Reder A, Meltzer H. Immune function, glucocorticoid receptor regu lation, and depression. In: Miller, A. (Ed.), Depressive Disorders & Immunity. American Psychiatric Press, Washington, DC, 1989, pp. 107133.
96. Moalli P & Rosen S. Glucocorticoid receptors and resistance to glucocorticoids in hematologia malignancies. Leuk Lymph 1994; 15: 363-374.
97. Cypcar D & Busse W. Steroid-resistant asthma. J Allergy Clin Immunol 1993; 92: 362-372.
98. Hennebold J & Daynes R. Microenvironmental control of glucocorticoid functions in immune regulation. In: Rook G & Lightman S. Steroid hormones and the T-cell cytokine profile. London: Springer-Verlag. 1997, pp. 101-134.
99. Rosner W. Plasma steroid-binding proteins. Endocr Metab Clin North Amer 1991; 20: 697-720.
100. Adcock I, Lane S, Brown C, Peters M, Lee T, Barnes P. Differences in binding of glucocorticoid receptor to DNA in steroid-resistant asthma. J Immunol 1995; 154; 3500-3505.
Sponsoring: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES (grant 1517023) and National Alliance for Research on Schizophrenia and Depression - NARSAD.
Received in 06/06/2004
Accepted in 06/30/2004
Original version accepted in English
This article has received corrections in agreement with the ERRATUM published in Volume 26 Number 4.
- 1. Checkley S. The neuroendocrinology of depression and chronic stress. Br Med Bull. 1996;52(3):597-617.
- 2. Nemeroff CB. The corticotropin-releasing factor (CRF hypothesis of depression: new findings and new directions. Mol Psychiatry. 1996;1(4):336.
- 3. Juruena MF, Cleare AJ, Bauer ME, Pariante CM. Molecular mechanism of GR sensitivity and relevance for affective disorders for special issue Acta Neuropsychiatrica. 2003;15(3):354-67.
- 4. de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19(3):269-301.
- 5. Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49(5):391-404.
- 6. Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestation of depression. Relation to the neurobiology of stress. N Engl J Med. 1988;319(7):;413-20.
- 7. Tsigos C, Chrousos GP. Hypothalamicpituitaryadrenal axis, neuroendocrine factors and stress. J Psychosom Res. 200253(4):865-71.
- 8. Kellner M, Yehuda R. Do panic disorder and posttraumatic stress disorder share a common psychoneuroendocrinology? Psychoneuroendocrinology. 1999;24(5):485-504.
- 9. Cleare AJ, Blair D, Chambers S, Wessely S. Urinary free cortisol in chronic fatigue syndrome. Am J Psychiatry. 2001;158(4):641-3.
- 10. Cleare AJ, Miell J, Heap E, Sookdeo S, Young L, Malhi GS, et al. Hypothalamo -pituitary-adrenal axis function in chronic fatigue syndrome, and the effects of low-dose hydrocortisone therapy. J Clin Endocrinol Metab. 2001;86(8):3545-54.
- 11. Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7(3):254-75.
- 12. Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23(5):477-501.
- 13. McQuade R, Young AH. Future therapeutic targets in mood disorders: the glucocorticoid receptor. Br J Psychiatry. 2000;177:390-5.
- 14. Owens MJ, Nemeroff CB. The ole of HLC in the pathophysiology of affective disorders. New York: John Wiley & Sons; 1993. (Laboratory and Clinical Studies, 172).
- 15. Holsboer F, Barden N. Antidepressants and hypothalamic-pituitaryadrenocortical regulation. Endocr Rev. 1996;17:187-205.
- 16. Axelson DA, Doraiswamy PM, Boyko OB, Rodrigo EP, McDonald WM, Ritchie JC, et al. In vivo assessment of pituitary volume with magnetic resonance imaging and systematic stereology: relationship to dexamethasone suppression test results in patients. Psychiatry Res. 1992;44(1):63-70.
- 17. Pariante CM, Vassilopoulou K, Velakoulis D, Phillips L, Soulsby B, Wood SJ, et al. Pituitary volume in psychosis. Br J Psychiatry. 2004;185:5-10.
- 18. Heuser IJ, Bissette G, Dettling M, Schweiger U, Gotthardt U, Schmider J, et al. Cerebrospinal fluid concentrations of corticotropin-releasing-hormone vasopressin, and somatostatin in depressed patients and healthy controls: response to amitriptyline treatment. Depress Anxiety. 1998;8(2):71-9.
