Abstract
Objective:
To evaluate the effects of Hypericum perforatum (hypericum) on cognitive behavior and neurotrophic factor levels in the brain of male and female rats.
Methods:
Male and female Wistar rats were treated with hypericum or water during 28 days by gavage. The animals were then subjected to the open-field test, novel object recognition and step-down inhibitory avoidance test. Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and glial cell-line derived neurotrophic factor (GDNF) levels were evaluated in the hippocampus and frontal cortex.
Results:
Hypericum impaired the acquisition of short- and long-term aversive memory in male rats, evaluated in the inhibitory avoidance test. Female rats had no immediate memory acquisition and decreased short-term memory acquisition in the inhibitory avoidance test. Hypericum also decreased the recognition index of male rats in the object recognition test. Female rats did not recognize the new object in either the short-term or the long-term memory tasks. Hypericum decreased BDNF in the hippocampus of male and female rats. Hypericum also decreased NGF in the hippocampus of female rats.
Conclusions:
The long-term administration of hypericum appears to cause significant cognitive impairment in rats, possibly through a reduction in the levels of neurotrophic factors. This effect was more expressive in females than in males.
Hypericum perforatum; avoidance memory; recognition memory; BDNF; NGF; GDNF
Introduction
Hypericum perforatum (hypericum), commonly known as St. John’s Wort, is a traditional medicinal plant possessing antidepressant activity.11. Marrelli M, Statti G, Conforti F, Menichini F. New potential pharmaceutical applications of hypericum species. Mini Rev Med Chem. 2016;16:710-20. A systematic review has shown that the effects of hypericum are similar to those of antidepressants in the treatment of major depressive episodes. In addition, hypericum had fewer side effects than conventional antidepressants.22. Linde K, Berner MM, Kriston L. St John's wort for major depression. Cochrane Database Syst Rev. 2008;4:CD000448. In a study by Linde et al.,22. Linde K, Berner MM, Kriston L. St John's wort for major depression. Cochrane Database Syst Rev. 2008;4:CD000448. the most frequently reported side effects or adverse events were gastrointestinal symptoms, increased sensitivity to light, and skin problems. It is important to emphasize that the most important risk associated with hypericum extracts is the potential for interactions with other drugs.33. Forsdike K, Pirotta M. St John's wort for depression: scoping review about perceptions and use by general practitioners in clinical practice. J Pharm Pharmacol. 2017 Jun 27. doi: 10.1111/jphp.12775. [Epub ahead of print]
10.1111/jphp.12775...
Some studies have demonstrated that the antidepressant mechanisms of hypericum are linked to the inhibition of serotonin reuptake.44. Apaydin EA, Maher AR, Shanman R, Booth MS, Miles JN, Sorbero ME, et al. A systematic review of St. John's wort for major depressive disorder. Syst Rev. 2016;5:148. Hypericum also has an effect on various neurotransmitter systems via the up-regulation of brain levels of serotonin, norepinephrine, and dopamine.55. Bano S, Ara I, Saboohi K, Moattar T, Chaoudhry B. St. John's Wort increases brain serotonin synthesis by inhibiting hepatic tryptophan 2, 3 dioxygenase activity and its gene expression in stressed rats. Pak J Pharm Sci. 2014;27:1427-35.,66. Calapai G, Crupi A, Firenzuoli F, Inferrera G, Squadrito F, Parisi A, et al. Serotonin, norepinephrine and dopamine involvement in the antidepressant action of hypericum perforatum. Pharmacopsychiatry. 2001;34:45-9. Furthermore, this medicinal plant causes down-regulation of D2 receptors and up-regulation of 5-HT2A and BDZ receptors,77. Muruganandam AV, Ghosal S, Bhattacharya SK. The role of xanthones in the antidepressant activity of hypericum perforatum involving dopaminergic and serotonergic systems. Biog Amines. 1999;15:553-67. and can increase the levels of intracellular calcium in presynaptic vagal afferent neurons, which, in turn, leads to a release of higher levels of neurotransmitters.88. Vance KM, Ribnicky DM, Hermann GE, Rogers RC. St. John's Wort enhances the synaptic activity of the nucleus of the solitary tract. Nutrition. 2014;30:S37-42. Studies have also reported that hypericum can act as protein kinase C (PKC) blocker,99. Takahashi I, Nakanishi S, Kobayashi E, Nakano H, Suzuki K, Tamaoki T. Hypericin and pseudohypericin specialty inhibit protein kinase C: possible relation to their antiretroviral activity. Biochem Biophys Res Commun. 1989;165:1207-12. competitively binding to the regulatory domain of PKC.1010. Kocanova S, Hornakova T, Hritz J, Jancura D, Chorvat D, Mateasik A, et al. Characterization of the interaction of hypericin with protein kinase C in U-87 MG human glioma cells. Photochem Photobiol. 2006;82:720-8.
There is a considerable amount of data in the literature demonstrating that hypericum exerts neuroprotective effects in the brain in distinct ways.1111. Gómez del Rio MA, Sánchez-Reus MI, Iglesias I, Pozo MA, García-Arencibia M, Fernández-Ruiz J, et al. Neuroprotective properties of standardized extracts of hypericum perforatum on rotenone model of Parkinson’s disease. CNS Neurol Disord Drug Targets. 2013;12:665-79.,1212. Kraus B, Wolff H, Elstner EF, Heilmann J. Hyperforin is a modulator of inducible nitric oxide synthase and phagocytosis in microglia and macrophages. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:541-53. Hypericum reduces formation of nitric oxide (NO), a pro-inflammatory mediator, by decreasing inducible NO synthase expression in mRNA.1212. Kraus B, Wolff H, Elstner EF, Heilmann J. Hyperforin is a modulator of inducible nitric oxide synthase and phagocytosis in microglia and macrophages. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:541-53. An animal model of Parkinson’s disease induced by rotenone showed that hypericum extract reduced both neuronal damage and dopaminergic cell death, and caused inhibition of the apoptotic cascade by decreasing the levels of Bax in the brain.1111. Gómez del Rio MA, Sánchez-Reus MI, Iglesias I, Pozo MA, García-Arencibia M, Fernández-Ruiz J, et al. Neuroprotective properties of standardized extracts of hypericum perforatum on rotenone model of Parkinson’s disease. CNS Neurol Disord Drug Targets. 2013;12:665-79. In addition, some studies have demonstrated the effects of hypericum against cognitive damage in several different models of chronic stress and Alzheimer’s disease.1313. Hofrichter J, Krohn M, Schumacher T, Lange C, Feistel B, Walbroel B, et al. Reduced Alzheimer's disease pathology by St. John's Wort treatment is independent of hyperforin and facilitated by ABCC1 and microglia activation in mice. Curr Alzheimer Res. 2013;10:1057-69.
14. Trofimiuk E, Walesiuk A, Braszko JJ. St John's wort (hypericum perforatum) diminishes cognitive impairment caused by the chronic restraint stress in rats. Pharmacol Res. 2005;51:239-46.
15. Trofimiuk E, Walesiuk A, Braszko JJ. St john's wort (hypericum perforatum) counteracts deleterious effects of the chronic restraint stress on recall in rats. Acta Neurobiol Exp (Wars). 2006;66:129-38.
16. Trofimiuk E, Braszko JJ. Alleviation by hypericum perforatum of the stress-induced impairment of spatial working memory in rats. Naunyn Schmiedebergs Arch Pharmacol. 2008;376:463-71.
17. Trofimiuk E, Holownia A, Braszko JJ. Activation of CREB by St. John's wort may diminish deletorious effects of aging on spatial memory. Arch Pharm Res. 2010;33:469-77.-1818. Trofimiuk E, Holownia A, Braszko JJ. St. John's wort may relieve negative effects of stress on spatial working memory by changing synaptic plasticity. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:415-22. However, there is little evidence in the literature demonstrating the long-term effects of hypericum in relation to alterations in memory and neurochemistry.
