ABSTRACT
This study was carried out to investigate the effects of eggshell colour and spot properties (colour and size of the spot area) on eggshell incubation temperature and hatching outcomes of Japanese quail eggs (Coturnix coturnix japonica). Study material was allocated to five groups according to their eggshell and spot colours: black spots on greyish white coloured eggshell (I), blue spots on greyish white coloured eggshell (II), diffuse brown spots on greyish brown coloured eggshell (III), brown spots on light green coloured eggshell (IV), and small brown spots on greyish brown coloured eggshell (V). The size of the spotted area was determined in each egg group using digital image analysis. Mean relative weight losses of hatched and unhatched eggs between days 0-10 and 0-14 of embryonic development were 4.76% and 10.48% and 9.17% and 15.46%, respectively. The mean eggshell temperatures of hatched and unhatched eggs measured at the equatorial region on days 10 and 14 during embryonic development were 36.92 and 37.79 ºC and 36.84 and 37.18 ºC, respectively. Eggshell temperatures at the equatorial region on days 10 (36.89 ºC) and 14 (37.57 ºC) of embryonic development were lower than the fixed temperature of the incubator (37.6 ºC). Fertility, hatchability of fertile eggs, and hatchability and embryonic mortality rates do not vary in relation to eggshell colour or the size of the spotted area.
Key Words:
quail; spot colour
Introduction
Eggshell colour, which has an effect on hatching results, is one of the eggshell traits. Literature reports indicate that in various bird species, eggshell pigmentation serves as an indicator for the selection of females as well as for genetic advance achieved by means of effective mating (Stoddard et al., 2012Stoddard, M. C.; Fayet, A. L.; Kilner, R. M.; Hinde, C. A. 2012. Egg speckling patterns do not advertise offspring quality or influence male provisioning in Great Tits. Plos One 7:1-12.). Eggshell pigmentation has also been reported to have an effect on the egg nutrient content and on the protection of the embryo from solar radiation during natural incubation (Moreno and Osorno, 2003Moreno, J. and Osorno, J. L. 2003. Avian egg colour and sexual selection: does eggshell pigmentation reflect female condition and genetic quality? Ecology Letters 6:803-806.; Lahti, 2008Lahti, D. C. 2008. Population differentiation and rapid evolution of egg color in accordance with solar radiation. The Auk 125:796-802.; Riehl, 2011Riehl, C. 2011. Paternal investment and the 'sexually selected hypothesis' fort he evolution of eggshell coloration: revisiting the assumptions. The Auk 128:175-179.; Cassey et al., 2012Cassey, P.; Thomas, G. H.; Portugal, S. J.; Maurer, G.; Hauber, M. E.; Grim, T.; Lovell, P. G. and Mikšík, I. 2012. Why are birds' eggs colourful? Eggshell pigments co-vary with life-history and nesting ecology among British breeding non-passerine birds. Biological Journal of the Linnean Society 106:657-672.). Eggshell colour is formed by pigments that are secreted by the shell gland (Liu and Cheng, 2010Liu, H. S. and Cheng, W. T. 2010. Eggshell pigmentatoin: a review. Journal of the Chinese Society of Animal Science 39:75-89.).
The major pigments in bird eggs are protoporphyrin-IX, biliverdin IX, and zinc chelate. It is suggested that eggshell pigments are derived from the disintegration of erythrocytes found in the mucous layer of the shell gland. Blood and the shell gland are considered to be the site of the biosynthesis of eggshell porphyrin (Liu and Cheng, 2010Liu, H. S. and Cheng, W. T. 2010. Eggshell pigmentatoin: a review. Journal of the Chinese Society of Animal Science 39:75-89.). Biliverdin, a green pigment formed during the breakdown of haemoglobin, gives the eggshell a blue or green colour, whilst protoporphyrin, an immediate precursor of heme molecule, gives the eggshell a reddish or brown colour (Miksik et al., 1994Mikšík, I.; Holáň, V. and Deyl, Z. 1994. Quantification and variability of eggshell pigment content. Comparative Biochemistry Physiology 109A:769-772.; Liu and Cheng, 2010Liu, H. S. and Cheng, W. T. 2010. Eggshell pigmentatoin: a review. Journal of the Chinese Society of Animal Science 39:75-89.). It has been reported that, of these pigments, biliverdin, which is found in blue-green coloured eggshells, has antioxidant properties (Moreno and Osorno, 2003Moreno, J. and Osorno, J. L. 2003. Avian egg colour and sexual selection: does eggshell pigmentation reflect female condition and genetic quality? Ecology Letters 6:803-806.; Soler et al., 2005Soler, J. J.; Moreno, J.; Avilés, J. M. and Møller, A. P. 2005. Blue and green egg-color intensity is associated with parental effort and mating system in passerines: support for the sexual selection hypothesis. Evolution 59:636-644.; Siefferman et al., 2006Siefferman, L.; Navara, K. J. and Hill, G. E. 2006. Egg coloration is correlated with female condition in eastern blue birds (Sialia sialis). Behavioral Ecology Sociobiology 59:651-656.; Zhao et al., 2006Zhao, R.; Xu, G. Y.; Liu, Z. Z.; Li, J. Y. and Yang, N. 2006. A study on eggshell pigmentation: biliverdin in blue-shelled chickens. Poultry Science 85:546-549.; Liu and Cheng, 2010; Morales et al., 2011Morales, J.; Velando, A. and Torres, R. 2011. Biliverdin-based egg coloration is enhanced by carotenoid supplementation. Behavioral Ecology Sociobiology 65:197-203.; Duval et al., 2013Duval, C.; Cassey, P.; Mikšík, I.; Reynolds, S. J. and Spencer, K. A. 2013. Condition-dependent strategies of eggshell pigmentation: an experimental study of Japanese quail (Coturnix coturnix japonica). The Journal of Experimental Biology 216:700-708.), whilst protoporphyrin increases the resistance of the eggshell to breakage (Mikšik et al., 1996Mikšík, I.; Holáň, V. and Deyl, Z. 1996. Avian eggshell pigments and their variability. Comparative Biochemistry Physiology 113B:607-612.; Gosler et al., 2011Gosler, A. G.; Connor, O. R. and Bonser, R. H. C. 2011. Protoporphyrin and eggshell strength: preliminary findings from a passerine bird. Avian Biology Research 4:214-223.; Cassey et al., 2012Cassey, P.; Thomas, G. H.; Portugal, S. J.; Maurer, G.; Hauber, M. E.; Grim, T.; Lovell, P. G. and Mikšík, I. 2012. Why are birds' eggs colourful? Eggshell pigments co-vary with life-history and nesting ecology among British breeding non-passerine birds. Biological Journal of the Linnean Society 106:657-672.). Furthermore, Moreno and Osorno (2003)Moreno, J. and Osorno, J. L. 2003. Avian egg colour and sexual selection: does eggshell pigmentation reflect female condition and genetic quality? Ecology Letters 6:803-806., Liu and Cheng (2010)Liu, H. S. and Cheng, W. T. 2010. Eggshell pigmentatoin: a review. Journal of the Chinese Society of Animal Science 39:75-89., and Holveck et al. (2012Holveck, M. J.; Grégoire, A.; Staszewski, V.; Guerreiro, R.; Perret, P.; Boulinier, T. and Doutrelant, C. 2012. Eggshell spottiness reflects maternally transferred antibodies in blue tits. Plos One 7:1-12.) have reported the protoporphyrin pigment to have a pro-oxidant effect.