- 19. Carroll J. Clinical applications of the dexamethasone suppression test for endogenous depression. Pharmacopsychiatria. 1982;15(1):19-24.
- 20. Vale W, Spiess I, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and betaendorphin. Science. 1981;213(4514):1394-7.
- 21. Gold PW, Loriaux DL, Roy A, Kling MA, Calabrese JR, Kellner CH, et al. Responses to corticotrophin-releasing hormone in the hyporcortisolism of depression and Cushing's disease. N Engl J Med.1986;314(21):1329-35.
- 22. Holsboer F, Gerken A, von Bardeleben U, Grimm W, Beyer H, Muller OA, Stalla GK. Human corticotropinreleasing hormone (HLC) in patients with depression, alcoholism and panic disorder. Biol Psychiatry. 1986;21(7):601-11.
- 23. von Bardeleben U, Holsboer F. Cortisol response to a combined dexamethasone- hHLC challenge in patients with depression. J Neuroendocrinol. 1989;1:485-8.
- 24. von Bardeleben U, Holsboer F. Effect of age upon the cortisol response to human corticotropin-releasing hormone in depressed patients pretreated with dexamethasone. Biol Psychiatry. 1991;29(10):1042-50.
- 25. Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: A refined laboratory test for psychiatric disorders. J Psychiatr Res. 1994;28(4):341-56.
- 26. Meijer OC, de Lange ECM, Breimer DD, de Boer AC, Workel JO, de Kloet ER. Penetration of dexamethasone into brain glucocorticoid targets is enhanced in mdrlAP-glycoprotein knockout mice. Endocrinology. 1998;139(4):1789-93.
- 27. von Bardeleben U, Holsboer F, Stalla GK, Muller OA. Combined administration of human corticotropin-releasing factor and lysine vasopressin induces cortisol escape from dexamethasone suppression in healthy subjects. Life Sci. 1985;37(17):1613-9.
- 28. Orth DN, Kovacs WJ. The adrenal cortex. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR, editors. Williams textbook of endocrinology, 9th ed. Philadelphia: W.B. Saunders 1998. p. 517-664.
- 29. Pariante CM, Papadopoulos AS, Poon L, Checkley SA, English J, Kerwin RW, et al. A novel prednisolone suppression test for the hypothalamic-pituitary-adrenal axis. Biol Psychiatry. 2002;51(11):922-30.
- 30. Karssen AM, Meijer OC, van der Sandt IC, Lucassen PJ, de Lange EC, de Boer AG, et al. Multidrug resistance P-glycoprotein hampers the access of cortisol but not of corticosterone to mouse and human brain. Endocrinology. 2001;142(6):2686-94.
- 31. Nemeroff CB, Widerlov E, Bissette C, Walleus H, Karlsson I, EkLund K, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226(4680):1342-4.
- 32. Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M. Reduced corticotropin- releasing factor (HLC) binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry. 1988;45(6):377-9.
- 33 Raadsheer FC, Hoogendijk WIG, Stam FC, Tilders FHJ, Swaab DF. Increased numbers of corticotropinreleasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436-44.
- 34. de Bellis MD, Gold PW, Geracioti TD Jr, Listwak SI, Kling MA. Association of fluoxetine treatment with reductions in CSF concentrations of corticotropinreleasing hormone and arginine vasopressin in patients with major depression. Am J Psychiatry. 1993;150(4):656-7.
- 35. Miller AH, Spencer RL, Pulera M, Kang S, McEwen BS, Stein M. Adrenal steroid receptor activation in rat brain and pituitary following dexamethasone: Implications for the dexamethasone suppression test. Biol Psychiatry. 1992;32(10):850-69.
- 36. Young EA, Haskett RF, Murphy.Weinberg V, Watson SI, Akil H. Loss of glucocorticoid fast feedback in depression. Arch Gen Psychiatry. 1991;48(8):693-9.
- 37. Glover V, O'Connor TG. Effects of antenatal stress and anxiety: Implications for development and psychiatry Br J Psychiatry. 2002;180:389-91.
- 38. Ribeiro SCM, Tandon R, Grunhaus L, Greden JF. The DST as a predictor of outcome in depression: a meta-analysis. Am J Psychiatry. 1993;150(11):1618-29.
