Abstracts
Patients with Hansen's disease (HD) may present themselves with clinical and laboratorial features that resemble rheumatic disorders. This requires a careful exercise of differential diagnosis. We describe here a case of a young woman with HD that presented with vasculitic like lesions, fibular vessels obstruction, ANA and antiphospholipid antibodies without typical signs of cutaneous disease, illustrating this clinical difficulty.
leprosy; arthritis; antiphospholipid antibodies
Pacientes com Mal de Hansen (MH) podem se apresentar com quadro clínico e laboratorial sugestivo de doenças reumáticas, o que exige um exercício cuidadoso de diagnósticos diferenciais. Descreve-se aqui o caso de uma jovem com MH que se apresentou com lesões cutâneas sugestivas de vasculite, obstrução de vasos fibulares, FAN e anticorpos antifosfolípides positivos sem muitos estigmas da doença cutânea, ilustrando essa dificuldade.
hanseníase; artrite; anticorpos antifosfolípides; vasculite
CASE REPORT
IResidents in the Rheumatology Service, Hospital Universitário Evangélico de Curitiba
Correspondence to
ABSTRACT
Patients with Hansen's disease (HD) may present themselves with clinical and laboratorial features that resemble rheumatic disorders. This requires a careful exercise of differential diagnosis. We describe here a case of a young woman with HD that presented with vasculitic like lesions, fibular vessels obstruction, ANA and antiphospholipid antibodies without typical signs of cutaneous disease, illustrating this clinical difficulty.
Keywords: leprosy, arthritis, antiphospholipid antibodies.
INTRODUCTION
Infectious diseases are part of the list of differential diagnosis of rheumatic diseases. There is not only a overlaying of signs and symptoms, but the autoantibodies can be positive in both situations. Among infectious diseases, Hansen's disease (HD) is one of the main diagnoses to be remembered, once it can present with clinical and laboratorial findings similar to the ones in a autoimmune disease vasculitis.1,2
This paper describes the case of a patient who was referred to the rheumatology department for treatment of vasculitis, whose investigation revealed a diagnosis of HD.
CASE REPORT
Female patient, 23 years old, caucasian, was referred to diagnosis investigation of skin lesions in the lower limbs. These lesions were red-violaceous, with ulcers and had initiated five years before, with periods of improvement and exacerbation. According to the patient, she had been hospitalized in another hospital one year before with the same complaints, having been submitted to skin biopsy that didn't clarify the diagnosis. Eight months before the current visit, she noticed febrile episodes up to 38ºC. She denied surgeries and transfusions. There was history of a motorcycle accident three years before, with nasal septum fracture resulting in a saddle deformity of the nose. She had one term gestation, without any complications, four years before. Nevertheless, during prenatal, a positive VDRL was found and the patient received six injections of benzathine penicillin.
In direct questioning, she denied oral, nasal or genital ulcers, alopecia, Raynaud's phenomenon, photosensitivity, weight loss, alteration in appetite, respiratory complaints, cardiac or any alteration in urinary color or volume.
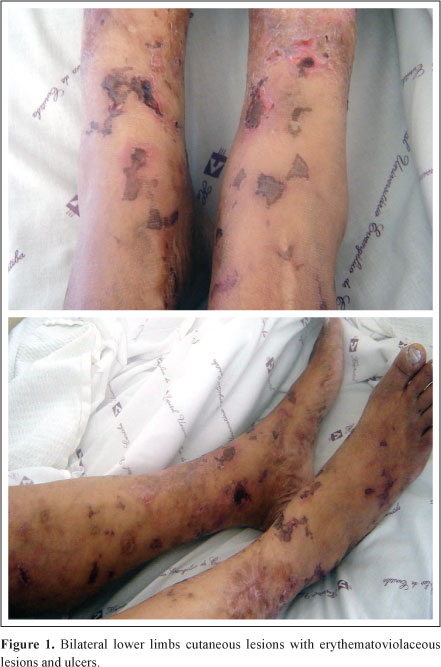
Physical examination showed a low grade fever, pale, hydrated, with a saddle nose patient. There were erythematoviolaceous skin lesions with ulcers in both lower limbs (Figure 1). She did not show nerve thickening at palpation or alteration of sensitivity. Lungs, heart and abdomen examination showed no abnormalities. Pedious pulses were present, although weak.
