ABSTRACT
Objective:
To evaluate the impact of body mass index on the short-term prognosis of non-surgical critically ill patients while controlling for performance status and comorbidities.
Methods:
We performed a retrospective analysis on a two-year single-center database including 1943 patients. We evaluated the impact of body mass index on hospital mortality using a gradient-boosted model that also included comorbidities and was assessed by Charlson’s comorbidity index, performance status and illness severity, which was measured by the SAPS3 score. The SAPS3 score was adjusted to avoid including the same variable twice in the model. We also assessed the impact of body mass index on the length of stay in the hospital after intensive care unit admission using multiple linear regressions.
Results:
A low value (< 20kg/m2) was associated with a sharp increase in hospital mortality. Mortality tended to subsequently decrease as body mass index increased, but the impact of a high body mass index in defining mortality was low. Mortality increased as the burden of comorbidities increased and as the performance status decreased. Body mass index interacted with the impact of SAPS3 on patient outcome, but there was no significant interaction between body mass index, performance status and comorbidities. There was no apparent association between body mass index and the length of stay at the hospital after intensive care unit admission.
Conclusion:
Body mass index does appear to influence the shortterm outcomes of critically ill medical patients, who are generally underweight. This association was independent of comorbidities and performance status.
Body mass index; Critical illness; Obesity; Prognosis
RESUMO
Objetivo:
Avaliar o impacto do índice de massa corporal no prognóstico em curto prazo de pacientes gravemente enfermos não cirúrgicos, ao mesmo tempo em que se controla em relação a performance status e comorbidades.
Métodos:
Análise retrospectiva da base de dados referente a 2 anos de um único centro, incluindo 1.943 pacientes. Avaliamos o impacto do índice de massa corporal na mortalidade hospitalar, utilizando um modelo gradiente boosted, que também incluiu comorbidades, analisadas pelo índice de comorbidades de Charlson; performance status; e gravidade da doença, que foi observada pelo escore SAPS3. O escore SAPS3 foi ajustado para evitar a inclusão duplicada de uma mesma variável no modelo. Também avaliamos o impacto do índice de massa corporal na duração da permanência no hospital, após a permanência na unidade de terapia intensiva, utilizando múltiplas regressões lineares.
Resultados:
Um valor baixo do índice de massa corporal (< 20kg/m2) se associou com um aumento abrupto na mortalidade hospitalar. A mortalidade subsequentemente tendeu a diminuir, à medida que o índice de massa corporal aumentou, mas o impacto de um índice alto de massa corporal na definição da mortalidade foi baixo. A mortalidade aumentou conforme aumentou o ônus de comorbidades e o performance status diminuiu. O índice de massa corporal interagiu com o impacto do SAPS3 no desfecho dos pacientes, mas não houve interação significante entre índice de massa corporal, performance status e comorbidades. Não houve associação aparente entre o índice de massa corporal e a duração da permanência no hospital após a admissão à unidade de terapia intensiva.
Conclusão:
O índice de massa corporal não pareceu influenciar nos desfechos em curto prazo de pacientes clínicos gravemente enfermos, que geralmente estão abaixo do peso. Essa associação foi independente de comorbidades e performance status.
Índice de massa corporal; Estado terminal; Obesidade; Prognóstico
INTRODUCTION
Critical care outcomes are related to both the patient’s illness and their
characteristics.(11 Pickkers P, de Keizer N, Dusseljee J, Weerheijm D, van der Hoeven JG,
Peek N. Body mass index is associated with hospital mortality in critically ill
patients: an observational cohort study. Crit Care Med.
2013;41(8):1878-83.,22 Robinson MK, Mogensen KM, Casey JD, McKane CK, Moromizato T, Rawn JD, et
al. The relationship among obesity, nutritional status, and mortality in the
critically ill. Crit Care Med. 2015;43(1):87-100.) The same
degree of illness severity may or may not be lethal, depending on the patient’s
background characteristics, such as their performance, nutritional status and
preexisting comorbidities.(33 Soares M, Salluh JI, Toscano L, Dias FL. Outcomes and prognostic factors
in patients with head and neck cancer and severe acute illnesses. Intensive Care Med.
2007;33(11):2009-13.
4 Soares M, Caruso P, Silva E, Teles JM, Lobo SM, Friedman G, Dal Pizzol
F, Mello PV, Bozza FA, Silva UV, Torelly AP, Knibel MF, Rezende E, Netto JJ, Piras C,
Castro A, Ferreira BS, Réa-Neto A, Olmedo PB, Salluh JI; Brazilian Research in
Intensive Care Network (BRICNet). Characteristics and outcomes of patients with
cancer requiring admission to intensive care units: a prospective multicenter study.