Neurotrophic factors – including the neurotrophin family, glial cell-line derived neurotrophic factor (GDNF), ciliary neurotrophic factor, nerve growth factor (NGF), and brain-derived neurotrophic factor (BDNF)1919. Kimura A, Namekata K, Guo X, Harada C, Harada T. Neuroprotection, growth factors and BDNF-TrkB signalling in retinal degeneration. Int J Mol Sci. 2016;17: pii: E1584. – play an important role in memory and in the development and survival of neurons. NGF is essential for the functional integrity of cholinergic neurons within the central nervous system (CNS).2020. Aloe L, Bracci-Laudiero L, Bonini S, Manni L. The expanding role of nerve growth factor: from neurotrophic activity to immunologic diseases. Allergy. 1997;52:883-94. In the CNS, NGF is mainly expressed in the cortex, hippocampus, basal ganglia, thalamus, spinal cord, and retina. BDNF belongs to the NGF family and is widely expressed throughout the CNS. It is the most abundant neurotrophin in the adult brain. BDNF is mainly expressed in the hippocampus and exerts its pro-survival effects by binding to its receptor.2121. Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358-62.
22. Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297-305.-2323. Galvez-Contreras AY, Campos-Ordonez T, Lopez-Virgen V, Gomez-Plascencia J, Ramos-Zuniga R, Gonzalez-Perez O. Growth factors as clinical biomarkers of prognosis and diagnosis in psychiatric disorders. Cytokine Growth Factor Rev. 2016;32:85-96. GDNF was originally identified as a potent neurotrophic factor that promotes the survival of midbrain dopaminergic neurons.2424. Deister C, Schmidt CE. Optimizing neurotrophic factor combinations for neurite outgrowth. J Neural Eng. 2006;3:172-9.,2525. Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130-2. The neurotrophic effects of GDNF have been related to neuronal atrophy, which causes cognitive deficits. Pertusa et al.2626. Pertusa M, García-Matas S, Mammeri H, Adell A, Rodrigo T, Mallet J, et al. Expression of GDNF transgene in astrocytes improves cognitive deficits in aged rats. Neurobiol Aging. 2008;29:1366-79. demonstrated that the increased expression of GDNF improves cognitive deficits in rats.
It is well described in the literature that depressive episodes decrease brain levels of BDNF,2727. Bocchio-Chiavetto L, Bagnardi V, Zanardini R, Molteni R, Nielsen MG, Placentino A, et al. Serum and plasma BDNF levels in major depression: a replication study and meta-analyses. World J Biol Psychiatry. 2010;11:763-73.,2828. Nomoto H, Baba H, Satomura E, Maeshima H, Takebayashi N, Namekawa Y, et al. Serum brain-derived neurotrophic factor levels and personality traits in patients with major depression. BMC Psychiatry. 2015;15:33. whereas antidepressants increase the levels of this neurotrophin.2929. Molendijk ML, Bus BA, Spinhoven P, Penninx BW, Kenis G, Prickaerts J, et al. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatry. 2011;16:1088-95.,3030. Amidfar M, Réus GZ, Quevedo J, Kim YK, Arbabi M. Effect of co-administration of memantine and sertraline on the antidepressant-like activity and brain-derived neurotrophic factor (BDNF) levels in the rat brain. Brain Res Bull. 2017;128:29-33. Molendijk et al.2929. Molendijk ML, Bus BA, Spinhoven P, Penninx BW, Kenis G, Prickaerts J, et al. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatry. 2011;16:1088-95. have demonstrated that treatment with antidepressants or hypericum induces up-regulation of serum BDNF in depressed patients when compared to an antidepressant-free depressed group. NGF and GDNF are also related to mood disorders, and antidepressant activity seems to be associated with the up-regulation of these neurotrophic factors.3131. Ogłodek EA, Just MJ, Szromek AR, Araszkiewicz A. Melatonin and neurotrophins NT-3, BDNF, NGF in patients with varying levels of depression severity. Pharmacol Rep. 2016;68:945-51. Previous studies from our research group have demonstrated that antidepressant substances improve cognition and enhance neurotrophic expression in animal models of depression.3232. Valvassori SS, Varela RB, Arent CO, Dal-Pont GC, Bobsin TS, Budni J, et al. Sodium butyrate functions as an antidepressant and improves cognition with enhanced neurotrophic expression in models of maternal deprivation and chronic mild stress. Curr Neurovasc Res. 2014;11:359-66. Therefore, studies evaluating the effects of hypericum on neurotrophic factors are important to improve our knowledge about the mechanisms of action of this plant.
There is some evidence to suggest that males and females have different types of cognitive functions, as well as different levels of estrogen and testosterone in the cortex, hippocampus, and amygdala, which are brain regions responsible for modulating cognition and memory. This might explain the differences in cognition seen between the sexes.3333. Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477-84.
34. Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847-55.-3535. Wilson CA, Davies DC. The control of sexual differentiation of the reproductive system and brain. Reproduction. 2007;133:331-59. Therefore, the effects of drugs should be tested in both male and female animals. In a systematic review, Leger & Neill3636. Leger M, Neill JC. A systematic review comparing sex differences in cognitive function in schizophrenia and in rodent models for schizophrenia, implications for improved therapeutic strategies. Neurosci Biobehav Rev. 2016;68:979-1000. demonstrated an advantage of males regarding working memory in a rat model of schizophrenia. In turn, female rats were at an advantage in terms of visual learning and memory and social cognition. Matyi et al.,3737. Matyi J, Tschanz JT, Rattinger GB, Sanders C, Vernon EK, Corcoran C, et al. Sex differences in risk for Alzheimer's disease related to neurotrophin gene polymorphisms: the cache county memory study. J Gerontol A Biol Sci Med Sci. 2017;72:1607-13. who evaluated sex differences in Alzheimer's disease risk and neurotrophin gene polymorphisms, suggest several sex differences in the association with Alzheimer's disease and BDNF gene polymorphisms.
It is possible to evaluate recognition memory in rodents, in particular through the use of object recognition tasks, which measure the spontaneous preference for novel objects in an open field.3838. Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13:93-110.,3939. Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav Brain Res. 1999;99:191-200. Some previous studies using rodents found that interactions between perirhinal cortex, hippocampus, and medial prefrontal cortex are necessary for recognition memory.4040. Warburton EC, Brown MW. Findings from animals concerning when interactions between perirhinal cortex, hippocampus and medial prefrontal cortex are necessary for recognition memory. Neuropsychologia. 2010;48:2262-72.,4141. Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between hippocampus and perirhinal cortex on tests of spatial and object recognition memory: heterogeneity of function within the medial temporal lobe. J Neurosci. 2004;24:5901-8. Aversive memory can also be easily measured in rodents in the inhibitory avoidance task. In this task, the animals learn not to step-down from a platform in order to avoid entering a place where they once received a foot shock. This task relies heavily on the dorsal hippocampus,4242. Lorenzini CA, Baldi E, Bicherelli C, Sacchetti B, Tassoni G. Role of dorsal hippocampus in acquisition, consolidation and retrieval of rat's passive avoidance response: a tetrodotoxin functional inactivation study. Brain Res. 1996;730:32-9.,4343. Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285-316. where it uses a sequence of molecular events very remindful of those of long-term potentiation.4343. Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285-316.
44. Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, et al. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc Natl Acad Sci USA. 1997;94:7041-6.-4545. Izquierdo I, Medina JH. Correlation between the pharmacology of long-term potentiation and the pharmacology of memory. Neurobiol Learn Mem. 1995;63:19-32.
The objective of the present study was to evaluate the pharmacological effects of administering chronic doses of hypericum on the cognitive behavior and the levels of neurotrophic factors in the frontal cortex and hippocampus of male and female rats.
