Abstracts
Blood and serum samples from 170 horses raised in the Jaboticabal microregion, São Paulo State, Brazil, were collected and tested by microscopic examination of blood smears, indirect fluorescent antibody test (IFAT) and nested polymerase chain reaction (nPCR) for Theileria equi infections. The association among the test results was verified by the McNemar test. During the examination of thin blood smears, parasites were detected in six (3.52%) horses. Anti-T. equi antibodies were detected in 100% sera samples, with titers ranging between 1:80 and 1:5120. The nPCR based on the T. equi merozoite antigen gene (EMA-1) allowed the visualization of specie-specific amplified product in 108 (63.53%) horses. All six samples judged positive microscopically were also positive for nPCR. Statistical analysis indicated general disagreement (p < 0.0001) between IFAT and nPCR; IFAT and blood smear; and nPCR and blood smear on the detection of parasite carriers. The results of the present study indicate that T. equi is widely spread among horses in the Jaboticabal microregion, Northeast region of São Paulo State, Brazil.
Theileria equi; horse; diagnosis; IFAT; nested PCR
Amostras de sangue e soro de 170 equinos criados na microrregião de Jaboticabal, Estado de São Paulo, Brasil, foram coletadas e avaliadas pelo exame direto em esfregaço sanguíneo, reação de imunofluorescência indireta (RIFI) e nested reação em cadeia da polimerase (nPCR) para a detecção de infecções por Theileria equi. A concordância dos resultados entre os testes de diagnóstico foi verificada pelo teste de McNemar. Durante o exame dos esfregaços sanguíneos, parasitos foram detectados em seis (3,52%) equinos. Anticorpos anti-T. equi foram detectados em 100% das amostras de soros, com títulos variando entre 1:80 e 1:5120. O nPCR, baseado na sequência do gene do antígeno de merozoíto de T. equi (EMA-1), permitiu a visualização de produtos de amplificação espécie-específico em 108 (63,53%) equinos. Houve diferença altamente significativa (p < 0,0001) entre RIFI e nPCR; RIFI e esfregaço sanguíneo; e nPCR e esfregaço na detecção do parasito. O resultado do presente estudo indica que a infecção por T. equi está amplamente distribuída entre os equinos na microrregião de Jaboticabal, região Nordeste do Estado de São Paulo, Brasil.
Theileria equi; equino; diagnóstico; RIFI; nested PCR
FULL ARTICLE
Occurrence of Theileria equi in horses raised in the Jaboticabal microregion, São Paulo State, Brazil
Ocorrência de Theileria equi em equinos criados na microrregião de Jaboticabal, Estado de São Paulo, Brasil
Cristiane Divan BaldaniI; Andrea Cristina Higa NakaghiII; Rosangela Zacarias MachadoII
IDepartamento de Medicina e Cirurgia Veterinária, Instituto de Veterinária, Universidade Federal Rural do Rio de Janeiro UFRRJ
IIDepartamento de Patologia Veterinária, Faculdade de Ciências Agrárias e Veterinárias, Universidade Estadual Paulista UNESP
Corresponding author Corresponding author: Cristiane Divan Baldani Laboratório de Patologia Clínica Departamento de Medicina e Cirurgia Veterinária Instituto de Veterinária Universidade Federal Rural do Rio de Janeiro UFRRJ Rod. Br 465, Km 47 CEP 23890-000, Seropédica - RJ, Brazil e-mail: crisbaldani@ufrrj.br
ABSTRACT
Blood and serum samples from 170 horses raised in the Jaboticabal microregion, São Paulo State, Brazil, were collected and tested by microscopic examination of blood smears, indirect fluorescent antibody test (IFAT) and nested polymerase chain reaction (nPCR) for Theileria equi infections. The association among the test results was verified by the McNemar test. During the examination of thin blood smears, parasites were detected in six (3.52%) horses. Anti-T. equi antibodies were detected in 100% sera samples, with titers ranging between 1:80 and 1:5120. The nPCR based on the T. equi merozoite antigen gene (EMA-1) allowed the visualization of specie-specific amplified product in 108 (63.53%) horses. All six samples judged positive microscopically were also positive for nPCR. Statistical analysis indicated general disagreement (p < 0.0001) between IFAT and nPCR; IFAT and blood smear; and nPCR and blood smear on the detection of parasite carriers. The results of the present study indicate that T. equi is widely spread among horses in the Jaboticabal microregion, Northeast region of São Paulo State, Brazil.