Crit Care Med. 2010;38(1):9-15.-55 Zampieri FG, Colombari F. The impact of performance status and
comorbidities on the short-term prognosis of very elderly patients admitted to the
ICU. BMC Anesthesiol. 2014;14:59.) Body
mass index (BMI) is frequently measured at intensive care unit (ICU) admission and
appears to be related not only to short-term outcomes but also to long-term resource
utilization.(11 Pickkers P, de Keizer N, Dusseljee J, Weerheijm D, van der Hoeven JG,
Peek N. Body mass index is associated with hospital mortality in critically ill
patients: an observational cohort study. Crit Care Med.
2013;41(8):1878-83.) BMI
has been associated with mortality in other scenarios, both in
general(66 Prospective Studies Collaboration, Whitlock G, Lewington S, Sherliker P,
Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality
in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet.
2009;373(9669):1083-96.) and in
specific populations.(77 Hutagalung R, Marques J, Kobylka K, Zeidan M, Kabisch B, Brunkhorst F,
et al. The obesity paradox in surgical intensive care unit patients. Intensive Care
Med. 2011;37(11):1793-9.)
However, complex relationships exist between the patient’s body mass index,
comorbidities, previous medications in use, performance status and outcomes.
Obesity, which is assessed by body mass index, has been associated with a protective
effect during a critical illness; this phenomenon is called the “obesity paradox,”
although evidence is inconclusive.(11 Pickkers P, de Keizer N, Dusseljee J, Weerheijm D, van der Hoeven JG,
Peek N. Body mass index is associated with hospital mortality in critically ill
patients: an observational cohort study. Crit Care Med.
2013;41(8):1878-83.,22 Robinson MK, Mogensen KM, Casey JD, McKane CK, Moromizato T, Rawn JD, et
al. The relationship among obesity, nutritional status, and mortality in the
critically ill. Crit Care Med. 2015;43(1):87-100.,88 Oliveros H, Villamor E. Obesity and mortality in critically ill adults:
a systematic review and meta-analysis. Obesity (Silver Spring).
2008;16(3):515-21.
9 Westerly BD, Dabbagh O. Morbidity and mortality characteristics of
morbidly obese patients admitted to hospital and intensive care units. J Crit Care.
2011;26(2):180-5.-1010 Mica L, Keel M, Trentz O. The impact of body mass index on the
physiology of patients with polytrauma. J Crit Care.
2012;27(6):722-6.) However, because body mass index may be affected by
comorbidities and performance status, it is unclear if obesity is just a marker of
physiological reserve or if it is independently linked to better
outcomes.(1111 Rahman A, Stapleton RD, Heyland DK. Not all critically ill obese
patients are the same: the influence of prior comorbidities. ISRN Obes.
2012;2012:743978.) There
is compelling evidence for both arguments: weight loss is known to be a marker of
both uncontrolled comorbidities and end-of-life;(1111 Rahman A, Stapleton RD, Heyland DK. Not all critically ill obese
patients are the same: the influence of prior comorbidities. ISRN Obes.
2012;2012:743978.,1212 Alley DE, Metter EJ, Griswold ME, Harris TB, Simonsick EM, Longo DL, et
al. Changes in weight at the end of life: characterizing weight loss by time to death
in a cohort study of older men. Am J Epidemiol. 2010;172(5):558-65.) conversely, body composition may independently influence
the inflammatory response to an acute-stress event, such as sepsis or
trauma.(1313 Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin
Immunol. 2005;115(5):911-9; quiz 20. Review.)
Therefore, the exact role of body mass index on the patient’s outcome after critical
illness is likely complex.
We therefore sought to evaluate the influence of body mass index, comorbidities, performance status and their relationships on the short-term outcome (i.e., hospital mortality) of a large clinical database of medical ICU admissions. We hypothesize that when relevant variables that affect body mass index where considered, their influence on the outcome would likely be limited to underweight patients. As a secondary goal, we assessed the impact of BMI on the length of stay in the hospital after ICU admission, which is the sum of the length of stay in the ICU and the length of stay in the hospital after ICU discharge, in hospital survivors.
Part of this analysis was previously presented as an abstract at the 28th Annual Congress of the European Society of Intensive Care Medicine, 2014 (LIVES 2014 - Barcelona).
METHODS
A retrospective single-center analysis of an administrative database in a tertiary ICU in São Paulo, Brazil was performed. All data were collected during ICU admission using an integrated database (Epimed Monitor®, Epimed, Rio de Janeiro, Brazil). Approval by the local Ethics Committee was obtained, and the need for informed consent was waived due to the retrospective nature of the study (approval number 820.311).
