Abstracts
Ninety specimens of Plagioscion squamosissimus captured using fishing tackle in the Outeiro district, state of Pará, were examined. Fish were placed in plastic bags containing water, under conditions of artificial aeration, and transported live to the Carlos Azevedo Research Laboratory (LPCA), in Belém, Pará. They were anesthetized, euthanized and necropsied; small fragments of the epaxial and hypaxial muscles were removed for examination of fresh histological sections by means of optical microscopy. In 100% of the specimens analyzed, parasitic pseudocysts were seen to be interspersed within and between the skeletal muscle. These contained pseudoquadrate and/or star-shaped spores that presented four valves and four polar capsules, which were identified from their morphology as belonging to the genus Kudoa. This is the first report of Kudoa in P. squamosissimus in the Amazon region, Pará, Brazil.
White fish; histology; amazonian region; Myxozoan; parasite; Plagioscion squamosissimus
Foram observados 90 exemplares de P. squamosissimus, capturados com apetrechos de pesca no distrito de Outeiro, estado do Pará. Os animais foram acondicionados em sacos plásticos com água do habitat e aeração artificial, transportados vivos até o Laboratório de Pesquisa Carlos Azevedo (LPCA), em Belém-PA. Foram anestesiados, eutanasiados e necropsiados; pequenos fragmentos da musculatura epi e hipoaxial foram retirados para observação, a fresco e para histologia em microscopia de luz (ML). Em 100% dos exemplares analisados, foi observada a presença de pseudocistos parasitários entremeados, dentro e entre as fibras musculares estriadas esqueléticas, onde observou-se a presença de esporos de formato pseudoquadrado e/ou estrelado, com quatro valvas e quatro cápsulas polares, sendo identificados pela sua morfologia como pertencentes ao gênero Kudoa. Este é o primeiro relato de Kudoa em P. squamosissimus na Amazônia, Pará, Brasil.
Pescada branca; histologia; região amazônica; myxosporídios; parasito; Plagioscion squamosissimus
Introduction
The Amazon region has an abundance and diversity of fish and is very important for commercial and artisanal fishing. The Outeiro district of Belém is located on the island of Caratateua, which is situated 18 km from the main urban area of Belém (the state capital of Pará) and is connected directly to the Icoaraci district. The island is surrounded by the murky, muddy freshwater of the Guajará bay (PEREIRA, 2001Pereira KRB. Caracterização geoquímica de sedimentos de fundo da orla de Belém - Pará [Dissertação]. Belém: Universidade Federal do Pará; 2001.). Because of the influence of the bay, a wide variety of fish is available on the island, thereby allowing local subsistence and artisanal fishing. Among the variety of fish species is Plagioscion squamosissimus Heckel, 1840, which is popularly known as hake and accounts for one of the largest productions of artisanal and industrial fishing (BARTHEM, 1985Barthem RB. Ocorrência, distribuição e biologia dos peixes da Baía de Marajó, Estuário Amazônico. Bol Mus Para Emilio Goeldi 1985; 2(1): 49-69.; BOEGER & KRITSKY, 2003Boeger WAB, Kritsky DC. Parasites, Fossils and Geologic History: Historical Biogeography of the South American Freshwater Croakers, spp. (Teleostei, Sciaenidae). Plagioscion Zool Scr 2003; 32(1): 3-11. http://dx.doi.org/10.1046/j.1463-6409.2003.00109.x.
http://dx.doi.org/10.1046/j.1463-6409.20...
; VIANA et al., 2006Viana AP, Frédou T, Lucena F. Aplicações de técnicas morfométricas no estudo da morfometria de pescada branca, Plagioscion squamosissimus, Heckel (1940), Perciformes, Sciaenidae, desembarcada na ilha de Mosqueiro-PA. Bol Lab Hidrobiol 2006; 19(1): 1-12.). This species has benthopelagic habits and is carnivorous (BARTHEM, 1985Barthem RB. Ocorrência, distribuição e biologia dos peixes da Baía de Marajó, Estuário Amazônico. Bol Mus Para Emilio Goeldi 1985; 2(1): 49-69.; BOEGER & KRITSKY, 2003Boeger WAB, Kritsky DC. Parasites, Fossils and Geologic History: Historical Biogeography of the South American Freshwater Croakers, spp. (Teleostei, Sciaenidae). Plagioscion Zool Scr 2003; 32(1): 3-11. http://dx.doi.org/10.1046/j.1463-6409.2003.00109.x.
http://dx.doi.org/10.1046/j.1463-6409.20...
; SANTOS et al., 2006Santos GM, Ferreira EJG, Zuanon JAS. Peixes comerciais de Manaus. Manaus: IBAMA/ProVárzea; 2006.; VIANA et al., 2006Viana AP, Frédou T, Lucena F. Aplicações de técnicas morfométricas no estudo da morfometria de pescada branca, Plagioscion squamosissimus, Heckel (1940), Perciformes, Sciaenidae, desembarcada na ilha de Mosqueiro-PA. Bol Lab Hidrobiol 2006; 19(1): 1-12.). In Pará, this species has high commercial value, both in freshwater fishing in the estuary and also possibly due to the changes in salinity in the dry season (BARTHEM, 1985Barthem RB. Ocorrência, distribuição e biologia dos peixes da Baía de Marajó, Estuário Amazônico. Bol Mus Para Emilio Goeldi 1985; 2(1): 49-69.).
