Abstracts
Introduction
The assessment of the activity of rheumatoid arthritis and juvenile idiopathic arthritis is made by means of the tools DAS-28 and JADAS, respectively.
Objective
To compare DAS-28 and JADAS with scores of 71, 27 and 10 joint counts in juvenile idiopathic arthritis.
Method
A secondary analysis of a phase III placebo-controlled trial, testing safety and efficacy of abatacept was conducted in 8 patients with 178 assessment visits. Joint count scores for active and limited joints, physician's and parents’ global assessment by 0–10 cm Visual Analog Scale, and erythrocyte sedimentation rate normalized to 0–10 scale, in all visits. The comparison among the activity indices in different observations was made through Anova or adjusted gamma model. The paired observations between DAS-28 and JADAS 71, 27 and 10, respectively, were analyzed by linear regression.
Results
There were significant differences among individual measures, except for ESR, in the first 4 months of biological treatment, when five of the eight patients reached ACR-Pedi 30, with improvement. The indices of DAS-28, JADAS 71, 27 and 10 also showed significant difference during follow-up. Linear regression adjusted model between DAS-28 and JADAS resulted in mathematical formulas for conversion: [DAS-28 = 0.0709 (JADAS 71) + 1.267] (R2 = 0.49); [DAS-28 = 0.084 (JADAS 27) + 1.7404] (R2 = 0.47) and [DAS-28 = 0.1129 (JADAS-10) + 1.5748] (R2 = 0.50).
Conclusion
The conversion of scores of DAS-28 and JADAS 71, 27 and 10 for this mathematical model would allow equivalent application of both in adolescents with arthritis.
Juvenile idiopathic arthritis; Rheumatoid arthritis; Disease Activity Score-28; Juvenile Arthritis Disease Activity Score
Introdução
A avaliação de atividade da artrite reumatoide e da artrite idiopática juvenil é feita por meio de instrumentos distintos, respectivamente pelo DAS-28 e pelo JADAS.
Objetivo
Comparar o DAS-28 e o JADAS com a pontuação de 71, 27 e 10 articulações, na artrite idiopática juvenil.
Método
Foram avaliadas 178 visitas em oito pacientes com artrite idiopática juvenil, participantes de um ensaio clínico controlado de fase III, testando eficácia e segurança do abatacepte. Pontuaram-se as articulações ativas e limitadas, a avaliação global pelo médico e pelos pais em escala analógica visual de 0-10 cm e a velocidade de hemossedimentação convertida em escala de 0-10, em todas as visitas. A comparação entre os índices de atividade entre diferentes observações foi por Anova ou modelo ajustado Gama. As observações pareadas entre o DAS-28 e o JADAS 71, 27 e 10, respectivamente, foram analisadas por meio de regressão linear.
Resultados
Houve diferença significativa entre as medidas individuais, exceto a VHS, nos primeiros quatro meses de tratamento com biológico, quando cinco entre os oito pacientes atingiram a resposta ACR-Pedi 30, com melhora. Os índices DAS-28, JADAS 71, 27 e 10 também apresentaram diferença relevante durante o período de observação. O ajustamento por meio de regressão linear entre o DAS-28 e o JADAS resultou em fórmulas matemáticas para conversão: [DAS-28 = 0,0709 (JADAS 71) + 1,267] (R2 = 0,49); [DAS-28 = 0,084 (JADAS 27) + 1,7404] (R2 = 0,47) e [DAS-28 = 0,1129 (JADAS-10) + 1,5748] (R2 = 0,50).
Conclusão
A conversão da pontuação do DAS-28 e do JADAS 71, 27 e 10 por esse modelo matemático permitiria a aplicação equivalente de ambos em adolescentes com artrite.
Artrite idiopática juvenil; Artrite reumatoide; Disease Activity Score-28; Juvenile Arthritis Disease Activity Score
Introduction
Juvenile idiopathic arthritis (JIA) has a chronic course and great variability of outcomes, it may progress to spontaneous remission or be refractory to available treatments.11 Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767-78. JIA subtypes represent different phenotypes, classified as oligoarticular (<5 joints), polyarticular (≥5 joints), systemic, arthritis related to enthesitis, psoriatic arthritis, and undifferentiated or unclassified arthritis.22 Petty RE, Southwood TR, Manners P,Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision. J Rheumatol. 2004;31:390-2.