Critically ill non-surgical patients admitted to a tertiary intensive care unit in Brazil during a two-year period (January 2012 through December 2013) were investigated in this study.
Patient height and weight are routinely measured at hospital admission using a standard method (i.e., a scale and measuring tape). When patients were admitted from the emergency department and could not stand, their weight was measured using the ICU bed’s built-in scale after removal of any object that could interfere with the measurement. BMI was calculated by the formula weight (kg)/height2 (m2). BMI was added as a continuous variable in the primary model, because categorization may lead to the loss of relevant information;(1414 Filardo G, Adams JP. Effect of body mass index on mortality in patients undergoing isolated coronary artery bypass grafting. Ann Thorac Surg. 2010;90(3):1060.) however, we also collected mortality data based on the World Health Organization BMI classification using an unadjusted odds ratio for illustrative purposes in a univariate analysis. The burden of comorbidities was measured with Charlson’s comorbidity index (CCI) without attribution to age.(1515 Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-83.) The performance status (PS) was categorized on a scale of 0-2 based on the patient’s capability to perform daily routine activities. Patients were categorized as fully independent (PS of 0; no need for assistance during routine daily activities), partially dependent (PS of 1; need for assistance with at least one daily activity) and fully dependent (PS of 2; need for assistance with all daily activities), as previously reported.(1616 Zampieri FG, Ladeira JP, Park M, Haib D, Pastore CL, Santoro CM, et al. Admission factors associated with prolonged (>14 days) intensive care unit stay. J Crit Care. 2014;29(1):60-5.)
Illness severity was measured using Simplified Acute Physiology Score III (SAPS3 scores); considering that SAPS3 scores include comorbidities, and to avoid correcting for the same variable twice, we removed the comorbidity points from the SAPS3 scores. We also removed the points for age, the length of stay in the hospital (LOS) before ICU admission and the temperature at ICU admission, because we planned to add them independently to the model. The resulting SAPS3 score after the removal of these points was called the adjusted SAPS3 (SAPS3adj). Previous corticosteroid use was defined as the use of any dose of corticosteroid for more than one month. Sepsis, regardless of its severity, was defined as proposed by the Surviving Sepsis Guidelines(1717 Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165-228.) and was measured as present or absent at the time of ICU admission. If the attending physician judged that sepsis was present at the time of ICU admission, we reported sepsis as present despite it not fulfilling the diagnostic criteria.
Statistical analysis
Continuous variables were assessed for normality between the survivors and non-survivors using the Kolmogorov-Smirnof test. Parametric variables were compared between the groups using t-tests. Non-parametric variables were compared using the Mann-Whitney test. Categorical variables were compared using a Chi-squared test. This analysis used a gradient-boosted model (GBM) to evaluate the influence of illness severity, burden of comorbidities, body mass index and performance status on hospital mortality.(1818 Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. J Anim Ecol. 2008;77(4):802-13.) GBM is thus an additive regression model in which the terms are decision trees that are obtained after simple recursive partitioning. After the first decision tree is built based on the data, another tree is fitted for the residuals of the first tree. This process continues until pre-specified boundaries are reached, producing hundreds or thousands of trees that are then included via a boosting algorithm that eventually produces the final model. GBM has several advantages over traditional logistic regression, such as having no need for prior variable transformations, insensitivity to the effects of outliers, the ability to fit non-linear relationships and the capability of handling missing data.(1818 Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. J Anim Ecol. 2008;77(4):802-13.) GBM is frequently used to describe findings and patterns(1818 Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. J Anim Ecol. 2008;77(4):802-13.) but is seldom used in critical care medicine. In some scenarios, the use of GBM provided additional data that were not obtained by other methods.(1919 Dodd S, Berk M, Kelin K, Zhang Q, Eriksson E, Deberdt W, et al. Application of the Gradient Boosted method in randomised clinical trials: Participant variables that contribute to depression treatment efficacy of duloxetine, SSRIs or placebo. J Affect Disord. 2014;168:284-93.) The GBM model considered age, SAPS3adj, PS, CCI, previous steroid use, LOS before ICU admission, temperature at admission, diagnosis of sepsis at admission and BMI. GBM settings were set to obtain at least one thousand trees.(1818 Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. J Anim Ecol. 2008;77(4):802-13.) The initial settings included a bag fraction of 0.5, a tree complexity of 8, and a learning rate of 0.001. Ten-fold cross validation was used. We report the relative influence of each variable on the model using the method suggested by Friedman,(2020 Friedman JH, Roosen CB. An introduction to multivariate adaptive regression splines. Stat Methods Med Res. 1995;4(3):197-217.) where the relative influence of each variable on the outcome is scaled from 0 to 100 with the relative influence of each variable being proportional to the number of times the variable was used in node splitting and weighted by the squared improvement to the model that results from the split and the average for all trees.(1818 Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. J Anim Ecol. 2008;77(4):802-13.,2020 Friedman JH, Roosen CB. An introduction to multivariate adaptive regression splines. Stat Methods Med Res. 1995;4(3):197-217.,2121 Hijmans RJ, Phillips S, Leathwick J, Elith J. dismo: Species distribution modeling. R package version 0.9-3; 2013.) The influence of each variable on the outcome is shown in partial dependence plots. The strength of the second-degree interactions was assessed by the mean value of the residuals of a linear model that relates the predictions of each predictor pair with the predictors fitted by the factors.(2121 Hijmans RJ, Phillips S, Leathwick J, Elith J. dismo: Species distribution modeling. R package version 0.9-3; 2013.) When necessary, interaction plots are shown.
