Abstracts
OBJECTIVE: To assess the association of the polymorphisms of the interleukin-18 (IL-18) gene with rheumatoid arthritis (RA) and with risk factors for cardiovascular diseases (CVD). METHODS: This sample comprised 97 patients with RA and 151 healthy controls. In the patients, risk factors for CVD were analyzed, such as cholesterol levels, arterial hypertension, smoking habit, C-reactive protein (CRP) level, and rheumatoid factor. DNA was extracted and the single nucleotide polymorphisms (SNP) at the -607C/A and -137G/C positions of the IL-18 gene were assessed in both groups. The Hardy-Weinberg equilibrium (HWE) was calculated and the odds ratio (OR) test performed, considering a 95% CI and P < 0.05. RESULTS: The frequencies of the -607A allele in patients with RA and in controls were 0,443 and 0.424, respectively, and of the -137C allele, 0.304 and 0.291, respectively. The genotype frequencies were in HWE, except for controls in the -137 locus (P = 0.006). Association of the polymorphisms of the IL-18 gene was found with neither RA nor risk factors for CVD, including cholesterol level and CRP (P > 0.05). In addition, more smokers were found among patients with RA as compared with controls (OR = 1.691; P = 0.088), and the CRP levels were slightly higher in patients who smoked than in patients who did not (OR = 2.673; P = 0.061). CONCLUSIONS: In this sample of patients with RA in the South of Brazil, association of the polymorphisms of the IL-18 gene was observed with neither RA nor risk factors for CVD.
Interleukin-18; Rheumatoid arthritis; Cardiovascular diseases; Gene polymorphism; Brazil
OBJETIVO: Analisar a associação dos polimorfismos do gene interleucina-18 (IL-18) com artrite reumatoide (AR) e com fatores de risco de doenças cardiovasculares (DCV). MÉTODOS: A amostra foi constituída por 97 pacientes com AR e 151 controles saudáveis. Nos primeiros, foram analisados fatores de risco de DCV, tais como níveis do colesterol, hipertensão arterial, tabagismo e fator reumatoide, bem como o nível da proteína C-reativa (CRP). O DNA foi extraído e foram analisados os polimorfismos de nucleotídeo único (SNP) nas posições -607C/A e -137G/C do gene IL-18 em ambos os grupos. O equilíbrio de Hardy-Weinberg (EHW) e o odds ratio (OR) foram realizados, considerando IC 95% e P < 0,05. RESULTADOS: As frequências do alelo -607A nos pacientes com AR e nos controles foram de 0,443 e 0,424 e do alelo -137C foram de 0,304 e 0,291, respectivamente. As frequências do genótipo estavam em EHW, exceto em controles no locus -137 (P = 0,006). Não foi encontrada associação dos polimorfismos do gene IL-18 com AR, nem com fatores de risco de DCV, incluindo o nível do colesterol e de CRP (P > 0,05). Além disso, observaram-se mais indivíduos fumantes entre pacientes com AR em comparação aos controles (OR = 1,691; P = 0,088), e os níveis de CRP eram ligeiramente mais elevados em pacientes fumantes quando comparados aos de pacientes não fumantes (OR = 2,673; P = 0,061). CONCLUSÕES: Ao analisar uma amostra de pacientes com AR no sul do Brasil, não foi encontrada associação dos polimorfismos do gene IL-18 com AR, nem com os fatores de risco de DCV.
Interleucina-18; Artrite reumatoide; Doenças cardiovasculares; Polimorfismo genético; Brasil
ORIGINAL ARTICLE
ILaboratory of genetic polymorphisms, Universidade Federal de Santa Catarina (LAPOGE/UFSC), Florianópolis, SC, Brazil
IIHospital das Clínicas, Medical School, Universidade do Estado de São Paulo (HC-FMUSP), São Paulo, SP, Brazil
IIIRheumatology Service, Hospital Universitário, Universidade Federal de Santa Catarina (HU-UFSC), Florianópolis, SC, Brazil
ABSTRACT
OBJECTIVE: To assess the association of the polymorphisms of the interleukin-18 (IL-18) gene with rheumatoid arthritis (RA) and with risk factors for cardiovascular diseases (CVD).