In order to assess arthritis activity, it is essential to measure the response to treatment, and early treatment is crucial to the outcome. In children, the response to treatment, evaluated in clinical trials, involves six primary outcome measures: physician's global assessment, global assessment by the parents or by the patient, joint count in absolute numbers of inflamed joints and joints with limited range of motion, erythrocyte sedimentation rate (ESR), and functional capacity index. The minimum criteria for response (ACR Pedi 30) are defined as improvement of at least 30% in three of six measures, with not more than 30% of worsening in no more than one of these parameters, representing a cutoff of response differentiation in the treated group and in the placebo group in clinical trials.33 Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997;40:1202-9. Currently, the improvements that are considered clinically significant are those in excess of 50, 70, 90%, or even the inactive state of arthritis.44 Wallace C, Ruperto N, Giannini EH. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31:2290-4. However, these measures are related to the response to treatment, and are not suitable as absolute measures of arthritis activity, because nature of calculation does not allow absolute comparison of response between groups of patients.
The most commonly used in rheumatoid arthritis (RA) are the DAS55 Van der Heijde DM, Van't Hof M, Van Riel PL, Van de Putte LB. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol. 1993;20:579-81. (Disease Activity Score) and DAS2866 Prevoo ML, Van't Hof MA, Kuper HH, Van Leeuwen MA, Van de Putte LB, Van Riel PL. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44-8. in its simplified version. JADAS77 Consolaro A, Ruperto N, Bazso A, Pistorio A, Magni-Manzoni S, Filocamo G, et al. Paediatric Rheumatology International Trials Organization. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Care Res. 2009;61:658-66. (Juvenile Arthritis Disease Activity Score), with three versions of joint scoring, was developed for JIA. Both use the same components for the absolute assessment of arthritis activity, including “active” joint count, physician's and patient's or his/her parents’ global assessment, and laboratory tests, which may be ESR or C-reactive protein (CRP), and is useful in clinical trials and in daily practice.88 Lurati A, Pontikaki I, Teruzzi B, Desiati F, Gerloni V,Gattinara M, et al. Comparison of response criteria to evaluate therapeutic response in patients with juvenile idiopathic arthritis treated with methotrexate and/or anti-tumor necrosis factor agents. Arthritis Rheum. 2006;54:1602-7.
DAS2866 Prevoo ML, Van't Hof MA, Kuper HH, Van Leeuwen MA, Van de Putte LB, Van Riel PL. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44-8. combines information on the number of painful and swollen joints, with 28 joints being selected, as well as ESR or CRP and patient's global assessment measured on a visual analog scale (VAS) from zero to 10 cm. DAS28 score is calculated using a mathematical formula, and the activity of arthritis can be interpreted in categorical scale.
JADAS score77 Consolaro A, Ruperto N, Bazso A, Pistorio A, Magni-Manzoni S, Filocamo G, et al. Paediatric Rheumatology International Trials Organization. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Care Res. 2009;61:658-66. is performed by adding the four individual measurements: global assessment of arthritis activity by the physician, in 10-cm VAS, global evaluation by the parents/patients as measured in the same 10-cm VAS, where 0 indicates no activity and 10, maximum activity, ESR and joint count. There are three versions, scoring from 0 to 71, 0 to 27 or 0 to 10 joints.
Functional capacity is often assessed through a health questionnaire, the Childhood Health Assessment Questionnaire (CHAQ),99 Singh G, Athreya B, Goldsmith DP. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis Rheum. 1994;37:1761-9.,1010 Machado CS, Ruperto N, Silva CH, Ferriani VP, Roscoe I, Campos LM, et al. The Brazilian version of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ). Clin Exp Rheumatol. 2001;19 Suppl 23:S25-30. the corresponding version of the Health Assessment Questionnaire (HAQ). Both evaluate the degree of difficulty and independence in activities of daily life in eight domains of functional capacity, also considering the pain and discomfort through aggregated VAS (0–10 cm). Functional capacity is included among the response measures of ACR Pedi 30.