Methods
Animals
Adult male and female Wistar rats (weighing 250-350 g) were obtained from our breeding colony. They were housed five animals to a cage, with food and water ad libitum, and maintained on a 12-h light/dark cycle (lights on at 7:00 a.m.) at a temperature of 22±1 °C. All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996. All efforts were made to minimize the number of animals used and their suffering. The experimental procedures were approval of, the local ethics committee for the use of animals (protocol no. 66/2010 UNESC). Experiments were performed during the day, always at the same time, to avoid circadian variations.
Drugs and pharmacological procedures
Dried hypericum extract containing 0.32% of total hypericin (Vitalis Farmácia de Manipulação, Criciúma, Brazil) was used. The dose of hypericum (300 mg/kg once daily) was based on a previous study by Galeotti et al.4646. Galeotti N, Maidecchi A, Mattoli L, Burico M, Ghelardini C. St. John's wort seed and feverfew flower extracts relieve painful diabetic neuropathy in a rat model of diabetes. Fitoterapia. 2014;92:23-33. Hypericum was suspended in water, prepared immediately before administration, and protected from light during the experimental sessions. The solution was homogenized throughout the administration period.
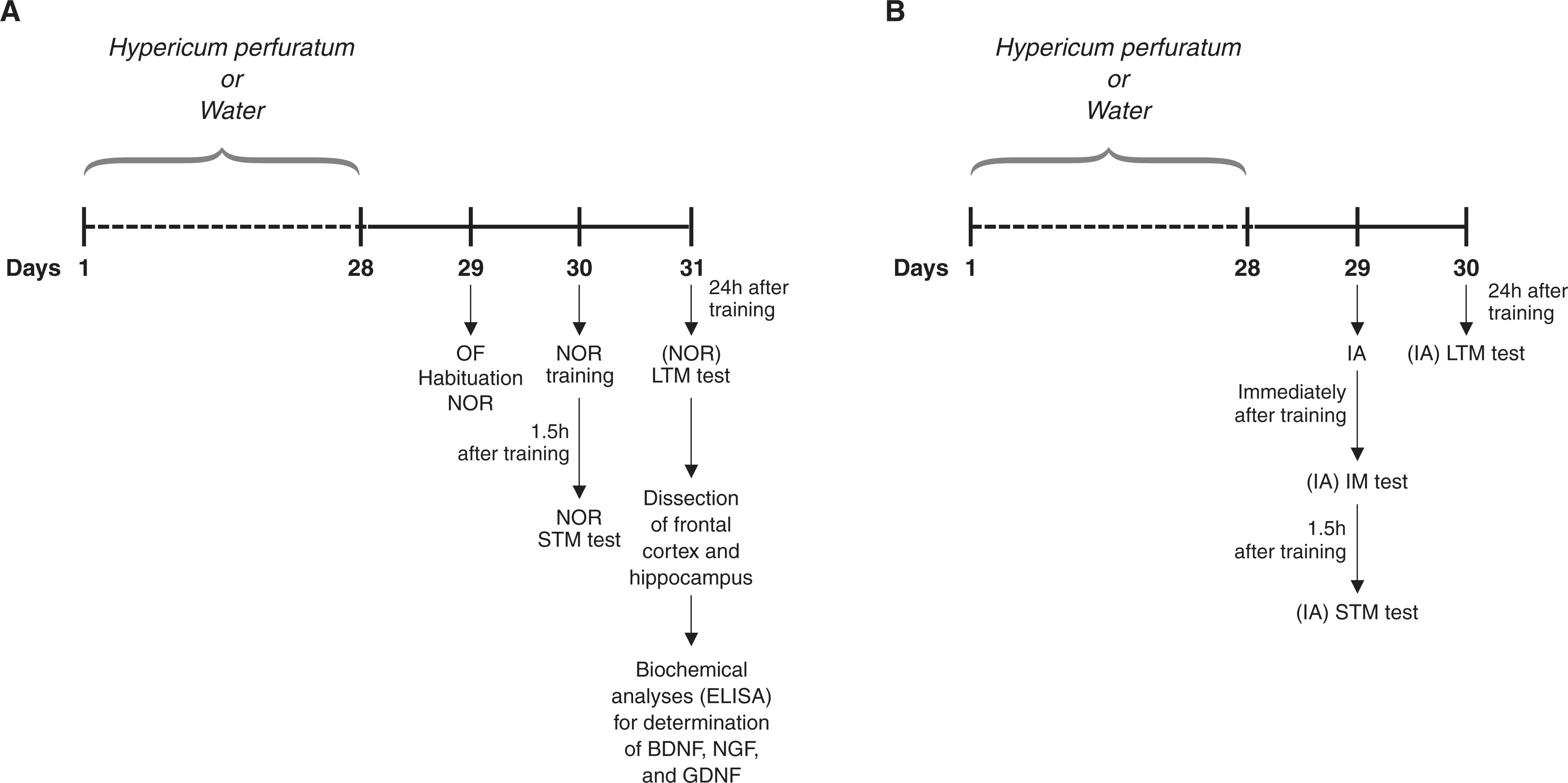
Eighty animals (40 males and 40 females) were randomly assigned to a treatment or a control group: the treatment group (20 males, 20 females) received hypericum (300 mg/kg once daily) during 28 days, for a volume of 1 mL/kg, administered by gavage once daily. Control animals (20 males, 20 females) received water (1 mL/kg). Behavioral tests were initiated 24 h after the last administration of drug/water.
The same animals were used for the open-field test and novel object recognition test [males receiving water or hypericum (n = 10 animals per group) and females receiving water or hypericum (n = 10 animals per group)]. The step-down inhibitory avoidance task was performed with a different set of males receiving water or hypericum (n = 10 animals per group) and females receiving water or hypericum (n = 10 animals per group). Further details about the test procedures are given below.
Behavioral tests
Open-field arena/test
The apparatus consisted of a brown plywood arena (surface area: 45 × 60 cm) surrounded by 50 cm high walls, three of which were made of wood. The fourth wall was made of glass. The floor was divided by black lines into nine 15 x 20 rectangles. Individual animals were gently placed on the left rear quadrant and allowed to explore the arena for 5 min. In order to evaluate locomotor and exploratory activities, the number of horizontal (crossings) and vertical (rearings) activities performed by each rat during the 5 min were counted. The open-field test was performed 24 h after the last injection of drug/water (Figure 1). The open-field test was also used for habituation of the animals to the environment for performance of the novel object recognition test.
Novel object recognition (NOR)
The NOR test was performed in the open-field apparatus. Habituation to the apparatus was made during the open-field test, as described above. No objects were placed in the box during the habituation trial. Twenty-four hours after habituation, training was conducted by placing individual rats for 5 min in the open field, in which two identical objects (objects A1 and A2; both being cubes) were positioned in two adjacent corners, at 10 cm from the walls. In a short-term recognition memory test given 1.5 h after training, the rats explored the open field for 5 min in the presence of one familiar (A) and one novel (B, a pyramid with a square-shaped base) object. All objects had similar textures (smooth), colors (blue), and sizes (weight 150-200 g), but distinctive shapes. A recognition index calculated for each animal is reported as the ratio TB/(TA + TB) (TA = time spent exploring the familiar object A; TB = time spent exploring the novel object B). Between trials the objects were washed with 10% ethanol solution. In a long-term recognition memory test given 24 h after training, the same rats were allowed to explore the field for 5 min in the presence of the familiar object A and a novel object C (a sphere with a square-shaped base). Long-term recognition memory was evaluated as described for short-term recognition memory. Exploration was defined as sniffing (exploring the object 3-5 cm away from it) or touching the object with the nose and/or forepaws.