METHODS: This sample comprised 97 patients with RA and 151 healthy controls. In the patients, risk factors for CVD were analyzed, such as cholesterol levels, arterial hypertension, smoking habit, C-reactive protein (CRP) level, and rheumatoid factor. DNA was extracted and the single nucleotide polymorphisms (SNP) at the -607C/A and -137G/C positions of the IL-18 gene were assessed in both groups. The Hardy-Weinberg equilibrium (HWE) was calculated and the odds ratio (OR) test performed, considering a 95% CI and P < 0.05.
RESULTS: The frequencies of the -607A allele in patients with RA and in controls were 0,443 and 0.424, respectively, and of the -137C allele, 0.304 and 0.291, respectively. The genotype frequencies were in HWE, except for controls in the -137 locus (P = 0.006). Association of the polymorphisms of the IL-18 gene was found with neither RA nor risk factors for CVD, including cholesterol level and CRP (P > 0.05). In addition, more smokers were found among patients with RA as compared with controls (OR = 1.691; P = 0.088), and the CRP levels were slightly higher in patients who smoked than in patients who did not (OR = 2.673; P = 0.061).
CONCLUSIONS: In this sample of patients with RA in the South of Brazil, association of the polymorphisms of the IL-18 gene was observed with neither RA nor risk factors for CVD.
Keywords: Interleukin-18, Rheumatoid arthritis, Cardiovascular diseases, Gene polymorphism, Brazil
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by chronic inflammation, leading to joint destruction and systemic complications which increase morbidity and mortality.1,2 This disease affects 0.5%-1% of the general population worldwide, with a higher incidence in women than in men.3 Although the incidence and clinical manifestations of RA have been shown to vary in many different geographical regions, in Latin America, especially in Brazil, this information is scarce.4,5 Therefore, it is very important to enlighten RA pathogenesis in heterogeneous population such as in Brazil.
The RA has a complex and unclear etiology, but in genetically susceptible individuals, specific environmental factors can potentially activate pathogenic immune reactions, including autoantibody formation and autoreactivity response.1-3,6 The onset of RA can be indicated by the development of antibodies against citrullinated protein antigens (ACPA) and rheumatoid factor (RF) related to the self-tolerance loss.7 Recently, it has been recognized two RA subsets based on presence or absence of ACPA. Patients with ACPA positive have more extra-articular manifestations, smoking habit, and worse prognosis.2 The main cause of mortality in RA patients is cardiovascular diseases (CVD). Once the risk of CVD in RA patients is 50% higher when compared with the general population, it is believed that other risk factors are present in RA disease.8,9 Thus, the pathogenesis of accelerated cardiovascular damage is caused by traditional cardiovascular risk factors in combination to disease-related inflammatory and autoimmune mechanisms.10,11
Inflammation has an important role in atherosclerotic lesion and RA patients have a higher prevalence of atherosclerosis.12 In immune-mediated diseases such as RA, the accelerated and early atherosclerotic vascular damage may partially be explained by humoral and cellular autoimmune response against antigens expressed on the endothelium.8,13
Cytokines are also implicated in many immune processes associated with the pathogenesis of RA, especially in maintaining the active chronic inflammatory response. Because cytokines are involved in immune-regulatory and tissuedestructive events, it is likely that they would influence the severity of RA manifestations.7 Interleukin-18 (IL-18), a proinflammatory cytokine produced in RA by several synovium cells such as macrophages, chondrocytes and osteoblasts, induces signaling pathways common to other IL-1 family members, such as activation of nuclear factor-κB (NF-κB) and interferon-γ expression.7,14-16
Administration of IL-18 to mice caused development of erosive, inflammatory arthritis, suggesting that this cytokine can play a pro-inflammatory role in vivo.14 Furthermore, IL-18 mRNA and its protein were detected in RA synovial tissues in higher levels than in osteoarthritic controls.14 The structure, levels and regulation of IL-18 can be due to genetic differences on IL-18 gene expression.16
The chronic inflammatory condition seen in RA increase the levels and expression of C-reactive protein (CRP), tumor necrosis factor alpha (TNFα), interleukins-1, -6, and also -18, which are relevant as CVD risk factors.11,13,19 IL-18 is considered pro-atherogenic, presumably as a mediator of vascular inflammation itself, leading to augmentation and vulnerability of atherosclerotic plaque and finally, to its rupture.18 Human adipocytes are also capable of producing IL-18, contributing to systemic IL-18 concentrations and development of the increased risk of diabetes and CVD that are associated with obesity and insulin resistance states.19