The present study is a secondary analysis of a placebo-controlled, phase III clinical trial, to evaluate the efficacy and safety of intravenous abatacept in patients with active polyarticular JIA and unresponsive to treatment with antirheumatic therapy with methotrexate (MTX).1111 Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Pérez N, Silva CA, et al. Abatacept in children with juvenile idiopathic arthritis: a randomized, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372:383-91.,1212 Ruperto N, Lovell DJ, Mouy R, Paz E, Rubio-Pérez N, Silva CA, et al. Long-term safety and efficacy of abatacept in children with juvenile idiopathic arthritis. Arthritis Rheum. 2010;62:1792-802. Patients selected for clinical trials have more intense activity and are resistant to conventional treatment, showing more enhanced differences of clinical response. Thus, this sample was considered optimal to compare different continuous measures of activity.
The aim was to explore score equivalence of the tool DAS28 and JADAS with scores of 71, 27 and 10 joints, respectively, in children and adolescents with JIA.
Subjects and method
One hundred and seventy-eight visits were assessed of eight patients with JIA who participated in a controlled clinical phase III trial testing the efficacy and safety of abatacept1111 Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Pérez N, Silva CA, et al. Abatacept in children with juvenile idiopathic arthritis: a randomized, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372:383-91.,1212 Ruperto N, Lovell DJ, Mouy R, Paz E, Rubio-Pérez N, Silva CA, et al. Long-term safety and efficacy of abatacept in children with juvenile idiopathic arthritis. Arthritis Rheum. 2010;62:1792-802. and using the same evaluations at intervals of four to 12 weeks of the original trial, a withdrawal study design,1111 Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Pérez N, Silva CA, et al. Abatacept in children with juvenile idiopathic arthritis: a randomized, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372:383-91.,1212 Ruperto N, Lovell DJ, Mouy R, Paz E, Rubio-Pérez N, Silva CA, et al. Long-term safety and efficacy of abatacept in children with juvenile idiopathic arthritis. Arthritis Rheum. 2010;62:1792-802. which included an open-label phase of 4 months, double-blind phase trial open-label extension phase of up to 5 years. In the double-blind period, assessments were monthly performed, and in other periods, complete assessments, including measures of activity, were performed every 3 months. The same clinical, laboratory and functional parameters of the clinical trial for the calculation of activity rates of DAS28 and JADAS-71, 27, 10 were used. The protocol of secondary study was approved by the Ethics Committee in Institutional Research (no. 345/2009) of 14 September 2009.
Data were collected from first to last visit with complete joint assessment. Of the eight subjects included, five completed the open phase of induction and the double-blind phase, extension open-label phase. Of the five who concluded the double-blind period, two were given placebo and three were given the study medication. Three subjects concluded the open period, but were not approved for the double-blind since they did not reach ACR-Pedi 30 response, staying in the open-label extension indicated by the protocol. Four subjects left the study in the extension phase in different periods, due to lack of medication efficacy, with change of treatment being necessary. Three subjects concluded the 5 years of extension phase.
Standardized joint assessment (it is more specific for the technical procedure) was performed by the same observer throughout the study. Within the same joint assessment of each valid visit, JADAS-71, 27 and 10 were scored alongside DAS28. To calculate JADAS 71, the score includes 71 joints, with more comprehensive examination including the joints of the lower and upper extremities, spine, and temporomandibular joint. In JADAS-27, the following joints are scored: cervical spine, elbows, wrists, metacarpophalangeal from 1 to 3, proximal interphalangeal, hips, knees and ankles. Regarding JADAS-10, the upper score is 10, that is, if a patient has 15 or 20 active joints, the maximum score to be assigned will be 10.
JADAS final score is calculated by the sum of four components: global assessment of arthritis activity by a physician, measured in a 10-cm VAS, where zero indicates no activity and 10, maximum activity; global assessment by parents/patients also measured on a 10-cm VAS, where zero indicates no activity and 10, the maximum activity perceived by parents or by the patient; active joints count of zero-71 joints and ESR converted to a scale from zero-10 = [VHS mm/h − 20)/10] with values over 120 mm/h being converted to 120.
