Abstracts
INTRODUCTION: Systemic lupus erythematosus (SLE) is an autoimmune disease that affects fertile-age women and of which treatment includes immunosuppressive agents that can affect the gonads. OBJECTIVE: To evaluate the frequency of menstrual alterations in lupus patient treated with immunosuppressive agents (ISA). PATIENTS AND METHODS: A totalof 87 patients, aged < 40 years, were studied. The patients were followed by outpatient management and the treatment used was verified. Only organic causes of menstrual alterations were excluded from the study. The menstrual alterationswere correlated with the type and time of use of different ISA. RESULTS: Age varied from 14 to 38 years, with a mean age of 28.01 ± 5.81 years; the mean age at menarche was 13.12 ± 1.77 years and the diagnosis of SLE was at 21.40 ±5.75 years. Corticoids were used as single therapy by 63.2% of them, for a mean time of 6.11 ± 5.14 years and the use of other immunosuppressive agents occurred with a mean time of 5.60 ± 3.59 years. Menstrual alterations occurred in 37.9% and amenorrhea in 11.5%. There was an association between menstrual alterations with the use of ISA (P = 0.034). CONCLUSION: The frequency of menstrual alterations was higher than that found in the general population, similar to what was observed in other publications on lupus treatment. The higher frequency of menstrual alterations in thesepatients was significantly associated with the use of ISA. The results justify caution when prescribing these medications,research indication and the use of techniques for ovarian preservation means.
premature ovarian failure; immunosuppressive agents; uterine hemorrhage; systemic lupus erythematosus; amenorrhea
INTRODUÇÃO: O lúpus eritematoso sistêmico (LES) é uma doença autoimune que afeta mulheres em idade fértil. Em seu tratamento são utilizados imunossupressores que podem afetar as gônadas. OBJETIVO: Avaliar a frequência de alterações menstruais de pacientes lúpicas tratadas com imunossupressores. MÉTODOS: Foram estudadas 87 pacientes, com idade inferior a 40 anos, com LES em seguimento ambulatorial e verificado o esquema terapêutico utilizado. Excluiu-se do estudo outras causas orgânicas de alteração menstrual. Foi feita correlação de alterações menstruais com o tipo e o tempo de uso dos diferentes imunossupressores. RESULTADOS: A idade variou de 14 a 38 anos, com média de idade 28,01 ± 5,81 anos; a média de idade da menarca foi de 13,12 ± 1,77 anos e o diagnóstico de lúpus 21,40 ± 5,75 anos. O corticoide estava em uso de forma individual por 63,2%, com média de tempo de 6,11 ± 5,14 anos e o uso de outros imunossupressores ocorreu com média de tempo de 5,60 ± 3,59 anos. Alterações menstruais ocorreram em 37,9% e amenorreia em 11,5%. Houve associação das alterações menstruais com o uso dos imunossupressores (IS) (P = 0,034). CONCLUSÃO: A frequência de alterações menstruais foi superior a encontrada na população em geral, semelhante ao observado em outras publicações sobre lúpus em tratamento. A maior frequência de alterações menstruais nessas pacientes foi significativamente associada ao uso de Imunesupressores. O resultado justifica a prudência no uso destes medicamentos, a orientação para pesquisa e uso de técnicas de meios de preservação dos ovários.
falência ovariana prematura; imunossupressores; hemorragia uterina; lúpus eritematoso sistêmico; amenorreia
ORIGINAL ARTICLE
IAssistant Professor of the Department of Gynecology and Obstetrics of FM-UFG - Master in Health Sciences - FM-UFG
IIAssistant Professor of the Service of Rheumatology of the Department of Internal Medicine of FM-UFG - Master in Tropical Medicine - Universidade Federal de Goiás
IIISixth-year Medical Student - Escola Superior de Ciências de Saúde do Distrito Federal
IVFifth-year Medical Student - Faculdade de Medicina - UFG
VFull Professor of Rheumatology of Faculdade de Medicina - Department of Internal Medicine of FM-UFG
Correspondence to
ABSTRACT
INTRODUCTION: Systemic lupus erythematosus (SLE) is an autoimmune disease that affects fertile-age women and of which treatment includes immunosuppressive agents that can affect the gonads.
OBJECTIVE: To evaluate the frequency of menstrual alterations in lupus patient treated with immunosuppressive agents (ISA).
