Abstract
The genus Cotylophoron belongs to the Paramphistomidae family and its definitive hosts are ruminants in general. This work describes the presence of a new species of the gender, a parasite in the rumen and reticulum of Bubalus bubalis, on Marajó Island in the Eastern Brazilian Amazon, using of light microscopy, scanning electronic microscopy and molecular biology techniques. One hundred and ten animals were analyzed, of which 4.54% were parasitized by flukes in their adult forms. The helminths were found fixed to the ruminal mucosa and present Liorchis-type pharynx, Cotylophoron-type genital sucker, oblique testicles larger than the ovary, uterus in rings full of eggs and Cotylophoron-type acetabulum. These morphologic characters do not fit into any previously described species. Thus, it is proposed that this is a new species in the genus Cotylophoron. The present work expands the record of parasitism by helminths in Bubalus bubalis, this being the first record of trematoda from the genus Cotylophoron for this host in the Brazilian Amazon.
Keywords:
Taxonomy; parasite; Trematoda; buffalo; Brazil
Resumo
O gênero Cotylophoron pertence à família Paramphistomidae e possui como hospedeiros definitivos ruminantes em geral. Este trabalho descreve a presença de uma espécie nova do gênero, parasito do rúmen e retículo de Bubalus bubalis, na Ilha de Marajó, Amazônia oriental brasileira, a partir das técnicas de microscopia de luz, microscopia eletrônica de varredura e biologia molecular. Foram analisados 110 animais, dos quais 4,54% estavam parasitados por trematódeos na sua forma adulta. Os helmintos foram encontrados fixados à mucosa ruminal, apresentando faringe do tipo Liorchis, ventosa genital do tipo Cotylophoron, testículos oblíquos maiores que o ovário, útero em alças repleto de ovos, e acetábulo do tipo Cotylophoron. Estes caracteres morfológicos não se enquadram em nenhuma espécie previamente descrita. Assim, propõe-se uma nova espécie ao gênero Cotylophoron. O presente trabalho amplia o registro do parasitismo por helmintos em Bubalus bubalis, sendo este o primeiro registro de trematódeos do gênero Cotylophoron nesse hospedeiro para a Amazônia brasileira.
Palavras-chave:
Taxonomia; parasito; Trematoda; búfalo; Brasil
Introduction
The Bubalus bubalis species is known as the “buffalo” throughout Brazil and is bred in several regions of the world because of its sturdiness, milk and dairy products and good-quality meat, besides being used for work (Damasceno et al., 2010Damasceno FA, Viana JM, Tinôco IFF, Gomes RCC, Schiassi L. Adaptação de bubalinos ao ambiente tropical. Rev Eletrônica Nutritime 2010; 7(5): 1370-1381.). In Brazil, buffalos have been imported from different countries such as Australia, Egypt, India, Italy, and Southwest Asia and were first introduced in the continent on Marajó Island in 1895, where the natural conditions of this region were excellent for their development (Marques, 2000Marques JRF. Búfalos: o produtor pergunta, a Embrapa responde. Belém: Embrapa Comunicação para Transferência de Tecnologia; 2000.). And although it is a domestic and economically important animal, little is known about its parasitic interactions.
The genus Cotylophoron (Stiles & Goldberger, 1910) belongs to the Paramphistomidae family (Fischoeder 1901). In general, they have ruminants as definite hosts, the rumen and reticulum being their sites of infection when adults. The immature forms inhabit the small intestine, where they perform the backward movement in their development process in the digestive tract, traveling from the small intestine to the rumen/reticulum (Forlano et al., 2001Forlano MD, Henríquez HR, Meléndez RD. Incidencia y prevalencia de Cotylophoron spp. (Trematoda: Digenea) en bovinos del Asentamiento Campesino” Las Majaguas”. Portuguesa-Venezuela 1996-1997. Gaceta Cienc Vet 2001; 7(1): 15-23.). This behavior is due to parasitic ability of genetic expression that allows migration through host’s tissues while triggering it’s immune responses (Pérez-Ponce de León & Hernández-Mena, 2019Pérez-Ponce de León G, Hernández-Mena D. Testing the higher-level phylogenetic classification of Digenea (Platyhelminthes, Trematoda) based on nuclear rDNA sequences before entering the age of the ‘next-generation’ Tree of Life. J Helminthol 2019; 93(3): 260-276. http://dx.doi.org/10.1017/S0022149X19000191. PMid:30973318.
http://dx.doi.org/10.1017/S0022149X19000...