Animals received chronic treatment with Hypericum perforatum (hypericum) (20 males, 20 females) or water (20 males, 20 females) once daily during 28 days. 24h after administration of substances, they were submitted to the OF test, NOR test or IA test. A) The same animals were used in the OF and NOR tests [males receiving water or hypericum (n = 10 animals per group) and females receiving water or hypericum (n = 10 animals per group)]. After the NOR test, the animals were killed by decapitation and the brains were removed, with dissection of the hippocampus and frontal cortex for determination of BDNF, NGF, and GDNF levels. Five male and female samples from the water and treatment groups were randomly selected for these analyses. B) A different set of male or female animals receiving water or hypericum (n = 10 animals per group) was used for the IA task. BDNF = brain-derived neurotrophic factor; GDNF = glial cell-line derived neurotrophic factor; IA = inhibitory avoidance; IM = immediate memory; LTM = long-term memory test; NGF = nerve growth factor; NOR = novel object recognition; OF = open-field; STM = short-term memory test.
Step-down inhibitory avoidance (IA) task
The step-down IA apparatus consisted of a 50 × 25 × 25-cm plastic box with a front glass wall. The floor was covered by parallel 10-mm bronze bars. The left end of the grid was occupied by a 7-cm wide, 2.5-cm high Formica platform. Individual rats were gently placed on the platform facing the rear wall and their latency to step-down with all four paws on the grid was recorded. In the training session (24 h after the last injection), after stepping down, the animals received a 0.4-mA, 2-s scrambled foot shock and were withdrawn immediately from the cage. There were three test sessions: 1) immediately after training, to evaluate the immediate memory (IM); 2) 1.5 h after training, to evaluate the short-term memory (STM), and; 3) 24 h after training session, to evaluate the long-term memory (LTM) (Figure 1). In the test sessions, the procedure was repeated, but foot shock was not given. Test session step-down latency was used as a measure of retention. A ceiling of 180 s was imposed on this measure, i.e., animals whose test latency was more than 180 s were considered to have a latency of 180 s, as previously proposed by Gold4747. Gold PE. The use of avoidance training in studies of modulation of memory storage. Behav Neural Biol. 1986;46:87-98. and Izquierdo & Medina.4343. Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285-316.
Measurement of NGF, BDNF, and GDNF levels
After NOR, the animals undergoing that test were killed by decapitation, and the brains were removed with dissection of the hippocampus and frontal cortex. NGF, BDNF, and GDNF levels were measured in the hippocampus and frontal cortex, using sandwich enzyme-linked immunosorbent assay (ELISA) and commercial kits according to the manufacturer's instructions (NGF and BDNF from Chemicon International Inc., California, USA; GDNF from Biosensis Pty Ltd., California, USA). Microtiter plates (96-well flat-bottom) were coated for 24 h with the samples diluted 1:2 in sample diluent. Standard curve ranged from 7.8 to 500 pg of BDNF or NGF. Then, plates were wash four times with sample diluents. Anti-BDNF rabbit monoclonal antibody, anti-NGF rabbit monoclonal antibody, or GDNF rat polyclonal antibody diluted 1:1,000 in sample diluent were incubated for 3 h at room temperature (RT). After washing, a second incubation was performed with peroxidase conjugated anti-rabbit antibody diluted 1:1,000 for 1h at RT. After addition of streptavidin-enzyme substrate and stop solution, the amount of BDNF, NGF, or GDNF was determined for absorbance in 450 nm. The standard curve demonstrates a direct relationship between optical density (OD) and BDNF, NGF, and GDNF concentration. Total protein was measured by Lowry's4848. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-75. method using bovine serum albumin (BSA) as standard.
Statistical analysis
Data from the open-field and neurotrophin (BDNF, NGF, and GDNF) levels were reported as means ± standard error of mean (SEM) and were analyzed by t test for independent samples. The data obtained in the novel object recognition test were reported as means ± SEM and the differences between groups in this behavioral analysis were verified using repeated measures analysis of variance followed by Tukey's post-hoc tests. The data obtained in the step-down IA task were reported as median ± interquartile ranges (25 and 75). The analysis of IA data was nonparametric because this procedure involved a cutoff score, and the Kruskal-Wallis test was performed followed by Mann-Whitney’s U test.
Results
Locomotor and exploratory activities
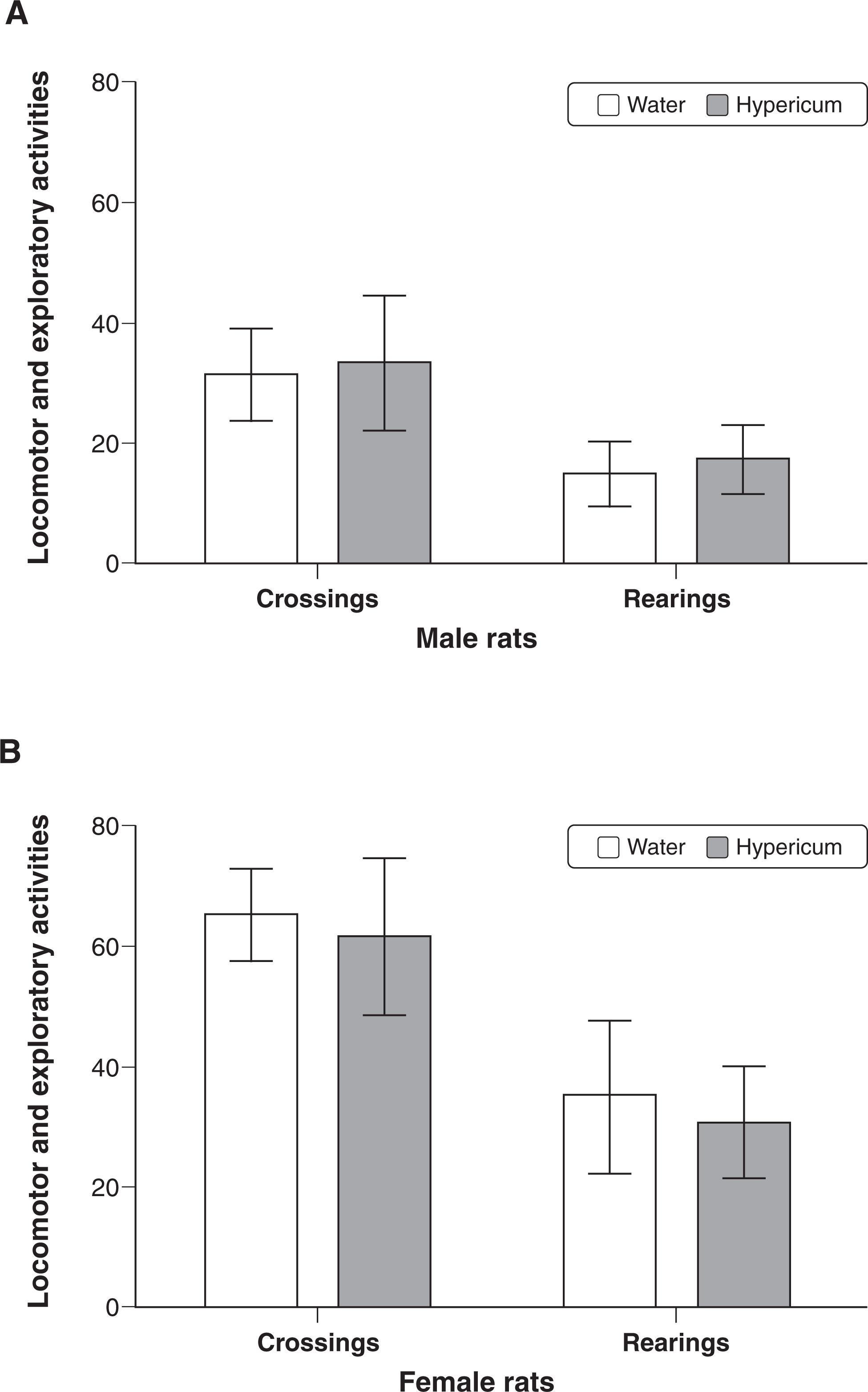
The open field test was used for assessing locomotor (crossings) and exploratory (rearings) activities in male (Figure 2A) or female (Figure 2B) rats after chronic treatment with hypericum or water. However, no alteration in spontaneous locomotion was observed in either males or females. The number of crossings (male: t-value(17) = -0.436, p = 0.67; female: t-value(21) = 0.807, p = 0.43) and the number of rearings (male: t-value(17) = 0,899, p = 0.38; female: t-value(21) = 0.94, p = 0.35) were also similar.