PATIENTS AND METHODS: A totalof 87 patients, aged < 40 years, were studied. The patients were followed by outpatient management and the treatment used was verified. Only organic causes of menstrual alterations were excluded from the study. The menstrual alterationswere correlated with the type and time of use of different ISA.
RESULTS: Age varied from 14 to 38 years, with a mean age of 28.01 ± 5.81 years; the mean age at menarche was 13.12 ± 1.77 years and the diagnosis of SLE was at 21.40 ±5.75 years. Corticoids were used as single therapy by 63.2% of them, for a mean time of 6.11 ± 5.14 years and the use of other immunosuppressive agents occurred with a mean time of 5.60 ± 3.59 years. Menstrual alterations occurred in 37.9% and amenorrhea in 11.5%. There was an association between menstrual alterations with the use of ISA (P = 0.034).
CONCLUSION: The frequency of menstrual alterations was higher than that found in the general population, similar to what was observed in other publications on lupus treatment. The higher frequency of menstrual alterations in thesepatients was significantly associated with the use of ISA. The results justify caution when prescribing these medications,research indication and the use of techniques for ovarian preservation means.
Keywords: premature ovarian failure; immunosuppressive agents; uterine hemorrhage; systemic lupus erythematosus; amenorrhea.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease that affects multiple organs, of unknown cause, characterized by the presence of several autoantibodies. It has a polymorphic clinical development, with periods of exacerbation and remissions. The development of the disease is associated with genetic predisposition and environmental factors and its etiology is yet to be fully clarified.1
The knowledge that rheumatologic diseases have an extensive impact on several organs and systems of patients and the studies aiming at the assessment of sexual and reproductive function show important results for clinical conduction due to the multifactorial origin and by the negative impact of the disease on the quality of life of patients of both sexes.2
SLE presents predominantly in female patients, at a proportion of 10:1 in relation to the male sex. The disease manifests mainly in menacme, when the ovarian function with a normal menstrual cycle and fertility are important for the woman's health.1 Female patients with SLE undergoing treatment frequently report menstrual irregularities, amenorrhea and premature ovarian failure. Causal explanations associate them to both the autoimmunity and the used medications, specially the immunosuppressive agents (ISA).3,4
Several studies and publications have attempted to evaluate menstrual alterations related to autoimmune diseases and, especially, SLE.3,4,5 A prospective study in 36 patients with SLE, who were not receiving cyclophosphamide, sought to determine the prevalence of menstrual alterations and evaluate the possible association with clinical, hormonal and therapeutic variables. Menstrual alterations were observed in 52.7% (19/36), and the ovarian function was preserved in all of them. The current use of azathioprine was not associated with the menstrual alteration. SLE activity was considered as the main risk factor for menstrual alterations in patients not receiving alkylating drugs.5
The immunosuppressive agents (ISA) broadly used in the treatment of SLE have a suppressive action through the capacity of inhibiting cell proliferation and producing apoptosis in immunological response cells. This action can extend beyond the immunological system and, among others, to the female reproductive organs. Corticoids, which are also part of this study, also present a immunosuppressive action and other possible consequences such as the retroinhibition of the hypothalamic axis.6
The ovaries present different sensitivities to toxic substances. This sensitivity varies according to the patient's age, the type and dose of the medication and the stage of evolution of the ovarian follicle. In the female ovary, the larger follicles, as well as antral and pre-ovulatory follicles, are more sensitive to toxicity, when the patient receives treatment with ionizing radiation and alkylating agents (cyclophosphamide). It has been established that the total dose of cyclophosphamide to produce amenorrhea is 20.4 g in women aged 20 to 29 years; 9.3 g between 30 and 39 years and 5.2 g between 40 and 49 years.7
In the male gender, the functional assessment of the gonads in patients with SLE is a concern on the part of researchers. In a functional analysis, the abnormalities in sperm cells and decrease in testis volume are researched variables. The use of intravenous cyclophosphamide is considered a risk factor for permanent damage according to published research.8
Publications have demonstrated the association between corticoid use and menstrual alterations, due to the crossed retroinhibition with the hypothalamus-pituitary-ovary axis. A randomized clinical study carried out in eumenorrheic patients aimed at verifying the alterations in the secretion of gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), of estrogens and progesterone. Cortisol injections and sequential measurements at the initial follicular phase showed a decrease in the pulsatile secretion of LH and FSH. The study demonstrated that cortisol directly alters the production of gonadotropins by interfering with the secretion of the gonadotropin-releasing hormone (GnRH). Only the excess of cortisol seems to interfere with and define the causes of menstrual alterations, such as, for instance, stress situations.9
The analysis of gonadal function carried out in 25 males with SLE showed that the incidence of the following gonadal dysfunctions: testicular atrophy, elevated levels of FSH and LH and sperm cell alterations were statistically higher in patients with SLE than in the controls. The study concludes that a multidisciplinary approach is essential to offer preventive measures to these patients.10
Considering the possible gonadotoxic effect, or the interference with the hypothalamus-pituitary-ovary (H-P-O) axis, it is important to verify whether the patients submitted to treatment with ISA present clinical signs of menstrual alterations.