).
Currently, the genus Cotylophoron comprises eight species: Cotylophoron cotylophorum (Fischoeder 1901); Cotylophoron jacksoni Nasmark 1937; Cotylophoron fulleborni Nasmark, 1938; Cotylophoron panamensis Prince & McIntosh 1953; Cotylophoron bareilliense Mukherjee & Chauhan 1965; Cotylophoron macrosphinetris Sey & Graber 1979; Cotylohoron xiangjiangense Wang 1979; and Cotylophoron travassosi (Martins & Pezzi, 1992).
This genus includes trematode parasites of rumen and reticulum in ruminants worldwide (Martínez & Velásquez, 2012Martínez JL, Velásquez LET. Cotylophoron panamensis (Digenea: Paramphistomidae) en bovinos del Meta y del Guaviare, Colombia. Acta Biol Colomb 2012; 17(2): 419-428.). Alarcón & Velásquez (2009)Alarcón EP, Velásquez LE. Descripción morfológica de Cotylophoron cotylophorum (Digenea: Paramphistomidae) hallado en bovinos de Rio Negro, Antioquia, Colombia. Rev Colomb Cienc Pecu 2009; 22(2): 168-177. described the C. cotylophorum species as also present in the rumen of Bos taurus in the region of Rio Negro, Colombia. Sánchez et al. (2009)Sánchez P, Tantalean VM, Chávez A, Soto AO. Presencia de Cotylophoron cotylophorum (Trematoda, Paramphistomidae) en bovinos de Loreto, Perú. Rev Peru Biol 2009; 16(1): 141-142. described the same species as parasitizing the rumen of bovines in Loreto in Peru. Morales et al. (2015)Morales G, Pino LA, Moreno L. Cotylophoron fulleborni Nasmark, 1937 (Trematoda: Paramphistomidae). Primer Reporte Para Venezuela. ACI Av Cienc Ing 2015; 7(2): 20-23. http://dx.doi.org/10.18272/aci.v7i2.253.
http://dx.doi.org/10.18272/aci.v7i2.253...
reported the presence of C. fulleborni in the ruminal mucosa of bovines in Venezuela.
In Brazil, the genus Cotylophoron is reported as parasitizing different ruminants in different locations. Costa & Guimarães (1990)Costa HMA, Guimarães MP. Cotylophoron bareilliense Mukherjee e Chauhan, 1965 (Trematoda-Paramphistomidae). Arq Bras Med Vet Zootec 1990; 42(1): 183-186. mention the first occurrence of C. bareilliensis parasitizing the rumen of sheep in the state of Pará and Costa & Guimarães (1992)Costa HMA, Guimarães MP. Cotylophoron travassosi sp. n. (Trematoda-Paramphistomidae) from cattle. Mem Inst Oswaldo Cruz 1992; 87(Suppl. 1): 69-72. http://dx.doi.org/10.1590/S0074-02761992000500013. PMid:1343800.
http://dx.doi.org/10.1590/S0074-02761992...
describe the C. travassosi species in bovines in the state of Maranhão. Miranda & Costa (1999)Miranda MA, Costa HMA. Report and redescription of some species of Cotylophoron (Trematoda: Paramphistomidae) in domestic ruminants of Brazil. Rev Bras Parasitol Vet 1999; 8(1): 1-15. http://dx.doi.org/10.4322/rbpv.01802003.
http://dx.doi.org/10.4322/rbpv.01802003...
report the incidence of C. panamensis parasitizing bovines of the state of Rondônia and C. fulleborni in bovines and goats in the states of Roraima, Pará and Maranhão and C. jacksoni also in bovines in the states of Pará, Roraima and Rondônia.
With this in mind, the present work describes the morphology, prevalence and molecular data of new species of Paramphistomidae that was found as a parasite of B. bubalis on Marajó Island, Brazil.
Material and Methods
Study area and collection
The sampling was composed of hosts deriving from the island of Marajó, from the municipality of Soure (00°4’00”S, 48°31’24”W), Breves (01°40’56”S, 50°28’49”W) and Cachoeira do Arari (01°00’41”S, 48°57’48”W), totaling 110, all rumens and reticulums of the examined buffalos obtained from slaughter in the Abatedouro Frigorífico, in Tapanã, at Cooperativa da Indústria Agropecuária do Pará (SOCIPE), in Belém and in the Matadouro Frigorífico Municipal de Soure, Pará. Each animal had its locality confirmed through its Animal Transport Guide (GTA). Fragments of rumen and reticulum were collected and transported cooled to the Laboratório de Histologia e Embriologia Animal (LHEA), Universidade Federal Rural da Amazônia (UFRA).