Interest in studying parasites in fish has increased over recent years because of the consequences and economic losses produced by their presence, as well as the hazards that they present to human health, with the possibility of food and/or allergic poisoning. Parasites of the phylum Myxozoa, which includes myxosporidians, have been the subject of many studies recently. The locations of aquatic myxosporidians in their hosts are varied, and they are found in nearly all tissues and organs. The species of this genus are mostly histozoic (intercellular or intracellular) and are often found in the somatic musculature of their hosts. They can also be found in the heart, intestines, gills, brain, kidneys, gallbladder and blood (LOM & DYKOVÁ, 2006Lom J, Dyková I. Myxozoan genera: definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitol (Praha) 2006; 53(1): 1-36. http://dx.doi.org/10.14411/fp.2006.001. PMid:16696428
http://dx.doi.org/10.14411/fp.2006.001...
; MACIEL et al., 2011Maciel PO, Affonso EG, Boijink CL, Tavares-Dias M, Inoue LAKA. sp. (Myxozoa) in the circulating blood of . Myxobolus
Colossoma macropomum (Osteichthyes, Characidae)Rev Bras Parasitol Vet 2011; 20(1): 82-84. http://dx.doi.org/10.1590/S1984-29612011000100018. PMid:21439240
http://dx.doi.org/10.1590/S1984-29612011...
).
Most species of myxosporidians have pathogenic effects on their hosts. For example, the genus Kudoa currently consists of more than 95 species (EIRAS et al., 2014Eiras JC, Saraiva A, Cruz C. Synopsis of the species of Kudoa Meglitsch, 1947 (Myxozoa: Myxosporea: Multivalvulida). Syst Parasitol 2014; 87(2): 153-180. http://dx.doi.org/10.1007/s11230-013-9461-4. PMid:24474038
http://dx.doi.org/10.1007/s11230-013-946...
) and causes lesions to the somatic musculature of fish. This may have a significant economic impact through softening of the flesh, with or without formation of macroscopic pseudocysts, which gives rise to postmortem myoliquefaction (ANDRADA et al., 2005Andrada CDG, Tortelly R, Nogueira PP, Andrade CL, Lima FC. Infecção por Kudoa Meglitsch, 1947 (Myxozoa: Multivalvulida) em musculatura esquelética de espada Trichiurus L. (Teleostei: Trichiuridae). lepturusParasitol latinoam 2005; 60(3-4): 150-153.). This muscle lysis effect is caused by the action of proteases that the parasites produce and use to soften the muscles of the host in order to promote their own development (ANDRADA et al., 2005Andrada CDG, Tortelly R, Nogueira PP, Andrade CL, Lima FC. Infecção por Kudoa Meglitsch, 1947 (Myxozoa: Multivalvulida) em musculatura esquelética de espada Trichiurus L. (Teleostei: Trichiuridae). lepturusParasitol latinoam 2005; 60(3-4): 150-153.). This effect compromises the acceptance of these fish among consumers. Furthermore, there is evidence that consumption of fish that have been infected by Kudoa may cause allergic symptoms (MARTÍNEZ DE VELASCO et al., 2007Martínez de Velasco G, Rodero M, Chivato T, Cuéllar C. Seroprevalence of anti- sp. (Myxosporea: Multivalvulida) antibodies in a Spanish population. KudoaParasitol Res 2007; 100(6): 1205-1211. http://dx.doi.org/10.1007/s00436-006-0390-x. PMid:17177059
http://dx.doi.org/10.1007/s00436-006-039...
; GRABNER et al., 2012Grabner DS, Yokoyama H, Shirakashi S, Kinami R. Diagnostic PCR assays to detect and differentiate and . Kudoa septempunctata, K. thyrsites
K. lateolabracis (Myxozoa, Multivalvulida) in muscle tissue of olive flounder (Paralichthys olivaceus)Aquaculture 2012; 338-341: 36-40. http://dx.doi.org/10.1016/j.aquaculture.2012.01.022.
http://dx.doi.org/10.1016/j.aquaculture....
; KAWAI et al., 2012Kawai T, Sekizuka T, Yahata Y, Kuroda M, Kumeda Y, Iijima Y, et al. Identification of as the causative agent of novel food poisoning outbreaks in Japan by consumption of in raw fish. Kudoa septempunctata
Paralichthys olivaceusClin Infect Dis 2012; 54(8): 1046-1052. http://dx.doi.org/10.1093/cid/cir1040. PMid:22281845
http://dx.doi.org/10.1093/cid/cir1040...
). The present study describes a infection in muscle tissue of Plagioscion
squamosissimus fish caught in the Amazon Region.