The following joints were assessed for scoring DAS 28: shoulders (2), elbows (2), wrists (2), metacarpophalangeal (10), proximal interphalangeal (10) and knees (2). The joints with pain and edema are independently scored, in addition to the global assessment of activity by the patient, which in this study was performed either by the parents or by the patient him/herself, according to age, being measured on a 10-cm VAS in which zero indicates no disease activity and 10, maximum activity, according to the patient's perception. In this study, scales scores were performed by the parents regardless of age. DAS28 score was calculated using the following formula in Microsoft Excel:
DAS28 = 0,56 √ number of joints with pain (28) + 0,28 √ number of joints with swelling + 0,70 log n (ESR) + 0,014 global VAS.
Functional capacity, as an integral parameter for the calculation of ACR-Pedi-30 response, was assessed by the CHAQ score with values of zero-3, with 3 meaning the maximum disability scale.
Statistics analysis
A descriptive analysis was carried out of baseline variables obtained during patient selection and with calculation of average, standard deviation, median and quartiles for quantitative variables, as well as frequencies and percentages for qualitative variables.
A longitudinal analysis of variables was performed using a repeated measure model through analysis of variance (ANOVA) followed by Tukey's multiple comparisons test for data showing symmetrical distribution. The adjustment of a generalized linear model for repeated measures, with Gamma distribution, was performed for the data that showed an asymmetric distribution.
For comparative evaluation between DAS28 and JADAS in three versions (71, 27 and 10), a linear regression was performed by applying the ANOVA test for data with normal distribution. As for the comparison between JADAS-71, JADAS-27 and JADAS-10, a model with Gamma distribution was adjusted.
All analyses were performed using SAS for Windows, v.9.2. In all tests, we used a significance level of 5% or the corresponding p-value.
Results
Three boys and five girls were assessed, all diagnosed with JIA and aged 7–17 years, with a case classified as systemic and seven as polyarticular, with two being positive for rheumatoid factor (latex test). Clinical, anthropometric, laboratory and activity variables of arthritis, including the functional indices in the first evaluation, are presented in Table 1.
Clinical, anthropometric, laboratorial, activity and functional parameters in eight patients during the first evaluation of selection for the clinical trial.
With the use of ANOVA a significant difference was found in the visits which took place for selection and those after 4 months of treatment for all indices, when five patients met the criteria of ACR Pedi 30 response, that is, there was improvement in 30% of at least three of the six key variables.
Longitudinal comparison showed that there was asymmetric distribution of CHAQ, DAS28, JADAS-71, 27 and 10 variables, and the adjustment of the model with Gamma distribution showed statistically significant difference within the assessments (p < 0.05), with the highest rates being in the first and in the second evaluation, respectively, at the selection and after 4 months of biological treatment in open phase. The other visits included a total of 30 serial evaluations, monthly, within 6 months of the double-blind phase, and quarterly in the evaluations that followed during the open-label extension. These evaluations were compared, but no significant difference was found in all the individual parameters for the calculation of the indices and items of DAS28 and JADAS-71, 27 and 10. No significant difference was observed within the respective versions of JADAS-71, 27 and 10. For this comparison, we also adjusted a model with Gamma (p = 0.5) distribution.