The effect of eggshell colour on hatching results has been investigated in previous research. In their study on chicken eggs with brown, light brown, and creamy white eggshell colour, Kumar et al. (2012Kumar, A.; Das, K.; Mukherjee, K.; Bharti, A. and Singh, A. K. 2012. Frequency of different shell color and its effect on the fertility and hatchability in black rock, gramapriya and vanaraja breeds of chicken. Veterinary World 5:594-598.) determined that eggs with dark eggshell colour displayed higher fertility and fertile-egg hatchability rates than those of eggs with light eggshell colour. Hulet et al. (1978Hulet, R. M.; Marquez, B. J.; Molle, S. and Flegal, C. J. 1978. Relationship of pheasant egg color and hatchability. Poultry Science 57:1146-1148.) demonstrated that, in pheasant eggs, eggshell colour and hatchability were correlated with each other. Richards and Deeming (2001Richards, P. D. G. and Deeming, D. C. 2001. Correlation between Shell colour and ultrastructure in pheasant eggs. British Poultry Science 42:338-343.) suggested that eggs with blue eggshell colour are structurally defective eggs with a thin eggshell. These researchers explained the low hatchability of blue coloured eggs as being due to the high weight loss resulting from their structural defection. In pheasant (ring-necked) eggs, aiming to determine the effect of eggshell colour on egg quality, hatching results, egg shape, and eggshell quality, eggs were grouped as dark brown, light brown, olive, and blue (Krystianiak et al., 2005Krystianiak, S.; Kożuszek, R.; Kontecka, H. and Nowaczewski, S. 2005. Quality and ultrastructure of eggshell and hatchability of eggs in relation to eggshell colour in pheasants. Animal Science Papers and Reports 23:5-14.; Kożuszek et al., 2009bKożuszek, R.; Kontecka, H.; Nowaczewski, S.; Leśnierowski, G.; Kijowski, J. and Rosiński, A. 2009b. Quality of pheasant (Phasianus colchicus L.) eggs with different shell colour. Archiv Für Geflügelkunde 73:201-207.; Nowaczewski et al., 2011Nowaczewski, S.; Stuper, K.; Szablewski, T. and Kontecka, H. 2011. Microscopic fungi in eggs of ring-necked pheasants kept in aviaries. Poultry Science 90:2467-2470.; Nowaczewski et al., 2013Nowaczewski, S.; Kontecka, H. and Rosiński, A. 2012. Effect of Japanese quail eggs location in the setter on their weight loss and eggshell temperature during incubation as well as hatchability results. Archiv Für Geflügelkunde76:168-175.). In an investigation conducted by Ristić et al. (2013Ristić, Z. A.; Marković, V.; Kovaćević, M.; Nad, I.; Matejević, M. and Jovanović, T. 2013. The significance of egg shell color on the pheasant hatching production results. Pakistan Journal of Zoology 45:1549-1553.) aiming to determine the effects of eggshell colour on hatching results, pheasant eggs were classified as dark brown, light brown, brown-green, and blue-green. In research on fertility, hatchability, embryonic mortality, malposition, and egg quality traits in quail eggs, eggs were classified as brown pigmented, black stained, white, spotted, and blue pigmented, according to the eggshell colour (Taha, 2011Taha, A. E. 2011. Analyzing of quail eggs hatchability, quality, embryonic mortality and malpositions in relation to their shell colors. Online Journal of Animal and Feed Research 1:267-273.). The correlation between 12 eggshell pigmentation patterns and breeder live weight, laying interval, mean egg weight, and hatching results was investigated in quail eggs, on the basis of region of interest (ROI) images (Fayeye et al., 2013Fayeye, T. R.; Ojo, V.; Alli, O. I. and Adebayo, B. K. 2013. Egg pigment pattern and association between hen body weight, oviposition interval, egg-weight and hatchability in Japanese quail. Niseb Journal 13:1-4.). In research carried out in quail eggs on the effect of eggshell colour on various traits, eggs were classified as white, spotted violet, and spotted brown (Farghly et al., 2015Farghly, M. F. A.; Mahrose, Kh. M. A. and Abou-Kassem, D. E. 2015. Pre and post hatch performance of different japanese quail egg colors incubated under photostimulation. Asian Journal of Poultry Science 9:19-30.); and sienna, rich brown, and chocolate (Idahor et al., 2015Idahor, K. O.; Akinola, L. A. F. and Chia, S. S. 2015. Predetermination of quail chick sex using egg indices in North Central Nigeria. Journal of Animal Production Advances 5:599-605.).
The direct measurement of embryo temperature, which has a significant role in embryonic development, is not possible. For this reason, eggshell temperature is used as an indicator of embryo temperature (van der Pol et al., 2013Van Der Pol, C. W.; Van Roovert-Reijrink, I. A. M.; Maatjens, C. M.; Van Der Brand, H. and Molenaar, R. 2013. Effect of relative humidity during incubation at a set eggshell temperature and brooding temperature posthatch on embryonic mortality and chick quality. Poultry Science 92:2145-2155.). The temperature of the developing embryo depends on the temperature of the setter, heat transfer between the embryo and the setter, and heat production by the embryo (French, 1997French, N. A. 1997. Modeling incubation temperature: the effects of incubator design, embryonic development, and egg size. Poultry Science 76:124-133.).
This study aimed to determine the effect of eggshell colour and spot properties (colour and size of spot area) on eggshell temperature during incubation and hatching results in eggs of Japanese quail (Coturnix coturnix japonica).
Material and Methods
Hatching eggs, collected within a three-day period from a private holding raising Japanese quails, constituted the material of the study. The eggs underwent macroscopic examination at the laboratory, and broken, cracked, abnormally shaped, and soiled eggs were excluded from the study. The experiment involved 1,062 eggs from 16-wk-old Japanese quail (Coturnix coturnix japonica), which reached 95% egg production.
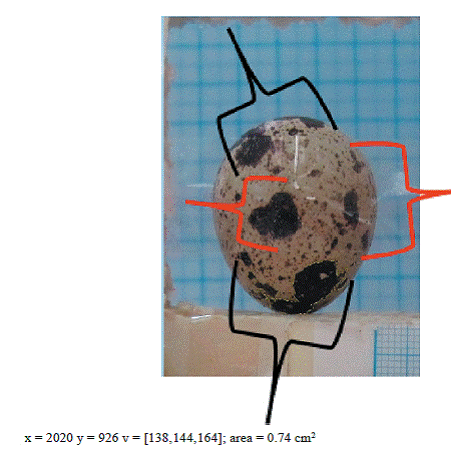
After being macroscopically examined prior to incubation, the eggs constituting the study material were allocated to five groups according to their eggshell and spot colours, and were individually numbered. The size of the spotted area was determined in each egg in all groups using the digital image analysis method (Table 1). A mechanism with a measurement scale was set up for the imaging process. The individually numbered eggs were placed in the mechanism and were imaged at an approximate distance of 20 cm. Each egg was photographed from one side, and then turned 180º to the other side to be photographed again (Figures 1 and 2). A total of 4,248 (1,062 × 4) images were taken and stored in a flash memory. Digital images were analyzed using the UTHSCSA Image Tool software at the laboratory of the Faculty of Engineering. The measurement unit used for the size of the spotted area was cm2.
No photo-manipulation was made to the images stored in the flash memory, prior to incubation. Digital images were analyzed using the sharpest images. Egg length and egg width were determined by digital image analysis, and these values were used for the calculation of the egg shape index (Figures 3 and 4). The formula given below was used for the calculation of the egg shape index:
Prior to incubation, all eggs included in each group were individually weighed to determine egg initial weight. The eggs were randomly placed in the setter with 3 repeats, such that each tray provided for one repeat. In the setter, the temperature was set at 37.6 ºC and the relative humidity was adjusted to 60-65%. On day 14 of embryonic development, individual compartments were established for the each egg included in the same group, and the hatching period was initiated by placing eggs of the same group in an individual compartment. In the hatching machine, the temperature was set at 37.2 ºC and the relative humidity was adjusted to 65-70%.