Materials and Methods
Ninety specimens of the freshwater fish P. squamosissimus Heckel, 1840 (Teleostei, Perciformes), Brazilian common name “pescada branca”, i.e. “white fish”, were collected from the Amazonian estuarine region of Outeiro (1° 14’ S; 48° 26’ W) near the city of Belém. The mean total length of the fish was 12.28 ± 3.67 cm (range: 7.5-18.30) and their mean weight was 25.14 ± 18.48 g (range: 6.12-61.8). They were lightly anesthetized using MS 222 Sigma (Sabdoz Laboratories) diluted in freshwater and after euthanize samples of infected epaxial and hypaxial muscles were taken for optical and electron microscopy studies. For optical microscopy, small fragments (0.5 cm) of parasitized tissue from the palate of the fish were fixed in Davidson solution (neutral buffered formalin, glacial acetic acid, 95% ethyl alcohol and distilled water) for 24 h and were then processed and stained with hematoxylin-eosin (HE), May-Grunwald-Giemsa and Ziehl-Neelsen (LUNA, 1968Luna LG. Manual of histologic staining methods of the armed forces institute of pathology. 3rd ed. New York: MacGraw-Hill; 1968.). The stained sections were documented using a Zeiss Primo Star optical microscope and Zeiss Axio Cam ERc 5s microscope camera, with micrometrics imaging software. Some fragments containing tissue cysts were analyzed using a differential interference contrast (DIC) microscope (Nomarski). Measurements on the spores were made in micrometers (µm), with minimum and maximum values in parentheses. The dimensions were expressed as means ± standard deviations. To calculate the statistical data, the BioStat 5.0 software was used (AYRES et al., 2007Ayres M, Ayres-Junior M, Ayres DL, Santos AS, Ayres LL. Bioestat 5.0 aplicações estatísticas nas áreas das ciências bi-médicas. Belém: IDSM; 2007.). Parasite prevalence was analyzed according to Bush et al. (1997)Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 1997; 83(4): 575-583. http://dx.doi.org/10.2307/3284227. PMid:9267395
http://dx.doi.org/10.2307/3284227...
. The measurements of the spores were made in accordance to Lom & Dyková (1992)Lom J, Dyková I. Development in aquaculture and fisheries science. Amsterdam: Elsevier; 1992. 315 p. vol. 26. Protozoan Parasites of Fishes., under 1000X magnification.
Results and Discussion
In fresh preparations of fragments of muscle tissue viewed under an optical microscope, pseudocysts were seen within and between the muscle fibers. When this material was squashed between the slide and cover slip, large numbers of spores of different shapes and sizes were observed (Figure 1). The rectangular or pseudoquadrate format and/or star shape, with four valves and four polar capsules (Figures 2 and 3), was identified from its morphology as belonging to the genus Kudoa. The taxonomic classification was as follows:
Optical micrographs: 1. Cysts of Kudoa spp. (*) in fresh muscle of P. squamosissimus Bar= 100 μm. Spores of Kudoa spp. in DIC: 2. stellate in format; 3. Pseudoquadrade in format. Bar = 5 μm.
Phylum: Myxozoa Grassé, 1970
Class: Myxosporea Bütschli, 1881
Order: Multivalvulidae Shulman, 1959
Family: Kudoidae Meglitsch, 1960
Genus: Kudoa Meglitsch, 1947
The information on the morphological data of the parasite and on the site of infection is corroborated by Heckmann (2012)Heckmann RA. Histopathology and fine structure of two myxosporidans, and . Kudoa clupeidae
Henneguya sebasta and a microsporidian glugea infecting fishes from the California, USA coast, histozoic parasitesProc Parasitol 2012; 54: 1-25.; Moran et al. (1999)Moran JDW, Whitaker DJ, Kent ML. A review of the myxosporean genusKudoaMeglitsch, 1947, and its impact on the international aquaculture industry and commercial fisheries. Aquaculture 1999; 172(1-2): 163-196. http://dx.doi.org/10.1016/S0044-8486(98)00437-2.
http://dx.doi.org/10.1016/S0044-8486(98)...
; Lom & Dyková (1992)Lom J, Dyková I. Development in aquaculture and fisheries science. Amsterdam: Elsevier; 1992. 315 p. vol. 26. Protozoan Parasites of Fishes., who reported that members of the genus Kudoa infect muscle tissues of their hosts and can present starry, square or rounded square shape in apical view.
Some studies on other host species have already recorded spores of stellate Kudoa format infecting the somatic musculature, like Kudoa histolytica Pérard, 1928; Kudoa cruciformum Matsumoto, 1954; Kudoa kabatai Shulman and Kovaleva, 1979; Kudoa bengalensis Sarkar and Mazumder, 1983; Kudoa mirabilis Naidenova and Gaevskaya, 1991; Kudoa cynoglossi Obiekezie and Lick, 1994; and Kudoa miniauriculata Whitaker et al., 1996; and also in pseudoquadrate format: Kudoa funduli Hahn, 1915; Kudoa clupeidae Hahn, 1917; Kudoa crumena Iversen and Van Meter, 1967; Kudoa alliaria Shulman and Kovaleva, 1979; Kudoa amamiensis Egusa and Nakajima, 1980; Kudoa caudata Kovaleva and Gaevskaya, 1983; and Kudoa leiostomi Dyková et al., 1994 (MORAN et al., 1999Moran JDW, Whitaker DJ, Kent ML. A review of the myxosporean genusKudoaMeglitsch, 1947, and its impact on the international aquaculture industry and commercial fisheries. Aquaculture 1999; 172(1-2): 163-196. http://dx.doi.org/10.1016/S0044-8486(98)00437-2.
http://dx.doi.org/10.1016/S0044-8486(98)...
).