Keywords:Theileria equi, horse, diagnosis, IFAT, nested PCR.
RESUMO
Amostras de sangue e soro de 170 equinos criados na microrregião de Jaboticabal, Estado de São Paulo, Brasil, foram coletadas e avaliadas pelo exame direto em esfregaço sanguíneo, reação de imunofluorescência indireta (RIFI) e nested reação em cadeia da polimerase (nPCR) para a detecção de infecções por Theileria equi. A concordância dos resultados entre os testes de diagnóstico foi verificada pelo teste de McNemar. Durante o exame dos esfregaços sanguíneos, parasitos foram detectados em seis (3,52%) equinos. Anticorpos anti-T. equi foram detectados em 100% das amostras de soros, com títulos variando entre 1:80 e 1:5120. O nPCR, baseado na sequência do gene do antígeno de merozoíto de T. equi (EMA-1), permitiu a visualização de produtos de amplificação espécie-específico em 108 (63,53%) equinos. Houve diferença altamente significativa (p < 0,0001) entre RIFI e nPCR; RIFI e esfregaço sanguíneo; e nPCR e esfregaço na detecção do parasito. O resultado do presente estudo indica que a infecção por T. equi está amplamente distribuída entre os equinos na microrregião de Jaboticabal, região Nordeste do Estado de São Paulo, Brasil.
Palavras-chave:Theileria equi, equino, diagnóstico, RIFI, nested PCR.
Introduction
Theileria equi is a protozoan of the phylum Apicomplexa that is biologically transmitted by ixodid ticks. It causes disease in equids characterized by fever, anemia, icterus, hepatosplenomegaly, intravascular hemolysis, hemoglobinuria and in some cases death can occur (SCHEIN, 1988). However, most of the animals that recover from an acute or primary infection are carriers of the parasite for several years, becoming reservoirs for vector ticks (CACCIÒ et al., 2000; KNOWLES, 1996). Additionally, T. equi infection can be suppressed by chemotherapy but it cannot be completely eliminated (DE WAAL; VAN HEERDEN, 1994).
The disease has a worldwide distribution and is endemic in most tropical and subtropical areas as well as in some temperate zones of the world (BRÜNING, 1996; DE WAAL, 1992; SCHEIN, 1988). It has caused important economic losses in the horse industry, being a serious threat to the horse raising industry and international movement of horses (FRIEDHOFF et al., 1990). Indeed, there are regulatory import restrictions in some disease-free countries such as United States, Canada, Japan, Australia and New Zealand (BRÜNING, 1996). Nowadays, regulations usually require serological testing of horses in order to confirm seronegativity and to identify seropositive animals whose movement is restricted (BÖSE et al., 1995; BRÜNING, 1996).
Equine piroplasmosis can be diagnosed by several different methods. Direct microscopic identification of the parasite in stained blood smears is confirmatory, but it is usually difficult to find the organism in carrier animals since parasites are generally present in very low numbers in the blood. Therefore, despite its high specificity, microscopic examination of blood smears has low sensitivity for the detection of carrier animals (BÖSE et al., 1995). Hence several serological tests have been developed and are of great interest for the detection of specific antibodies, such as complement fixation test (CFT), indirect fluorescent antibody test (IFAT) and enzyme-linked immunosorbent assays (ELISA). The World Organization for Animal Health (2008) currently recommends IFAT or ELISA as tests of choice for the diagnosis of T. equi infection. Several studies have showed low sensitivity and specificity of CFT in identifying equine piroplasmosis carriers, and while they rarely give false positive results, it may occasionally result in a negative response in horses with latent infections (WEILAND et al., 1986). IFAT, on the other hand, is commonly used to detect specific antibodies to T. equi, especially in cases where CFT has proven to be inconclusive (BRÜNING, 1996). Although IFAT is more sensitive than CFT and rarely renders false negative results (TENTER; FRIEDHOFF, 1986), standardization is difficult, considering the subjectivity of the reader in assessing the results (BÖSE et al., 1995; BRÜNING, 1996). ELISA is nowadays an alternative for increased specificity and sensitivity in the detection of acute and latent babesial infections and several procedures of ELISA using recombinant antigen or crude antigen have been standardized (BALDANI et al., 2007; KUMAR et al., 2003; HIRATA et al., 2003; XUAN et al., 2001; KATZ et al., 2000).