The menstrual alterations with increased bleeding impair the social and work activities and can result in iron-deficiency anemia. The loss of fertility is a concern for these patients, as SLE occurs during a period when the woman often yearns for a child. Even more severe, premature menopause is a condition that anticipates health alterations in the woman with a higher tendency toward chronic degenerative diseases, gynecological cancer and early mortality.11
The aim of the present study was to evaluate possible effects of immunosuppressive drugs used in the treatment of SLE on the ovarian function, here evaluated through clinical manifestations of menstrual irregularities. The importance of the present study is emphasized by the scarcity of existing studies in the literature and the present is the first report of the assessment of patients in our region with this focus. We can now develop comparative studies with other patients from our country of continental dimensions and on several factors that can modify the disease evolution.
PATIENTS AND METHODS
The cross-sectional study was carried out in a population of patients with a diagnosis of systemic lupus erythematosus (SLE) treated at the service of rheumatology of Hospital das Clínicas da Faculdade de Medicina da Universidade Federal do Estado de Goiás. The women that came to the service from January 2007 and November 2008 were sequentially included in the study. A protocol was used to collect all the information on the patients, including the demographic data, the criteria that justified the diagnosis of SLE, gynecological and obstetric data and information related to the type of treatment they were receiving.
The inclusion criteria were: 1) to voluntarily agree to participate in the study and sign the Free and Informed Consent Form; 2) to have a established diagnosis of SLE according to the criteria established by the American College of Rheumatology;12,13 3) to be at an age range between menarche and younger than 40 years; and 4) to have an active disease or not.
The exclusion criteria included: 1) refusal to participate at any moment during the research process; 2) use of contraceptives; 3) to have organic causes of uterine bleeding; 4) to have been submitted to a hysterectomy.
According to the clinical indication of each case, the patients were submitted to the treatment with immunosuppressive agents (ISA) as a single or associated therapy (prednisone, cyclophosphamide, azathioprine and methotrexate), with doses being defined by the protocol of the service of rheumatology. Prednisone was used at an initial dose of 1 mg/Kg/day with a maintenance dose of 5-10 mg/day via oral route and cyclophosphamide at a dose of 0.75 g/m2 up to 1 g/m2 via IV route, monthly. The dose of azathioprine was 2-3 mg/Kg/day via oral route and methotrexate at a dose of 10-25 mg via oral, intramuscular or IV route, weekly.
The diagnosis of altered uterine bleeding followed the classification suggested by Machado ,14 carried out according to the following criteria: normality, identified as eumenorrhea, characterized by bleeding between 21 and 35 days and amount between 20 and 80 mL, for 2 to 7 days. The alterations were grouped as they manifested either by excessive or decreased menstrual flow, increased time gap or reduced duration of the bleeding:
Hypermenorrhea, with menstrual bleeding for more than seven days;
Menorrhagia, with a volume of bleeding > 80 mL during the menstrual period;
Polymenorrhea, in case of bleeding within an interval < 18 days;
Hypomenorrhea, when the menstrual period lasts < 3 days;
Oligomenorrhea, when there is an interval > 45 days between menstrual periods;
Secondary amenorrhea, when there is an interval > 90 days between the bleeding episodes after the occurrence of menarche.14
The normality of the uterine and ovarian anatomy, in order to consider the hemorrhage as dysfunctional, was also evaluated by ultrasonography. A normal uterine volume was considered as the one between 30 and 120 cm3, using as the measuring method the product of the longitudinal, antero-posterior and lateral diameters by the constant 0.5233 (calculation of volume as of an ellipsoid structure). Measurements of 3 to 9cm3 were accepted as normal ovarian volume, using as the measuring method the product of the longitudinal, antero-posterior and lateral diameters by the constant 0.4233 (calculation of volume as of an elliptical structure).15
The comparative study of the menstrual characteristics with the time of ISA use and of those with the different groups of medications used was carried out. Considering the presence of these continuous and nominal variables and due to the fact that they were samples with normal distributions, they were submitted to analysis of variance (ANOVA). Fisher's test was used to test differences between nominal variables, of menstrual alterations or not with the use of ISA in both groups, due to the fact that the size of the sample presented by categories became insufficient for the Chi-square test to be used. The level of confidence was set at 95% and a P value < 0.05 was considered significant.