The infection site observed was limited to the skeletal muscles of the host (P. squamosissimus), and pseudocysts could not be viewed macroscopically in any infected animal. According to Abdel-Ghaffar et al. (2012)Abdel-Ghaffar F, Morsy K, Mehlhorn H, Bashtar AR, Shazly MA, Saad AH, et al. First report of Kudoa species (Myxozoa: Kudoidae) infecting the spotted coral grouper Plectropomus from the Red Sea. A light and ultrastructural study. maculatesParasitol Res 2012; 111(4): 1579-1585. http://dx.doi.org/10.1007/s00436-012-3011-x. PMid:22740296
http://dx.doi.org/10.1007/s00436-012-301...
, most of the genus Kudoa form macroscopic pseudocysts in muscles and cause economic problems with regard to sale of infected fish. On the other hand, like in the results from the present study, little or no inflammatory reaction has been found associated with parasitosis (ANDRADA et al., 2005Andrada CDG, Tortelly R, Nogueira PP, Andrade CL, Lima FC. Infecção por Kudoa Meglitsch, 1947 (Myxozoa: Multivalvulida) em musculatura esquelética de espada Trichiurus L. (Teleostei: Trichiuridae). lepturusParasitol latinoam 2005; 60(3-4): 150-153.; CASAL et al., 2008Casal G, Matos E, Matos P, Azevedo C. Ultrastructural Description of a New Myxosporean Parasite sp. n. (Myxozoa, Myxosporea), found in the Sub-Opercular Musculature of . Kudoa aequidens
Aequidens plagiozonatus (Teleostei) from the Amazon RiverActa Protozool 2008; 47: 135-141.; BURGER & ADLARD, 2010Burger MAA, Adlard RD. Four new species of Meglitsch, 1947 (Myxosporea: Multivalvulida) from Australia with recommendations for species descriptions in the Kudoidae. KudoaParasitology 2010; 137(5): 793-814. http://dx.doi.org/10.1017/S0031182009991557. PMid:20025820
http://dx.doi.org/10.1017/S0031182009991...
; HEINIGER & ADLARD, 2012Heiniger H, Adlard RD. Host specificity and local infection dynamics of n. sp. (Multivalvulida: Kudoidae) from the pericardial cavity of two spp. (Perciformes: Apogonidae) at Lizard Island lagoon, Queensland, Australia. Kudoa leptacanthae
ZoramiaParasitol Int 2012; 61(4): 697-706. http://dx.doi.org/10.1016/j.parint.2012.08.001. PMid:22922116
http://dx.doi.org/10.1016/j.parint.2012....
; GRABNER et al., 2012Grabner DS, Yokoyama H, Shirakashi S, Kinami R. Diagnostic PCR assays to detect and differentiate and . Kudoa septempunctata, K. thyrsites
K. lateolabracis (Myxozoa, Multivalvulida) in muscle tissue of olive flounder (Paralichthys olivaceus)Aquaculture 2012; 338-341: 36-40. http://dx.doi.org/10.1016/j.aquaculture.2012.01.022.
http://dx.doi.org/10.1016/j.aquaculture....
; HEINIGER et al., 2013Heiniger H, Cribb TH, Adlard RD. Intra-specific variation of Kudoa spp. (Myxosporea: Multivalvulida) from apogonid fishes (Perciformes), including the description of two new species, K. cheilodipteri n. sp. and n. sp., from Australian waters. K. cookiiSyst Parasitol 2013; 84(3): 193-215. http://dx.doi.org/10.1007/s11230-012-9400-9. PMid:23404757
http://dx.doi.org/10.1007/s11230-012-940...
). According to Grabner et al. (2012)Grabner DS, Yokoyama H, Shirakashi S, Kinami R. Diagnostic PCR assays to detect and differentiate and . Kudoa septempunctata, K. thyrsites
K. lateolabracis (Myxozoa, Multivalvulida) in muscle tissue of olive flounder (Paralichthys olivaceus)Aquaculture 2012; 338-341: 36-40. http://dx.doi.org/10.1016/j.aquaculture.2012.01.022.
http://dx.doi.org/10.1016/j.aquaculture....
, Kudoa species were found in the muscle tissue of Paralichthys olivaceus, but no cyst formation or myoliquefaction was visible. On the other hand, myoliquefaction was found in Paralichthys orbignyanus parasitized by Myxobolus sp. (EIRAS et al., 2007Eiras JC, Júnior JP, Sampaio LA, Robaldo R, Abreu PC. sp. can cause in vivo myoliquefaction in the host . Myxobolus
Paralichthys orbignyanus (Osteichthyes, Paralichthydae)Dis Aquat Organ 2007; 77(3): 255-258. http://dx.doi.org/10.3354/dao01852. PMid:18062475
http://dx.doi.org/10.3354/dao01852...