The open field test was used for assessing locomotor (crossings) and exploratory (rearings) activities after chronic treatment with water or Hypericum perforatum in (A) males (n=10 animals per group) or (B) females (n=10 animals per group). Data represent the mean ± standard error of mean.
Novel object recognition (NOR)
In all experimental protocols (with both males and females), there were no differences among groups in the total time exploring either object during the retention test trial. Also, there was no significant performance difference among groups in the training trial. These results indicate that pre-training treatment with hypericum did not affect sensorimotor parameters, such as locomotion and motivation. Male (Figure 3A) and female (Figure 3B) animals treated with hypericum showed impaired retention (STM and LTM), as shown by decreased preference for the novel object compared to controls given water. Despite a decrease in recognition index during STM and LTM, male rats treated with hypericum showed a significantly higher preference for the novel object during LTM compared to the training trial. However, female rats given hypericum showed no significant preference for the new object during STM and LTM tests.
Repeated measures analysis of variance for drugs administration: males: F(1.14) = 150.168, p < 0.001; females: F(1.14) = 251.261, p < 0.001; and for the behavioral repetitions: males: F(2.28) = 96.21, p < 0.001; females: F(2.28) = 76.848, p < 0.001.
The NOR task was used to evaluate recognition memory. Recognition index for the objects in the training and test sessions after treatment with water or Hypericum perforatum appears in (A) for males (n= 10 animals per group) and (B) for females (B) (n= 10 animals per group). Results are presented as means ± standard error of mean of the recognition index. The test session was performed 24 h after the training session. IM = immediate memory; STM = short-term memory; LTM = long-term memory; NOR = novel object recognition. * p < 0.001 different from training test. † p < 0.01 different from control group, according to one-way repeated measures ANOVA followed by Tukey's post-hoc test.
Step-down inhibitory avoidance (IA) task
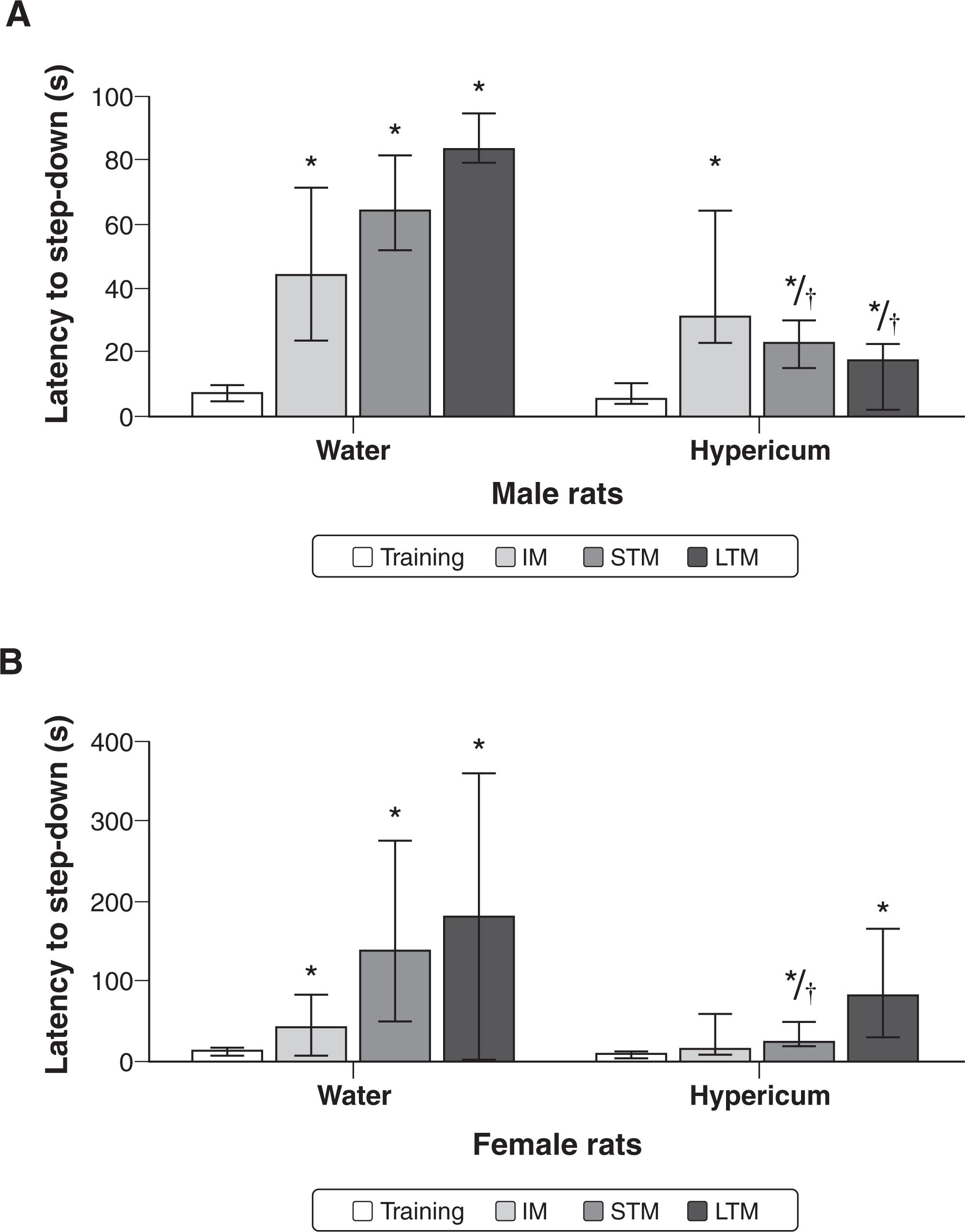
In the step-down IA test, control and hypericum animals of both sexes had higher latency in the STM and LTM tests as compared to the training session, indicating acquisition of memory. However, treatment with hypericum induced impairment of the acquisition of memory in males (Figure 4A) and females (Figure 4B), since the latencies in the test sessions were decreased for STM as well as for LTM, as compared with the control group. It should be noted that hypericum administration in female rats also induced impairment of the IM, since there were no statistical differences between training and test latencies.
Data from Kruskal-Wallis test: difference between groups for training (male: chi-square = 0.292, degrees of freedom [df] = 1, p = 0.589; female: chi-square = 2.494, df = 1, p = 0.114), for IM (male: chi-square = 0.494, df = 1, p = 0.482; female: chi-square = 0.991, df = 1, p = 0.320), for STM (male: chi-square = 10.38, df = 1, p < 0.001; female: chi-square = 5.258, df = 1, p = 0.022), and for LTM (male: chi-square = 13.223, df = 1, p < 0.001; female: chi-square = 0.617, df = 1, p = 0.432).