- 39. Zobel AW, Yassouridis A, Frieboes RM, Holsboer F. Prediction of medium-term outcome by cortisol response to the combined dexamethasone-CRH test in patients with remitted depression. Am J Psychiatry. 1999;156(6):949-51.
- 40. Zobel AW, Nickel T, Sonntag A, Uhr M, Holsboer F, Ising M. Cortisol response in the combined dexamethasone/CRH test as predictor of relapse in patients with remitted depression a prospective study. J Psychiatr Res. 2001;35(2):83-94.
- 41. Sapolsky RM. Glucocorticoid toxicity in the hippocampus: reversal by supplementation with brain fuels. J Neurosci. 1986;6(8):2240-4.
- 42. McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30-47.
- 43. Sapolsky RM, Romero M, Munck AU. How do glucocorticoids influence stress responses? integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55-89.
- 44. Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160(9):1554-65.
- 45. McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886(1-2):172-89.
- 46. Spencer RL, Kim PJ, Kalman BA, Cole MA. Evidence for mineralocorticoid receptor facilitation of glucocorticoid receptor-dependent regulation of hypothalamic-pituitary-adrenal axis activity. Endocrinology. 1998;139(6):2718-26.
- 47. Pratt WB. The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor. J Biol Chem. 1993;268(29):21455-8.
- 48. Guiochon-Mantel A, Delabre K, Lescop P, Milgrom E. The Ernst Schering Poster Award. Intracellular traffic of steroid hormone receptors. J Steroid Biochem Mol Biol. 1996;56(1-6):3-9.
- 49. Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117(6):2505-11.
- 50. Modell S, Yassouridis A, Huber I, Holsboer F. Corticosteroid receptor function is decreased in depressed patients. Neuroendocrinology. 1997;65(3):216-22.
- 51. Murphy BEP. Treatment of major depression with steroid suppressive drugs. J Steroid Biochem Mol Biol. 1991;39(92):239-44.
- 52. Gametchu B. Glucocorticoid receptor-like antigen in lymphoma cell membranes: correlation to cell lysis. Science. 1987;236(4800):456-61.
- 54. Cotter D, Pariante CM. Stress on the progress of the developmental hypothesis of schizophrenia? Br J Psychiatry. 2002;181:363-5.
- 55. Lopez JF, Chalmers DT, Little KI, Watson SJ. E. Bennett Research Award. Regulation of serotonin 1A, glucocorticoid and mineralocorticoid receptor in rat and human hippocampus: Implications for the neurobiology of depression. Biol Psychiatry. 1998;43(8):547-73.
- 56. Bauer M, Vedhara K, Perks P, Wilcock G, Lightman S, Shanks N. Chronic stress in caregivers of dementia patients is associated with reduced lymphocyte sensitivity to glucocorticoids. J Neuroimmunol. 2000;103(1):84-92.
- 57. Kok F, Heijnen C, Bruijn J, Westenberg H, Van Ree J. Immunoglobulin production in vitro in major depression: a pilot study on the modulating action of endogenous cortisol. Biol Psychiatry. 1995;38(4):217-26.
- 58. Bauer ME, Papadopoulus A, Poon L, Perks P, Lightman SL, Checkley S, et al. Altered glucocorticoid immunoregulation in treatment resistant depression. Psychoneuroendocrinology. 2003 ;28(1):49-65.
- 59. Bauer M, Papadopoulos A, Poon L, Perks P, Lightman S, Checkley S, Shanks N. Dexamethasone-induced effects on lymphocyte distribution and expression of adhesion molecules in treatment resistant major depression. Psychiatry Res. 2002;113(1-2):1-15.
- 60. Asnis GM, Haibreich U, Ryan ND, Rabinowicz H, Puig-Antich J, Nelson B, et al. The relationship of the dexamethasone suppression test (1 mg and 2 mg) to basal plasma cortisol levels in endogenous depression. Psychoneuroendocrinology. 1987;12(4):295-301.
- 61. Miller AH, Sastry G, Speranza Jr AJ, Lawlor BA, Mohs RC, Ryan TM, et al. Lack of association between cortisol hypersecretion and nonsuppression on the DST in patients with Alzheimer's disease. Am J Psychiatry. 1994;151(2):267-70.