). In Trichiurus lepturus, parasitic cysts were found in somatic muscle fibers, but with no inflammatory reaction (ANDRADA et al., 2005Andrada CDG, Tortelly R, Nogueira PP, Andrade CL, Lima FC. Infecção por Kudoa Meglitsch, 1947 (Myxozoa: Multivalvulida) em musculatura esquelética de espada Trichiurus L. (Teleostei: Trichiuridae). lepturusParasitol latinoam 2005; 60(3-4): 150-153.). In Aequidens plagiozonatus, Kudoa
aequidens was reported with ultrastructural data; this was first reported in Brazilian aquatic fauna in the subopercular muscles, thus emphasizing that no direct inflammatory responses were found in the fibers but, rather, free spores that had disintegrated between myofibrils, which indicated that muscle liquefaction was associated with presence of spores (CASAL et al., 2008Casal G, Matos E, Matos P, Azevedo C. Ultrastructural Description of a New Myxosporean Parasite sp. n. (Myxozoa, Myxosporea), found in the Sub-Opercular Musculature of . Kudoa aequidens
Aequidens plagiozonatus (Teleostei) from the Amazon RiverActa Protozool 2008; 47: 135-141.).
The biometric data on the spores of Kudoa spp. in apical view and stellate format comprised length of 9.70 ± 0.14 μm and width of 9.33 ± 0.25 μm. The four polar capsules in piriform shape consisted of two larger and two smaller capsules, with lengths of 4.05 ± 0.07 μm and 3.63 ± 0.18 μm and widths of 1.28 ± 0.32 μm and 1.15 ± 0.14 μm, respectively. Spores of pseudoquadrate square format presented length of 5.63 ± 0.18 μm and width of 5.60 ± 0.07 μm; the four polar capsules were equal in size and had a piriform shape, with length of 1.75 ± 0.24 μm and width of 0.98 ± 0.06 μm. Table 1 shows measurement data relating to spore sizes, shapes, lengths and widths of some polar Kudoa species capsules, in comparison with the data of the present study.
From comparisons of morphological species of the genus Kudoa, it could be seen that the length, width and polar capsule measurements of the Kudoa species of the present study differed from the species previously studied , thus giving evidence that there must be two new species. Previously, in fish from the Amazon River, only K. aequidens had been described by Casal et al. (2008)Casal G, Matos E, Matos P, Azevedo C. Ultrastructural Description of a New Myxosporean Parasite sp. n. (Myxozoa, Myxosporea), found in the Sub-Opercular Musculature of . Kudoa aequidens Aequidens plagiozonatus (Teleostei) from the Amazon RiverActa Protozool 2008; 47: 135-141. in Aequidens plagiozonatus (common name: cará pixuna).
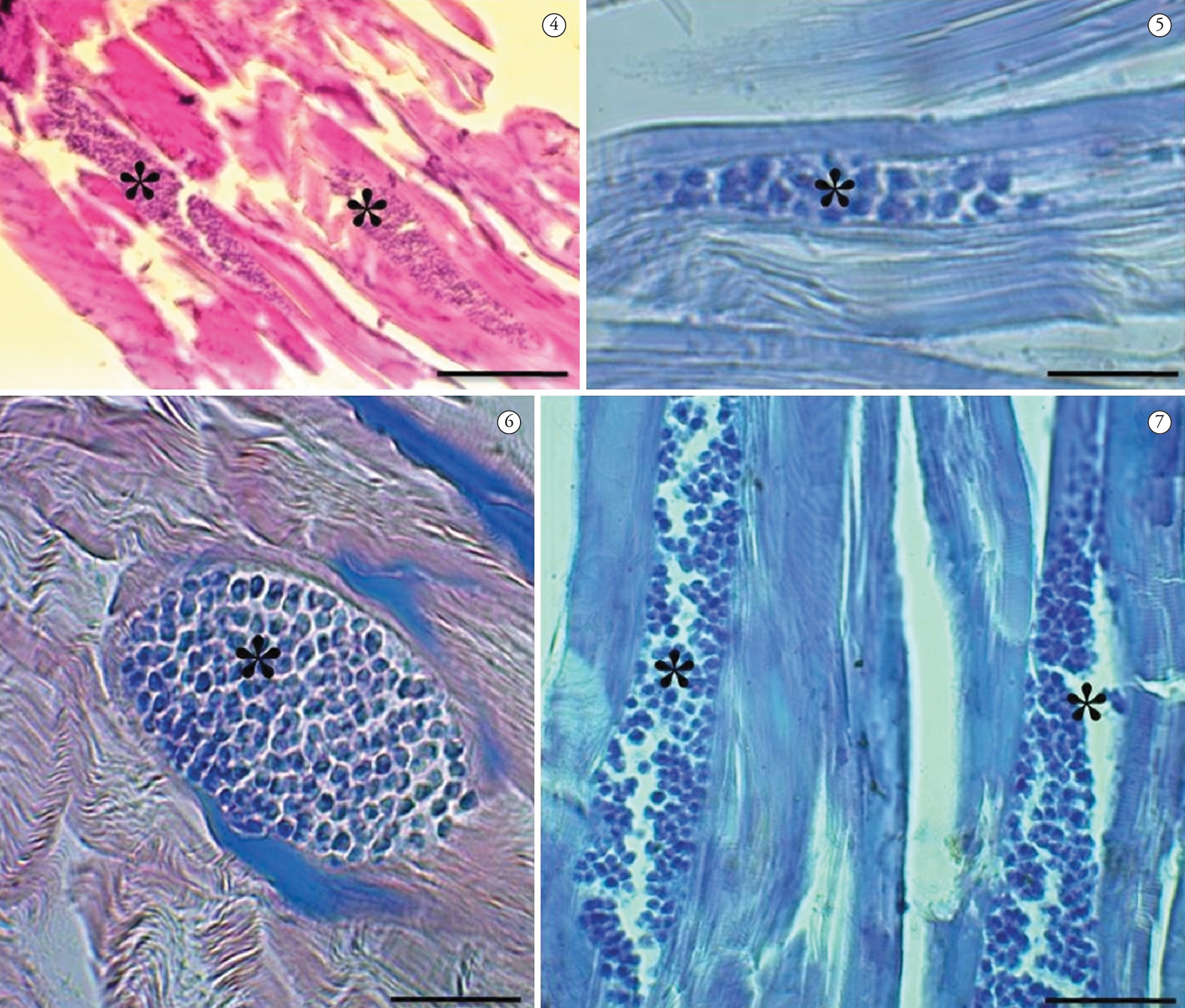
Examination of the histological sections revealed that the parasite Kudoa spp. was presented within and between the muscle fibers (Figure 4-7), with 100% prevalence. The extent of the damage and the fate of the infected muscle fibers shows that it is possible that full replacement of mature spores may occur, within and between skeletal muscle fibers, thus causing changes to the swimming behavior of the fish and making them more vulnerable to predators. This was also found by Dyková et al. (2009)Dyková I, Buron I, Fiala I, Roumillat WA. Kudoa inornata sp. n. (Myxosporea: Multivalvulida) from the skeletal muscles of (Teleostei: Sciaenidae). Cynoscion nebulosusFolia Parasitol 2009; 56(2): 91-98. http://dx.doi.org/10.14411/fp.2009.014. PMid:19606785