Data from Wilcoxon test: training session vs. test sessions for control group (training vs. IM of male rats: z = 2.524, p = 0.012; training vs. IM of female rats: z = 2.106, p = 0.035; training vs. STM of male rats: z = 2.521, p = 0.012; training vs. STM of female rats: z = 2.521, p = 0.012; training vs. LTM of male rats: z = 2.524, p = 0.012; training vs. LTM of female rats: z = 2.38, p = 0.017), for hypericum group (training vs. IM of male rats: z = 2.934, p = 0.003; training vs. IM of female rats: z = 1.557, p = 0.119; training vs. STM of male rats: z = 2.936, p = 0.003; training vs. STM of female rats: z = 2.314, p = 0.021; training vs. LTM of male rats: z = 2.803, p = 0.05; training vs. LTM of female rats: z = 2.845, p = 0.004).
The inhibitory avoidance task was used to evaluate aversive memory. Latency time was recorded in training and test sessions after treatment with water or Hypericum perforatum for (A) males (n=10 animals per group) and (B) females (B) (n=10 animals per group). Results are presented as medians ± interquartile ranges (25 and 75). The test session was performed 24 h after the training session. IM = immediate memory; LTM = long-term memory; STM = short-term memory. * p < 0.001 different from training test. † p < 0.01 different from control group, according to Kruskal-Wallis test followed by Mann-Whitney U test.
Neurotrophic factors levels
The chronic treatment with hypericum decreased BDNF levels in the hippocampus of male (Figure 5A) and female (Figure 5B). Treatment with hypericum decreased NGF levels in hippocampus of female rats (Figure 5C). No changes in NGF levels were observed in the hippocampus or frontal cortex of male rats after chronic administration hypericum (Figure 5D). Hypericum treatment in male (Figure 5E) or female (Figure 5F) rats did not change GDNF levels in the hippocampus or frontal cortex.
Data from t test to neurotrophic factors levels: BDNF levels in hippocampus (male: t-value(8) = 7.124, p < 0.001; female: t-value(8) = 4.820, p < 0.01), BDNF levels in frontal cortex (male: t-value(8) = 0.276, p = 0.79; female: t-value(8) = 0.970, p = 0.36), NGF levels in hippocampus (male: t-value(8) = 0.310, p = 0.76; female: t-value(8) = 4.425, p < 0.001), NGF levels in frontal cortex (male: t-value(8) = -0.428, p = 0.68; female: t-value(8) = 1.640, p = 0.14), GDNF levels in hippocampus (male: t-value(8) = 0.638, p = 0.54; female: t-value(8) = -0.217, p = 0.83), GDNF levels in frontal cortex (male: t-value(8) = -0.417, p = 0.69; female t-value(8) = -1.544, p = 0.16).
Effects of water or Hypericum perforatum on levels of BDNF (A and B), NGF (C and D) and GNDF (E and F) in hippocampus and frontal cortex of male (n = 5 animals per group) (A, C, E) and female (n = 5 animals per group) (B, D, F) rats. Data represent the mean ± standard error of mean. BDNF = brain-derived neurotrophic factor; GNDF = glial cell-line derived neurotrophic factor; NGF = nerve growth factor; * p < 0.05 according to Student’s t test.
Discussion
Our results demonstrate that the chronic administration of hypericum did not alter locomotor and exploratory activities as compared to controls. Reis et al.4949. Reis EM, Röpke J, Busanello A, Reckziegel P, Leal CQ, Wagner C, et al. Effect of hypericum perforatum on different models of movement disorders in rats. Behav Pharmacol. 2013;24:623-7. had previously reported that seven days of treatment with hypericum had no effect on the levels of locomotor activity.4949. Reis EM, Röpke J, Busanello A, Reckziegel P, Leal CQ, Wagner C, et al. Effect of hypericum perforatum on different models of movement disorders in rats. Behav Pharmacol. 2013;24:623-7. In the present study, male and female rats that were chronically treated with hypericum showed impairments in memory retention (STM and LTM) in the object recognition test; however, these memory impairments were more significant in females than in males. Additionally, treatment with hypericum induced impairments in the acquisition of aversive memory (STM and LTM) in both male and female rats in the avoidance step-down test. Interestingly, hypericum administration also induced impairments in the IM in females, but not in male rats.
Our results contradict several studies in the literature relating to improvements in cognitive performance in male rats after administration of hypericum. Trofimiuk et al.1414. Trofimiuk E, Walesiuk A, Braszko JJ. St John's wort (hypericum perforatum) diminishes cognitive impairment caused by the chronic restraint stress in rats. Pharmacol Res. 2005;51:239-46.,1818. Trofimiuk E, Holownia A, Braszko JJ. St. John's wort may relieve negative effects of stress on spatial working memory by changing synaptic plasticity. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:415-22. demonstrated that three weeks of hypericum (350 mg kg[-1]) treatment enhanced cognitive performance in male rats performing object recognition, water maze, and Barnes maze tasks following chronic restraint stress or administration of exogenous corticosterone. Widy-Tyszkiewicz et al.5050. Widy-Tyszkiewicz E, Piechal A, Joniec I, Blecharz-Klin K. Long term administration of hypericum perforatum improves spatial learning and memory in the water maze. Biol Pharm Bull. 2002;25:1289-94. also showed that the administration of hypericum (4.3 or 13 µg/kg) in heath rats over a nine-week period decreased the escape latency time and increased the number of platform area crossings in the water maze test, indicating enhanced cognitive performance. Furthermore, Hasanein & Shahidi5151. Hasanein P, Shahidi S. Effects of hypericum perforatum extract on diabetes-induced learning and memory impairment in rats. Phytother Res. 2011;25:544-9. demonstrated that 30 days of treatment with hypericum (6, 12, or 25 mg/kg) improved the learning parameters in the passive avoidance learning test in an animal model of diabetes mellitus. However, this discrepancy can be explained, at least in part, by methodological differences such as study duration, doses used, and method of administration used for hypericum.
It is important to note that in the present study, hypericum decreased the levels of BDNF in the hippocampus of both male and female rats. BDNF plays an important role in the survival, differentiation, and outgrowth of peripheral and central neurons during development and in adulthood. BDNF has also been shown to play an important role in synaptic plasticity, mainly within the hippocampus.5252. McAllister AK. Subplate neurons: a missing link among neurotrophins, activity, and ocular dominance plasticity? Proc Natl Acad Sci USA. 1999;96:13600-2.,5353. Sohrabji F, Lewis DK. Estrogen-BDNF interactions: implications for neurodegenerative diseases. Front Neuroendocrinol. 2006;27:404-14. Therefore, the memory impairments induced by hypericum might be associated with decreased levels of BDNF in the hippocampus of rats.
It is important to note that the cognitive impairments induced by hypericum were more expressive in females than in males. Some evidence from the literature supports these findings. A preclinical study evaluating cognitive parameters in the offspring of mice treated in the prenatal period with hypericum found that only female offspring had cognitive impairment, which was evaluated in the water maze test. The authors found that adult female offspring exposed to hypericum, rather than to a placebo, required more time to learn the Morris maze task.5454. Rayburn WF, Gonzalez CL, Christensen HD, Harkins TL, Kupiec TC. Impact of hypericum (St.-John's-wort) given prenatally on cognition of mice offspring. Neurotoxicol Teratol. 2001;23:629-37. In the present study, while there BDNF levels seem to have been impaired in both sexes, only females expressed decreased levels of NGF. This neurotrophic factor is responsible for repairing functions in cholinergic neurons within specific regions of the brain that are related to memory formation.5555. Williams BJ, Bimonte-Nelson HA, Granholm-Bentley AC. ERK-mediated NGF signaling in the rat septo-hippocampal pathway diminishes with age. Psychopharmacology (Berl). 2006;188:605-18.,5656. Yeiser EC, Rutkoski NJ, Naito A, Inoue J, Carter BD. Neurotrophin signaling through the p75 receptor is deficient in traf6-/- mice. J Neurosci. 2004;24:10521-9. Thus, these sex-related differences can be explained, at least in part, by the fact that hypericum only decreased the levels of NGF in the hippocampus of female rats.