Preparation of parasites for light microscopy
The trematodes found were fixed and processed according to Giese et al. (2015)Giese EG, Silva MVO, Videira MN, Furtado AP, Matos ER, Gonçalves EC, et al. Rohdella amazonica n. sp. (Aspidogastrea: Aspidogastridae) from the Amazoninan banded puffer fish Colomesus psittacus (Bloch & Schneider, 1801). J Helminthol 2015; 89(3): 288-293. http://dx.doi.org/10.1017/S0022149X14000054. PMid:24572176.
http://dx.doi.org/10.1017/S0022149X14000...
. Ten trematodes were used for morphologic and morphometric analysis. The measures were obtained in millimeters and are presented in the form of average and amplitude between parentheses. The images in light microscopy were obtained by photomicroscope with connected clear vision camera (LEICA DM2500). For scanning electron microscopy, the helminths specimens were washed in distilled water, post-fixed in 1% osmium tetroxide, dehydrated to the critical point of CO2, metallized with gold+palladium, and analyzed using a TESCAN scanning electron microscope (VEGA 3) in the Scanning Electron Microscopy Laboratory, Universidade Federal Rural da Amazônia.
Molecular analysis
Molecular characterization was based on partial 18S small subunit ribosomal rRNA gene (18S SSU), which after DNA extraction through the Invisorb Spin Tissue Mini Kit (Stratec Molecular), was amplified using the primers cc18sf (5’- cggtgaaaccgcgaatggctc - 3’) and cc18sr (5’- gacgggcggtgtgtacaaagg - 3’). The polymerase chain reactions (PCRs) were carried out in 25 µL final volume, containing 5-10 ng of DNA, 50 mM KCl, 2 mM MgCl2, 10 mM Tris-HCl, 50 µM of each DNTP, 0.5 µM of each oligonucleotide and one unit of Taq DNA polymerase (Invitrogen). The amplification reaction consisted of 35 cycles of 1 min at 95 °C, 1 min at 67 °C, and 1 min 30 sec at 72 °C, preceded by 5 min at 95 °C and followed by 10 min at 72 °C. Amplicons were enzymatically purified with Illustra ExoProStar (GE Healthcare). Nucleotide sequencing was performed in an ABI 3500 xL Genetic Analyzer (Thermo Fisher Scientific), according to the manufacturer’s specifications. BioEdit software (Hall, 1999Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 1999; 41: 95-98.) was used to align forward and reverse sequences.
Results
Paramphistomidae (Fischoeder 1901)
Genus Cotylophoron (Stiles & Goldberger 1910)
Cotylophoron marajoensis n. sp.
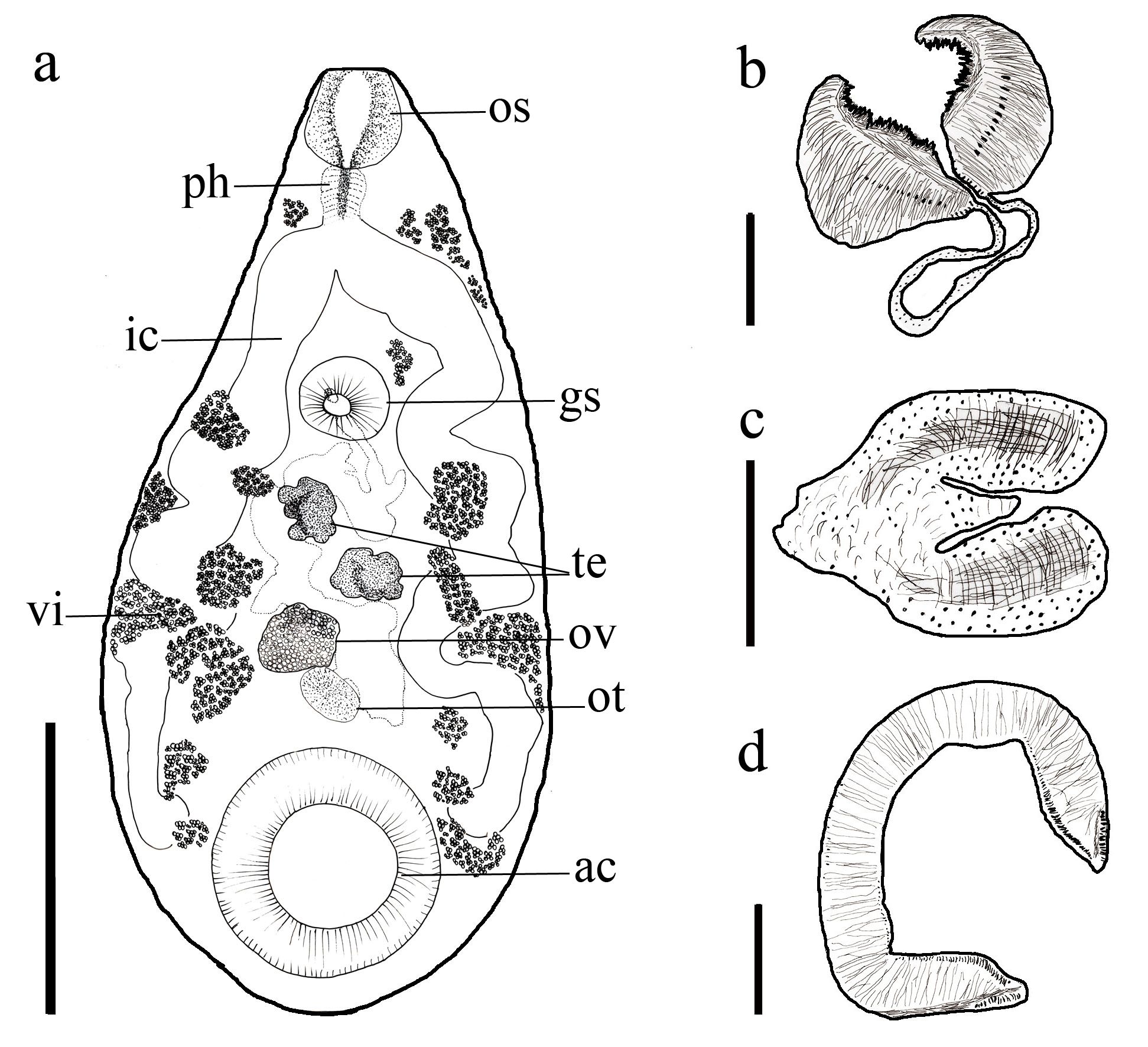
Morphology of Cotylophoron marajoensis n. sp.: (a) entire helminth, ventral view with oral sucker (os), pharynx (ph) of Liorchis type, intestinal cecum (ic), genital sucker (gs); ootype (ot), acetabulum (ac), ovary (ov), testicles (te) and vitellaria (vi); (b) pharynx of Liorchis type; (c) genital pore of Cotylophoron type; (d) acetabulum of Cotylophoron type. Scale-bars: A 200 μm, B 50 μm, C 100 μm, D 50 μm.
Scanning electron micrographs of Cotylophoron marajoensis n. sp.: (a) external morphology with genital pore (arrow) in the anterior portion of the helminth and acetabulum (ac) in the posterior portion of the helminth; (b) internal morphology with pharynx (ph) along with oral sucker (*), genital papillae along with genital pore (gp), uterus in handles full of eggs (arrow), and ovary (ov) central, acetabulum (ac) in the posterior portion of the helminth; (c) detail of the pharynx’s papillae (pa); (d) detail of the genital papillae (*) around the genital pore (gp); (e) detail of the muscular bands from acetabulum (*) and oblique muscular band (arrow). Scale-bars: A 500 μm, B 500 μm, C 50 μm, D 200 μm, E 50 μm.