http://dx.doi.org/10.14411/fp.2009.014...
, who observed that the muscle fibers infected with K. inornata were completely replaced by spores.
Optical micrographs: Cysts of Kudoa spp. parasitizing muscle fibers (*) P. squamosissimus. 4 - Staining with hematoxylin-eosin, 5 – Staining in Ziehl-Neelsen, 6 and 7 – Staining in May Grunwald-Giemsa. Bar = 5 µm.
Since presence of Kudoa spores in the fish host can cause lesions in its muscles, this disease has a significant economic impact because of postmortem myoliquefaction. This muscle lysis effect comes from the action of proteases, thereby leaving the muscle of the host softened (ANDRADA et al., 2005Andrada CDG, Tortelly R, Nogueira PP, Andrade CL, Lima FC. Infecção por Kudoa Meglitsch, 1947 (Myxozoa: Multivalvulida) em musculatura esquelética de espada Trichiurus L. (Teleostei: Trichiuridae). lepturusParasitol latinoam 2005; 60(3-4): 150-153.) and compromising the acceptance of the fish among consumers. Furthermore, there is evidence that consumption of fish infected with Kudoa may cause allergy symptoms or food poisoning (MARTÍNEZ DE VELASCO et al., 2007Martínez de Velasco G, Rodero M, Chivato T, Cuéllar C. Seroprevalence of anti- sp. (Myxosporea: Multivalvulida) antibodies in a Spanish population. KudoaParasitol Res 2007; 100(6): 1205-1211. http://dx.doi.org/10.1007/s00436-006-0390-x. PMid:17177059
http://dx.doi.org/10.1007/s00436-006-039...
; GRABNER et al., 2012Grabner DS, Yokoyama H, Shirakashi S, Kinami R. Diagnostic PCR assays to detect and differentiate and . Kudoa septempunctata, K. thyrsites
K. lateolabracis (Myxozoa, Multivalvulida) in muscle tissue of olive flounder (Paralichthys olivaceus)Aquaculture 2012; 338-341: 36-40. http://dx.doi.org/10.1016/j.aquaculture.2012.01.022.
http://dx.doi.org/10.1016/j.aquaculture....
; KAWAI et al., 2012Kawai T, Sekizuka T, Yahata Y, Kuroda M, Kumeda Y, Iijima Y, et al. Identification of as the causative agent of novel food poisoning outbreaks in Japan by consumption of in raw fish. Kudoa septempunctata
Paralichthys olivaceusClin Infect Dis 2012; 54(8): 1046-1052. http://dx.doi.org/10.1093/cid/cir1040. PMid:22281845
http://dx.doi.org/10.1093/cid/cir1040...
).
The observations under the microscope in the present study provide the first confirmation that the presence of infection in the musculature of P. squamosissimus is related to parasitism by two different spores of the genus Kudoa. There is a high possibility that these two new species are treatable, but analyses using transmission electron microscopy and molecular biology are necessary in order to determine the species. Studies of this nature are of great relevance, since parasitism by Kudoa may damage the muscles of the host, which is considered to be the finest area of the fish, thus causing commercial losses.
Acknowledgements
We are grateful to CNPq, CAPES (colleger PPG AqRAT-UFRA), Bank Santander, ICMBIO-SISBIO IBAMA (license 27119-1), FAPESPA.