The association between the LOS in the hospital after ICU admission in hospital survivors was assessed with multiple linear regression analyses. The same variables included in the mortality model were used in this analysis, and interactions were allowed; however, no stepwise analysis was performed.
All analyses were performed using R project v 3.0.2 (www.r-project.org) with the gbm, dismo and ggplot2 packages. We used the documentation of the dismo package and a previous review on the subject as a guide to these analyses.(1818 Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. J Anim Ecol. 2008;77(4):802-13.,2121 Hijmans RJ, Phillips S, Leathwick J, Elith J. dismo: Species distribution modeling. R package version 0.9-3; 2013.)
RESULTS
A total of 1943 patients were included in the analysis. A study flowchart is shown in figure 1. A histogram and density plot of BMI in the population is shown in the electronic supplementary material (Figure S1). The general characteristics and the comparison between the survivors and non-survivors is shown in table 1. The number of non-survivors and the unadjusted odds ratio for BMI categorized based on the World Health Organization criteria is shown in table 2.
Study flowchart.
SAPS3 - Simplified Acute Physiology Score III; BMI - body mass index; ICU - intensive care unit.
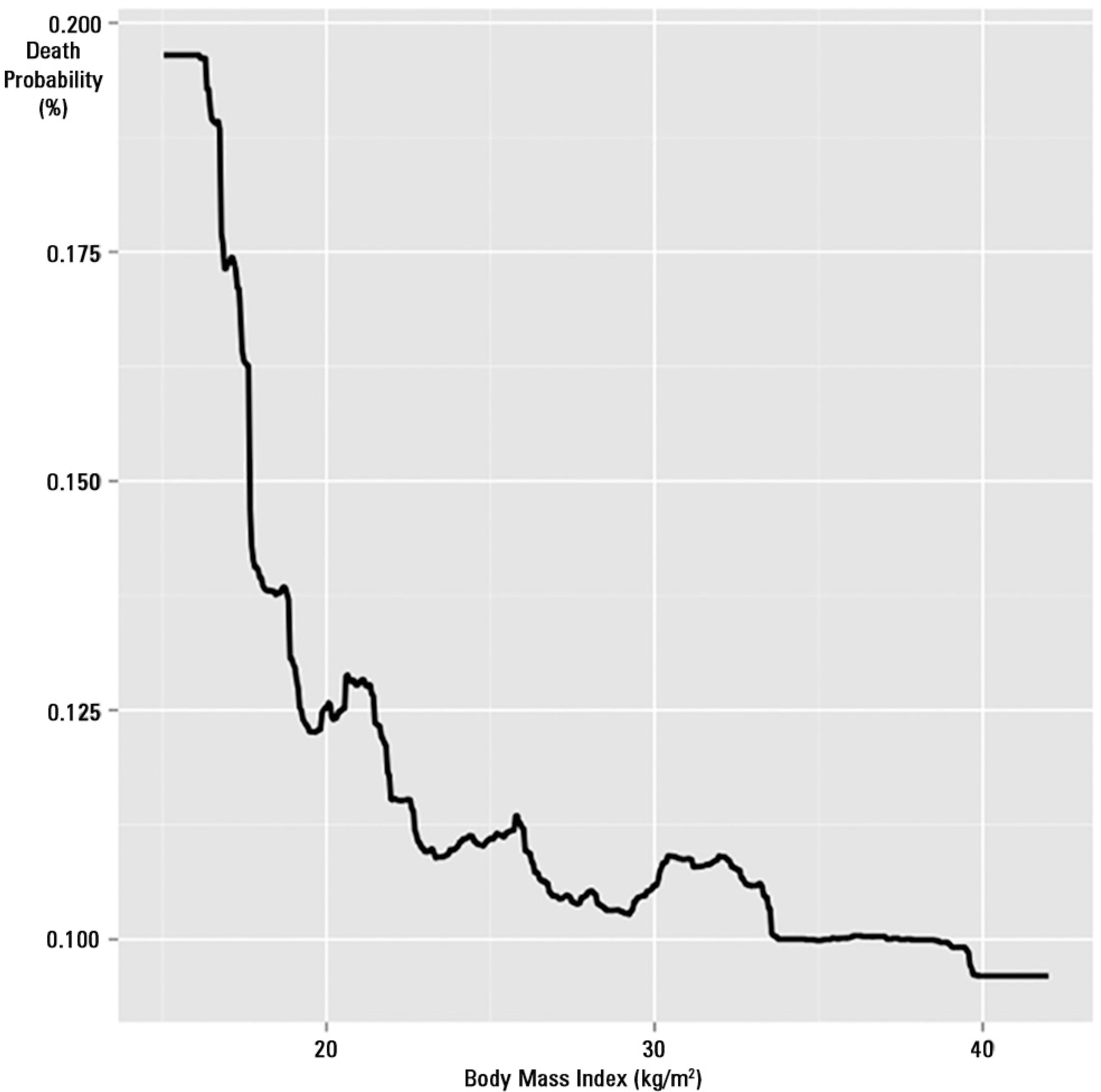
All variables elected for the GBM were retained by the model after the analysis. The final model had 3550 trees. The model had a high discriminative capability with an area under the receiver operator characteristic (ROC) curve of 0.91. The relative influence of each variable on the outcome is shown in figure 2. BMI was the third most important determinant of the outcome after SAPS3adj and age, accounting for 9.48% of all the influence in the model. SAPS3adj had a relative influence above 50%, and age had a relative influence of 10.4%. Comorbidities, temperature at admission and LOS before ICU admission had similar influences on the outcome (7.43%, 7.3% and 7.19%, respectively). The effect of BMI on death probability, with all other variables fixed at their mean values, is shown in figure 3. The death probability increased significantly for a BMI below 20kg/m2 and then tended to marginally decrease as BMI increased, although its influence was small beyond 22kg/m2. The partial dependence plots for age, SAPS3adj, CCI, temperature at admission, LOS before ICU admission, PS, sepsis and steroid use are shown in the electronic supplementary material (Figures S1 to S9, respectively).