In the present study, the estrous cycle was not evaluated in female rats during the experiments, even though some studies have demonstrated sex differences in some behavioral response displayed between estrous cycle phases (proestrus, estrous, early diestrus, and late diestrus cycles).5757. Devall AJ, Liu ZW, Lovick TA. Hyperalgesia in the setting of anxiety: sex differences and effects of the oestrous cycle in Wistar rats. Psychoneuroendocrinology, 1995;34:587-96.,5858. Devall AJ, Santos JM, Fry JP, Honour JW, Brandão ML, Lovick TA. Elevation of brain allopregnanolone rather than 5-HT release by short term, low dose fluoxetine treatment prevents the estrous cycle-linked increase in stress sensitivity in female rats. Eur Neuropsychopharmacol. 2015;25:113-23. According to Millad,5959. Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164:887-95. females in the estrous cycle group (with high estrogen level) presented a quicker extinction of aversive memory than females in the diestrus group (with low estrogen and progesterone levels), suggesting changes of memory and learning tasks in female rats due gonadal hormone influence. Scharfman & MacLusky6060. Scharfman HE, MacLusky NJ. Sex differences in hippocampal area CA3 pyramidal cells. J Neurosci Res. 2017;95:563-75. recently published a review about sex structural brain differences in the hippocampus and the influence of hormonal modulation on cell expression during the estrous cycle. In this same review, the authors suggest that drug treatment could have a variety of effects due to hormonal modulation. However, it is important to emphasize that, in clinical studies, investigators do not assess hormone levels in women’s blood in all experiments, and drugs should work in all phases of the cycle. Therefore, the present experiment reflect the procedures of clinical studies. In addition, a clinical study has demonstrated that the menstrual cycle does not always concur with plasma hormone levels.6161. Weiser M, Vega-Saenz de Miera E, Kentros C, Moreno H, Franzen L, Hillman D, et al. Differential expression of Shaw-related K+ channels in the rat central nervous system. J Neurosci. 1994;14:949-72.
Regarding limitations, it is important to note that animals can be affected by a novel stimulus in the object recognition task. It could change behavior, provoke stress responses, and elicit approach behavior. Besides, some substances can change the preference for novelty in rats.3838. Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13:93-110. Therefore, it is plausible to suggest that chronic treatment with hypericum could lead to a change in preference for novelty in rats. However, the present study also detected memory impairment in the inhibitory avoidance task; this supports the notion that chronic hypericum treatment caused cognitive damage. Finally, we did not evaluate the estrous cycle in females during the experiments. However, as demonstrated in a clinical study, the menstrual cycle does not always concur with the hormone plasma levels.6161. Weiser M, Vega-Saenz de Miera E, Kentros C, Moreno H, Franzen L, Hillman D, et al. Differential expression of Shaw-related K+ channels in the rat central nervous system. J Neurosci. 1994;14:949-72.
In conclusion, treatment with hypericum induced impairments in both aversive and recognition memories in male and female rats. Chronic administration of hypericum was shown to decrease the levels of BDNF in the hippocampus of both male and female rats when compared to controls. It can be suggested that long-term treatments with PKC inhibitors may lead to significant cognitive impairments by reducing the levels of neurotrophic factors in the brain of rats. Besides, the cognitive damage induced by hypericum was more significant in female than in male rats. This difference can be explained, at least in part, by the fact that treatment with hypericum decreased the levels of NGF only in the hippocampus of females. Additionally, the results suggest that hypericum is more harmful to memory formation in females than in male animals.
References
-
1Marrelli M, Statti G, Conforti F, Menichini F. New potential pharmaceutical applications of hypericum species. Mini Rev Med Chem. 2016;16:710-20.
-
2Linde K, Berner MM, Kriston L. St John's wort for major depression. Cochrane Database Syst Rev. 2008;4:CD000448.
-
3Forsdike K, Pirotta M. St John's wort for depression: scoping review about perceptions and use by general practitioners in clinical practice. J Pharm Pharmacol. 2017 Jun 27. doi: 10.1111/jphp.12775. [Epub ahead of print]
» 10.1111/jphp.12775 -
4Apaydin EA, Maher AR, Shanman R, Booth MS, Miles JN, Sorbero ME, et al. A systematic review of St. John's wort for major depressive disorder. Syst Rev. 2016;5:148.
-
5Bano S, Ara I, Saboohi K, Moattar T, Chaoudhry B. St. John's Wort increases brain serotonin synthesis by inhibiting hepatic tryptophan 2, 3 dioxygenase activity and its gene expression in stressed rats. Pak J Pharm Sci. 2014;27:1427-35.
-
6Calapai G, Crupi A, Firenzuoli F, Inferrera G, Squadrito F, Parisi A, et al. Serotonin, norepinephrine and dopamine involvement in the antidepressant action of hypericum perforatum. Pharmacopsychiatry. 2001;34:45-9.
-
7Muruganandam AV, Ghosal S, Bhattacharya SK. The role of xanthones in the antidepressant activity of hypericum perforatum involving dopaminergic and serotonergic systems. Biog Amines. 1999;15:553-67.
-
8Vance KM, Ribnicky DM, Hermann GE, Rogers RC. St. John's Wort enhances the synaptic activity of the nucleus of the solitary tract. Nutrition. 2014;30:S37-42.
-
9Takahashi I, Nakanishi S, Kobayashi E, Nakano H, Suzuki K, Tamaoki T. Hypericin and pseudohypericin specialty inhibit protein kinase C: possible relation to their antiretroviral activity. Biochem Biophys Res Commun. 1989;165:1207-12.
-
10Kocanova S, Hornakova T, Hritz J, Jancura D, Chorvat D, Mateasik A, et al. Characterization of the interaction of hypericin with protein kinase C in U-87 MG human glioma cells. Photochem Photobiol. 2006;82:720-8.
-
11Gómez del Rio MA, Sánchez-Reus MI, Iglesias I, Pozo MA, García-Arencibia M, Fernández-Ruiz J, et al. Neuroprotective properties of standardized extracts of hypericum perforatum on rotenone model of Parkinson’s disease. CNS Neurol Disord Drug Targets. 2013;12:665-79.
-
12Kraus B, Wolff H, Elstner EF, Heilmann J. Hyperforin is a modulator of inducible nitric oxide synthase and phagocytosis in microglia and macrophages. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:541-53.
-
13Hofrichter J, Krohn M, Schumacher T, Lange C, Feistel B, Walbroel B, et al. Reduced Alzheimer's disease pathology by St. John's Wort treatment is independent of hyperforin and facilitated by ABCC1 and microglia activation in mice. Curr Alzheimer Res. 2013;10:1057-69.
-
14Trofimiuk E, Walesiuk A, Braszko JJ. St John's wort (hypericum perforatum) diminishes cognitive impairment caused by the chronic restraint stress in rats. Pharmacol Res. 2005;51:239-46.
-
15Trofimiuk E, Walesiuk A, Braszko JJ. St john's wort (hypericum perforatum) counteracts deleterious effects of the chronic restraint stress on recall in rats. Acta Neurobiol Exp (Wars). 2006;66:129-38.
-
16Trofimiuk E, Braszko JJ. Alleviation by hypericum perforatum of the stress-induced impairment of spatial working memory in rats. Naunyn Schmiedebergs Arch Pharmacol. 2008;376:463-71.
-
17Trofimiuk E, Holownia A, Braszko JJ. Activation of CREB by St. John's wort may diminish deletorious effects of aging on spatial memory. Arch Pharm Res. 2010;33:469-77.
-
18Trofimiuk E, Holownia A, Braszko JJ. St. John's wort may relieve negative effects of stress on spatial working memory by changing synaptic plasticity. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:415-22.