References
- Abdel-Ghaffar F, Morsy K, Mehlhorn H, Bashtar AR, Shazly MA, Saad AH, et al. First report of Kudoa species (Myxozoa: Kudoidae) infecting the spotted coral grouper Plectropomus from the Red Sea. A light and ultrastructural study. maculatesParasitol Res 2012; 111(4): 1579-1585. http://dx.doi.org/10.1007/s00436-012-3011-x. PMid:22740296
» http://dx.doi.org/10.1007/s00436-012-3011-x - Andrada CDG, Tortelly R, Nogueira PP, Andrade CL, Lima FC. Infecção por Kudoa Meglitsch, 1947 (Myxozoa: Multivalvulida) em musculatura esquelética de espada Trichiurus L. (Teleostei: Trichiuridae). lepturusParasitol latinoam 2005; 60(3-4): 150-153.
- Ayres M, Ayres-Junior M, Ayres DL, Santos AS, Ayres LL. Bioestat 5.0 aplicações estatísticas nas áreas das ciências bi-médicas. Belém: IDSM; 2007.
- Barthem RB. Ocorrência, distribuição e biologia dos peixes da Baía de Marajó, Estuário Amazônico. Bol Mus Para Emilio Goeldi 1985; 2(1): 49-69.
- Boeger WAB, Kritsky DC. Parasites, Fossils and Geologic History: Historical Biogeography of the South American Freshwater Croakers, spp. (Teleostei, Sciaenidae). Plagioscion Zool Scr 2003; 32(1): 3-11. http://dx.doi.org/10.1046/j.1463-6409.2003.00109.x.
» http://dx.doi.org/10.1046/j.1463-6409.2003.00109.x - Burger MAA, Adlard RD. Four new species of Meglitsch, 1947 (Myxosporea: Multivalvulida) from Australia with recommendations for species descriptions in the Kudoidae. KudoaParasitology 2010; 137(5): 793-814. http://dx.doi.org/10.1017/S0031182009991557. PMid:20025820
» http://dx.doi.org/10.1017/S0031182009991557 - Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 1997; 83(4): 575-583. http://dx.doi.org/10.2307/3284227. PMid:9267395
» http://dx.doi.org/10.2307/3284227 - Casal G, Matos E, Matos P, Azevedo C. Ultrastructural Description of a New Myxosporean Parasite sp. n. (Myxozoa, Myxosporea), found in the Sub-Opercular Musculature of . Kudoa aequidens Aequidens plagiozonatus (Teleostei) from the Amazon RiverActa Protozool 2008; 47: 135-141.
- Dyková I, Buron I, Fiala I, Roumillat WA. Kudoa inornata sp. n. (Myxosporea: Multivalvulida) from the skeletal muscles of (Teleostei: Sciaenidae). Cynoscion nebulosusFolia Parasitol 2009; 56(2): 91-98. http://dx.doi.org/10.14411/fp.2009.014. PMid:19606785
» http://dx.doi.org/10.14411/fp.2009.014 - Eiras JC, Júnior JP, Sampaio LA, Robaldo R, Abreu PC. sp. can cause in vivo myoliquefaction in the host . Myxobolus Paralichthys orbignyanus (Osteichthyes, Paralichthydae)Dis Aquat Organ 2007; 77(3): 255-258. http://dx.doi.org/10.3354/dao01852. PMid:18062475
» http://dx.doi.org/10.3354/dao01852 - Eiras JC, Saraiva A, Cruz C. Synopsis of the species of Kudoa Meglitsch, 1947 (Myxozoa: Myxosporea: Multivalvulida). Syst Parasitol 2014; 87(2): 153-180. http://dx.doi.org/10.1007/s11230-013-9461-4. PMid:24474038
» http://dx.doi.org/10.1007/s11230-013-9461-4 - Grabner DS, Yokoyama H, Shirakashi S, Kinami R. Diagnostic PCR assays to detect and differentiate and . Kudoa septempunctata, K. thyrsites K. lateolabracis (Myxozoa, Multivalvulida) in muscle tissue of olive flounder (Paralichthys olivaceus)Aquaculture 2012; 338-341: 36-40. http://dx.doi.org/10.1016/j.aquaculture.2012.01.022.
» http://dx.doi.org/10.1016/j.aquaculture.2012.01.022 - Heckmann RA. Histopathology and fine structure of two myxosporidans, and . Kudoa clupeidae Henneguya sebasta and a microsporidian glugea infecting fishes from the California, USA coast, histozoic parasitesProc Parasitol 2012; 54: 1-25.
- Heiniger H, Adlard RD. Host specificity and local infection dynamics of n. sp. (Multivalvulida: Kudoidae) from the pericardial cavity of two spp. (Perciformes: Apogonidae) at Lizard Island lagoon, Queensland, Australia. Kudoa leptacanthae ZoramiaParasitol Int 2012; 61(4): 697-706. http://dx.doi.org/10.1016/j.parint.2012.08.001. PMid:22922116
» http://dx.doi.org/10.1016/j.parint.2012.08.001 - Heiniger H, Cribb TH, Adlard RD. Intra-specific variation of Kudoa spp. (Myxosporea: Multivalvulida) from apogonid fishes (Perciformes), including the description of two new species, K. cheilodipteri n. sp. and n. sp., from Australian waters. K. cookiiSyst Parasitol 2013; 84(3): 193-215. http://dx.doi.org/10.1007/s11230-012-9400-9. PMid:23404757
» http://dx.doi.org/10.1007/s11230-012-9400-9 - Kawai T, Sekizuka T, Yahata Y, Kuroda M, Kumeda Y, Iijima Y, et al. Identification of as the causative agent of novel food poisoning outbreaks in Japan by consumption of in raw fish. Kudoa septempunctata Paralichthys olivaceusClin Infect Dis 2012; 54(8): 1046-1052. http://dx.doi.org/10.1093/cid/cir1040. PMid:22281845
» http://dx.doi.org/10.1093/cid/cir1040 - Lom J, Dyková I. Myxozoan genera: definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitol (Praha) 2006; 53(1): 1-36. http://dx.doi.org/10.14411/fp.2006.001. PMid:16696428
» http://dx.doi.org/10.14411/fp.2006.001 - Lom J, Dyková I. Development in aquaculture and fisheries science. Amsterdam: Elsevier; 1992. 315 p. vol. 26. Protozoan Parasites of Fishes.