Relative influence of each variable of interest on patient outcome.
SAPS3adj - Simplified Acute Physiology Score 3 adjusted; BMI - body mass index; CCI - Charlson´s comorbidity index; LOS - length of stay; PS - performance status.
Influence of body mass index on death probability with other variables kept constant. Note the significant increase in mortality for body mass indices lower than 20kg/m2.
The most important interactions were those found between SAPS3adj and age, between SAPS3adj and CCI, and between CCI and age (Figures S10 - S12, interaction plots), with mean values of the residuals for the interactions of 17.45, 14.13 and 12.10, respectively. BMI was associated with SAPS3adj and age, with mean values of the residuals for the interactions of 6.25 and 4.92, respectively. A lower BMI increased the death probability in all SAPS3adj ranges (Figure 4). No significant interactions were found for PS.
Interaction plot showing the association between body mass index and SAPS3adj on patient outcome.
SAPS3adj - Simplified Acute Physiology Score 3 adjusted.
Only SAPS3adj (p < 0.001) and temperature (p = 0.009) were found to be associated with the LOS in the hospital after ICU admission; estimates were 0.56 for SAPS3adj and -1.61 for temperature at admission.
DISCUSSION
In this retrospective cohort analysis, we were able to show that BMI is independently associated with higher mortality even when illness severity, comorbidities, performance status and other markers of poor prognosis, such as LOS before ICU admission, are considered. Additionally, our analysis shows that applying machine learning techniques, such as the gradient-boosted model, is appropriate for the analysis of clinical databases and may provide relevant clinical information. In this sense, the constraints imposed by the unicentric nature of this analysis are validated by the robustness of the analysis, which results in a more adequate picture of the real impact of BMI on hospital mortality by eliminating bias that could arise from variable interactions.