Egg weight loss was calculated using the initial egg weight and the egg weights measured on days 10 and 14 of the incubation period. Egg weight measurements were performed separately for each egg. The formulae used for egg weight measurements are shown next:
in which W0 = initial egg weight; and W1 = egg weight on day of measurement.
On days 10 and 14 of embryonic development, eggshell temperature was measured in all eggs included in each group at three different regions of the eggs, including the pointed end, blunt end, and the centre. Measurements were performed using a thermometer (Braun Thermoscan meter). During the measurement of eggshell temperature, the temperature of the room, where the setting machine was placed, was set to a temperature equal to that of the setter. Eggshell temperature was measured at the pointed and blunt ends and centre of the eggs, and the arithmetic mean of these three measurements was calculated as the regional temperature (pointed end, blunt end, and centre) of the eggshell. The average temperature of the whole egg was calculated using the formula given below:
General eggshell temperature (ºC) = (Average eggshell temperature at pointed end + Average eggshell temperature at centre + Average eggshell temperature at blunt end)/3
Eggshell thickness was calculated according to the formula given below, using the egg weight.
Eggshell thickness (mm) = 0.0546 × (Egg weight)0.441 (Rahn and Paganelli, 1989Rahn, H. and Paganelli, C. V. 1989. Shell mass, thickness and density of avian eggs derived from the tables of Schönwetter. Journal of Ornithology 130:59-68.).
The hatching weights of the chicks were determined for all eggs included in each group. During these measurements, the eggshell weight of each hatched egg was also determined. In each group, the unhatched eggs were separated for the determination of fertilization, and fertilized eggs were examined for embryonic mortality between days 0-6 of embryonic development (black eye and conspicuous beak), days 6-17 of embryonic development (embryo with feathers and embryo with yolk out), and days 17-18 of embryonic development (full-grown embryo dead and with yolk subtracted and pipped eggs) (Aygun and Sert, 2013Aygun, A. and Sert, D. 2013. Effects of prestorage application of propolis and storage time on eggshell microbial activity, hatchability, and chick performance in Japanese quail (Coturnix coturnix japonica) eggs. Poultry Science 92:3330-3337.). These data were used for the calculation of the hatching results given below:
Fertility (%) = (Number of fertilized eggs/Number of eggs placed in the setter) × 100
Hatchability of fertile eggs (%) = (Number of hatched chicks/Number of fertilized eggs) × 100
Hatchability (%) = (Number of hatched chicks/Number of eggs placed in the setter) × 100
Relative weight loss in unhatched eggs (%) = 100 × ((W0 - W2)/W0),
in which W0 = initial egg weight; and W2 = weight of unhatched eggs.
The statistical analysis of the data was performed using the SPSS software. The chi-square test was used to determine the effect of the eggshell colour of hatching eggs on hatchability, fertility, hatchability of fertile eggs, and embryonic mortality during incubation. The other data were compared using analysis of variance, and the groups differing from each other were determined by Duncan's multiple comparison test.
Results
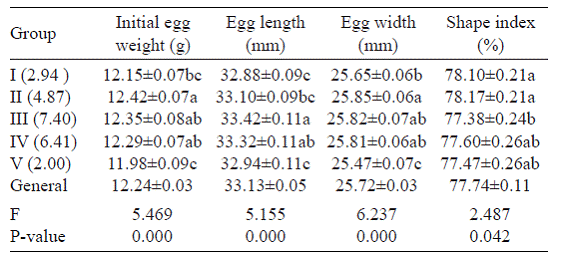
The eggs with the same eggshell colour (Groups I and II and Groups III and V) demonstrated that egg weight was higher in the groups with a larger size of spotted area (P<0.001) (Table 2). Thus, of the groups with a greyish white eggshell colour, Group I (a spotted area of 2.94 cm2) presented an egg weight of 12.15 g, whilst Group II (a spotted area of 4.87 cm2) presented an egg weight of 12.42 g. Similarly, of the groups with a greyish brown eggshell colour, Group III (a spotted area of 7.40 cm2) presented an egg weight of 12.35 g, whilst Group V (a spotted area of 2.00 cm2) presented an egg weight of 11.98 g, and thus displayed higher egg weight with larger spotted area.
Egg shape index values were highest in Groups I (78.10%) and II (78.17%), and the difference between these two groups was statistically insignificant. On the other hand, egg shape index values were lowest in Groups III (77.38%) and V (77.47%), which had a greyish brown eggshell colour, and these two groups were determined to significantly differ from each other (P<0.05).
The differences between groups for the relative weight losses between days 0-10 and 0-14 of embryonic development were statistically significant (P<0.05) (Table 3). The mean relative weight losses of the groups between days 0-10 and 0-14 of embryonic development were determined as 7.38% and 12.33%, respectively. Relative weight loss between days 0-10 and 0-14 of embryonic development was highest in Group V and lowest in Group II.
Mean relative weight losses of hatched eggs between days 0-10 and 0-14 of embryonic development were 4.76% and 10.48%, respectively. Throughout the investigated periods, egg weight loss in the hatched eggs was highest in Group V. The lowest relative weight losses were determined in Group I between days 0-10 and 0-14 of embryonic development.
In the unhatched eggs, mean relative weight losses between days 0-10 and 0-14 of embryonic development were 9.17% and 15.46%, respectively. Egg weight losses between days 0-10 and 0-14 of embryonic development were highest in Group IV (19.15%) and lowest in Group II (11.83%).
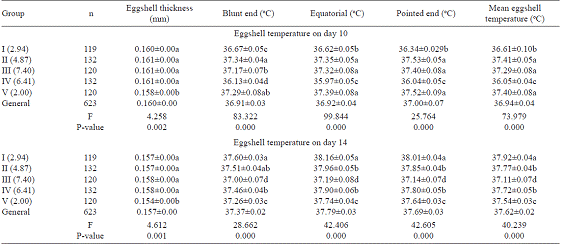
The mean eggshell temperature measured at the equatorial region on days 10 and 14 during incubation in all groups was 36.89 ºC and 37.57 ºC, respectively (Table 4) and the differences between groups were statistically significant (P<0.001). The lowest eggshell temperatures at equatorial region on days 10 and 14 during embryonic development were 35.92 ºC in Group IV and 36.95 ºC in Group III.
In the hatched eggs (Table 5), the mean eggshell temperatures measured at the equatorial region on days 10 and 14 during embryonic development were 36.92 ºC and 37.79 ºC, respectively. The differences between groups were statistically significant (P<0.001).
In the unhatched eggs (Table 6), the equatorial eggshell temperatures on days 10 and 14 during embryonic development differed significantly between the groups (P<0.001). The highest equatorial eggshell temperatures on days 10 and 14 during the incubation period were 37.32 ºC in Group II and 37.68 ºC in Group I.
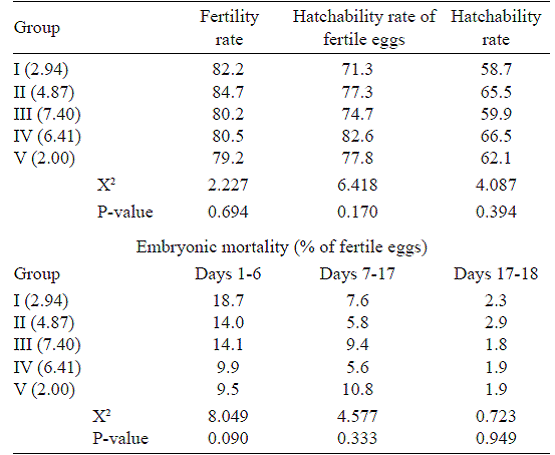
In the present study, the differences observed between the groups for fertility rates, hatchability rates, and the hatchability of fertile eggs were found to be statistically insignificant (P>0.05) (Table 7). The highest fertility rate was determined as 84.7% in Group II, whereas the lowest fertility rate was determined as 79.2% in Group V. The hatchability of fertile eggs was highest in Group IV (82.6%), followed by Groups V (77.8%), II (77.3%), III (74.7%), and I (71.3%).