Moreover, molecular diagnostic tools such as polymerase chain reaction (PCR) have been proven useful for the detection of equine piroplasmosis. The assays are mainly based on ribosomal 18S RNA sequence or EMA-1 gene and have showed to have high sensitivity and specificity (BALDANI et al., 2008; CACCIÒ et al., 2000; FRITZ, 2010; HEIM et al., 2007; NICOLAIEWSKY et al., 2001; RAMPERSAD et al., 2003). Therefore, this direct diagnostic method has now been incorporated to routine diagnosis, especially in association with serological assays. Another diagnostic technique available for equine piroplasmosis is in vitro blood culture, which has been used for the detection of carrier host animals. Although considered reliable and species-specific, it is a very time-consuming technique and unsuitable for routine diagnosis (BALDANI et al., 2008; HOLMAN et al., 1997). Therefore, the advantages and disadvantages of each test should be considered while choosing the best test for the diagnosis of equine piroplasmosis.
The objective of this study was to apply IFAT, nPCR and direct microscopic examination to determine the occurrence of T. equi infections in horses from stud farms in the Jaboticabal microregion, São Paulo State, Brazil, as well as to compare the performance of these diagnostic techniques.
Material and Methods
1. Horses and sample collection
A total of 170 horses were tested for T. equi, which were randomly selected from 8 stud farms of variable size in the Jaboticabal microregion (21º 15' 17" S and 48º 19' 20" W), Northeast region of São Paulo State, Brazil. The region is mainly hilly and is 0-605 m above sea level (INSTITUTO BRASILEIRO DE GEOGRAFIA E ESTATÍSTICA, 2007). The average annual temperature is 22 ºC. According to the Koppen classification, the climate is of Cwa type, i.e., mesothermic with hot and rainy summers and dry winters. The study was carried out between October 2003 and June 2004. Horse owners participated voluntarily and signed a consent form which clarified the objective of the study. At the moment of sample collection no clinical signs characteristic of equine babesiosis were seen and all owners stated that despite a tick control program in their farms, horses had been exposed to tick infestation.
Blood samples were collected from jugular venipuncture and placed in sterile tubes with and without anticoagulant EDTA. Serum samples were obtained by centrifugation at room temperature (25 ºC) at 3000 rpm for 10 minutes and were stored at 20 ºC until serological analysis. Tubes containing EDTA were either used immediately for blood smears or stored at 20 ºC for later use in nPCR.
2. Blood smears
Thin blood smears were prepared from anticoagulated blood, stained with Giemsa and then examined under light microscopy (magnification 1000x; 100 fields) to check for the presence of intracellular parasites.
3. Indirect fluorescent antibody test (IFAT)
IFAT antigen was prepared from blood of horses experimentally infected with Jaboticabal T. equi strain (GenBank accession no. AF255730) and its sensitivity and specificity were assessed in a previous study (BALDANI et al., 2007). IFAT procedure essentially followed those described by Baldani et al. (2007). Sera were considered positive if they showed strong fluorescence of parasites at a dilution of 1:80 or above. The size, appearance and density of staining were compared with a positive control in which a positive reaction was seen as apple green fluorescent inclusions within infected erythrocytes while a negative reaction showed a uniform red counter stain.
4. Nested PCR (nPCR)
DNA extraction was performed from EDTA-whole blood sample by the Puregene Kit (Gentra Systems Inc., USA) in accordance with the manufacturer's protocol. DNA concentrations were determined using the NanoDrop ND-1000 spectrometer (NanoDrop Technologies, DE, USA). Nested PCR assays were performed as described previously by Nicolaiewsky et al. (2001) and Baldani et al. (2008). The primers sets EMAE-F (5'-CCGCCCTTCACCTCGTTCTCAA-3')/EMAE-R (5'-TCTCGGCGGCATCCTTGACCTC-3') and EMAI-F (5'-CCGTCTCCGTTGACTTGGCCG-3')/EMAI-R (5'-GGACGCGCTTGCCTGGAGCCT-3') were chosen to flank 396 and 102 bp regions of the EMA-1 gene sequence (GenBank accession number L13784), respectively. PCR products were detected by electrophoresis on 1% agarose gel stained with ethidium bromide.