Plasma IL-18 concentration has shown to be increased in post myocardial infarction patients and was associated with coronary atherosclerosis.20 Indeed, variations in the IL18 gene were associated with raised IL-18 serum concentrations and higher cardiovascular mortality among coronary artery disease (CAD) patients.21 Other studies showed that IL-18 gene polymorphisms are involved in the development of ischemic stroke,22 myocardial infarction (MI),23 and higher cardiovascular mortality risk.24 Moreover, IL-18 serum levels were associated with traditional risk factors such as LDL- and HDL-cholesterol abnormal values, obesity, insulin resistance and cell dysfunction.20
Even though the endogenous production of IL-18 is affected by multiple factors, individual differences could also be determined by genetic polymorphisms, potentially affecting the balance between Th1 and Th2 cytokine responses. This mechanism could be responsible for an increased resistance to microbial infections, but also for a higher susceptibility to autoimmune disorders in individuals carrying more active IL-18 alleles.15
The IL-18 gene is regulated by its promoter region polymorphisms, which variability could lead to differences in transcription factor binding. Two single nucleotide polymorphisms (SNPs) in the promoter region at -607C/A and -137G/C position have been studied, and those changes disrupts a potential binding site of the cAMP-responsive element binding (CREB) protein and the H4TF-1 nuclear factor, respectively.15,25
In RA patients, the higher frequency of -607A allele and/or higher frequency of -137C allele are related to deficiency in gene transcription; that would be beneficial for the individual, protecting against the development of RA. Accordingly, one study demonstrates that the -607AA genotype is associated with lower prevalence of RA in a Chinese population.25
On the other hand, the homozygous for C at position -607 and G at position -137 promote higher levels of IL-18 mRNA compared to the other genotypes, and the resulting elevated levels of the pro-inflammatory IL-18 protein mediate many acute and chronic inflammatory processes.15,25
The aim of this study was to analyze the influence of the IL-18 polymorphisms on RA pathogenesis, as well as in CVD risk factors (dyslipidemia, blood pressure, smoking).
Methods
Ninety seven RA patients diagnosed according to 1987 ACR classification criteria, from the outpatient clinic of the Rheumatology Division at the University Hospital of the Federal University of Santa Catarina, Florianópolis, Brazil, were enrolled. Control group was composed by 151 healthy volunteers without personal or family history of autoimmune diseases.The study was approved by the local Ethics Committee (CEP/UFSC - case number 172/06). All participants gave their written informed consent. Familial and epidemiologic data were collected using structured questionnaires. Clinical data were obtained from medical records.
As traditional CVD risk factors, we considered: high total (> 200 mg/dL) and LDL (> 100 mg/dL) cholesterol levels, systemic arterial hypertension (systolic arterial pressure (AP) > 140 mmHg and/or dyastolic AP > 90 mmHg), current smoking habit, RF positivity (> 20 IU/mL), and levels of CRP above the reference value (> 5mg/L).
Peripheral blood samples were collected for DNA extraction.26The SNP -607C/A (rs1946518) of IL-18 gene was detected by the polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) technique, amplifying a 301 base pair (bp) segment covering the polymorphic site by using the primers sequences on Table 1.27 To detect the polymorphism, the PCR products were followed by MseI restriction enzyme (BioLabs Inc., New England) digestion, at 37ºC for 12 hours, then subjected to electrophoresis in a 3% agarose gel and stained with ethidium bromide (1%). The digested PCR products were identified as CC homozygous individuals when cut into 199 and 73bp fragments, and as AA homozygous individuals when cut into 101, 98 and 73 bp fragments. Thus, the CA heterozygous individuals were identified when showing the expected fragments: 199, 101, 98 and 73 bp (Fig. 1a).
The SNP -137G/C (rs187238) of the same gene was detected by sequence specific PCR (PCR-SSP) method, according to Takada et al. (2002).27 In this method were used a common reverse primer (R) and two sequence-specific forwards primers, specific F1 for C allele and specific F2 for G allele, amplifying a product of 261 bp (Table 1). As an internal positive amplification control, a control forward primer (F) was used to amplify a 446 bp fragment which covers the polymorphic site (Table 1) (Fig. 1b). For confirmation of typing, we used negative and positive controls for each genotype in all experiments in both SNP detection methods.