The effect of eggshell colour on embryonic mortality was insignificant (P>0.05). Embryonic mortality between days 1-6 was highest in Group I (18.7%) and lowest in Group V (9.5%). Embryonic mortality between days 7-17 was highest in Groups V (10.8%) and followed by Groups III (9.4%), I (7.6%), II (5.8%), and IV (5.6%). Embryonic mortality ranged from 1.8% to 2.9% between days 17 and 18.
The shell weight of hatched eggs was higher in Groups II (0.97 g) and I (0.95 g), which had the same eggshell colour, than in other groups (Table 8). The chick weight/egg weight ratio was highest in Groups I (70.55%) and V (70.33%), followed by Groups IV (68.88%), III (68.80%), and II (68.45%) (P<0.001).
Discussion
The demonstration of eggshell colour having an effect on egg weight, egg length, and egg width in the present study was in agreement with the report of Taha (2011Taha, A. E. 2011. Analyzing of quail eggs hatchability, quality, embryonic mortality and malpositions in relation to their shell colors. Online Journal of Animal and Feed Research 1:267-273.), indicating eggshell colour to have a significant effect on egg weight, egg length, and egg width in quails (Table 2). Egg weight and egg width values were determined to be highest in the blue spotted eggs and lowest in the black spotted eggs. Taha (2011)Taha, A. E. 2011. Analyzing of quail eggs hatchability, quality, embryonic mortality and malpositions in relation to their shell colors. Online Journal of Animal and Feed Research 1:267-273. reported that egg weight, egg length, and egg width were higher in black spotted eggs when compared with blue spotted eggs. The different results obtained in these studies are attributed to differences in the trial groups established. In another study carried out in quails (Farghly et al., 2015Farghly, M. F. A.; Mahrose, Kh. M. A. and Abou-Kassem, D. E. 2015. Pre and post hatch performance of different japanese quail egg colors incubated under photostimulation. Asian Journal of Poultry Science 9:19-30.), eggshell colour (white, spotted violet, spotted brown) was reported to have no effect on egg weight. Other studies conducted in different avian species suggest that eggshell colour has an effect on egg weight. In research conducted on pheasant eggs of varying eggshell colour (Kırıkcı et al., 2005Kirikci, K.; Gunlu, A. and Garip, M. 2005. Some quality characteristics of pheasant (Phasianus colchicus) eggs with different shell colors. Turkish Journal of Veterinary and Animal Sciences 29:315-318.; Kożuszek et al., 2009aKożuszek, R.; Kontecka, H.; Nowaczewski, S. and Rosiński, A. 2009a. Storage time and eggshell colour of pheasant eggs vs. the number of blastodermal cells and hatchability results. Folia Biologica 57:121-130.), eggshell colour was determined to have a significant effect on egg weight. On the other hand, Kożuszek et al. (2009b)Kożuszek, R.; Kontecka, H.; Nowaczewski, S.; Leśnierowski, G.; Kijowski, J. and Rosiński, A. 2009b. Quality of pheasant (Phasianus colchicus L.) eggs with different shell colour. Archiv Für Geflügelkunde 73:201-207. and Nowaczewski et al. (2013Nowaczewski, S.; Szablewski, T.; Cegielska-Radziejewska, R. and Kontecka, H. 2013. Egg morphometry and eggshell quality in ring-necked pheasants kept in cages. Annals of Animal Science 13:531-541.) reported that the impact of eggshell colour on egg weight was statistically insignificant. While reports by Shafey et al. (2004Shafey, T. M.; Ghannam, M. M.; Al-Batshan, H. A. and Al-Ayed, M. S. 2004. Effect of pigment intensity and region of eggshell on the spectral transmission of light that passes the eggshell of chickens. International Journal of Poultry Science 3:228-233.) and Shafey et al. (2005)Shafey, T. M.; Al-Batshan, H. A.; Ghannam, M. M. and Al-Ayed, M. S. 2005. Effect of intensity of eggshell pigment and illuminated incubation on hatchability of brown eggs. British Poultry Science 46:190-198. suggest that the effect of the pigment density of brown chicken eggs on egg weight is statistically insignificant, Yang et al. (2009Yang, H. M.; Wang, Z. Y. and Lu, J. 2009. Study on the relationship between eggshell colors and egg quality as well as shell ultrastructure in yangzhou chicken. African Journal of Biotechnology 8:2898-2902.) suggested the effect of pigment density to be significant.
In the present study, while egg shape index was the same in blue spotted (Group II, 78.17%) and black spotted (Group I, 78.10%) eggs of the same eggshell colour, the differences observed between these two groups and the brown spotted groups (Groups III, IV and V) were statistically significant. This result, suggesting egg shape index to vary with spot colour, disagrees with the reports of Taha (2011Taha, A. E. 2011. Analyzing of quail eggs hatchability, quality, embryonic mortality and malpositions in relation to their shell colors. Online Journal of Animal and Feed Research 1:267-273.) and Farghyl et al. (2015)Farghly, M. F. A.; Mahrose, Kh. M. A. and Abou-Kassem, D. E. 2015. Pre and post hatch performance of different japanese quail egg colors incubated under photostimulation. Asian Journal of Poultry Science 9:19-30. suggesting egg shape index not to vary with eggshell colour. In the present study, egg shape index values were highest in the blue coloured eggs, whilst Taha (2011)Taha, A. E. 2011. Analyzing of quail eggs hatchability, quality, embryonic mortality and malpositions in relation to their shell colors. Online Journal of Animal and Feed Research 1:267-273. and Farghyl et al. (2015)Farghly, M. F. A.; Mahrose, Kh. M. A. and Abou-Kassem, D. E. 2015. Pre and post hatch performance of different japanese quail egg colors incubated under photostimulation. Asian Journal of Poultry Science 9:19-30. reported these values to be highest in brown and spotted violet quail eggs. While Kirikci et al. (2005Kirikci, K.; Gunlu, A. and Garip, M. 2005. Some quality characteristics of pheasant (Phasianus colchicus) eggs with different shell colors. Turkish Journal of Veterinary and Animal Sciences 29:315-318.) and Kozuszek et al. (2009bKożuszek, R.; Kontecka, H.; Nowaczewski, S.; Leśnierowski, G.; Kijowski, J. and Rosiński, A. 2009b. Quality of pheasant (Phasianus colchicus L.) eggs with different shell colour. Archiv Für Geflügelkunde 73:201-207.) reported that eggshell colour had a significant effect on egg shape index in pheasants, Nowaczewski et al. (2013Nowaczewski, S.; Szablewski, T.; Cegielska-Radziejewska, R. and Kontecka, H. 2013. Egg morphometry and eggshell quality in ring-necked pheasants kept in cages. Annals of Animal Science 13:531-541.) suggested that egg shape index did not vary with eggshell colour.