[Based on 10 adult specimens: metrical data in Table 1]. Pear-shaped, smooth body, slightly recurved in the ventral direction, measuring 6.30 (3.42-754) × 2.98 (2.62-3.42) mm, maximum width to the testis level. Oral sucker, adorned with innumerable ciliate papillae in the anterior extremity of the trematode. Pharynx with well-defined muscular portion and evidencing papillae of varied sizes in its mucosa, characteristic of pharynx of Liorchis genus, measuring 0.56 (0.43-0.65) × 0.63 (0.33-0.80) mm. Muscular oesophagus 0.58 (0.42-0.79) × 0.68 (0.15-0.45) mm, with muscular streaks in an uniformly thin layer, absent bulb. Caeca with undulations, finishing to the level of the anterior edge of the acetabulum. Acetabulum in the posterior fraction of the body, subterminal adorned with numerous ciliate papillae in a well-developed concentric arrangement, demonstrating definite transversal muscular bands, as well as external oblique muscular band, characterizing it as a Cotylophoron acetabulum, measuring 1.40 (0.74-1.77) × 1.15 (0.43-1.67) mm. Genital sucker, located in the anterior fraction of the body, the genital papillae and sucker below the oral sucker, with conspicuous genital papilla, characteristic of Cotylophoron, measuring 0.59 (0.43-0.78) × 0.74 (0.46-1.66) mm. Oblique, lobe-segmented testes, smaller in relation to the ovary, located in the middle fraction of the body, anterior measuring 0.38 (0.29-0.56) × 0.39 (0.32-0.55), posterior measuring 0.36 (0.26-0.50) × 0.42 (0.31-0.74) mm, Ovary located after the testes in the middle fraction of the body with 0.76 (0.32-0.95) × 0.49 (0.37-0.63) mm. Uterine loops packed with ascending eggs, located in the middle fraction, in the middle of the parasite. Mehlis’ gland (Ootype) measuring 0.46 (0.33-0.59) × 0.38 (0.27-0.43) mm. Vitellogenic glands, situated in the lateral parts from the anterior fraction going until the posterior fraction, sometimes invade the intracaecal area. Eggs operculate 0.130 (0.08-0.14) × 0.05 (0.04-0.06) mm.
Morphological and morphometric comparison of Cotylophoron marajoensis n. sp. collected from Bubalus bubalis commercialized in the state of Pará and compared with other authors.
Excluding primers, 1712 base pairs were obtained for 18S SSU rRNA gene (Genbank accession number: SUB8164216 Cotylophoron MW024900). In comparison to Cotylophoron cotylophorum (JX678230) a BLAST search resulted in maximum score = 3068, query coverage = 95% and percent identity = 99.88%.
Taxonomy summary
Cotylophoron marajoensis n. sp.
Type-host:Bubalus bubalis (Linnaeus 1758). Common name: Water Bufallo
Site of infection: rumen and reticulum.
Location type: Soure (00°4’00”S, 48°31’24”W), Breves (01°40'56”S, 50°28'49”W) and Cachoeira do Arari (01° 00' 41” S, 48° 57' 48” W), Marajó Island, Pará, Brazil.
Prevalence: 4.54% (5 infected hosts of 110 analyzed).
ZooBank registration: SUB8164216 Cotylophoron MW024900
Etymology: The specific name marajoensis refers to the geographical region of the distribution (i.e., Marajó Island, state of Pará, Amazon, Brazil).
Deposit of Specimens: Holotype MPE 000261 and paratype MPEG 00262 a 00265, were deposited in an invertebrate collection at Museu Paranse Emílio Goeldi (MPEG), Belém, Pará, Brazil.
Discussion
Morphological delimitation
The characteristics presented for the taxon studied here such as pear-shaped body, smooth tegument without papillae, oral sucker in the anterior extremity of the body, genital sucker with genital papilla in the anterior fraction of the body and sub-ventral acetabulum of average size, fit it into the Cotylophoron genus in accordance with the keys and descriptions of the genus (Eduardo, 1985Eduardo SL. The taxonomy of the family Paramphistomidae Fischoeder, 1901 with special reference to the morphology of species occurring in ruminants. V. Revision of the genus Cotylophoron Stiles & Goldberger, 1910. Syst Parasitol 1985; 7(1): 3-26. http://dx.doi.org/10.1007/BF00010157.
http://dx.doi.org/10.1007/BF00010157...
). The morphologic characteristics of Cotylophoron, in the present work, are similar to those of the of genus Paramphistomum (Fischoeder 1901) and genus Balanorchis (Fischoeder 1901), but they are distinguished mainly in relation to the Paramphistomum because of the presence of genital papilla and, in relation to the Balanorchis, because of the absence of long papillae in the oral sucker (Jones et al., 2005Jones A, Bray RA, Gibson DI. Keys to the Trematoda. 1st ed. London: CABI Publishing and The Natural History Museum; 2005. (vol. 2). http://dx.doi.org/10.1079/9780851995878.0000.
http://dx.doi.org/10.1079/9780851995878....
).