- 62. Cotter P, Mulligan O, Landau S, Papadopoulos A, Lightman S, Checkley S. Vasoconstrictor response to topical beclomethasone in major depression. Psychoneuroendocrinology. 2002;27(4):475-87.
- 63. Maguire TM, Thakore J, Dinan TG, Hopwood S, Breen KC. Plasma sialyltransferase levels in psychiatric disorders as a possible indicator of HPA axis function. Biol Psychiatry. 1997;41(11):1131-6.
- 64. Thakore JH, Richards PJ, Reznek RH, Martin A, Dinan TG. Increased intra-abdominal fat deposition in patients with major depression illness as measured by computed tomography. Biol Psychiatry. 1997;41(11):1140-2.
- 65. Schweiger U, Deuschle M, Korner A, Lammers CH, Schmider J, Gotthard U, et al. Low lumbar bone mineral density in patients with major depression. Am J Psychiatry. 1994;151(11):1691-3.
- 66. Michelson D, Stratakis C, Hill L, Reynolds J, Galliven E, Chrousos G, Gold P. Bone mineral density in women with depression. N Engl J Med.1996;335(16):1176-81.
- 67. O'Malley BW, Schrader WT, Mani S, Smith C, Weigel NL, Conneely OM, et al. An alternative ligand independent pathway for activation of steroid receptors. Recent Prog Horm Res. 1995;50:333-47.
- 68. Miller AH, Pariante CM, Pearce BD. Effects of cytokines on glucocorticoid receptor expression and function: Glucocorticoid resistance and relevance to depression. Adv Exp Med Biol. 1999;461:107-16.
- 69 Pariante CM, Pearce BD, Pisell TL, Sanchez CI, Po C, Su C, Miller AH. The proinflammatory cytokine, interleukin-1 alpha, reduces glucocorticoid receptor translocation and function. Endocrinology. 1999;140(9):4359-66.
- 70. Rangarajan PN, Umesono K, Evans RM. Modulation of glucocorticoid receptor function by protein kinase A Mol Endocrinol. 1992;6(9):1451-7.
- 71. Avissar S, Nechamkin Y, Roitman G, Schreiber G. Reduced G protein functions and immunoreactive levels in mononuclear leukocyts of patients with depression. Am J Psychiatry. 1997;154(2):211-7.
- 72. Shelton RC, Mainer DH, Sulser F. cAMP-dependent protein kinase activity in major depression. Am J Psychiatry. 1996;153(8):1037-42.
- 73. Castro M, Elliot S, Kino T, Bamberger C, Karl M, Webster E, Chrousos G. The non-ligand binding beta-isoform of the human glucocorticoid receptor (hGC-beta): tissue levels, mechanism of action, and potential physiologic role. Mol Med. 1996;2(5):597-607.
- 74. Bronnegard M, Stierna P, Marcus C. Glucocorticoid resistant syndromes - molecular basis and clinical presentations. J Neuroendocrinol. 1996;8(6):405-15.
- 75. Pariante CM, Makoff A, Lovestone S, Feroli S, Heyden A, Miller AH, Kerwin RW. Antidepressants enhance glucocorticoid receptor function in vitro by modulating the membrane steroid transporters. Br J Pharmacol. 2001;13496):1335-43.
- 76. Barden N. Regulation of corticosteroid receptor gene expression in depression and antidepressant action. J Psychiatry Neurosci. 1999;24(1):25-39.
- 78. Pariante CM, Pearce BD, Pisell TL, Owens MJ, Miller AH. Steroid-independent translocation of the glucocorticoid receptor by the antidepressant desipramine. Mol Pharmacol. 1997;52(4):571-81.
- 79. Pariante CM, Pearce BD, Pisell TL, Su C, Miller AH. The steroid receptor antagonists, RU486 and RU40555, activate glucocorticoid receptor translocation and are not excreted by the steroid hormone transporter in L929 cells. J Endocrinol. 2001;169(2):309-20.
- 80. Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci. 2000;11(4):265-83.
- 81. Ueda K, Okamura N, Hirai M, Tanigawara Y, Saeki T, Kioka N, / et al. Human P-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. J Biol Chem. 1992;267(34):24248-52.
- 82. de Kloet ER, Meijer OC, Vreugdenhil E, Joels M.The Yin and Yang of nuclear receptors: symposium on nuclear receptors in brain, Oegstgeest, The Netherlands, 13-14 April 2000. Trends Endocrinol Metab. 2000;11(6):245-8.