Egg weight loss during the incubation period is a major parameter, which affects hatching results. In the present study, the highest egg weight loss between days 0-14 of embryonic development was determined at 13.70% in Group V, whilst the lowest loss that occurred in the same period was determined at 11.15% in Group II. This difference observed between the groups for egg weight loss was attributed to the eggs included in Group V having a thinner eggshell and a spotted area of smaller size, and the eggs included in Group II having a thicker eggshell and a spotted area of medium size. These results are in support of the reports of Ar et al. (1974Ar, A.; Paganelli, C. V.; Reeves, R. B.; Greene, D. G. and Rahn, H. 1974. The avian egg: water vapor conductance, shell thickness, and functional pore area. The Condor 76:153-158.) and Rahn and Ar (1974Rahn, H. and Ar, A. 1974. The avian egg: incubation time and water loss. The Condor 76:147-152.) suggesting that eggs with a thicker eggshell have lower gas permeability. In an investigation carried out in meat-type and egg-type quails, Romao et al. (2008Romao, J. M.; Moraes, T. G. V.; Teixeira, R. S. C.; Cardoso, W. M. and Buxade, C. C. 2008. Effect of egg storage length on hatchability and weight loss in incubation of egg and meat type Japanese quails. Brazilian Journal of Poultry Science 10:143-147.) reported egg weight losses in the embryonic development period to have occurred at the rates of 8.27 and 9.31%, respectively. Furthermore, egg weight loss during incubation was reported as 9.72% in meat-type Pharaoh quails (Genchev, 2009Genchev, A. 2009. Influence of hatching eggs storage period upon the incubation parameters in Japanese quails. Journal Central European Agriculture 10:167-174.). In another study conducted by Nowaczewski (2010Nowaczewski, S.; Witkiewicz, K.; Kontecka, H.; Krystianiak, S. and Rosiński, A. 2010. Eggs weight of Japanese quail vs. eggs quality after storage time and hatchability results. Archiv Tierzucht 53:720-730.) in groups of varying egg weight (small: 10.5 g, medium: 10.51-11.50 g, large: 11.51-12.50 g, and extra large: 12.51 g), egg weight losses were reported to have occurred at the rates of 11.0, 10.4, 9.9, and 9.5%, respectively, between days 0-15 of embryonic development. In an investigation on the effects of varying propolis concentrations and storage periods on microbial load and hatching results in hatching quail eggs, Aygun and Sert (2013Aygun, A. and Sert, D. 2013. Effects of prestorage application of propolis and storage time on eggshell microbial activity, hatchability, and chick performance in Japanese quail (Coturnix coturnix japonica) eggs. Poultry Science 92:3330-3337.) reported egg weight loss to have occurred at a rate of 10.59% between days 0-14 of embryonic development in the control group. Nowaczewski et al. (2012)Nowaczewski, S.; Kontecka, H. and Rosiński, A. 2012. Effect of Japanese quail eggs location in the setter on their weight loss and eggshell temperature during incubation as well as hatchability results. Archiv Für Geflügelkunde76:168-175. reported the weight loss (0-14 days of embryonic development) of quail eggs at the upper levels in the setter at 9.07% (P>0.05). In another study on the effect of eggshell colour in quail eggs (Farghly et al., 2015Farghly, M. F. A.; Mahrose, Kh. M. A. and Abou-Kassem, D. E. 2015. Pre and post hatch performance of different japanese quail egg colors incubated under photostimulation. Asian Journal of Poultry Science 9:19-30.), weight loss was reported as 15.70% in eggs with white coloured eggshell, 15.42% in eggs with spotted brown eggshell, and 14.04% in eggs with spotted violet eggshell. The weight losses determined in the present study are higher than those reported for quail eggs by Romao et al. (2008)Romao, J. M.; Moraes, T. G. V.; Teixeira, R. S. C.; Cardoso, W. M. and Buxade, C. C. 2008. Effect of egg storage length on hatchability and weight loss in incubation of egg and meat type Japanese quails. Brazilian Journal of Poultry Science 10:143-147., Genchev (2009)Genchev, A. 2009. Influence of hatching eggs storage period upon the incubation parameters in Japanese quails. Journal Central European Agriculture 10:167-174., Nowaczewski et al. (2010)Nowaczewski, S.; Witkiewicz, K.; Kontecka, H.; Krystianiak, S. and Rosiński, A. 2010. Eggs weight of Japanese quail vs. eggs quality after storage time and hatchability results. Archiv Tierzucht 53:720-730., Nowaczewski et al. (2012)Nowaczewski, S.; Kontecka, H. and Rosiński, A. 2012. Effect of Japanese quail eggs location in the setter on their weight loss and eggshell temperature during incubation as well as hatchability results. Archiv Für Geflügelkunde76:168-175., and Aygun and Sert (2013)Aygun, A. and Sert, D. 2013. Effects of prestorage application of propolis and storage time on eggshell microbial activity, hatchability, and chick performance in Japanese quail (Coturnix coturnix japonica) eggs. Poultry Science 92:3330-3337. and lower than those reported by Farghly et al. (2015)Farghly, M. F. A.; Mahrose, Kh. M. A. and Abou-Kassem, D. E. 2015. Pre and post hatch performance of different japanese quail egg colors incubated under photostimulation. Asian Journal of Poultry Science 9:19-30.. The differences between the results of these studies were attributed to the use of hatching eggs from animals of varying genotypes and ages, the use of different setters for embryonic development, and different environmental conditions having been adjusted in the setters.
In the present study, weight losses that occurred between days 0-14 of embryonic development in the hatched eggs with a greyish white eggshell colour were determined as 9.58% in Group I and 10.81% in Group II. Weight losses that occurred in the same period in the eggs with a greyish brown eggshell colour were determined as 12.14% in Group V and 9.93% in Group III (P<0.05) (Table 3). These values demonstrated that, in Groups III and V, egg weight loss had decreased with an increased size of spotted area on the same eggshell background colour. This result was in agreement with that reported by Higham and Gosler (2006Higham, J. P. and Gosler, A. G. 2006. Speckled eggs: water-loss and incubation behaviour in the great tit Parus majör. Oecologia 149:561-570.) for eggs of tit (Parus major) suggesting an increased spotted area of hatched eggs to be associated with increased resistance of the eggshell to breakage and decreased eggshell permeability. However, the results obtained in Groups I and II, which included eggs of the same eggshell colour, differed from those reported by Higham and Gosler (2006)Higham, J. P. and Gosler, A. G. 2006. Speckled eggs: water-loss and incubation behaviour in the great tit Parus majör. Oecologia 149:561-570.. In the present study, the eggs included in Group I being black spotted and the eggs included in Group II being blue spotted demonstrated that egg weight loss was affected by spot colour rather than by the size of the spotted area. Furthermore, in an investigation on eggs of Houbara bustard (Chlamydotis undulata macqueennii), Baggott et al. (2002Baggott, G. K.; Deeming, D. C.; Hemon, S. and Paillat, P. 2002. Relationships between eggshell pigmentation, ultrastructure and water vapour conductance in the houbara bustard (Chlamydotis undulata macqueennii). Avian and poultry Biology Reviews 13:234-235.) reported that light and very light coloured eggs had a thinner eggshell with a greater number of pores and greater water permeability, when compared with medium-coloured and dark coloured eggs.
Fertility rates having been determined not to differ with eggshell colour were in agreement with the report of Hassan et al. (2013Hassan, H. A.; El-Nesr, S. S.; Osman, A. M. R. and Arram, G. A. 2013. Ultrastructure of eggshell, egg weight loss and hatching traits of Japanese quail varding in eggshell color and pattern using image analysis. Egyptian Poultry Science 34:1-17.) suggesting light, dotted, spotted, and dark eggshell colour not to have any effect on fertility, but diverged from the results of Taha (2011Taha, A. E. 2011. Analyzing of quail eggs hatchability, quality, embryonic mortality and malpositions in relation to their shell colors. Online Journal of Animal and Feed Research 1:267-273.) suggesting that fertility varied among eggs with black, blue, brown, spotted, and white eggshell colour. In research carried out in pheasant eggs, while Hulet et al. (1985Hulet, R. M.; Flegal, C. J.; Carpenter, G. H. and Champion, L. R. 1985. Effect of eggshell color and thickness on hatchability in Chinese ring-necked pheasants. Poultry Science 64:235-237.) reported that olive, dark brown, grey-white, tan, and blue eggshell colour had no effect on fertility rate, Krystianiak et al. (2005Krystianiak, S.; Kożuszek, R.; Kontecka, H. and Nowaczewski, S. 2005. Quality and ultrastructure of eggshell and hatchability of eggs in relation to eggshell colour in pheasants. Animal Science Papers and Reports 23:5-14.) and Kożuszek et al. (2009aKożuszek, R.; Kontecka, H.; Nowaczewski, S. and Rosiński, A. 2009a. Storage time and eggshell colour of pheasant eggs vs. the number of blastodermal cells and hatchability results. Folia Biologica 57:121-130.) reported that fertility rate varied with dark brown, light brown, olive, and blue eggshell colour.