In the investigation, a blood count revealed hematocrit of 36,5%, 13,800 leukocytes/mm3, normal renal function, normal transaminases, gamma GT of 145.2U/l (RV = 9-36U/l), negative serologies for HIV, hepatitis A, B and C was verified. One VDRL was reagent in the titer of 1:2 with negative FTA-ABS. ANCA-p and ANCA-c were negative. ANA was positive (titer of 1/160, fine speckled pattern) with negative anti-Ro and anti-La; anticardiolipin antibody (aCl) IgG = 24.5 GPL (RV = up to 15 GPL), IgM = 121.20 MPL (RV = up to 15 MPL); lupus anticoagulant (LAC) by dRVVT test was positive. The complements were: CH50 (dosed by complement activity enzyme immunoassay) = 108 CAE U/mL (RV = 60-144U CAE U/mL), C4 = 37.2 mg/dl (RV = 10-40 mg/dl), C3 = 227.9 mg/dl (RV = 90-180 mg/dl). Thorax radiography and cardiac ECHO were normal. An angiography of the lower limbs showed bilateral fibular artery occlusion in the distal third (Figure 2).
Ziehl-Gabbet bacilloscopy in skin slit smears from the ear lobule revealed 90% of entire bacillus. Skin biopsy showed granulomatous lesion with positive BAAR. The patient received the diagnosis of HD, lepromatous form, questioning the presence of secondary antiphospholipid antibody syndrome. She was anticoagulated and received rifampicin, dapsone and clofazimine with disappearance of the cutaneous lesions. About six months after the treatment antibodies were repeated and findings were aCl IgG <11 GPL; aCl IgM = 161 MPL. LAC couldn't be evaluated because the patient was anticoagulated.
DISCUSSION
Autoantibodies and muscle-skeletal complaints appear as much in HD as in many rheumatic diseases. In HD, joint pain is the third more common complaint (superseded only by cutaneous and neuritic manifestations) and it is present in up to 84% of the cases.1,2 They include lepra reaction arthralgia and arthritis, arthritis nonrelated to lepra reactions, swollen hands syndrome, myositis and Charcot arthropathy.1,3
Arthritides related to reactions are sterile, involve large and small joints and have a synovial fluid which can be non-inflammatory, inflammatory, or even purulent. They respond to thalidomide and corticosteroids.3 Cases of sacroiliac onset1 and involvements of similar pattern to rheumatoid arthritis3-5 are described. Arthritis nonrelated to lepra reactions are in great majority infectious, i. e., the bacillus is present in synovial tissues.3,4 They tend to be monoarticular, indolent, chronic and with poor response to the use of non-hormonal anti-inflammatories and specific treatment for HD.4 Enthesitis is also described.5
Another form of skeletal muscle dysfunction is the swollen hands syndrome, in which there is a granulomatous inflammation of the subcutaneous tissue, more common in lepra reactions, giving the hand a juicy aspect, in glove, similar to the one seen in RS3PE syndrome (Remitting Seronegative Symmetrical Synovitis with Pitting Edema).6 Lastly, myositis are also found, as well as Charcot arthropathy, which is seen in more advanced cases, being secondary to neurological damage.1
In addition to the muscle-skeletal complaints, other manifestations that simulate rheumatic diseases can be found, such as vasculitis associated with cryoglobulins or Lucio's phenomenon.7,8 In Lucio's phenomenon there is a very large load of bacilli seen in endothelial cells.8 It is, therefore, an infectious vasculitis, which runs with focal proliferation of endothelial cells, necrosis and consequently ischemia of the subjacent epidermis.8 Deep tissues or extremities gangrene is not common in this entity.8
Autoantibodies that can be found in HD include rheumatoid factor (RF),9 ANA,9 ANCA10 and the antiphospholipid antibodies.11-13 In a study involving 120 patients (77 of the lepromatous form and 43 of turberculoid form) the presence of RF was seen in 35% and ANA was seen in 55,8% of the patients.9 The presence of ANCA was studied in 68 patients with Hansen's disease and ANCA-p was found in 31% of those with lepromatous form and 16% of those with borderline form; ANCA-c was seen in 5% of those with the lepromatous form. None of the patients with the tuberculoid form had a positive ANCA.10 Anticardiolipin antibodies are found in 8,3% of the paucibacillary patients and in 80,7% of the multibacillary, without any change in the titers after treatment.12
In the reported case, some aspects draw attention. The first one was the appearance of cutaneous lesions of similar in aspect to vasculitis in lower limbs without previous history of other lesions or cutaneous infiltrations. Within the forms of HD presentation, one of them, denominated pure and primitive diffuse lepromatosis (Latapi's leprosy), characterized by a diffuse infiltration of all the skin which never is transformed into nodule, giving the patient a healthy aspect, and consequently the diagnosis is difficult.14
The second one was the presence of autoantibodies (ANA and anticardiolipin) that could indicate a rheumatic entity as the cause of the illness. Besides the presence of positive anticardiolipin antibodies, the finding of fibular artery obstruction raised the possibility of a secondary antiphospholipid syndrome (APS). Vasculitis such as Takayasu and Wegener's granulomatosis with secondary APS15,16 could be included in the differential diagnosis.