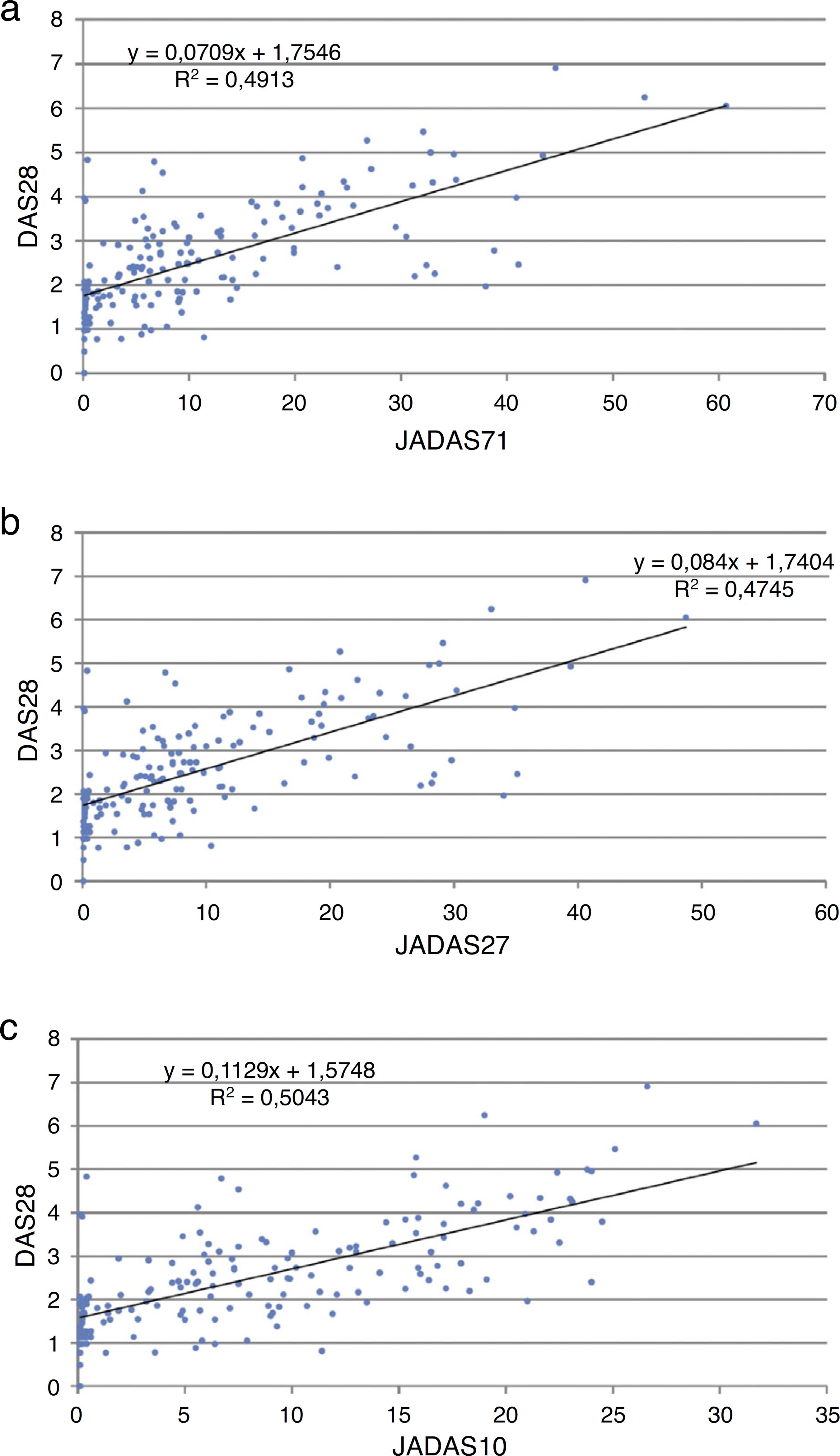
The linear regression analysis of JADAS-71, 27 and 10 and DAS 28 resulted in conversion formulas among the scales, the regression analysis of which is shown in Fig. 1:
Linear regression plots within values of DAS28 andJADAS-71 (a), JADAS-27 (b) and JADAS-10 (c) and theirrespective conversion equations (plots a, b, c).
[DAS 28 = 0.0709 (JADAS-71) + 1.267] (R2 = 0.49).
[DAS 28 = 0.084 (JADAS-27) + 1.7404] (R2 = 0.47).
[DAS 28 = 0.1129 (JADAS-10) + 1.5748] (R2 = 0.50).
Discussion
The presented results support the equivalence between the DAS-28 and JADAS in three versions, with joint counts of 71, 27 or 10, respectively, through longitudinal observation made during a controlled clinical trial in polyarticular JIA. Besides DAS28, there are other instruments used for RA, such as the Clinical Disease Activity Index (CDAI), among others,1313 Fransen J, Van Riel PL. The disease activity score and the EULAR response criteria. Rheum Dis Clin N Am. 2009;35:745-57. but of limited use in pediatric patients.