In the present study, the effect of eggshell colour on hatchability and the hatchability of fertile eggs having been determined to be statistically insignificant was in agreement with the report of Fayeye et al. (2013Fayeye, T. R.; Ojo, V.; Alli, O. I. and Adebayo, B. K. 2013. Egg pigment pattern and association between hen body weight, oviposition interval, egg-weight and hatchability in Japanese quail. Niseb Journal 13:1-4.) suggesting that pigment density has no effect on the hatchability of fertile eggs in quails. On the other hand, the results obtained for hatchability and the hatchability of fertile eggs in the present study diverged from those reported by Farghly et al. (2015Farghly, M. F. A.; Mahrose, Kh. M. A. and Abou-Kassem, D. E. 2015. Pre and post hatch performance of different japanese quail egg colors incubated under photostimulation. Asian Journal of Poultry Science 9:19-30.) and Hassan et al. (2013Hassan, H. A.; El-Nesr, S. S.; Osman, A. M. R. and Arram, G. A. 2013. Ultrastructure of eggshell, egg weight loss and hatching traits of Japanese quail varding in eggshell color and pattern using image analysis. Egyptian Poultry Science 34:1-17.) suggesting eggshell colour to have an effect on both hatchability and hatchability of fertile eggs. In research conducted by Hulet et al. (1985Hulet, R. M.; Flegal, C. J.; Carpenter, G. H. and Champion, L. R. 1985. Effect of eggshell color and thickness on hatchability in Chinese ring-necked pheasants. Poultry Science 64:235-237.) and Richards and Deeming (2001Richards, P. D. G. and Deeming, D. C. 2001. Correlation between Shell colour and ultrastructure in pheasant eggs. British Poultry Science 42:338-343.) on pheasant eggs, it was ascertained that blue and tan coloured eggs had a thinner eggshell and presented a lower hatchability, in comparison with green, dark brown, and grey/white eggs. Furthermore, Kożuszek et al. (2009aKożuszek, R.; Kontecka, H.; Nowaczewski, S. and Rosiński, A. 2009a. Storage time and eggshell colour of pheasant eggs vs. the number of blastodermal cells and hatchability results. Folia Biologica 57:121-130.) reported that the hatchability of fertile eggs was lower in blue eggs compared with dark brown eggs.
In the present study, it was determined that the effect of eggshell colour on embryonic mortality was statistically insignificant, which was in agreement with the report of Farghly et al. (2015Farghly, M. F. A.; Mahrose, Kh. M. A. and Abou-Kassem, D. E. 2015. Pre and post hatch performance of different japanese quail egg colors incubated under photostimulation. Asian Journal of Poultry Science 9:19-30.) suggesting that in white, spotted violet, and spotted brown quail eggs, eggshell colour had no effect on embryonic mortality. On the contrary, Taha (2011Taha, A. E. 2011. Analyzing of quail eggs hatchability, quality, embryonic mortality and malpositions in relation to their shell colors. Online Journal of Animal and Feed Research 1:267-273.) and Hassan et al. (2013Hassan, H. A.; El-Nesr, S. S.; Osman, A. M. R. and Arram, G. A. 2013. Ultrastructure of eggshell, egg weight loss and hatching traits of Japanese quail varding in eggshell color and pattern using image analysis. Egyptian Poultry Science 34:1-17.) reported that eggshell colour has an impact on embryonic mortality rates. In their research on pheasant eggs, Hulet et al. (1985Hulet, R. M.; Flegal, C. J.; Carpenter, G. H. and Champion, L. R. 1985. Effect of eggshell color and thickness on hatchability in Chinese ring-necked pheasants. Poultry Science 64:235-237.) and Ristić et al. (2013Ristić, Z. A.; Marković, V.; Kovaćević, M.; Nad, I.; Matejević, M. and Jovanović, T. 2013. The significance of egg shell color on the pheasant hatching production results. Pakistan Journal of Zoology 45:1549-1553.) determined that eggshell colour had no effect on embryonic mortality.
The mean eggshell temperatures measured at the equatorial region of the eggs on days 10 and 14 of embryonic development, in all of the eggs incubated in the present study, were 36.89 and 37.57 ºC, respectively, and were found to be approximately 0.7 and 0.3 ºC lower than the temperature of the incubator, respectively (Table 4). These results are in support of the results reported by French (1997French, N. A. 1997. Modeling incubation temperature: the effects of incubator design, embryonic development, and egg size. Poultry Science 76:124-133.) suggesting that during early embryonic development, embryo temperature is lower than the temperature of the incubator due to evaporative cooling. Nowaczewski et al. (2012Nowaczewski, S.; Kontecka, H. and Rosiński, A. 2012. Effect of Japanese quail eggs location in the setter on their weight loss and eggshell temperature during incubation as well as hatchability results. Archiv Für Geflügelkunde76:168-175.) determined that, in quail eggs, while eggshell temperature increased between days 2-12 of incubation, it decreased on the 14th day of incubation. The results of the present study, which demonstrated that the eggshell temperatures measured until days 10 and 14 of incubation were lower than the fixed incubator temperature, are in agreement with the results reported by Nowaczewski et al. (2012)Nowaczewski, S.; Kontecka, H. and Rosiński, A. 2012. Effect of Japanese quail eggs location in the setter on their weight loss and eggshell temperature during incubation as well as hatchability results. Archiv Für Geflügelkunde76:168-175. for eggshell temperature change on day 14 of incubation. In a study carried out in chicken eggs, Romijn and Lokhorst (1956Romijn, C. and Lokhorst, W. 1956. The caloric equilibrium of the chicken embryo. Poultry Science 35:829-834.) determined that eggshell temperature was 0.2-0.1 ºC lower than the temperature of the setter (38.0 ºC) during the first half of the incubation period, but was higher than the setter temperature during the second half of the incubation period.