-
19Kimura A, Namekata K, Guo X, Harada C, Harada T. Neuroprotection, growth factors and BDNF-TrkB signalling in retinal degeneration. Int J Mol Sci. 2016;17: pii: E1584.
-
20Aloe L, Bracci-Laudiero L, Bonini S, Manni L. The expanding role of nerve growth factor: from neurotrophic activity to immunologic diseases. Allergy. 1997;52:883-94.
-
21Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358-62.
-
22Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297-305.
-
23Galvez-Contreras AY, Campos-Ordonez T, Lopez-Virgen V, Gomez-Plascencia J, Ramos-Zuniga R, Gonzalez-Perez O. Growth factors as clinical biomarkers of prognosis and diagnosis in psychiatric disorders. Cytokine Growth Factor Rev. 2016;32:85-96.
-
24Deister C, Schmidt CE. Optimizing neurotrophic factor combinations for neurite outgrowth. J Neural Eng. 2006;3:172-9.
-
25Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130-2.
-
26Pertusa M, García-Matas S, Mammeri H, Adell A, Rodrigo T, Mallet J, et al. Expression of GDNF transgene in astrocytes improves cognitive deficits in aged rats. Neurobiol Aging. 2008;29:1366-79.
-
27Bocchio-Chiavetto L, Bagnardi V, Zanardini R, Molteni R, Nielsen MG, Placentino A, et al. Serum and plasma BDNF levels in major depression: a replication study and meta-analyses. World J Biol Psychiatry. 2010;11:763-73.
-
28Nomoto H, Baba H, Satomura E, Maeshima H, Takebayashi N, Namekawa Y, et al. Serum brain-derived neurotrophic factor levels and personality traits in patients with major depression. BMC Psychiatry. 2015;15:33.
-
29Molendijk ML, Bus BA, Spinhoven P, Penninx BW, Kenis G, Prickaerts J, et al. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatry. 2011;16:1088-95.
-
30Amidfar M, Réus GZ, Quevedo J, Kim YK, Arbabi M. Effect of co-administration of memantine and sertraline on the antidepressant-like activity and brain-derived neurotrophic factor (BDNF) levels in the rat brain. Brain Res Bull. 2017;128:29-33.
-
31Ogłodek EA, Just MJ, Szromek AR, Araszkiewicz A. Melatonin and neurotrophins NT-3, BDNF, NGF in patients with varying levels of depression severity. Pharmacol Rep. 2016;68:945-51.
-
32Valvassori SS, Varela RB, Arent CO, Dal-Pont GC, Bobsin TS, Budni J, et al. Sodium butyrate functions as an antidepressant and improves cognition with enhanced neurotrophic expression in models of maternal deprivation and chronic mild stress. Curr Neurovasc Res. 2014;11:359-66.
-
33Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477-84.
-
34Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847-55.
-
35Wilson CA, Davies DC. The control of sexual differentiation of the reproductive system and brain. Reproduction. 2007;133:331-59.
-
36Leger M, Neill JC. A systematic review comparing sex differences in cognitive function in schizophrenia and in rodent models for schizophrenia, implications for improved therapeutic strategies. Neurosci Biobehav Rev. 2016;68:979-1000.
-
37Matyi J, Tschanz JT, Rattinger GB, Sanders C, Vernon EK, Corcoran C, et al. Sex differences in risk for Alzheimer's disease related to neurotrophin gene polymorphisms: the cache county memory study. J Gerontol A Biol Sci Med Sci. 2017;72:1607-13.
-
38Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13:93-110.
-
39Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav Brain Res. 1999;99:191-200.
-
40Warburton EC, Brown MW. Findings from animals concerning when interactions between perirhinal cortex, hippocampus and medial prefrontal cortex are necessary for recognition memory. Neuropsychologia. 2010;48:2262-72.
-
41Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between hippocampus and perirhinal cortex on tests of spatial and object recognition memory: heterogeneity of function within the medial temporal lobe. J Neurosci. 2004;24:5901-8.
-
42Lorenzini CA, Baldi E, Bicherelli C, Sacchetti B, Tassoni G. Role of dorsal hippocampus in acquisition, consolidation and retrieval of rat's passive avoidance response: a tetrodotoxin functional inactivation study. Brain Res. 1996;730:32-9.
-
43Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285-316.
-
44Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, et al. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc Natl Acad Sci USA. 1997;94:7041-6.
-
45Izquierdo I, Medina JH. Correlation between the pharmacology of long-term potentiation and the pharmacology of memory. Neurobiol Learn Mem. 1995;63:19-32.
-
46Galeotti N, Maidecchi A, Mattoli L, Burico M, Ghelardini C. St. John's wort seed and feverfew flower extracts relieve painful diabetic neuropathy in a rat model of diabetes. Fitoterapia. 2014;92:23-33.
-
47Gold PE. The use of avoidance training in studies of modulation of memory storage. Behav Neural Biol. 1986;46:87-98.
-
48Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-75.
-
49Reis EM, Röpke J, Busanello A, Reckziegel P, Leal CQ, Wagner C, et al. Effect of hypericum perforatum on different models of movement disorders in rats. Behav Pharmacol. 2013;24:623-7.
-
50Widy-Tyszkiewicz E, Piechal A, Joniec I, Blecharz-Klin K. Long term administration of hypericum perforatum improves spatial learning and memory in the water maze. Biol Pharm Bull. 2002;25:1289-94.
-
51Hasanein P, Shahidi S. Effects of hypericum perforatum extract on diabetes-induced learning and memory impairment in rats. Phytother Res. 2011;25:544-9.
-
52McAllister AK. Subplate neurons: a missing link among neurotrophins, activity, and ocular dominance plasticity? Proc Natl Acad Sci USA. 1999;96:13600-2.
-
53Sohrabji F, Lewis DK. Estrogen-BDNF interactions: implications for neurodegenerative diseases. Front Neuroendocrinol. 2006;27:404-14.
-
54Rayburn WF, Gonzalez CL, Christensen HD, Harkins TL, Kupiec TC. Impact of hypericum (St.-John's-wort) given prenatally on cognition of mice offspring. Neurotoxicol Teratol. 2001;23:629-37.
-
55Williams BJ, Bimonte-Nelson HA, Granholm-Bentley AC. ERK-mediated NGF signaling in the rat septo-hippocampal pathway diminishes with age. Psychopharmacology (Berl). 2006;188:605-18.
-
56Yeiser EC, Rutkoski NJ, Naito A, Inoue J, Carter BD. Neurotrophin signaling through the p75 receptor is deficient in traf6-/- mice. J Neurosci. 2004;24:10521-9.
-
57Devall AJ, Liu ZW, Lovick TA. Hyperalgesia in the setting of anxiety: sex differences and effects of the oestrous cycle in Wistar rats. Psychoneuroendocrinology, 1995;34:587-96.
-
58Devall AJ, Santos JM, Fry JP, Honour JW, Brandão ML, Lovick TA. Elevation of brain allopregnanolone rather than 5-HT release by short term, low dose fluoxetine treatment prevents the estrous cycle-linked increase in stress sensitivity in female rats. Eur Neuropsychopharmacol. 2015;25:113-23.
-
59Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164:887-95.
-
60Scharfman HE, MacLusky NJ. Sex differences in hippocampal area CA3 pyramidal cells. J Neurosci Res. 2017;95:563-75.
-
61Weiser M, Vega-Saenz de Miera E, Kentros C, Moreno H, Franzen L, Hillman D, et al. Differential expression of Shaw-related K+ channels in the rat central nervous system. J Neurosci. 1994;14:949-72.
Publication Dates
-
Publication in this collection
09 Aug 2018 -
Date of issue
Oct-Dec 2018
History
-
Received
9 Mar 2017 -
Accepted
21 Sept 2017