5. Statistical analysis
Statistical analysis was performed using the McNemar test in which the association between direct microscopic identification of the parasite in stained blood smears, IFAT and nPCR were assessed comparing positive rates obtained for T. equi diagnosis.
Results
Of 170 blood samples examined by microscopic observation of Giemsa-stained blood smears, merozoites of T. equi were detected in six horses (3.52%). Intraerythrocytic stages of T. equi appeared typical and care was taken to differentiate B. caballi from T. equi in smears of horses.
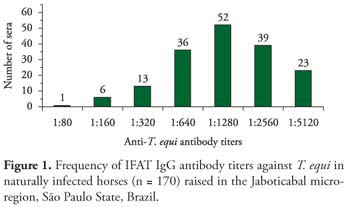
The overall antibody frequencies at titers 1:80 or greater for T. equi in 170 horses from the stud farms studied are showed in Figure 1. Anti-T. equi antibodies were detected in all 170 (100%) horses, with antibody titers ranging between 1:80 and 1:5120. The highest frequencies of titers were 1:1280 and 1:2560 in 52 (30.58%) and 39 (22.94%) horses, respectively.
The specific T. equi merozoite 102 pb gene fragment (EMA-1) was detected in 108 (63.53%) horses. The results of nPCR in horse blood samples are showed in Figure 2. All 6 samples judged positive microscopically were also positive for nPCR with T. equi specific primers. However, 102 horses found to be negative by microscopic examination were positive by nPCR.
Statistical analysis showed differences (p < 0.0001) between IFAT and nPCR; IFAT and blood smear; and nPCR and blood smear in the diagnosis of T. equi infection.
Discussion
Equine piroplasmosis is of great importance due to the international movement of horses, especially for horses that travel to equestrian sport events. And because of the upcoming V Military World Games in 2011 and the 2016 Olympic Games, both in the city of Rio de Janeiro, there is an increased concern in Brazil due to risk of transmission of the parasite from carrier horses (FRIEDHOFF, 1988). Moreover, some countries maintain stringent restrictions that prevent import of horses serologically positive for piroplasma species (BRÜNING, 1996).
In the present study, the occurrence of T. equi infection in horses of the Jaboticabal microregion was investigated by blood smear identification, IFAT and nPCR. The results demonstrate that T. equi is widespread in the region studied, suggesting high levels of transmission as there was found a high rate of positive animals by serological and molecular methods. Additionally, high titers of specific antibodies were detected in the IFAT test.
The positive rate of T. equi seen in the present study on direct microscopic identification was very low and is consistent with previous studies (RAMPERSAD et al., 2003; HEIM et al., 2007; SEVINC et al., 2008; MORETTI et al., 2010). Despite its high specificity, this method lacks sensitivity, especially in the diagnosis of subclinical infections when parasitemia becomes too low to detect positive cases (BÖSE et al., 1995). Statistical analysis showed significant differences (p < 0.0001) between IFAT and blood smear in the diagnosis of T. equi infection. All horses in the present study were adult and had been exposed to ticks and consequently to the parasite several times as demonstrated by IFAT results in which all horses were seropositive. As so the majority probably developed protective immunity and became chronically infected carriers, explaining the results found in the study. Similarly, the horse population studied did not have clinical signs of piroplasmosis.