The data were stored in a database that used an Excel spreadsheet (MS Office 2003). The tests were carried out with the SPSS software package, release 17.0 and the results were presented in tables and descriptive and comparative analysis in MS Word files.
The study project was approved by the Committee of Ethics in Research of institution under protocol CEPMHA/ HC/UFG/142/06, of 11/30/2006 and carried out according to Resolution # 196/96 of the National Health Council (Conselho Nacional de Saúde), which supervises researches that involve human beings.
RESULTS
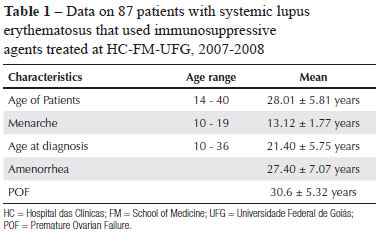
The sample consisted of 87 women, with a mean age of 28.01 ± 5.81 years, who were distributed among the following age ranges: 14 to 19 years, 8 patients, amounting to 9% of total; 20 to 25 years, 20 (23%), 26 to 30 years, 27 (31%), 31 to 35 years, 23 (26.4%), 36 to 38 years, 9 (10.4%). It was observed in the distribution that 9.2% of the patients were still adolescents and that 63.2% of the patients were younger than 30 years (Table 1).
In the studied group, the predominant ethnicity was Caucasian (55.2%) and Table 1 shows data regarding the age of occurrence of some of the study variables.
These patients were submitted to treatment with ISA, individually or in combination with other drugs (prednisone, azathioprine, cyclophosphamide and methotrexate), according with the clinical indications. Throughout the treatment, the patients used ISA according to the different clinical indications (Table 2).
The way the medications were used throughout the treatment are different from what is obtained at the moment of the research in a cross-sectional study, as shown in Table 2.
The patients undergoing treatment with prednisone were the predominant group, with 63.2% receiving this type of therapy. The second group consisted of patients that used antiproliferative immunosuppressant drugs (azathioprine, methotrexate, or cyclophosphamide) individually, or in combination with the other drugs or the corticoid prednisone (Table 3).
The time of medication use is shown in Table 3 and it can be observed that most used the medications for more than one year, with the mean time of prednisone use being 6.11 ± 5.14 years and the mean time of the other ISA being 5.60 ± 3.59 years (Table 4).
Table 4 shows the distribution of women that used immunosuppressive medications according to the behavior of the menstrual cycle. It can be observed that 23 women, 37.9% of the total assessed, presented alterations in their menstrual cycles. Of the 17 patients that presented a decrease in the menstrual bleeding, ten were characterized by the presence of amenorrhea, which corresponds to 11.5% of the total number of assessed women that used immunosuppressive drugs.
When comparing the patients that presented and the ones that did not present menstrual alterations in relation to corticoid use, it can be observed that there is no statistically significant difference (P = 0.225). However, when the same comparison is made in relation to the use of other ISA, a significant difference can be observed (P = 0.034).
The analysis of the occurrence of menstrual alterations and the patients' age showed the following frequency: in patients younger than 20 years, the alteration was 15.2%; from 21 to 25 years, 18.2%; from 26 to 30 years, 36.4%; from 31 to 35 years, 15.2%; and from 36 to 40 years, 15.2%. The frequency of patients with no menstrual alterations and the age ranges were: in those younger than 20 years, 5.6%; from 21 to 25 years, 22.2%; from 26 to 30 years, 24.1%; from 31 to 35 years, 31.5%; and from 36 to 40 years, 16.7%. There was no significant difference between the groups, with P = 0.242 (Chi-square) (Table 5).