The frequencies of alleles and genotypes in RA patients and controls of both SNPs -607 and -137 was performed by direct counting. To verify the genotype distribution, the Hardy-Weinberg Equilibrium (HWE) test was calculated using GENEPOP software.28 Since both SNPs are located in the same gene, we have verified if they were inherited together due to linkage disequilibrium (LD) using GENEPOP software. Once both SNPs are linked, they can be considered as one haplotype, and frequency of its combination was calculated by PHASE software.29
The odds ratio (OR) with 95% confidence interval was obtained for association analysis30 of the polymorphisms (alleles, genotypes and haplotypes).The analysis was performed separately for clinical and epidemiological data as well as for RA susceptibility and further confirmed using the HDS Epi-Max Calculator.31 The -607A and -137G alleles were considered as risk alleles to RA susceptibility. A P value below 0.05 was considered significant.
Results
Women were 88.66% of the RA patients and 96.40% of the controls (P > 0.05). Mean age was 54.63 (± 12.48) in patients and 48.00 (± 15.56) years old in controls (P > 0.05). Although in RA and controls the predominant ethnicity was Euro-Brazilian, frequency of Afro-Brazilians and Amerindian-Brazilians was higher in RA patients than in controls (P = 0.027) (Table 2).
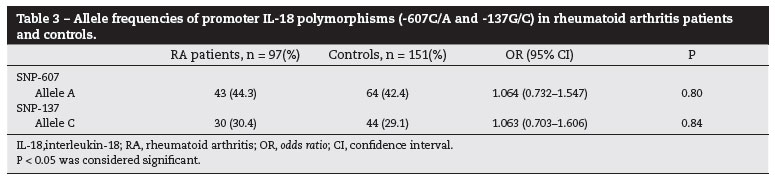
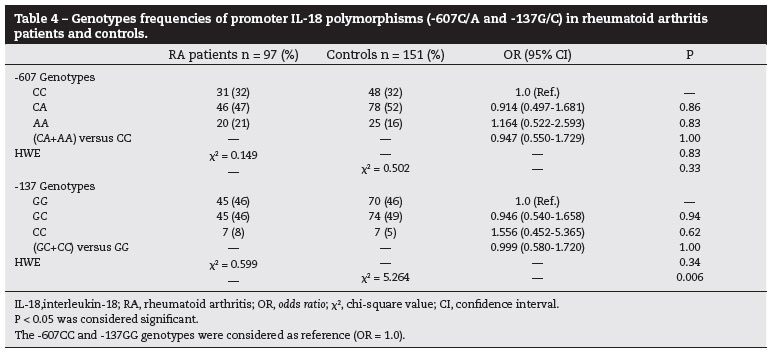
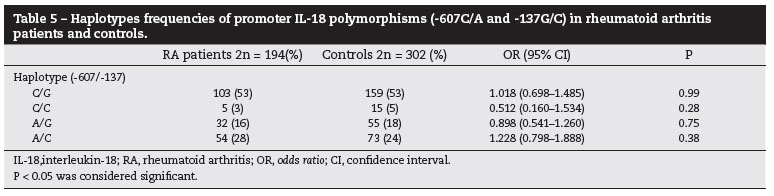
The allele, genotype and haplotypes frequencies of IL-18 gene polymorphisms were estimated in case and controls. The allele frequencies were showed in Table 3. The genotype frequencies distribution for both SNPs were in HWE in RA patients and controls, except for the genotype frequencies for -137 SNP in controls (P = 0.006) (Table 4). Furthermore, because we found that the polymorphisms -607 and -137 were in linkage disequilibrium in RA patients and controls (P < 0.001), both SNPs were analyzed as haplotypes (Table 5). We showed that the allele, genotype and haplotype frequencies of IL-18 gene were similar in RA patients and controls, and the IL-18 polymorphisms were not associated with the development of RA (P > 0.05).
Among the prevalence of CVD risk factors in RA patients, it was observed the presence of hypercholesterolemia (54.95%), arterial hypertension (56.99%), smoking habit (41.76%), positive RF (66.27%), and high CRP levels (52.38%) (Table 2). High cholesterol levels and arterial hypertension were not associated with IL-18 gene polymorphisms. Also, we observed that smoking habit in RA patients have a tendency to be associated with levels of positive RF (OR = 2.199; 0.739-6.677 95 CI%; P = 0.182), and CRP high levels (OR = 2.673; 0.961-7.546 95% CI; P = 0.061). The frequency of smokers was higher among RA patients than in controls (OR = 1.691; 0.930-3.079 95% CI; P = 0.088), however not statistically significant. Furthermore, neither RF nor smoking was associated with -607 or -137 polymorphisms.