Our model demonstrates the importance of BMI in determining the short-term outcomes of critical illnesses.(11 Pickkers P, de Keizer N, Dusseljee J, Weerheijm D, van der Hoeven JG, Peek N. Body mass index is associated with hospital mortality in critically ill patients: an observational cohort study. Crit Care Med. 2013;41(8):1878-83.) BMI was the third most important variable, after the adjusted SAPS3 and age, and therefore more important than the performance status, presence of sepsis and LOS before ICU admission. BMI interacts with illness severity on the in-hospital mortality; this association was found to be independent of comorbidities and the performance status. As shown in figure 4, BMI retained its effects throughout the range of SAPS3 scores. Therefore, BMI, specifically a low BMI, may be considered a self-determining modulator of patient outcomes and not only a marker of health status or physiological reserve.(1111 Rahman A, Stapleton RD, Heyland DK. Not all critically ill obese patients are the same: the influence of prior comorbidities. ISRN Obes. 2012;2012:743978.,2222 Tremblay A, Bandi V. Impact of body mass index on outcomes following critical care. Chest. 2003;123(4):1202-7.,2323 Wunsch H. Comorbidity or covert advantage? The obesity conundrum*. Crit Care Med. 2014;42(8):1935-6.)
Low BMI might be used as a surrogate for malnutrition in ambulatory patients, and therefore, our findings regarding the higher mortality in low BMI patients may indicate that malnutrition is the cause of the less favorable outcomes in this subgroup.(2424 Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, et al. Diagnostic criteria for malnutrition - An ESPEN Consensus Statement. Clin Nutr. 2015. pii: S0261-5614(15)00075-8.) However, the association between low BMI and malnutrition might not be as straightforward as it may seem, limiting the generality of such a statement.(2424 Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, et al. Diagnostic criteria for malnutrition - An ESPEN Consensus Statement. Clin Nutr. 2015. pii: S0261-5614(15)00075-8.) Previous studies suggest that low BMI may be associated with poor prognosis in surgical patients.(2525 Gupta R, Knobel D, Gunabushanam V, Agaba E, Ritter G, Marini C, et al. The effect of low body mass index on outcome in critically ill surgical patients. Nutr Clin Pract. 2011;26(5):593-7.) Low weight has also been suggested to be associated with higher mortality, more visits to the emergency department and hospitalization, although the performance status was not accounted for in this analysis.(2626 Takahashi PY, Sauver JL, Olson TC, Huber JM, Cha SS, Ebbert JO. Association between underweight and hospitalization, emergency room visits, and mortality among patients in community medical homes. Risk Manag Healthc Policy. 2013;6:1-6.)
Although mortality tended to decrease for high values of BMI, its impact was small, as shown in figure 3, and fluctuated marginally with a small increase at BMI between 30 - 35, followed by further decrease. Therefore, despite its existence, the “obesity paradox” likely has little influence on short-term mortality. The influence of BMI on the outcomes of critically ill patients has also been investigated by other groups. A meta-analysis on the subject reported that a BMI between 30 - 39.9kg/m2 can be associated with reduced mortality when compared with non-obese patients.(2727 Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med. 2008;36(1):151-8. Review.) In critically ill surgical patients, obesity also appeared to indicate a survival benefit in a large study;(77 Hutagalung R, Marques J, Kobylka K, Zeidan M, Kabisch B, Brunkhorst F, et al. The obesity paradox in surgical intensive care unit patients. Intensive Care Med. 2011;37(11):1793-9.) a subsequent and smaller study confirmed that this benefit could persist even when a specific subset of patients with peritonitis was evaluated.(2828 Utzolino S, Ditzel CM, Baier PK, Hopt UT, Kaffarnik MF. The obesity paradox in surgical intensive care patients with peritonitis. J Crit Care. 2014;29(5):887.e1-5.) Our results were similar to those recently observed by Picckers et al. in a larger cohort of critically ill patients.(11 Pickkers P, de Keizer N, Dusseljee J, Weerheijm D, van der Hoeven JG, Peek N. Body mass index is associated with hospital mortality in critically ill patients: an observational cohort study. Crit Care Med. 2013;41(8):1878-83.) However, in this latter study, the authors did not correct their analysis for performance status and limited the evaluation of comorbidities to a smaller number of conditions.
The absence of a clear association between PS, BMI and CCI was not expected but may be related to the fact that a single major event secondary to one comorbidity (i.e., a hemorrhagic stroke secondary to long-term hypertension) may result in a patient with a low CCI, but with a lower PS. In the same way, when there was a short period of time between the events that ultimately reduced the performance status, the patient may remain overweight for a period of time. This highlights the complexity of the evaluation of performance status in the critically ill.(55 Zampieri FG, Colombari F. The impact of performance status and comorbidities on the short-term prognosis of very elderly patients admitted to the ICU. BMC Anesthesiol. 2014;14:59.) Additionally, the absence of significant interactions between BMI, PS and CCI in determining patient outcomes shows that the effect of BMI on short-term outcomes does not mitigate the effects of other relevant clinical variables. Determining the cause of the beneficial effects of higher BMI is beyond the scope of this manuscript, but inflammatory changes induced by obesity may play a role.(1313 Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115(5):911-9; quiz 20. Review.) The reasons for these findings should be explored in both physiological and prospective clinical studies.