Antiphospholipid antibodies have been associated with numerous bacterial and viral infections.17,18 Although, in this context, anticardiolipins of the IgM isotype are more common, anticardiolipin IgG have also been detected.19 Initial descriptions associated infections with antiphospholipid nonpathogenic antibodies and not associated to ²2-glicoprotein I (²2GPI).20 However, the reactivity of these autoantibodies with ²2GPI was demonstrated later and clinical association with APS have been observed in some situations.19 To explain why antiphospholipid antibodies can remain in the system but cause thrombotic events only in determined situations, a two hit hypothesis has been proposed by Shoenfeld et al.19 In the first moment, antiphospholipid antibodies would be produced by molecular mimetism between microbian structures and ²2GPI. So, a propitious environment to thrombosis is created. However, this will only happen if there is a second hit. This new stimulus - which could be another encounter of infectious agents - would activate the toll-like receptors (mainly type 4), which would act in a synergistic way with the anti-²2GPI linked to endothelial cellular structures,19 unleashing the thrombotic event. Toll-like receptor type 4 genetically deficient animals are protected against the thrombogenic action of the antiphospholipid.21
In the reported case, besides the possibility of APS, there are other possible explanations for the vascular occlusion finding. As it was previously mentioned, the endothelial lesions of Lucio's phenomenon can cause obliteration. Arteriographic occlusion abnormalities, narrowing, tortuosity, dilations, wall irregularities and filling flaws are described in the digital circulation of patients with HD in up to 94% of the cases.11 The literature shows APS patients misdiagnosed as Lucio's phenomenon and vice-versa, as well as patients in which both processes were thought to exist.8,11 Loizou et al. describe that, in HD, the most common isotype of antiphospholipid antibody is IgA,22 which was not investigated in the referred patient. In the reported patient, the decision was to keep the patient anticoagulated.
HD should always be remembered in patients with cutaneous lesion which resemble vasculitis, as in the presented case. The presence of autoantibodies and articular complaints can be confusing factors, and skin biopsy is of great help for the diagnosis. In a country like Brazil, in which HD is considered common and there are 45 thousand new cases per year,23 rheumatologists should always be vigilant to this possibility.
REFERENCES
-
1Hayata ALS, Gonçalves CR, Souza APTC, Abreu AC, Dulcine M, Gelbert C et al Artrite causada pelo "Mycobacterium leprae": causa rara de envolvimento articular na hanseníase? Rev Bras Reumatol 1999;39:245-7.
-
2Freire M, Carneiro HE, Teodoro RB, Cecin HA. Manifestações reumáticas da hanseníase: dificuldades no diagnóstico precoce. Rev Bras Reumatol 1996;38:210-3.
-
3Gibson T, Ahsan Q, Hussein K. Arthritis of leprosy. Br J Rheumatol 1994;33:63-6.
-
4Al-Raqum HA., Uppal SS, Abdalghani RAR, Lasheen I. First report of leprosy presenting as acute polyarthritis in the setting of type I downgrading lepra reaction. Clin Rheumatol 2005;25:101-5.