Discussion
This is an important study that analyses for the first time in a Brazilian population the genetic features of the pro-inflammatory cytokine IL-18, and its role in RA. This cytokine can be involved in the characteristic inflammatory condition that causes so many injuries in RA patients.
The genotype frequencies of both -607 and -137 SNPs found in the present study resemble to other case-control studies including Caucasian RA patients, such as Polish,32 and Spanish.33 On the other hand, the genotype frequencies found in Asian studies differ from ours.25,34 Thus, the similarity to the European population regarding genotype frequencies could be explained by the high prevalence of Euro-descendants in our sample, since many immigrants from Europe were settled in Southern Brazil.35
A Chinese study found that the AA genotype of -607 SNP confers protection against RA development.25 However, this study showed no association between both -607 and -137 SNPs of IL-18 gene and susceptibility for RA development. In the same way, a recent meta-analysis on autoimmune diseases36 concluded that these polymorphisms were not related to RA development, similar to other studies done in Poland,32 Spain,33 and China.34 The latter study also showed that -607 SNP did not change IL-18 serum levels.34
Although RA is a disease of complex etiology, it is believed to be caused by the combination of genetic susceptibility and several environmental factors such as infections and lifestyle characteristics, the exact contribution of each of these factors for RA development in distinct populations are not well understood and need further investigation.3,37
We demonstrated that the IL-18 SNPs were in linkage disequilibrium in RA patients, as it was ever previously shown by other studies.25,32,33 Also, we found no association of haplotypes with RA susceptibility, in agreement to results from Spanish34 and Chinese studies.25 Nevertheless, a study from Poland found a significant decreased number of subjects with AC/AC and AG/AG diplotypes among RA patients as compared with controls, suggesting these diplotypes are related to RA development.32 The polymorphisms in the IL-18 promoter gene affect its activity, but this effect depends on cell types and local cytokine environment. Thus, the interaction between genotype and cellular environment remains to be evaluated.15
Although dyslipidemia is a controversial factor of CVD in RA, studies showed that low levels of HDL-cholesterol are common and could contribute for increased CV morbidity in these patients.8,17,38,39 Dyslipidemia has been associated with high levels of IL-18.20 However, our study showed that high levels of total cholesterol were not associated with the presence of the IL18 SNPs analyzed, which was found by other studies as well.24,40
Arterial hypertension is common among RA patients, but the cause of this increased prevalence compared with controls is unclear. There are multiple factors that influence blood pressure, such as obesity, physical inactivity, specific genetic polymorphisms, and some antirheumatic medications.8,37 Arterial hypertension is associated with increased IL-18 serum levels,20 and the expression of mRNA of this cytokine in atherosclerotic tissue samples was augmented when the -137GC polymorphisms and arterial hypertension were both present.41 Furthermore, a study about metabolic risk factors for CVD found that -137GC genotype confers increased risk of arterial hypertension development to African women when compared with -137GG subjects.41 In our study, however, arterial hypertension was not associated with IL-18 polymorphisms, as also demonstrated by Szeto et al.24
Smoking is a known risk factor for development of RA, and smokers with RA appear to have higher RF titers and worse prognosis in terms of disability, radiographic damage, and treatment response.38 We found no association between smoking habit and the IL-18 polymorphisms, nor between smoking habit and RF in RA patients (OR = 2.2; P > 0.05), which differs from data of a recent meta-analysis.38 In the same way, the presence of RF was not associated with the polymorphisms of IL-18 gene, as showed by other studies in RA.15,33 High levels of CRP were more frequent in RA patients who smoke, but this association was not statistically significant (OR = 2.67; P = 0.061). CRP levels were not associated with IL-18 gene polymorphisms, similarly to the results of another study.24
Although other studies have demonstrated that expression of IL-18 gene is higher in RA patients and that this cytokine might be relevant in the pathogenesis of the disease, we found that the polymorphisms -607 and -137 of IL-18 gene do not play a major role in RA susceptibility in our population. In addition, these polymorphisms are not associated with CVD risk factors in our RA patients.