Table 5 shows the time (mean) in years of immunosuppressive agent use (corticoids and other ISA) in relation to the type of menstruation of the study patients.
DISCUSSION
The study showed menstrual alterations and amenorrhea with an absolute frequency that was higher than the one expected in the general population. This study group, although restricted to the inclusion of patients at the age range after menarche and before menopause, shows that the disease concentrates on the fertile period of the women, with a mean age of 28.01 ± 5.81 years, which supports the concerns of the clinicians regarding ovarian function.
Menstrual alterations were observed in 37.9% of the patients that used medications. The result of the present study is higher than the statistical data of 15% of menstrual alterations observed in patients that seek gynecological consultations, as seen in the literature. The frequency of menstrual alterations in the population is variable, according to the study base. The studies carried out through population survey, thus different from the groups that voluntarily seek the medical offices, present a higher frequency. As it is more comprehensive, the research by survey shows that the frequency of menstrual alterations can be as high as 21.7%.16
When comparing with a cross-sectional study analyzing the prevalence and risk factors, menstrual alterations were observed in 49% of 61 patients with SLE. The group had as characteristics a mean age of 33.23 ± 10.96 years, 75% presented the severe manifestation of the disease. A group with 120 women without lupus was constituted by random selection for reference as a control group. Of the patients with SLE, 65.6% used a corticoid associated with another immunosuppressive agent and 34.4% used only a corticoid. Among the patients that used a combination with immunosuppressive agents, 90% used cyclophosphamide, 5% azathioprine and 5% used cyclosporine. Most of the patients with menstrual alterations were between 31 and 50 years. The authors concluded that the menstrual irregularity was a dependent variable and the patient age, immunosuppressive therapy and cyclophosphamide were considered predictive for this adverse effect. The control group presented 16.7% of menstrual alterations. The results were consistent with previous studies that showed a prevalence of menstrual alterations among patients with SLE between 15% and 40%.17 Our study showed a lower mean age, but regarding the type of therapy used, the ones that received corticoid were the predominant group. Prednisone was used by 63.2%, whereas 6.9% used cyclophosphamide individually and 9.1% used it in association with other immunosuppressive agents.
Without considering the SLE, dysfunctional menstrual alterations have different etiologies and frequencies according to the age range. The dysfunctional uterine hemorrhage that has an anovulatory cause is more frequent at the extremes of the reproductive life - in the early menarche and in the perimenopausal period - with 20% occurring in adolescence and 50% occurring at menopause; of the ones that have an ovulatory cause, 30% of them occur in menacme.14
In our study 69.8% of the patients that presented menstrual alterations were 20 to 35 years. As our study did not include patients older than 40 years, an age at which physiological menopause is not yet established, does not have the capacity to offer conclusions on the correlations of these characteristics.
These variables, however, must be taken into account when one analyzes not only menstrual alterations, but reproductive health as a whole, of the female patient with juvenile systemic lupus erythematosus (JSLE) or adult-age SLE.
A study carried out with 30 patients with JSLE undergoing treatment with cyclophosphamide, presenting a mean age of 17.4 ± 3.2 years and mean age at menarche of 13.13 ± 1.4 years, observed menstrual alterations in 63% of the patients with lupus and 23% in those from the control group.18
A Brazilian study on the sexual function and reproductive health of adolescent women with JSLE observed that sexual dysfunction with decreased vaginal lubrication, decreased orgasms and lack of satisfaction with the sexual life were significantly higher in these patients. The study with 52 patients with JSLE and the same number of control individuals paired for age, however, did not observe any difference regarding pubertal alterations, menstrual cycle abnormalities and cervicalvaginal cytology with P > 0.05. The mean age of this group was 16.7 ± 1.94 years.19
The results of cervical cytology studies show differences when the sample refers to older patients. The study of the prevalence of alterations in colpocytological examinations of SLE patients with an older mean age presented differences in relation to the control group. A study of 76 examinations of patients with lupus, with a mean age of 39 ± 9.7 years showed a higher prevalence of cytological alterations in these patients when compared to the control group, with results of 9.2% and 1.2%, with P = 0.03. These alterations, however, did not correlate with the time of disease, use of ISA, presence of anti-Ro, anti-La or anti-DNA.20
The group with menstrual alterations in our series, when segmented, shows that 26.4% of the cases occurred in patients receiving some type of corticoid. The mechanism of action of corticoids influences several organs and functions of the human body. Currently, it is considered more important for the immunosuppressive action, the genomic action of production of the protein that inhibits the transcription factor kappa B. Another of its actions occurs at the hypothalamic-pituitary axis, inhibiting gonadal action.6 That can justify the interference on the production of gonadotropins, which stimulate the follicular development and hormone production, for endometrial proliferation and subsequent regular desquamation in time and quantity.