In our study, nPCR revealed that 108 (63.53%) horses were positive for T. equi, and all were positive by serology (108/108). All six samples positive microscopically were also positive for nPCR. Molecular methods have been extensively used to detect T. equi infection (CACCIÒ et al., 2000; NICOLAIEWSKY et al., 2001; RAMPERSAD et al., 2003; HEIM et al., 2007; BALDANI et al., 2008; FRITZ, 2010). The nPCR using oligonucleotides designed on the sequence of a T. equi merozoite antigen gene (EMA-1) used in the present study was able to detect the parasite in blood with a corresponding parasitemia of 0.000008% (BALDANI et al., 2008) or 0.000006%, equal to six infected cells out of 108 erythrocytes (NICOLAIEWSKY et al., 2001). As expected, significant differences (p < 0.0001) were detected between nPCR and blood smear for the detection of parasite carriers. The enhanced sensitivity of this direct diagnosis method contributes for high prevalent rate of T. equi in blood samples and is comparable to those reported in several other studies conducted in South Africa (BHOORA et al., 2009) and France (FRITZ, 2010), although with different set of primers or using real-time PCR. In Brazil, Heim et al. (2007) evaluated horses from different endemic regions and reported a prevalence of 59.7% for T. equi by a multiplex real-time PCR. And Battsetseg et al. (2002) evaluated blood samples from horses of Mato Grosso state and found 96% of positive rate for T. equi by nPCR.
Moreover, 62 (36.47%) horses were positive only by IFAT, being negative by both direct diagnosis methods, nPCR and stained blood smears. As so significant differences (p < 0.0001) between the three tests were detected. Antibodies can still be found after the parasite has been eliminated (JAFFER et al., 2010), but T. equi parasites are usually not completely eliminated from blood of horses after treatment or natural recovery, and horses may remain as lifelong carriers (DE WAAL, 1992). Additionally, molecular detection of these agents requires DNA isolation from parasites that are physically present in the blood sample to a detectable level above the sensitivity threshold of the method used (SALIM et al., 2008). Therefore, failure to detect T. equi by nPCR is most probably due to parasite clearance from the circulating blood or reduction to a level beyond the detection sensitivity of the assay used.
The study of equine piroplasmosis in Brazilian horses showed that the disease is a serious problem in the country and it is considered to be endemic. Previous epidemiological studies carried out in several Brazilian states show prevalence rates ranging from 17 to 100% (PFEIFER BARBOSA et al., 1995; HEUCHERT et al., 1999; KERBER et al., 1999; XUAN et al., 2001). Our results showed high prevalence for T. equi in the northeast region of São Paulo, and although relatively higher, they are consistent with those reported by Xuan et al. (2001) and Baldani et al. (2004) in the state, with a prevalence of 81 and 75% for T. equi, respectively. Heim et al. (2007) reported seroprevalence of 91.0% for T. equi in samples collected from horses at a slaughterhouse in the state of Minas Gerais, Southeast Brazil, in which some horses were originally from São Paulo State. However, Heuchert et al. (1999) tested 752 serum samples from the state of São Paulo by IFAT and CFT and reported prevalence rates of 29.6 and 17.6%, respectively. More recently, Kerber et al. (2009) reported an overall prevalence of 21.6% for T. equi by CFT and cELISA in horses of stud farms also in the State of São Paulo. The rate of equine piroplasmosis found in this study is significantly higher and is probably due to differences in the management of horses, which appears to be an important factor for the prevalence of T. equi infections, and, to a lesser extent, due to the number of serum samples examined. It has been demonstrated that when horses have direct or indirect contact with cattle and there is no rigorous tick control program, T. equi infection rates are much higher (HEUCHERT et al., 1999; KERBER et al., 1999). Indeed, the horses studied had history of contact with cattle and although there was tick control, several of them had tick infestations. The detection of high titers in more than half of the horses suggests that these animals are constantly exposed to ticks infected by T. equi. Considering that T. equi may remain as a lifelong infection (DE WAAL, 1992), it is likely that the horses of the present study are carriers, transmitting the parasite to ticks.
It has been pointed out that serological and molecular assays can be a more objective tool for the diagnosis of equine piroplasmosis (PERSING; CONRAD, 1995). Thus, it can be reasonably concluded that T. equi infections are highly prevalent in the Jaboticabal microregion, Northeast region of São Paulo State, and therefore the establishment of an appropriate and effective control program is of great importance. Despite the healthy appearance of horses in the present study, these carriers can transmit the parasite to ticks and are a potential continuous source for maintaining and disseminating the organism in horse population. Given the territorial extension of Brazil, further investigations on equine piroplasmosis are required for providing baseline information about its epidemiology, distribution and tick vectors.