IL-18 exhibits pleiotropic activities in RA, with a wide variety of effects that are influenced by the overall cytokine network. Future studies considering novel genetic markers within IL-18 or other genes involved in the cytokine network should be performed to evaluate their relevance in the context of RA and other inflammatory diseases.
Financial support
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento Pessoal de Ensino Superior (CAPES) and Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC).
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento Pessoal de Ensino Superior (CAPES) and Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC). The authors wish to thank the patients and volunteers for their cooperation, and the colleagues that helped in this work.
REFERENCES
-
1Firestein GS. Envolving concepts or rheumatoid arthritis. Nature. 2003;423:356-61.
-
2Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009;373(9664):659-72.
-
3Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev. 2005;4(3):130-6.
-
4Harney S, Wordsworth BP. Genetic epidemiology of rheumatoid arthritis. Tissue Antigens. 2002;60(6):465-73.
-
5Coenen MJ, Grefersen PK. Rheumatoid arthritis: a view of the current genetic landscape. Genes Immun. 2009;10(2):101-11.
-
6de Souza AW, Mesquita Júnior D, Araújo JA, Catelan TT, Cruvinel W de M, Andrade LE, et al. Immune system: part III. The delicate balance of the immune system between tolerance and autoimmunity. Rev Bras Reumatol. 2010;50(6):665-79.
-
7McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7(6):429-42.
-
8Gabriel SE, Crowson CS. Risk factors for cardiovascular disease in rheumatoid arthritis. Curr Opin Rheumatol. 2012;24(2):171-6.
-
9Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59(12):1690-7.
-
10Boyer JF, Gourraud PA, Cantagrel A, Davignon JL, Constantin A. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Jt, Bone Spine. 2011;78(2):179-83.
-
11Bartoloni E, Alunno A, Bistoni O, Gerli R. Cardiovascular Risk in Rheumatoid Arthritis and Systemic Autoimmune Rheumatic Disorders: a Suggested Model of Preventive Strategy. Clin Rev Allergy Immunol. 2013;44(1):14-22.
-
12Gonzalez-Juanatey C, Llorca J, Testa A, Revuelta J, Garcia-Porrua C, Gonzalez-Gay MA. Increased prevalence of severe subclinical atherosclerotic findings in long-term treated rheumatoid arthritis patients without clinically evident atherosclerotic disease. Medicine (Baltimore). 2003;82(6):407-13.
-
13Pereira IA, Borba EF. The role of inflammation, humoral and cell mediated autoimmunity in the pathogenesis of atherosclerosis. Swiss Med Wkly. 2008;138(37-38):534-9.
-
14Gracie JA, Forsey RJ, Chan WL, Gilmour A, Leung BP, Greer MR, et al. A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest. 1999;104(10):1393-401.
-
15Giedraitis V, He B, Huang WX, Hillert J. Cloning and mutation analysis of the human IL-18 promoter: a possible role of polymorphisms in expression regulation. J Neuroimmunol. 2001;112(1-2):146-52.
-
16Thompson SR, Humphries SE. Interleukin-18 genetics and inflammatory disease susceptibility. Genes Immun. 2007;8(2):91-9.
-
17Nurmohamed MT. Cardiovascular risk in rheumatoid arthritis. Autoimmun Rev. 2009;8(8):663-7.
-
18Blankenberg S, Tiret L, Bickel C, Peetz D, Cambien F, Meyer J, et al. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation. 2002;106:24-30.
-
19Skurk T, Kolb H, Müller-Scholze S, Röhrig K, Hauner H, Herder C. The proatherogenic cytokine interleukin-18 is secreted by human adipocytes. Eur J Endocrinol. 2005;152:863-8.
-
20Hulthe J, McPheat W, Samnegård A, Tornvall P, Hamsten A, Eriksson P. Plasma interleukin (IL)-18 concentrations is elevated in patients with previous myocardial infarction and related to severity of coronary atherosclerosis independently of C-reactive protein and IL-6. Atherosclerosis. 2006;188(2):450-4.
-
21Hernesniemi JA, Karhunen PJ, Oksala N, Kähönen M, Levula M, Rontu R, et al. Interleukin 18 gene promoter polymorphism: a link between hypertension and prehospital sudden cardiac death: the Helsinki Sudden Death Study. Eur Heart J. 2009;30(23):2939-46.
-
22Zhang N, Yu JT, Yu NN, Lu RC, Ma T, Wang ND, et al. Interleukin-18 promoter polymorphisms and risk of ischemic stroke. Brain Res Bull. 2010;81(6):590-4.