Continuous measures such as DAS28 and JADAS have the advantage of establishing absolute values, identifying changes in clinical status by a number on a continuous scale.1313 Fransen J, Van Riel PL. The disease activity score and the EULAR response criteria. Rheum Dis Clin N Am. 2009;35:745-57. The straightforward calculation makes the method feasible in daily practice, just as in clinical trials. However, there are few publications reporting the use of DAS28 in JIA.77 Consolaro A, Ruperto N, Bazso A, Pistorio A, Magni-Manzoni S, Filocamo G, et al. Paediatric Rheumatology International Trials Organization. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Care Res. 2009;61:658-66.,88 Lurati A, Pontikaki I, Teruzzi B, Desiati F, Gerloni V,Gattinara M, et al. Comparison of response criteria to evaluate therapeutic response in patients with juvenile idiopathic arthritis treated with methotrexate and/or anti-tumor necrosis factor agents. Arthritis Rheum. 2006;54:1602-7.
Measures in absolute values provide better consistency of assessment among physicians and allow patients to understand the significance of their disease activity via an absolute number. The corresponding measures for JIA were recently developed,77 Consolaro A, Ruperto N, Bazso A, Pistorio A, Magni-Manzoni S, Filocamo G, et al. Paediatric Rheumatology International Trials Organization. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Care Res. 2009;61:658-66. and three versions of the tool JADAS allowed to equate the different presentations of JIA according to the ILAR classification.22 Petty RE, Southwood TR, Manners P,Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision. J Rheumatol. 2004;31:390-2.
One must also consider that the joint counts of the DAS28 omit the lower limb joints,1414 Landewé R, Van der Hayde, Van der Linden, Boers M. 28-joint counts invalidate the DAS-28 remission definition owing to the omission of the lower extremity joints: a comparison with the original DAS remission. Ann Rheum Dis. 2006;65:637-41. but in the JIA the involvement of the lower extremities is predominant. Measures of perceived activity of arthritis by the physician, the patient him/herself or their parents, as well as ESR or CRP, implement the composite measures, weighing up several competing factors for the activity status.
In JADAS validation study,77 Consolaro A, Ruperto N, Bazso A, Pistorio A, Magni-Manzoni S, Filocamo G, et al. Paediatric Rheumatology International Trials Organization. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Care Res. 2009;61:658-66. as well as in a recent study, which used CRP to replace ESR,1515 Nordal EB, Zak M, Aalto K, Bernston L, Fasth A, Herlin T, et al. Validity and predictive ability of the juvenile arthritis disease activity score based on CRP versus ESR in a Nordic population-based setting. Ann Rheum Dis. 2012;71:1122-7. results of JADAS-71, 27 and 10 kept the correlation among them and with the other activity parameters. Also, McErlane et al.1616 McErlane F, Beresford ME, Baildam EM, ChiengA, Davidson J, Foster HE, et al. Validity of three-variable Juvenile Arthritis Disease Activity Score in children with new onset-juvenile idiopathic arthritis. Ann Rheum Dis. 2013;72:1983-8. recently calculated JADAS with only three variables, excluding ESR for broader applicability, and reported a correlation of measures and their metric equivalence.
One must consider, however, that in this study VAS scores by the patient him/herself as conceived in DAS28 was replaced by the score of the scale performed by the parents. It is also known that, regardless of age, caregiver's perception may substantially differ from the perception of the patient at any age.
Among the other limitations of this analysis we find the small sample that limits the power of the study, and the selection of children enrolled in clinical trials. If, on one hand, the population sample would provide greater variability of activity, strict control of all measures and standardized joint examination, by the same observer at regular intervals, in addition to the parallel evaluation of response measures (ACR-Pedi-30) to establish responders, the response pattern in the period of greatest activity when selecting for testing, were the favorable points to test this equivalence.1717 Ringold S, Bittner R, Neogi T, Wallace CA, Singer NG. Performance of Rheumatoid Arthritis Disease Activity Measures and Juvenile Arthritis Disease Activity Scores in polyarticular-course juvenile idiopathic arthritis: analysis of their ability to classify the American College of Rheumatology Pediatric measures of response and the preliminary criteria for flare and inactive disease. Arhtritis Care Res. 2010;62:1095-102.