Cotylophoron marajoensis n. sp. differs from C. cotylophorum in possessing a Liorchis pharynx, whereas in C. cotylophorum it is Calicophoron; still, absence of esophageal bulb in C. cotylophorum. Oblique and smaller testes in relation to the ovary presented by Cotylophoron marajoensis n. sp. distinguish it from C. cotylophorum, which presents larger in tandem testes in relation to the ovary (Alarcón & Velásquez, 2009Alarcón EP, Velásquez LE. Descripción morfológica de Cotylophoron cotylophorum (Digenea: Paramphistomidae) hallado en bovinos de Rio Negro, Antioquia, Colombia. Rev Colomb Cienc Pecu 2009; 22(2): 168-177.). Associated to these morphologic characteristics, C. cotylophorum has not been found in B. bubalis until now, nor in Brazilian territory.
In Brazil, C. fulleborni e C. jacksoni had been only described in Bos indicus e Capra hircus (Miranda & Costa, 1999Miranda MA, Costa HMA. Report and redescription of some species of Cotylophoron (Trematoda: Paramphistomidae) in domestic ruminants of Brazil. Rev Bras Parasitol Vet 1999; 8(1): 1-15. http://dx.doi.org/10.4322/rbpv.01802003.
http://dx.doi.org/10.4322/rbpv.01802003...
), without occurrence in B. bubalis. These species differ from Cotylophoron marajoensis n. sp. in possessing Calicophoron pharynx and larger testes in relation to the ovary (Morales et al., 2015Morales G, Pino LA, Moreno L. Cotylophoron fulleborni Nasmark, 1937 (Trematoda: Paramphistomidae). Primer Reporte Para Venezuela. ACI Av Cienc Ing 2015; 7(2): 20-23. http://dx.doi.org/10.18272/aci.v7i2.253.
http://dx.doi.org/10.18272/aci.v7i2.253...
). Around the world, C. panamensis has been described only in Bos indicus. It is shorter in length than Cotylophoron marajoensis n. sp., although larger in width. It possesses Calicophoron pharynx and, differently from Cotylophoron marajoensis n. sp. (whose testes are oblique and smaller than the ovary), it possesses horizontally parallel and larger testes in relation to the ovary, with its vitellarium exceeding the edge of the caeca, also differing from Cotylophoron marajoensis n. sp. because these do not exceed the caecal edges (Martínez & Velásquez, 2012Martínez JL, Velásquez LET. Cotylophoron panamensis (Digenea: Paramphistomidae) en bovinos del Meta y del Guaviare, Colombia. Acta Biol Colomb 2012; 17(2): 419-428.).
The species Cotylophoron bareilliense occurs in B. bubalis in the Phillipines. In Brazil, however, it is mentioned only as parasitizing Bos indicus, Capra hircus and Ovis aries (Miranda & Costa, 1999Miranda MA, Costa HMA. Report and redescription of some species of Cotylophoron (Trematoda: Paramphistomidae) in domestic ruminants of Brazil. Rev Bras Parasitol Vet 1999; 8(1): 1-15. http://dx.doi.org/10.4322/rbpv.01802003.
http://dx.doi.org/10.4322/rbpv.01802003...
). Moreover, it is longer in length than Cotylophoron marajoensis n. sp., and it possesses a Calicophoron pharynx (Eduardo, 1985Eduardo SL. The taxonomy of the family Paramphistomidae Fischoeder, 1901 with special reference to the morphology of species occurring in ruminants. V. Revision of the genus Cotylophoron Stiles & Goldberger, 1910. Syst Parasitol 1985; 7(1): 3-26. http://dx.doi.org/10.1007/BF00010157.
http://dx.doi.org/10.1007/BF00010157...
), differing from the Liorchis pharynx of the new species here being described.
Cotylophoron macrosphinctris is parasitic on buffalo, but of the species Syncerus caffer, the African buffalo; it has not been described in B. bubalis, nor in the Brazilian territory it is differentiated from the Cotylophoron marajoensis n. sp. by its Calicophoron pharynx, larger esophageal bulb and testicles in relation to the ovary. Furthermore, it possesses a Schistocotyle genital sucker, whereas Cotylophoron marajoensis n. sp. possesses a Cotylophoron genital sucker (Eduardo, 1985Eduardo SL. The taxonomy of the family Paramphistomidae Fischoeder, 1901 with special reference to the morphology of species occurring in ruminants. V. Revision of the genus Cotylophoron Stiles & Goldberger, 1910. Syst Parasitol 1985; 7(1): 3-26. http://dx.doi.org/10.1007/BF00010157.
http://dx.doi.org/10.1007/BF00010157...