Regarding LOS in the hospital, we were unable to find any association between BMI and the length of stay after ICU admission. We have chosen to use LOS in the hospital after ICU admission and not LOS in the ICU as an outcome, because BMI could influence the ICU discharge decision-making process. Previous analyses present different results with some analyses showing higher LOS for higher BMI and others suggesting no association.(2222 Tremblay A, Bandi V. Impact of body mass index on outcomes following critical care. Chest. 2003;123(4):1202-7.,2929 Ray DE, Matchett SC, Baker K, Wasser T, Young MJ. The effect of body mass index on patient outcomes in a medical ICU. Chest. 2005;127(6):2125-31.) Also, the association between admission temperature in LOS has been previously suggested.(1616 Zampieri FG, Ladeira JP, Park M, Haib D, Pastore CL, Santoro CM, et al. Admission factors associated with prolonged (>14 days) intensive care unit stay. J Crit Care. 2014;29(1):60-5.)
There are several limitations to our analysis. First, this study is a unicentric retrospective analysis and is therefore subject to an intrinsic bias; the measurement of BMI itself may be biased depending on the circumstances of height and weight measurement.(3030 Oud L. Reporting the methodology of height and weight acquisition in studies of body mass index-based prognosis in critically ill patients. J Crit Care. 2013;28(5):640-6.) For example, a patient directly admitted from the emergency department may have had her first weight measured in the ICU after fluid expansion, which may bias the BMI result. This bias would probably result in an effect where more severe patients, who received more fluids, would have a higher initial BMI due to the weight of infused fluids but also a higher mortality since they were more critically ill, which is the opposite of our findings. Therefore, it is possible, at least for the patients admitted from the emergency department, that the association between a higher BMI and a lower mortality would be even more pronounced. Despite the recommendation to record the first available body weight in the hospital recording system, we did not audit how many patients had their initial weight measured in the ICU or the mean elapsed time between the weight measurement and ICU admission. Second, there may still be an inherent bias in the admission pattern based on BMI that could not be measured. Third, we measured the performance status using a simplified scale that cannot account for all of the facets of daily living impairment. Other scales, such as ECOG or Karnosfky scales, could have provided more relevant information;(3131 Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32A(7):1135-41.) however, they were not available for this analysis. Thus, although the GBM is a powerful tool for exploring associations and patterns, it does not provide a straightforward numerical interpretation in this study and relies on association plots for interpretation; this nevertheless may be considered an advantage in exploratory analysis. The GBM may have poorer performance compared to logistic regression for the construction of propensity score models.(3232 Ellis AR, Dusetzina SB, Hansen RA, Gaynes BN, Farley JF, Stürmer T. Confounding control in a nonexperimental study of STAR*D data: logistic regression balanced covariates better than boosted CART. Ann Epidemiol. 2013;23(4):204-9.) As a result, we did not evaluate the influence of BMI on post-ICU outcomes. Finally, we have no data regarding the withholding of life-sustaining therapies in the included sample.
CONCLUSION
Our model highlights the importance of body mass index in determining the short-term outcomes of critical illness. A low body mass index is shown to be associated with a worse prognosis in critically ill medical patients, and there may be a survival advantage for patients with a higher body mass index. The reasons for these findings should be explored in both physiological and prospective clinical studies.
-
Responsible editor: Luciano César Pontes de Azevedo
REFERÊNCIAS
-
1Pickkers P, de Keizer N, Dusseljee J, Weerheijm D, van der Hoeven JG, Peek N. Body mass index is associated with hospital mortality in critically ill patients: an observational cohort study. Crit Care Med. 2013;41(8):1878-83.
-
2Robinson MK, Mogensen KM, Casey JD, McKane CK, Moromizato T, Rawn JD, et al. The relationship among obesity, nutritional status, and mortality in the critically ill. Crit Care Med. 2015;43(1):87-100.
-
3Soares M, Salluh JI, Toscano L, Dias FL. Outcomes and prognostic factors in patients with head and neck cancer and severe acute illnesses. Intensive Care Med. 2007;33(11):2009-13.
-
4Soares M, Caruso P, Silva E, Teles JM, Lobo SM, Friedman G, Dal Pizzol F, Mello PV, Bozza FA, Silva UV, Torelly AP, Knibel MF, Rezende E, Netto JJ, Piras C, Castro A, Ferreira BS, Réa-Neto A, Olmedo PB, Salluh JI; Brazilian Research in Intensive Care Network (BRICNet). Characteristics and outcomes of patients with cancer requiring admission to intensive care units: a prospective multicenter study. Crit Care Med. 2010;38(1):9-15.