There is practical applicability of the results to patients with JIA, because, besides the simple and direct score, the individual measures of clinical parameters can be conducted in daily practice. The use of metric conversion may also be useful in specific situations of transition from adolescent to adult condition. As an example, a patient diagnosed with JIA at 15 years and another diagnosed with RA at 17 could be evaluated by calculating the equivalence of the instruments used.
Referências
-
1Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767-78.
-
2Petty RE, Southwood TR, Manners P,Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision. J Rheumatol. 2004;31:390-2.
-
3Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997;40:1202-9.
-
4Wallace C, Ruperto N, Giannini EH. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31:2290-4.
-
5Van der Heijde DM, Van't Hof M, Van Riel PL, Van de Putte LB. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol. 1993;20:579-81.
-
6Prevoo ML, Van't Hof MA, Kuper HH, Van Leeuwen MA, Van de Putte LB, Van Riel PL. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44-8.
-
7Consolaro A, Ruperto N, Bazso A, Pistorio A, Magni-Manzoni S, Filocamo G, et al. Paediatric Rheumatology International Trials Organization. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Care Res. 2009;61:658-66.
-
8Lurati A, Pontikaki I, Teruzzi B, Desiati F, Gerloni V,Gattinara M, et al. Comparison of response criteria to evaluate therapeutic response in patients with juvenile idiopathic arthritis treated with methotrexate and/or anti-tumor necrosis factor agents. Arthritis Rheum. 2006;54:1602-7.
-
9Singh G, Athreya B, Goldsmith DP. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis Rheum. 1994;37:1761-9.
-
10Machado CS, Ruperto N, Silva CH, Ferriani VP, Roscoe I, Campos LM, et al. The Brazilian version of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ). Clin Exp Rheumatol. 2001;19 Suppl 23:S25-30.
-
11Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Pérez N, Silva CA, et al. Abatacept in children with juvenile idiopathic arthritis: a randomized, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372:383-91.
-
12Ruperto N, Lovell DJ, Mouy R, Paz E, Rubio-Pérez N, Silva CA, et al. Long-term safety and efficacy of abatacept in children with juvenile idiopathic arthritis. Arthritis Rheum. 2010;62:1792-802.
-
13Fransen J, Van Riel PL. The disease activity score and the EULAR response criteria. Rheum Dis Clin N Am. 2009;35:745-57.
-
14Landewé R, Van der Hayde, Van der Linden, Boers M. 28-joint counts invalidate the DAS-28 remission definition owing to the omission of the lower extremity joints: a comparison with the original DAS remission. Ann Rheum Dis. 2006;65:637-41.
-
15Nordal EB, Zak M, Aalto K, Bernston L, Fasth A, Herlin T, et al. Validity and predictive ability of the juvenile arthritis disease activity score based on CRP versus ESR in a Nordic population-based setting. Ann Rheum Dis. 2012;71:1122-7.
-
16McErlane F, Beresford ME, Baildam EM, ChiengA, Davidson J, Foster HE, et al. Validity of three-variable Juvenile Arthritis Disease Activity Score in children with new onset-juvenile idiopathic arthritis. Ann Rheum Dis. 2013;72:1983-8.
-
17Ringold S, Bittner R, Neogi T, Wallace CA, Singer NG. Performance of Rheumatoid Arthritis Disease Activity Measures and Juvenile Arthritis Disease Activity Scores in polyarticular-course juvenile idiopathic arthritis: analysis of their ability to classify the American College of Rheumatology Pediatric measures of response and the preliminary criteria for flare and inactive disease. Arhtritis Care Res. 2010;62:1095-102.
Publication Dates
-
Publication in this collection
Jan-Feb 2015
History
-
Received
20 Feb 2014 -
Accepted
17 Aug 2014