Our study shows that the individual use of prednisone demonstrates a higher incidence of menstrual alterations than the one observed in patients with lupus nephritis. Although our study is limited, as it does not specify these patients, it is important to compare it with the randomized and controlled study of methylprednisolone and cyclophosphamide use, either individually or in combination, in patients with lupus nephritis. In the aforementioned study, 82 patients with lupus nephritis were followed for a period of up to five years, distributed in three groups and submitted to pulse therapy. A group of 27 patients received methylprednisolone via IV route, 1 g/m2 of body surface administered as rapid infusion for 60 minutes, during 3 consecutive days, followed by a monthly infusion during at least 12 months. The second group of 27 patients was treated with a cyclophosphamide infusion via IV route at a dose of 0.5 to 1 g/m2 of body surface, once a month for 6 consecutive months and thereafter, every three months for at least two years. The third group used a combination of the two medications. The analysis of the adverse events showed uterine cervical dysplasia, avascular necrosis, herpes zoster, infection, and the most frequent, amenorrhea. Amenorrhea was verified in 7.4% of the patients in the group that used only methylprednisolone, 43% in the group that used a combination of methylprednisolone and cyclophosphamide and 41% in the group that used only cyclophosphamide.21
Our results in which the immunosuppressive treatment was carried out exclusively with prednisone led to the highest frequency of menstrual alterations, deserve some considerations. When we analyze the time of corticoid use at the study, we verify that the treatment can last more than 15 years. It is a long-term use and maintenance medication in order to keep the disease inactive. The use of corticoids that improves the disease has a long-term action, to act by inhibiting the production of gonadotropins or inhibiting cell proliferation in the development of ovarian follicles and even of the endometrium.
However, no significant association was observed (P = 0.115) between the use of corticoid (prednisone) as independent medication and e menstrual cycle alterations.
Cyclophosphamide, azathioprine and methotrexate were included in the other sample group of our study and these drugs were called other immunosuppressive agents.
Cyclophosphamide is the most often found immunosuppressive agent in studies and publications. The studies more frequently show the analysis of amenorrhea in relation to the use of these ISA. Regarding amenorrhea, a review study of the main publications analyzing the use of cyclophosphamide and ovarian function in adult and pediatric patients has shown that variations range from 11.7 to 37.3%.22 A cohort study with eight years of follow-up in eumenorrheic patients analyzed the predictive variable in amenorrhea occurrence when cyclophosphamide was used. The age of the patients at the time of the treatment was considered as a predictive variable for amenorrhea. Age > 32 years was considered determinant, in a study with 67 patients treated with cyclophosphamide pulse and therefore, this type of treatment has been considered unadvisable in patients older than 31 years.23 In the present study, amenorrhea was verified in 11.5% of the patients. Cyclophosphamide was used by 15.1% of the patients and presented 5.7% of menstrual alterations. Lower doses and adequate time of use can justify these differences among the publications, in addition to other factors that might be considered.
A statistical analysis associating the use of other ISA and comparing the patients that presented alterations with those that did not present menstrual alterations showed that there was significant difference. These results are similar to most studies following this direction, in which the cyclophosphamide use as ISA can be a risk factor for ovarian toxicity and dysfunction.
On the other hand, the immunosuppressive drugs azathioprine and methotrexate are less frequently associated with ovarian alterations in the analyzed publications. The few publications are inconclusive on the ovarian toxicity of these medications. Studies carried out in mice with azathioprine at a dose of 100 mg/kg/day for 9 days inhibited weight gain, increased mortality, but had no influence on the number of oocytes or follicles in the ovaries of these animals.24 A study with azathioprine used in the control group for assessment of the toxicity of cyclophosphamide, did not demonstrate alterations in the ovarian function of the patients in the control group.23
A study with 46 patients that analyzed anti-corpus luteum antibodies did not observe an association between the aforementioned antibody and menstrual alterations or hormonal disorders. The anti-corpus luteum antibody was not specific for SLE, or considered a marker of premature ovarian failure.25 On the other hand, studies have verified an association between disease activity and menstrual alterations.