- 83. Bourgeois S, Gruol DJ, Newby RF, Rajah FM. Expression of an mdr gene is associated with a new form of resistance to dexamethasone-induced apoptosis. Mol Endocrinol. 1993;7(5):840-51.
- 84. Varga A, Nugel H, Baehr R, Marx U, Hever A, Nacsa J, Ocsovsky I, Molnar J. Reversal of multidrug resistance by amitriptyline in vitro. Anticancer Res. 1996;16(1):209-12.
- 85. Merry S, Hamilton TG, Flanigan P, Freshney RI, Kaye SB. Circumvention of pleiotropic drug resistance in subcutaneous tumours in vivo with verapamil and clomipramine. Eur J Cancer. 1991; 27(1):31-4.
- 86. Szabó D, Szabó Jr G, Ocsovszki I, Aszalos A, Molnár J. Anti-psychotic drugs reverse multidrug resistance of tumor cell lines and human AML cell ex-vivo. Cancer Lett. 1999;1399(1):115-9.
- 88. Kralli A, Yamamoto KR. An FK506-sensitive transporter selectively decreases intracellular levels and potency of steroid hormones. J Biol Chem. 1996;271(29):17152-6.
- 89. Marsaud V, Mercier-Bodard C, Fortin D, Le Bihan S, Renoir JM. Dexamethasone and triamcinolone acetonide accumulation in mouse fibroblasts is differently modulated by the immunosuppressants cyclosporin A, FK506, rapamycin and their analogues, as well as by other p-glycoprotein ligands. J Steroid Biochem Molec Biol. 1998;66(1-2):11-25.
- 90. Medh RD, Lay RH, Schmidt TJ. Agonist-speciffic modulation of glucocorticoid receptor-mediated transcription by immunosuppressants. Mol Cell Endocrinol. 1998;138(1-2):11-23.
- 91. Przegalinski E, Budziszewska B, Siwanowicz J, Jaworska L. The effect of repeated combined treatment with nifedipine and antidepressant drugs or electroconvulsive shock on the hippocampal corticosteroid receptors in rats. Neuropharmacology. 1993;32(12):1397-400.
- 92. Pariante CM, Thomas SA, Lovestone S, Makoff A, Kerwin RW. Do antidepressants regulate how cortisol affects the brain? Psychoneuroendocrinology. 2004;29(4):423-47.
- 93. Pariante CM, Kim RB, Makoff A, Kerwin RW. Antidepressant fluoxetine enhances glucocorticoid receptor function in vitro by modulating membrane steroid transporters. Br J Pharmacol. 2003;139(6):1111-8.
- 94. Pariante CM, Hye A, Williamson R, Makoff A, Lovestone S, Kerwin RW. The antidepressant clomipramine regulates cortisol intracellular concentrations and glucocorticoid receptor expression in fibroblasts and rat primary neurones. Neuropsychopharmacology. 2003;28(9):1553-61.
- 95. Lowy M, Gormley G, Reder A, Meltzer H. Immune function, glucocorticoid receptor regu lation, and depression. In: Miller, A. (Ed.), Depressive Disorders & Immunity. American Psychiatric Press, Washington, DC, 1989, pp. 107133.
- 96. Moalli P & Rosen S. Glucocorticoid receptors and resistance to glucocorticoids in hematologia malignancies. Leuk Lymph 1994; 15: 363-374.
- 97. Cypcar D & Busse W. Steroid-resistant asthma. J Allergy Clin Immunol 1993; 92: 362-372.
- 98. Hennebold J & Daynes R. Microenvironmental control of glucocorticoid functions in immune regulation. In: Rook G & Lightman S. Steroid hormones and the T-cell cytokine profile. London: Springer-Verlag. 1997, pp. 101-134.
- 99. Rosner W. Plasma steroid-binding proteins. Endocr Metab Clin North Amer 1991; 20: 697-720.
- 100. Adcock I, Lane S, Brown C, Peters M, Lee T, Barnes P. Differences in binding of glucocorticoid receptor to DNA in steroid-resistant asthma. J Immunol 1995; 154; 3500-3505.
Publication Dates
-
Publication in this collection
23 Feb 2005 -
Date of issue
Sept 2004
History
-
Accepted
30 June 2004 -
Received
06 June 2004