-
5Zampieri FG, Colombari F. The impact of performance status and comorbidities on the short-term prognosis of very elderly patients admitted to the ICU. BMC Anesthesiol. 2014;14:59.
-
6Prospective Studies Collaboration, Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083-96.
-
7Hutagalung R, Marques J, Kobylka K, Zeidan M, Kabisch B, Brunkhorst F, et al. The obesity paradox in surgical intensive care unit patients. Intensive Care Med. 2011;37(11):1793-9.
-
8Oliveros H, Villamor E. Obesity and mortality in critically ill adults: a systematic review and meta-analysis. Obesity (Silver Spring). 2008;16(3):515-21.
-
9Westerly BD, Dabbagh O. Morbidity and mortality characteristics of morbidly obese patients admitted to hospital and intensive care units. J Crit Care. 2011;26(2):180-5.
-
10Mica L, Keel M, Trentz O. The impact of body mass index on the physiology of patients with polytrauma. J Crit Care. 2012;27(6):722-6.
-
11Rahman A, Stapleton RD, Heyland DK. Not all critically ill obese patients are the same: the influence of prior comorbidities. ISRN Obes. 2012;2012:743978.
-
12Alley DE, Metter EJ, Griswold ME, Harris TB, Simonsick EM, Longo DL, et al. Changes in weight at the end of life: characterizing weight loss by time to death in a cohort study of older men. Am J Epidemiol. 2010;172(5):558-65.
-
13Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115(5):911-9; quiz 20. Review.
-
14Filardo G, Adams JP. Effect of body mass index on mortality in patients undergoing isolated coronary artery bypass grafting. Ann Thorac Surg. 2010;90(3):1060.
-
15Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-83.
-
16Zampieri FG, Ladeira JP, Park M, Haib D, Pastore CL, Santoro CM, et al. Admission factors associated with prolonged (>14 days) intensive care unit stay. J Crit Care. 2014;29(1):60-5.
-
17Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165-228.
-
18Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. J Anim Ecol. 2008;77(4):802-13.
-
19Dodd S, Berk M, Kelin K, Zhang Q, Eriksson E, Deberdt W, et al. Application of the Gradient Boosted method in randomised clinical trials: Participant variables that contribute to depression treatment efficacy of duloxetine, SSRIs or placebo. J Affect Disord. 2014;168:284-93.
-
20Friedman JH, Roosen CB. An introduction to multivariate adaptive regression splines. Stat Methods Med Res. 1995;4(3):197-217.
-
21Hijmans RJ, Phillips S, Leathwick J, Elith J. dismo: Species distribution modeling. R package version 0.9-3; 2013.
-
22Tremblay A, Bandi V. Impact of body mass index on outcomes following critical care. Chest. 2003;123(4):1202-7.
-
23Wunsch H. Comorbidity or covert advantage? The obesity conundrum*. Crit Care Med. 2014;42(8):1935-6.
-
24Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, et al. Diagnostic criteria for malnutrition - An ESPEN Consensus Statement. Clin Nutr. 2015. pii: S0261-5614(15)00075-8.
-
25Gupta R, Knobel D, Gunabushanam V, Agaba E, Ritter G, Marini C, et al. The effect of low body mass index on outcome in critically ill surgical patients. Nutr Clin Pract. 2011;26(5):593-7.
-
26Takahashi PY, Sauver JL, Olson TC, Huber JM, Cha SS, Ebbert JO. Association between underweight and hospitalization, emergency room visits, and mortality among patients in community medical homes. Risk Manag Healthc Policy. 2013;6:1-6.
-
27Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med. 2008;36(1):151-8. Review.
-
28Utzolino S, Ditzel CM, Baier PK, Hopt UT, Kaffarnik MF. The obesity paradox in surgical intensive care patients with peritonitis. J Crit Care. 2014;29(5):887.e1-5.
-
29Ray DE, Matchett SC, Baker K, Wasser T, Young MJ. The effect of body mass index on patient outcomes in a medical ICU. Chest. 2005;127(6):2125-31.
-
30Oud L. Reporting the methodology of height and weight acquisition in studies of body mass index-based prognosis in critically ill patients. J Crit Care. 2013;28(5):640-6.
-
31Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32A(7):1135-41.
-
32Ellis AR, Dusetzina SB, Hansen RA, Gaynes BN, Farley JF, Stürmer T. Confounding control in a nonexperimental study of STAR*D data: logistic regression balanced covariates better than boosted CART. Ann Epidemiol. 2013;23(4):204-9.
Data availability
Publication Dates
-
Publication in this collection
Apr-Jun 2015
History
-
Received
24 Feb 2015 -
Accepted
09 Apr 2015