Acknowledgements
The authors are grateful to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for its financial support.
Received July 21, 2010
Accepted August 31, 2010
- BALDANI, C. D. et al. An enzyme-linked immunosorbent assay for the detection of IgG antibodies against Babesia equi in horses. Ciência Rural, v. 34, n. 5, p. 1525-1529, 2004.
- BALDANI, C. D. et al. Serodiagnosis of Babesia equi in horses submitted to exercise stress. Pesquisa Veterinária Brasileira, v. 27, n. 4, p. 179-183, 2007.
- BALDANI, C. D. In vitro culture, PCR, and nested PCR for the detection of Theileria equi in horses submitted to exercise. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, v. 60, n. 3, p. 550-558, 2008.
- BATTSETSEG, B. et al. Detection of natural infection of Boophilus microplus with Babesia equi and Babesia caballi in Brazilian horses using nested polymerase chain reaction. Veterinary Parasitology, v. 107, n. 4, p. 351-357, 2002.
- BHOORA, R. et al. Sequence heterogeneity in the 18S r RNA gene within Theileria equi and Babesia caballi from horses in South Africa. Veterinary Parasitology, v. 159, n. 2, p. 112-120, 2009.
- BÖSE, R. et al. Current state and future trends in the diagnosis of babesiosis. Veterinary Parasitology, v. 57, n. 1-3, p. 61-74, 1995.
- BRÜNING, A. Equine piroplasmosis an update on diagnosis, treatment and prevention. British Veterinary Journal, v. 152, n. 2, p. 139-151, 1996.
- CACCIÒ, S. et al. The beta-tubulin gene of Babesia and Theileria parasites is an informative marker for species discrimination. International Journal for Parasitology, v. 30, n. 11, p. 1181-1185, 2000.
- DE WAAL, D. T. Equine piroplasmosis: a review. British Veterinary Journal, v. 148, n. 1, p. 6-14, 1992.
- DE WAAL, D. T.; HEERDEN, J. V. Equine babesiosis. In: JAW, C., THOMSON, G. R., TUSTIN, R. C. (Ed.). Infectious diseases of livestock with special reference to South Africa. vol. 1. Oxford: University Press, 1994. p. 293-304.
- FRIEDHOFF, K. T. Transmission of Babesia In: RISTIC, M. Babesiosis of domestic animals and man Boca Raton: CRC Press, 1988. p. 23-52.
- FRIEDHOFF, K. T.; TENTER, A. M.; MULLER, I. Haemoparasites of equines: impact on internacional trade of horses. Revue Scientifique et Technique, v. 9, n. 4, p. 1187-1194, 1990.
- FRITZ, D. A PCR study of piroplasms in 166 dogs and 111 horses in France (March 2006 to March 2008). Parasitology Research, v. 106, n. 6, p. 1339-1342, 2010.
- HEIM, A. et al. Detection and molecular characterization of Babesia caballi and Theileria equi isolates from endemic areas of Brazil. Parasitology Research, v. 102, n. 1, p. 63-68, 2007.
- HEUCHERT, C. M. et al. Seroepidemiologic studies on Babesia equi and Babesia caballi infections in Brazil. Veterinary Parasitology, v. 85, n. 1, p. 1-11, 1999.
- HIRATA, H. et al. Identification of a specific antigenic region of the P82 protein of Babesia equi and its potential use in serodiagnosis. Journal of Clinical Microbiology, v. 41, n. 2, p. 547-551, 2003.
- HOLMAN, P. J. et al. Case report: field-acquired subclinical Babesia equi infection confirmed by in vitro culture. Journal of Clinical Microbiology, v. 35, n. 2, p. 474-476, 1997.
- INSTITUTO BRASILEIRO DE GEOGRAFIA E ESTATÍSTICA - IBGE. Produção da pecuária municipal, 2006 Malha municipal digital do Brasil: situação em 2006. Rio de Janeiro: IBGE, 2007.
- JAFFER, O. et al. A comparative study of serological tests and PCR for the diagnosis of equine piroplasmosis. Parasitology Research, v. 106, n. 3, p. 709-713, 2010.