-
23Bis JC, Heckbert SR, Smith NL, Reiner AP, Rice K, Lumley T, et al. Variation in inflammation-related genes and risk of incident nonfatal myocardial infarction or ischemic stroke. Atherosclerosis. 2008;198(1):166-73.
-
24Szeto CC, Chow KM, Poon PY, Kwan BC, Li PK. Association of interleukin-18 promoter polymorphism and atherosclerotic diseases in Chinese patients with diabetic nephropathy. Nephrology. 2009;14(6):606-12.
-
25Sivalingam SP, Yoon KH, Koh DR, Fong KY. Single-nucleotide polymorphisms of the interleukin-18 gene promoter region in rheumatoid arthritis patients: protective effect of AA genotype. Tissue Antigens. 2003;62(2):498-504.
-
26Sambrook J, Russel DW. Molecular cloning: a laboratory manual. 3.ed. New York: Cold Spring Harbour Laboratory Press; 2001.
-
27Takada T, Suzuki E, Morohashi K, Gejyo F. Association of single nucleotide polymorphisms in the IL-18 gene with sarcoidosis in a Japanese population. Tissue Antigens. 2002;60(1):36-42.
-
28Raymond M, Rousset F. GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 1995; 86(3):248-9. Available from: http://genepop.curtin.edu.au/ [Access in May 2011]
-
29Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68(4):978-89.
-
30Woolf B. On estimating the relation between blood groups and disease. Ann Hum Genet 1955;19(4):251-3.
-
31HDS EpiMax Table Calculator. Available from: http://www.healthstrategy.com/epiperl/epiperl.htm [Access in May 2011]
-
32Pawlik A, Kurzawski M, Czerny B, Gawronska-Szklarz B, Drozdzik M, Herczynska M. Interleukin-18 promoter polymorphism in patients with rheumatoid arthritis. Tissue Antigens. 2006;67(5):415-8.
-
33Rueda B, González-Gay MA, Mataran L, López-Nevot MA, Martín J. Interleukin-18-promoter polymorphisms are not relevant in rheumatoid arthritis. Tissue Antigens. 2005;65(6):544-8.
-
34Ying B, Shi Y, Pan X, Song X, Huang Z, Niu Q, et al. Association of polymorphisms in the human IL-10 and IL-18 genes with rheumatoid arthritis. Mol Biol Rep. 2011;38(1):379-58.
-
35Salzano FM, Freire-Maia SM. Problems in Human Biology. A Study of Brazilian Populations. Detroit: Wayne State University Press; 1970.
-
36Pan HF, Leng RX, Ye DQ. Lack of association of interleukin-18 gene promoter -607 A/C polymorphism with susceptibility to autoimmune diseases: a meta-analysis. Lupus. 2011;20(9):945-51.
-
37Mota LM, Cruz BA, Brenol CV, Pereira IA, Rezende-Fronza LS, Bertolo MB, et al. 2012 Brazilian Society of Rheumatology Consensus for the treatment of rheumatoid arthritis. Rev Bras Reumatol. 2012;52(2):152-74.
-
38Boyer JF, Gourraud PA, Cantagrel A, Davignon JL, Constantin A. Traditional cardiovascular risk factors in rheumatoid arthritis: A meta-analysis. Jt, Bone Spine. 2011;78(2):179-83.
-
39Torigoe, DY, Laurindo, IMM. Artrite Reumatoide e Doenças Cardiovasculares. Rev Bras Reumatol. 2006;46(1):60-6.
-
40Evans J, Collins M, Jennings C, van der Merwe L, Söderström I, Olsson T, et al. The association of interleukin-18 genotype and serum levels with metabolic risk factors for cardiovascular disease. Eur J Endocrinol. 2007;157(5):633-40.
-
41Hernesniemi JA, Anttila K, Nieminen T, Kähönen M, Mononen N, Nikus K, et al. IL-18 gene polymorphism, cardiovascular mortality and coronary artery disease. Eur J Clin Invest. 2010;40(11):994-1001.
Lack of association between interleukin-18 polymorphisms and rheumatoid arthritis
Publication Dates
-
Publication in this collection
30 Oct 2015 -
Date of issue
Apr 2013
History
-
Received
02 Feb 2012 -
Accepted
13 Dec 2012