Chick hatching weight, which is described as a certain percentage of the weight of an egg that has undergone incubation, has been extensively studied in several studies. While the chick weight/egg weight ratio determined in the present study (69.37%) (Table 8) fell within the range (62-78%) reported by Wilson (1991Wilson, H. R. 1991. Interrelationships of egg size, chick size, posthatching growth and hatchability. World's Poultry Science Journal 47:5-20.) for chickens, it was higher than the ratios reported for turkeys (63.5%), ducks (57.8%), geese (58.9%), and pheasants (61.9%) (Shanaway, 1987Shanawany, M. M. 1987. Hatching weight in relation to egg weight in domestic birds. World's Poultry Science Journal 43:107-115.). Furthermore, the results obtained for chick weight/egg weight ratio in the present study were in agreement with those reported by Uddin (1994Uddin, M. S.; Paul, D. C. and Huque, Q. M. E. 1994. Effect of egg weight and pre-incubation holding periods on hatchability of Japanese quail eggs in different seasons. Asian-Australasian Journal of Animal Sciences 7:499-503.) for quail eggs suggesting the chick weight percentage of small (8.59 g), medium (9.52 g), and large (10.56 g) eggs to be 68.77, 70.28, and 69.64%, respectively. The results obtained in the present study were also in agreement with those reported by Moraes et al. (2008Moraes, T. G. V.; Romao, J. M.; Teixeira, R. S. C. and Cardoso, W. M. 2008. Effects of egg position in artificial incubation of Japanese quail eggs (Coturnix japonica). Animal Reproduction 5:50-54.) (69.90%) and Genchev (2009Genchev, A. 2009. Influence of hatching eggs storage period upon the incubation parameters in Japanese quails. Journal Central European Agriculture 10:167-174.) (70.84%). These results were also similar to those reported by Sadeghi et al. (2013Sadeghi, R.; Pakdel, A. and Shahrbabak, M. M. 2013. Effects of divergent selection and egg status in artificial incubator on reproductive trait in Japanese quail. World Applied Sciences Journal 24:463-466.) indicating the chick weight percentage of eggs weighing 14.19, 12.02, and 10.20 g to be 76.4, 74.8, and 70.5%, respectively. The mean chick weight/egg weight ratio determined in the present study was higher than the ratios reported by Islam et al. (2014Islam, M. S.; Faruque, S.; Khatun, H. and Islam, M. N. 2014. Effects of quail genotypes on hatchability traits, body weight and egg production. Journal of Bangladesh Academy of Sciences 38:219-224.) as 62.68, 62.85, 58.23, and 46.35% for Japanese, white, brown, and black quails, respectively.
In the present study, in Groups I and II, which were of the same eggshell colour, chick weight/egg weight ratio was higher in Group I (70.55%) than Group II (68.45%). This difference was attributed to differences in eggshell weight. Eggshell weight was greater in Group II (0.97 g) in comparison with Group I (0.95 g). Similarly, in Groups III and V, which were of the same eggshell colour, the group with a higher eggshell weight presented a lower chick weight/egg weight ratio. Hassan et al. (2013Hassan, H. A.; El-Nesr, S. S.; Osman, A. M. R. and Arram, G. A. 2013. Ultrastructure of eggshell, egg weight loss and hatching traits of Japanese quail varding in eggshell color and pattern using image analysis. Egyptian Poultry Science 34:1-17.) determined that in light coloured (66.51%), dotted (68.73%), spotted (68.62%), and dark coloured (66.51%) quail eggs stored at room temperature, eggshell colour and egg shape had a significant effect on chick weight (%). Similarly, in the present study, it was determined that in the groups of the same eggshell colour (Groups I/II and Groups III/V), the chick weight/egg weight ratio was lower in the eggs with a larger spotted area, such that this ratio was 70.55, 68.45, 68.80, and 70.33% in Groups I, II, III and V, respectively.
In the present study, while egg shape index did not differ with spot colour in eggs of the same eggshell colour, it differed with the size of the spotted area in eggs of the same eggshell and spot colour. Egg weight loss between days 0-14 of incubation decreased with an increased spotted area in eggs of the same eggshell colour. Fertility, hatchability of fertile eggs, and hatchability and embryonic mortality rates did not vary with eggshell colour or the size of the spotted area. Eggshell temperatures measured on days 10 (36.89 ºC) and 14 (37.57 ºC) of embryonic development were lower than the fixed temperature of the incubator (37.6 ºC). Chick weight/egg weight ratios varied with eggshell weight in the groups with the same eggshell colour.
Conclusions
Fertility, hatchability of fertile eggs, and hatchability and embryonic mortality rates do not vary in relation to eggshell colour or the size of the spotted area.
Acknowledgments
This study was conducted between February and April, 2013. Present study was supported by the Scientific Research Project Unit of Mustafa Kemal University, Turkey (Project number: 1206 M0117).
References
- Ar, A.; Paganelli, C. V.; Reeves, R. B.; Greene, D. G. and Rahn, H. 1974. The avian egg: water vapor conductance, shell thickness, and functional pore area. The Condor 76:153-158.
- Aygun, A. and Sert, D. 2013. Effects of prestorage application of propolis and storage time on eggshell microbial activity, hatchability, and chick performance in Japanese quail (Coturnix coturnix japonica) eggs. Poultry Science 92:3330-3337.
- Baggott, G. K.; Deeming, D. C.; Hemon, S. and Paillat, P. 2002. Relationships between eggshell pigmentation, ultrastructure and water vapour conductance in the houbara bustard (Chlamydotis undulata macqueennii). Avian and poultry Biology Reviews 13:234-235.
- Cassey, P.; Thomas, G. H.; Portugal, S. J.; Maurer, G.; Hauber, M. E.; Grim, T.; Lovell, P. G. and Mikšík, I. 2012. Why are birds' eggs colourful? Eggshell pigments co-vary with life-history and nesting ecology among British breeding non-passerine birds. Biological Journal of the Linnean Society 106:657-672.
- Duval, C.; Cassey, P.; Mikšík, I.; Reynolds, S. J. and Spencer, K. A. 2013. Condition-dependent strategies of eggshell pigmentation: an experimental study of Japanese quail (Coturnix coturnix japonica). The Journal of Experimental Biology 216:700-708.
- Farghly, M. F. A.; Mahrose, Kh. M. A. and Abou-Kassem, D. E. 2015. Pre and post hatch performance of different japanese quail egg colors incubated under photostimulation. Asian Journal of Poultry Science 9:19-30.
- Fayeye, T. R.; Ojo, V.; Alli, O. I. and Adebayo, B. K. 2013. Egg pigment pattern and association between hen body weight, oviposition interval, egg-weight and hatchability in Japanese quail. Niseb Journal 13:1-4.
- French, N. A. 1997. Modeling incubation temperature: the effects of incubator design, embryonic development, and egg size. Poultry Science 76:124-133.
- Genchev, A. 2009. Influence of hatching eggs storage period upon the incubation parameters in Japanese quails. Journal Central European Agriculture 10:167-174.
- Gosler, A. G.; Connor, O. R. and Bonser, R. H. C. 2011. Protoporphyrin and eggshell strength: preliminary findings from a passerine bird. Avian Biology Research 4:214-223.
- Hassan, H. A.; El-Nesr, S. S.; Osman, A. M. R. and Arram, G. A. 2013. Ultrastructure of eggshell, egg weight loss and hatching traits of Japanese quail varding in eggshell color and pattern using image analysis. Egyptian Poultry Science 34:1-17.
- Higham, J. P. and Gosler, A. G. 2006. Speckled eggs: water-loss and incubation behaviour in the great tit Parus majör. Oecologia 149:561-570.
- Holveck, M. J.; Grégoire, A.; Staszewski, V.; Guerreiro, R.; Perret, P.; Boulinier, T. and Doutrelant, C. 2012. Eggshell spottiness reflects maternally transferred antibodies in blue tits. Plos One 7:1-12.
- Hulet, R. M.; Marquez, B. J.; Molle, S. and Flegal, C. J. 1978. Relationship of pheasant egg color and hatchability. Poultry Science 57:1146-1148.
- Hulet, R. M.; Flegal, C. J.; Carpenter, G. H. and Champion, L. R. 1985. Effect of eggshell color and thickness on hatchability in Chinese ring-necked pheasants. Poultry Science 64:235-237.
- Idahor, K. O.; Akinola, L. A. F. and Chia, S. S. 2015. Predetermination of quail chick sex using egg indices in North Central Nigeria. Journal of Animal Production Advances 5:599-605.
- Islam, M. S.; Faruque, S.; Khatun, H. and Islam, M. N. 2014. Effects of quail genotypes on hatchability traits, body weight and egg production. Journal of Bangladesh Academy of Sciences 38:219-224.
- Kirikci, K.; Gunlu, A. and Garip, M. 2005. Some quality characteristics of pheasant (Phasianus colchicus) eggs with different shell colors. Turkish Journal of Veterinary and Animal Sciences 29:315-318.