).
Besides having B. bubalis as host and the same type of pharynx (Liorchis) of the C. xiangjiangense species, Cotylophoron marajoensis n. sp. differs by the description of its biome, found in Amazon, while for C. xiangjiangense has only been described as occurring in China. In addition, Cotylophoron marajoensis n. sp. is longer in length and it does not possess an esophageal bulb, which present in the C. xiangjiangense. To strengthen this difference, the testis of Cotylophoron marajoensis n. sp. has a lobe format, whereas C. xiangjiangense possesses an oval testis format (Eduardo, 1985Eduardo SL. The taxonomy of the family Paramphistomidae Fischoeder, 1901 with special reference to the morphology of species occurring in ruminants. V. Revision of the genus Cotylophoron Stiles & Goldberger, 1910. Syst Parasitol 1985; 7(1): 3-26. http://dx.doi.org/10.1007/BF00010157.
http://dx.doi.org/10.1007/BF00010157...
).
The Cotylophoron travassosi species has not been described in B. bubalis up until now, being found, in Brazil, only in Bos indicus, Capra hircus and Ovis aries. It is shorter in length than Cotylophoron marajoensis n. sp., and it possesses a Paramphistomum pharynx (Miranda & Costa, 1999Miranda MA, Costa HMA. Report and redescription of some species of Cotylophoron (Trematoda: Paramphistomidae) in domestic ruminants of Brazil. Rev Bras Parasitol Vet 1999; 8(1): 1-15. http://dx.doi.org/10.4322/rbpv.01802003.
http://dx.doi.org/10.4322/rbpv.01802003...
). Additional morphometric comparisons between Cotylophoron marajoensis n. sp. and the other species of the genus Cotylophoron are presented in Table 1.
When analyzed by the SEM, the external morphology of Cotylophoron marajoensis n. sp. disclosed a smooth body, presence of genital papilla, oral sucker and acetabulum, these structures being common to the Cotylophoron (Miranda & Costa, 1999Miranda MA, Costa HMA. Report and redescription of some species of Cotylophoron (Trematoda: Paramphistomidae) in domestic ruminants of Brazil. Rev Bras Parasitol Vet 1999; 8(1): 1-15. http://dx.doi.org/10.4322/rbpv.01802003.
http://dx.doi.org/10.4322/rbpv.01802003...
). The internal morphology visualized by SEM has supplied data that have guaranteed diagnosis of the species, disclosing more details about the structures for type of pharynx and acetabulum.
Conclusion
In view of the above, the morphological evidence presented here for the new species falls within the genus Cotylophoron and the absence of all its valid species. Thus, we have added a species to this genus, named Cotylophoron marajoensis n. sp. Additionally we present its partial 18S small subunit ribosomal rRNA gene sequence which may contribute to future phylogenetics studies.
Acknowledgements
The authors are grateful the Laboratório de Microscopia Eletrônica de Varredura – ISPA – UFRA, Pará, Brazil for the use of the scanning electron microscope. This study is part of the Ph.D. of Vanessa Silva do Amaral, developed for the Programa de Pós-Graduação Saúde e Produção Animal na Amazônia (PPGSPAA), Instituto da Saúde e Produção Animal (ISPA), Universidade Federal Rural da Amazônia (UFRA). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES – BRASIL) - Finance Code 001 and and Project Pró-Amazônia: Biodiversidade e Sustentabilidade (Edital Nº 047/ 2012 – Ministério da Educação do Brasil). Raul Henrique da Silva Pinheiro was supported by a research fellowship from the “Programa de Pós-Graduação em Sociedade, Natureza e Desenvolvimento (PPGSND), Universidade Federal do Oeste do Pará – UFOPA/ CAPES/ BRASIL) – Finance Code 001”.
-
How to cite: Amaral VS, Sousa DF, Benigno RNM, Pinheiro RHS, Gonçalves EC, Giese EG. Cotylophoron marajoensis n. sp. (Digenea: Paramphistomidae) a parasite of Bubalus bubalis on Marajó Island, Pará, Brazilian Amazon. Braz J Vet Parasitol 2020; 29(4): e018320. https://doi.org/10.1590/S1984-29612020101
References
- Alarcón EP, Velásquez LE. Descripción morfológica de Cotylophoron cotylophorum (Digenea: Paramphistomidae) hallado en bovinos de Rio Negro, Antioquia, Colombia. Rev Colomb Cienc Pecu 2009; 22(2): 168-177.