Our study had as limitation the absence of analysis of aspects related to the disease activity, either by SLEDAI or another criterion. However, an association between disease activity and menstrual alterations was observed. Of the 36 patients studied according to this orientation, it was observed that menstrual alterations occurred in 71% of those that presented SLEDAI > 8.5 Future studies including factors related to disease activity and antibody research, which were found to be altered in the present study, can improve the analysis of the results related to the menstrual alterations.
In a new study concerns were raised regarding the treatment of lupus in male patients and toxicity for the gonads. A study of analysis of function of seminiferous tubules in male patients with lupus aged 15 to 45 years, was carried out using the levels of inhibin B as a functional marker of spermatogenesis, considering the negative feedback of inhibin in the pituitary production of FSH and LH. The study was carried out with 34 patients divided in 2 groups according to the measurement of inhibin levels. The patients used prednisone, azathioprine, cyclophosphamide, methotrexate and mycophenolate mofetil. The study observed that inhibin levels have a positive correlation with normal sperm regarding quantity and motility and a negative correlation with FSH levels, which is typical of negative feedback. This study, which was the first to address this subject, identified a high frequency of testicular dysfunction in Sertoli cells of male patients with lupus. It concludes that it is necessary to perform prospective studies to determine whether the concentration of inhibin and the association between inhibin B and FSH can be used as an early marker of intravenous cyclophosphamide toxicity in these patients.26
The results of our study, aimed at female patients, also point out to the importance of being cautious when prescribing immunosuppressive agents.
Menstrual alterations can be the prelude to a more severe outcome for ovarian function. Patient's fertility and the use of medications with potential ovarian toxicity are of great concern and mainly now, as to date there are no effective means of preserving oocytes, as can be done with spermatozoids.27,28
It is necessary to stimulate the studies on fertility preservation and suggest to the multiprofessional team to pay close attention to these patients, with the objective of attaining better treatment results. It is also crucial to study menstrual alterations and amenorrhea, as well as the several factors related to the disease and the treatment, to recognize and anticipate the adequate conduct to treat the most severe alteration, i.e., premature ovarian failure.
REFERENCES
-
1Borba EF, Latorre LC, Brenol JCT, Kayse C, Silva NA, Zimmermann AF et al Consenso de Lúpus Eritematoso Sistêmico. Rev Bras Reumatol 2008; 48:196-207.
-
2Onsten M. Função sexual comprometida em pacientes com doença reumática independente da atividade da doença, tratamento e função gonadal. Revista Brasileira de Reumatologia 2009; 49:639-42.
-
3Pardini DP, Silva RC, Clapauch R. (Org.) Sociedade Brasileira de Endocrinologia e Metabologia, Associação Médica Brasileira, Conselho Federal de Medicina. Projeto Diretrizes. Falência Ovariana Precoce; 2006.
-
4Goswami D, GConway GS. Premature ovarian failure. Human reproduction update 2005; 11:391-410.
-
5Pasoto SG, Mendonça BB, Bonfá EF. Menstrual disturbances in patients with systemic lupus erythematosus without alkylating therapy: clinical, hormonal and therapeutic associations. Lupus 2002; 11:175-180.
-
6Krensky MA, Vicentini F, Bennett ME. Imunossupressores, tolerógenos e imunoestimulantes. In: As Bases Farmacológicas da Terapêutica. Brunton LL, Lazo SJ, Parker LK, editors. Goodmam & Gilmam, 11.ª ed. 2007; pp. 1265-89.
-
7Mattinson DR, Nightingale MS, Shiromizu K. Effects of toxic substances on female reproduction. Environmental Health Perspectives 1983; 48:43-52.
-
8Soares PM, Borba EF, Bonfa E, Hallak J, Correa AL, Silva CA. Gonad evaluation in male systemic lupus erythematosus. Arthritis Rheum 2007; 56:2352-61.
-
9Saketos M, Sharma N, Santoro NF. Suppression of the hipothalamicpituitary-ovarian axis in normal woman by glucocorticoids. Biology of Reproduction (Madison) 1993; 49:1270-76.