- KATZ, J.; DEWALD, R.; NICHOLSON, J. Procedurally similar competitive immunoassay systems for the serodiagnosis of Babesia equi, Babesia caballi, Trypanosoma equiperdum, and Burkholderia mallei infection in horses. Journal of Veterinary Diagnostic Investigation, v. 12, n. 1, p. 46-50, 2000.
- KERBER, C. E. et al. Prevalence of equine piroplasmosis and its association with tick infestation in the State of São Paulo, Brazil. Revista Brasileira de Parasitologia Veterinária, v. 18, n. 4, p. 1-8, 2009.
- KERBER, C. E.; FERREIRA, F.; PEREIRA, M. C. Control of equine piroplasmosis in Brazil. Onderstepoort Journal of Veterinary Research, v. 66, n. 2, p. 123-127, 1999.
- KNOWLES, D. P. Control of Babesia equi parasitemia. Parasitology Today, v. 12, n. 5, p. 195-198, 1996.
- KUMAR, S. et al. Standardisation and comparison of serial dilution and single dilution enzyme linked immunosorbent assay (ELISA) using different antigenic preparations of the Babesia (Theileria) equi parasite. Veterinary Research, v. 34, n. 1, p. 71-83, 2003.
- MORETTI, A. et al. Prevalence and diagnosis of Babesia and Theileria infections in horses in Italy: a preliminary study. Veterinary Journal, v. 184, n. 3, p. 346-350, 2010.
- NICOLAIEWSKY, T. B. et al. Detection of Babesia equi (Laveran, 1901) by nested polymerase chain reaction. Veterinary Parasitology, v. 101, n. 1, p. 9-21, 2001.
- PERSING, D. H.; CONRAD, P. A. Babesiosis: new insights from phylogenetic analysis. Infectious Agents and Disease, v. 4, n. 4, p. 182-195, 1995.
- PFEIFER BARBOSA, I. et al. Epidemiological aspects of equine babesioses in a herd of horses in Brazil. Veterinary Parasitology, v. 58, n. 1-2, p. 1-8, 1995.
- RAMPERSAD, J. et al. A field evaluation of PCR for the routine detection of Babesia equi in horses. Veterinary Parasitology, v. 114, n. 2, p. 81-87, 2003.
- SALIM, B. O. M. et al. Diagnosis of Babesia caballi and Theileria equi infections in horses in Sudan using ELISA and PCR. Parasitology Research, v. 103, n. 5, p. 1145-1150, 2008.
- SCHEIN, E. Equine babesiosis. In: RISTIC, M. (Ed.). Babesiosis of domestic animals and man Boca Raton: CRS Press, 1988. p. 197-208.
- SEVINC, F. et al. A comparative study on the prevalence of Theileria equi and Babesia caballi infections in horse sub-populations in Turkey. Veterinary Parasitology, v. 156, n. 3-4, p. 173-177, 2008.
- TENTER, A. M.; FRIEDHOFF, K. T. Serodiagnosis of experimental and natural Babesia equi and B. caballi infections. Veterinary Parasitology, v. 20, n. 1-3, p. 49-61, 1986.
- WEILAND, G. Species-specific serodiagnosis of equine piroplasma infections by means of complement fixation test (CFT), immunofluorescence (IIF), and enzyme-linked immunosorbent assay (ELISA). Veterinary Parasitology, v. 20, n. 1-3, p. 43-48, 1986.
- WORLD ORGANIZATION FOR ANIMAL HEALTH - OIE. Equine piroplasmosis 2008. Disponível em: <http://www.oie.int/esp/.../mmanual/2008/.../2.05.08_EQUINE_PIROPLASMOSIS.pdf.htm>.Acesso em: 17 jun. 2010.
- XUAN, X. et al. Diagnosis of equine piroplasmosis in Brazil by serodiagnostic methods with recombinant antigens. Journal of Veterinary Medical Science, v. 63, n. 10, p. 1159-1160, 2001.
Corresponding author:
Publication Dates
-
Publication in this collection
07 Feb 2011 -
Date of issue
Dec 2010
History
-
Accepted
31 Aug 2010 -
Received
21 July 2010