- Kożuszek, R.; Kontecka, H.; Nowaczewski, S. and Rosiński, A. 2009a. Storage time and eggshell colour of pheasant eggs vs. the number of blastodermal cells and hatchability results. Folia Biologica 57:121-130.
- Kożuszek, R.; Kontecka, H.; Nowaczewski, S.; Leśnierowski, G.; Kijowski, J. and Rosiński, A. 2009b. Quality of pheasant (Phasianus colchicus L.) eggs with different shell colour. Archiv Für Geflügelkunde 73:201-207.
- Krystianiak, S.; Kożuszek, R.; Kontecka, H. and Nowaczewski, S. 2005. Quality and ultrastructure of eggshell and hatchability of eggs in relation to eggshell colour in pheasants. Animal Science Papers and Reports 23:5-14.
- Kumar, A.; Das, K.; Mukherjee, K.; Bharti, A. and Singh, A. K. 2012. Frequency of different shell color and its effect on the fertility and hatchability in black rock, gramapriya and vanaraja breeds of chicken. Veterinary World 5:594-598.
- Lahti, D. C. 2008. Population differentiation and rapid evolution of egg color in accordance with solar radiation. The Auk 125:796-802.
- Liu, H. S. and Cheng, W. T. 2010. Eggshell pigmentatoin: a review. Journal of the Chinese Society of Animal Science 39:75-89.
- Mikšík, I.; Holáň, V. and Deyl, Z. 1994. Quantification and variability of eggshell pigment content. Comparative Biochemistry Physiology 109A:769-772.
- Mikšík, I.; Holáň, V. and Deyl, Z. 1996. Avian eggshell pigments and their variability. Comparative Biochemistry Physiology 113B:607-612.
- Moraes, T. G. V.; Romao, J. M.; Teixeira, R. S. C. and Cardoso, W. M. 2008. Effects of egg position in artificial incubation of Japanese quail eggs (Coturnix japonica). Animal Reproduction 5:50-54.
- Morales, J.; Velando, A. and Torres, R. 2011. Biliverdin-based egg coloration is enhanced by carotenoid supplementation. Behavioral Ecology Sociobiology 65:197-203.
- Moreno, J. and Osorno, J. L. 2003. Avian egg colour and sexual selection: does eggshell pigmentation reflect female condition and genetic quality? Ecology Letters 6:803-806.
- Nowaczewski, S.; Witkiewicz, K.; Kontecka, H.; Krystianiak, S. and Rosiński, A. 2010. Eggs weight of Japanese quail vs. eggs quality after storage time and hatchability results. Archiv Tierzucht 53:720-730.
- Nowaczewski, S.; Stuper, K.; Szablewski, T. and Kontecka, H. 2011. Microscopic fungi in eggs of ring-necked pheasants kept in aviaries. Poultry Science 90:2467-2470.
- Nowaczewski, S.; Kontecka, H. and Rosiński, A. 2012. Effect of Japanese quail eggs location in the setter on their weight loss and eggshell temperature during incubation as well as hatchability results. Archiv Für Geflügelkunde76:168-175.
- Nowaczewski, S.; Szablewski, T.; Cegielska-Radziejewska, R. and Kontecka, H. 2013. Egg morphometry and eggshell quality in ring-necked pheasants kept in cages. Annals of Animal Science 13:531-541.
- Rahn, H. and Ar, A. 1974. The avian egg: incubation time and water loss. The Condor 76:147-152.
- Rahn, H. and Paganelli, C. V. 1989. Shell mass, thickness and density of avian eggs derived from the tables of Schönwetter. Journal of Ornithology 130:59-68.
- Richards, P. D. G. and Deeming, D. C. 2001. Correlation between Shell colour and ultrastructure in pheasant eggs. British Poultry Science 42:338-343.
- Riehl, C. 2011. Paternal investment and the 'sexually selected hypothesis' fort he evolution of eggshell coloration: revisiting the assumptions. The Auk 128:175-179.
- Ristić, Z. A.; Marković, V.; Kovaćević, M.; Nad, I.; Matejević, M. and Jovanović, T. 2013. The significance of egg shell color on the pheasant hatching production results. Pakistan Journal of Zoology 45:1549-1553.
- Romao, J. M.; Moraes, T. G. V.; Teixeira, R. S. C.; Cardoso, W. M. and Buxade, C. C. 2008. Effect of egg storage length on hatchability and weight loss in incubation of egg and meat type Japanese quails. Brazilian Journal of Poultry Science 10:143-147.
- Romijn, C. and Lokhorst, W. 1956. The caloric equilibrium of the chicken embryo. Poultry Science 35:829-834.
- Sadeghi, R.; Pakdel, A. and Shahrbabak, M. M. 2013. Effects of divergent selection and egg status in artificial incubator on reproductive trait in Japanese quail. World Applied Sciences Journal 24:463-466.
- Shafey, T. M.; Ghannam, M. M.; Al-Batshan, H. A. and Al-Ayed, M. S. 2004. Effect of pigment intensity and region of eggshell on the spectral transmission of light that passes the eggshell of chickens. International Journal of Poultry Science 3:228-233.
- Shafey, T. M.; Al-Batshan, H. A.; Ghannam, M. M. and Al-Ayed, M. S. 2005. Effect of intensity of eggshell pigment and illuminated incubation on hatchability of brown eggs. British Poultry Science 46:190-198.
- Shanawany, M. M. 1987. Hatching weight in relation to egg weight in domestic birds. World's Poultry Science Journal 43:107-115.
- Siefferman, L.; Navara, K. J. and Hill, G. E. 2006. Egg coloration is correlated with female condition in eastern blue birds (Sialia sialis). Behavioral Ecology Sociobiology 59:651-656.
- Soler, J. J.; Moreno, J.; Avilés, J. M. and Møller, A. P. 2005. Blue and green egg-color intensity is associated with parental effort and mating system in passerines: support for the sexual selection hypothesis. Evolution 59:636-644.
- Stoddard, M. C.; Fayet, A. L.; Kilner, R. M.; Hinde, C. A. 2012. Egg speckling patterns do not advertise offspring quality or influence male provisioning in Great Tits. Plos One 7:1-12.
- Taha, A. E. 2011. Analyzing of quail eggs hatchability, quality, embryonic mortality and malpositions in relation to their shell colors. Online Journal of Animal and Feed Research 1:267-273.
- Uddin, M. S.; Paul, D. C. and Huque, Q. M. E. 1994. Effect of egg weight and pre-incubation holding periods on hatchability of Japanese quail eggs in different seasons. Asian-Australasian Journal of Animal Sciences 7:499-503.
- Van Der Pol, C. W.; Van Roovert-Reijrink, I. A. M.; Maatjens, C. M.; Van Der Brand, H. and Molenaar, R. 2013. Effect of relative humidity during incubation at a set eggshell temperature and brooding temperature posthatch on embryonic mortality and chick quality. Poultry Science 92:2145-2155.
- Wilson, H. R. 1991. Interrelationships of egg size, chick size, posthatching growth and hatchability. World's Poultry Science Journal 47:5-20.
- Yang, H. M.; Wang, Z. Y. and Lu, J. 2009. Study on the relationship between eggshell colors and egg quality as well as shell ultrastructure in yangzhou chicken. African Journal of Biotechnology 8:2898-2902.
- Zhao, R.; Xu, G. Y.; Liu, Z. Z.; Li, J. Y. and Yang, N. 2006. A study on eggshell pigmentation: biliverdin in blue-shelled chickens. Poultry Science 85:546-549.
Publication Dates
-
Publication in this collection
May 2016
History
-
Received
13 Jan 2016 -
Accepted
16 Feb 2016