- Costa HMA, Guimarães MP. Cotylophoron bareilliense Mukherjee e Chauhan, 1965 (Trematoda-Paramphistomidae). Arq Bras Med Vet Zootec 1990; 42(1): 183-186.
- Costa HMA, Guimarães MP. Cotylophoron travassosi sp. n. (Trematoda-Paramphistomidae) from cattle. Mem Inst Oswaldo Cruz 1992; 87(Suppl. 1): 69-72. http://dx.doi.org/10.1590/S0074-02761992000500013 PMid:1343800.
» http://dx.doi.org/10.1590/S0074-02761992000500013 - Damasceno FA, Viana JM, Tinôco IFF, Gomes RCC, Schiassi L. Adaptação de bubalinos ao ambiente tropical. Rev Eletrônica Nutritime 2010; 7(5): 1370-1381.
- Eduardo SL. The taxonomy of the family Paramphistomidae Fischoeder, 1901 with special reference to the morphology of species occurring in ruminants. V. Revision of the genus Cotylophoron Stiles & Goldberger, 1910. Syst Parasitol 1985; 7(1): 3-26. http://dx.doi.org/10.1007/BF00010157
» http://dx.doi.org/10.1007/BF00010157 - Forlano MD, Henríquez HR, Meléndez RD. Incidencia y prevalencia de Cotylophoron spp. (Trematoda: Digenea) en bovinos del Asentamiento Campesino” Las Majaguas”. Portuguesa-Venezuela 1996-1997. Gaceta Cienc Vet 2001; 7(1): 15-23.
- Giese EG, Silva MVO, Videira MN, Furtado AP, Matos ER, Gonçalves EC, et al. Rohdella amazonica n. sp. (Aspidogastrea: Aspidogastridae) from the Amazoninan banded puffer fish Colomesus psittacus (Bloch & Schneider, 1801). J Helminthol 2015; 89(3): 288-293. http://dx.doi.org/10.1017/S0022149X14000054 PMid:24572176.
» http://dx.doi.org/10.1017/S0022149X14000054 - Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 1999; 41: 95-98.
- Jones A, Bray RA, Gibson DI. Keys to the Trematoda 1st ed. London: CABI Publishing and The Natural History Museum; 2005. (vol. 2). http://dx.doi.org/10.1079/9780851995878.0000
» http://dx.doi.org/10.1079/9780851995878.0000 - Marques JRF. Búfalos: o produtor pergunta, a Embrapa responde Belém: Embrapa Comunicação para Transferência de Tecnologia; 2000.
- Martínez JL, Velásquez LET. Cotylophoron panamensis (Digenea: Paramphistomidae) en bovinos del Meta y del Guaviare, Colombia. Acta Biol Colomb 2012; 17(2): 419-428.
- Miranda MA, Costa HMA. Report and redescription of some species of Cotylophoron (Trematoda: Paramphistomidae) in domestic ruminants of Brazil. Rev Bras Parasitol Vet 1999; 8(1): 1-15. http://dx.doi.org/10.4322/rbpv.01802003
» http://dx.doi.org/10.4322/rbpv.01802003 - Morales G, Pino LA, Moreno L. Cotylophoron fulleborni Nasmark, 1937 (Trematoda: Paramphistomidae). Primer Reporte Para Venezuela. ACI Av Cienc Ing 2015; 7(2): 20-23. http://dx.doi.org/10.18272/aci.v7i2.253
» http://dx.doi.org/10.18272/aci.v7i2.253 - Pérez-Ponce de León G, Hernández-Mena D. Testing the higher-level phylogenetic classification of Digenea (Platyhelminthes, Trematoda) based on nuclear rDNA sequences before entering the age of the ‘next-generation’ Tree of Life. J Helminthol 2019; 93(3): 260-276. http://dx.doi.org/10.1017/S0022149X19000191 PMid:30973318.
» http://dx.doi.org/10.1017/S0022149X19000191 - Sánchez P, Tantalean VM, Chávez A, Soto AO. Presencia de Cotylophoron cotylophorum (Trematoda, Paramphistomidae) en bovinos de Loreto, Perú. Rev Peru Biol 2009; 16(1): 141-142.
Publication Dates
-
Publication in this collection
20 Nov 2020 -
Date of issue
2020
History
-
Received
06 Aug 2020 -
Accepted
01 Oct 2020