-
5Haroon N, Agarwal V, Aggarwal A, Kumari N, Krishnani N, Misra R. Arthritis as presenting manifestation of pure neuritic leprosy - a rheumatologist's dilemma. Rheumatology 2007;46:653-6.
-
6Helling CA, Locursio A, Manzur ME, Fonseca MLS. Remitting seronegative symmetrical synovitis with pitting edema in leprosy. Clin Rheumatol 2005;25:95-7.
-
7Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev 2006;19:338-81.
-
8Bakos L, Corre CC, Bergmann L. Bonaligo RR, Muller LFB. Antiphospholipid antibodies thrombotic syndrome misdiagnosed as Lucio's phenomenon. Int J Lepr Other Mycobact Dis 1996;64:320-3.
-
9Dacas P, Picanso M, Mouchaileh G, Percegona L, Schultz MT, Silva MGB et al autoanticorpos e manifestações reumáticas em pacientes com Mal de Hansen. An Bras Dermatol 2000;75(5):553-61.
-
10Medina F, Camargo A, Moreno J, Zonana-Nacach A, Aceves-Avila J, Fraga A. Anti-neutrophil cytoplasmic autoantibodies in leprosy. Br J Rheumatol 1998;37:270-3.
-
11Akerkar SM, Bichile LS. Leprosy & gangrene: a rare association. Role of antiphospholipid antibodies. BMC Infectious Diseases 2005;5:74-6.
-
12Repka JCD, Skare, TL, Salles Jr G, Paul, GM. Anticorpo anticardiolipina em pacientes com Mal de Hansen. Rev Bras Reumatol 2001;41:1-6.
-
13Forastiero RR, Martinuzzo ME, Larranaga, MR. Circulating levels of tissue factor and proinflammatory cytokines in patients with primary antiphospholipid syndrome or leprosy related antiphospholipid antibodies. Lupus 2005;14:129-36.
-
14Azulai-Abulafia L, Spinelli L. Revendo a hanseníase de Lúcio e o fenômeno de Lúcio. Med Cutan Iber Lat AM 2005;33:125-33.
-
15Santiago MB,Paz O. Rare association of antiphospholipid syndrome and Takayasu arteritis. Clin Rheumatol 2007;26:821-2.
-
16Castellino G, LA Corte R, Santilli D, Trota F. Wegener's granulomatosis associated with antiphospholipid syndrome. Lupus 2000;9:717-20.
-
17Baker WF, Bick RL. The clinical spectrum of antiphospholipid syndrome. Hematol Oncol Clin N Am 2008;22:33-52.
-
18Amin NM. Antiphospholipid syndromes in infectious diseases. Hematol Oncol Clin N Am 2008;22:131-43.
-
19Shoenfeld Y, Blank M, Cervera R, Font J, RAShi E, Meroni P-L. Infectious origin of the antiphospholipid syndrome. Ann Rheum Dis 2006;65:2-6.
-
20Loizou S, Cazaban JK, Walport MJ, Tait D, So AK. Similarities of specificity and co fator dependence in serum antiphospholipid antibodies from patients with parvovirus B19 infection and those with systemic lupus erythematosus. Arthritis & Rheum 1997;40:103-8.
-
21Pierangeli SS, Vega-Ostertag ME, Raschi E, Liu X, Romay-Penabad Z, De Micheli V et al Toll like receptor and antiphospholipid mediated thrombosis: in vivo studies. Ann Rheum Dis 2007;66:1327-33.
-
22Loizou S, Singh S, Wypkema E, Asherson RA. Anticardiolipin, anti β2 glycoprotein 1 and antiprotrombin antibodies in black South African patients with infectious diseases. Ann Rheum Dis 2003;62:1106-11.
-
23Site do Ministério da Saúde. Disponível em http://portalweb05.saude.gov.br /portal/aplicacoes/noticias/noticias_detalhe.cfm?co_seq_noticia=9535 Acesso em 3 de abril de 2008.
Leprosy, antiphospholipid antibodies and bilateral fibular arteries obstruction
Publication Dates
-
Publication in this collection
07 Apr 2009 -
Date of issue
Apr 2009
History
-
Accepted
12 Jan 2009 -
Received
10 Apr 2008