- Lom J, Dyková I. Sporogenesis and spore structure in (Myxosporea, Multivalvulida). Kudoa lunataParasitol Res 1988; 74(6): 521-530. http://dx.doi.org/10.1007/BF00531629. PMid:3194365
» http://dx.doi.org/10.1007/BF00531629 - Luna LG. Manual of histologic staining methods of the armed forces institute of pathology. 3rd ed. New York: MacGraw-Hill; 1968.
- Maciel PO, Affonso EG, Boijink CL, Tavares-Dias M, Inoue LAKA. sp. (Myxozoa) in the circulating blood of . Myxobolus Colossoma macropomum (Osteichthyes, Characidae)Rev Bras Parasitol Vet 2011; 20(1): 82-84. http://dx.doi.org/10.1590/S1984-29612011000100018. PMid:21439240
» http://dx.doi.org/10.1590/S1984-29612011000100018 - Mansour L, Thabet A, Chourabi K, Harrath AH, Gtari M, Al Omar SY, et al. Kudoa azevedoi n. sp. (Myxozoa, Multivalvulida) from the oocytes of the Atlantic horse mackerel (Perciformes, Carangidae) in Tunisian coasts. Trachurus trachurusParasitol Res 2013; 112(4): 1737-1747. http://dx.doi.org/10.1007/s00436-013-3332-4. PMid:23435961
» http://dx.doi.org/10.1007/s00436-013-3332-4 - Martínez de Velasco G, Rodero M, Chivato T, Cuéllar C. Seroprevalence of anti- sp. (Myxosporea: Multivalvulida) antibodies in a Spanish population. KudoaParasitol Res 2007; 100(6): 1205-1211. http://dx.doi.org/10.1007/s00436-006-0390-x. PMid:17177059
» http://dx.doi.org/10.1007/s00436-006-0390-x - Matsukane Y, Sato H, Tanaka S, Kamata Y, Sugita-Konishi Y. Kudoa iwatai and two novel . Kudoa spp., K. trachuri n. sp. and K. thunni n. sp. (Myxosporea: Multivalvulida), from daily consumed marine fish in western JapanParasitol Res 2011; 108(4): 913-926. http://dx.doi.org/10.1007/s00436-010-2133-2. PMid:21053015
» http://dx.doi.org/10.1007/s00436-010-2133-2 - Miller TL, Adlard RD. Brain infecting kudoids of Australia’s coral reefs, including a description of Kudoa lemniscati n. sp. (Myxosporea: Kudoidae) from Lutjanus lemniscatus (Perciformes: Lutjanidae) off Ningaloo Reef, Western Australia. Parasitol Int 2012; 61(2): 333-342. http://dx.doi.org/10.1016/j.parint.2012.01.002. PMid:22260905
» http://dx.doi.org/10.1016/j.parint.2012.01.002 - Moran JDW, Whitaker DJ, Kent ML. A review of the myxosporean genusKudoaMeglitsch, 1947, and its impact on the international aquaculture industry and commercial fisheries. Aquaculture 1999; 172(1-2): 163-196. http://dx.doi.org/10.1016/S0044-8486(98)00437-2.
» http://dx.doi.org/10.1016/S0044-8486(98)00437-2 - Pereira KRB. Caracterização geoquímica de sedimentos de fundo da orla de Belém - Pará [Dissertação]. Belém: Universidade Federal do Pará; 2001.
- Santos GM, Ferreira EJG, Zuanon JAS. Peixes comerciais de Manaus. Manaus: IBAMA/ProVárzea; 2006.
- Viana AP, Frédou T, Lucena F. Aplicações de técnicas morfométricas no estudo da morfometria de pescada branca, Plagioscion squamosissimus, Heckel (1940), Perciformes, Sciaenidae, desembarcada na ilha de Mosqueiro-PA. Bol Lab Hidrobiol 2006; 19(1): 1-12.
- Yokoyama H, Yanagida T, Shirakashi S. Kudoa ogawai n. sp. (Myxozoa: Multivalvulida) from the trunk muscle of Pacific barrelfish Hyperoglyphe japonica (Teleostei: Centrolophidae) in Japan. Parasitol Res 2012; 110(6): 2247-2254. http://dx.doi.org/10.1007/s00436-011-2756-y. PMid:22173453
» http://dx.doi.org/10.1007/s00436-011-2756-y
Publication Dates
-
Publication in this collection
Apr-Jun 2015
History
-
Received
13 Nov 2014 -
Accepted
19 Feb 2015