-
10Silva CAA, Bonfa E, Borba EF, Braga AP, Soares PMF, Moraes AJP et al Saúde reprodutiva em homens com lúpus eritematoso sistêmico. Revista Brasileira de Reumatologia 2009; 49:207-22
-
11Luborsky JL, Meyer P, Sowers MF, Gold EB, Santoro N. Premature menopause in a multi-ethnic population study of the menopause transition. Human Reproduction 2003; 18:199-206.
-
12Tam EM, Cohen AS, Fries JF, Masi AT, Mcshane DJ, Rothfield NF et al Special article: the 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25:1271-77
-
13Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40:1725.
-
14Machado LV. Sangramento uterino disfuncional. Arquivos Brasileiros de Endocrinologia & Metabologia 2001; 45:375-82.
-
15Pastore AR, Souza EV, Cerri GG. Sistematização do Exame Pélvico Ginecológico. In: Ultra-sonografia, Obstetrícia e Ginecologia. Pastore AR, Cerri GG. 1.a ed. São Paulo: Sarvier; 2000.
-
16Ribeiro CP, Hardy E, Hebling EM. Preferências de mulheres brasileiras quanto a mudanças na menstruação. Rev Bras Ginecol Obstet 2007; 29:74-79.
-
17Fatnoon NNA, Azarisman SMS, Zainal D. Prevalence and risk factor for menstrual disorders among systemic lupus erythematosus patients. Singapore Med 2008; 49:413-18.
-
18Medeiros PB, Febronio MV, Bonfá EF, Borba Ef, Takiuti AD, Silva CA. Menstrual and hormonal alterations in juvenile systemic lupus erythematosus. Lupus 2009; 18:38-43.
-
19Silva CAA, Febronio MV, Bonfa E, Pereira RMR, Pereira EAG, Takiuiti AD. Função sexual e saúde reprodutiva em mulheres adolescentes com lúpus eritematoso sistêmico juvenil. Revista Brasileira de Reumatologia 2009; 49:690-702.
-
20Barros BRC, Matschinske R, Silva MB, Skare TL. Prevalência de alterações no exame citológico do colo do útero em pacientes com lúpus eritematoso sistêmico. Revista Brasileira de Reumatologia 2007; 47:325-329.
-
21Gourley MF, Austin HA 3rd, Scott D, Yarboro CH, Vaughan EM, Muir J et al Methylprednisolone and cyclophosphamide, alone or in combination, in patients with lupus nephrits - A randomized, controlled trial. Ann Intern Med 2002; 137:545-6.
-
22Brito OM, Guimarães MFB, Lanna CCD. Ciclofosfamida e função ovariana. Rev Bras Reumatol 2008; 48:39-45.
-
23Ioanides JPA, Katsifis GE, Tzioufas AG, Moutsopoulos HM,. Predictors of sustained amenorrhea from pulsed intravenous cyclophosphamide in premenopausal women with systemic lupus erythematosus. J Rheumatol 2002; 29:2129-35.
-
24Mattinson DR, Chang L, Thorgeirisson SS, Shiromizu K. The effects of cyclophosphamide, azathioprine, and 6-mercaptopurine on oocyte and follicle number in C57BL/6N mice. Res Commun Chem Pathol Pharmacol 1981; 31:155-61.
-
25Sousa DC, Medeiros MMC, Viana VST, Salani Mota RM. Anticorpus luteum antibody and menstrual irregularity in patients with systemic lupus erythematosus and Hasimoto's thyroiditis. Lupus 2005; 14:618-24.
-
26Suehiro RM, Borba EF, Bonfa E, Okay TS, Cocuzza M, Soares PMF et al Testicular Sertoli cell function in male systemic lupus erythematosus. Rheumatology (Oxford) 2008; 47:1692-7.
-
27Blumenfeld Z, Eckman A. Preservation of fertility and ovarian function and minimization of chemotherapy-induced gonadotoxicity in young women by GnRH-a. Journal of the National Cancer Institute Monographs 2005; 34:40-3.
-
28Beck-Fruchter R, Weiss A, Shaley E. GnRH agonist therapy as ovarian protectants in female patients undergoing chemotherapy: a review of the clinical data. Human Reproduction Update 2008; 14:553-61.
Menstrual disturbances in systemic lupus erythematosus patients using immunossuppressants
Publication Dates
-
Publication in this collection
12 Nov 2010 -
Date of issue
Oct 2010
History
-
Received
28 Jan 2010 -
Accepted
25 Aug 2010