ABSTRACT
The objective of this study was to evaluate the intake and ruminal parameters of goats fed diets supplemented with vegetable oils. Four rumen-cannulated Saanen goats were allocated to four treatments, which consisted of a control diet and diets with the inclusion of 30 g of canola, sunflower, or soybean oils per kilogram of diet dry matter (DM). The experiment lasted 40 days, which were divided into four 10-day periods. Forage intake was estimated using chromium oxide as an external marker, and supplement intake was determined as the difference between the daily amount supplied and orts. Rumen fermentation parameters were evaluated from samples of rumen fluid collected every 2 h, for 12 h. Rumen pH and short-chain fatty acid and ammonia nitrogen concentrations were measured. There was no effect of lipid supplementation on DM intake. Ether extract intake was highest in the treatments with oil inclusion, and the highest acid detergent fiber intake was obtained with the diet containing canola oil. The pH was highest in the group fed soybean oil and responded quadratically to the collection times. Total short-chain fatty acid and acetic acid concentrations also responded quadratically to the collection times. Propionic and butyric acid concentrations and acetic:propionic acid ratio showed a cubic behavior with the increasing collection times. Canola, sunflower, or soybean oils can be included at 30 g/kg of the diet DM as a strategy to increase the energy density of goat diets.
Capra hircus; fatty acids; fermentation; pH; rumen ammonia nitrogen
1. Introduction
Lipid supplementation in the form of vegetable oils in the diet of ruminants has been adopted as a strategy to improve the fatty acid profile of the fat in the food product (meat and milk). However, changes in the characteristics of ruminant diets, which are naturally forage-based, may induce alterations in rumen metabolism, digestive processes (Maia et al., 2006Maia, F. J.; Branco, A. F.; Mouro, G. F.; Coneglian, S. M.; Santos, G. T.; Minella, T. F. and Macedo, F. A. F. 2006. Inclusão de fontes de óleo na dieta de cabras em lactação: digestibilidade dos nutrientes e parâmetros ruminais e sanguíneos. Revista Brasileira de Zootecnia 35:1496-1503. https://doi.org/10.1590/S1516-35982006000500032
https://doi.org/10.1590/S1516-3598200600...
), and feed intake, possibly causing damage to the animal. Therefore, studies are warranted to prove the efficiency of the technique.
According to Nagaraja et al. (1997)Nagaraja, T. G.; Newbold, C. J.; van Nevel, C. J. and Demeyer, D. I. 1997. Manipulation of ruminal fermentation. p.523-632. In: The rumen microbial ecosystem. 2nd ed. Hobson, P. N. and Stewart, C. S., eds. Blackie Academic & Professional, London., the following effects of lipid inclusion are expected on rumen fermentation: reduced fermentation of fibrous carbohydrates; increased microbial efficiency, with consequent greater intestinal flow of microbial protein and decreased rumen ammonia concentration resulting from proteolysis and/or recycling of bacteria, both due to the decrease in ciliated protozoa; increased propionate production; and, oftentimes, reduced methanogenesis.
Vegetable oils contain a larger proportion of unsaturated than saturated fatty acids and have a higher apparent digestibility than animal fat sources (Costa et al., 2009Costa, R. G.; Queiroga, R. C. R. E. and Pereira, R. A. G. 2009. Influência do alimento na produção e qualidade do leite de cabra. Revista Brasileira de Zootecnia 38(supl. especial):307-321. https://doi.org/10.1590/S1516-35982009001300031
https://doi.org/10.1590/S1516-3598200900...
). When included in the ruminant diet, unsaturated lipids are modified in the rumen environment by the biohydrogenation process, which consists of the addition of hydrogen to the double bonds of unsaturated fatty acids, increasing the degree of saturation. According to Palmquist and Mattos (2011)Palmquist, D. L. and Mattos, W. R. S. 2011. Metabolismo de lipídios. p.299-321. In: Nutrição de ruminantes. Berchielli, T. T.; Pires, A. V.; Oliveira, S. G., eds. Funep, Jaboticabal., this is because some fatty acids are toxic to ruminal microorganisms, e.g., medium-chain fatty acids (4-10 carbons) and long-chain polyunsaturated fatty acids, suggesting that not only the chain size, but also the degree of unsaturation can alter rumen fermentation, but also can bring positives changes in the animal products.
Results of research in this area not only vary largely but are also conflicting. Jenkins (1993)Jenkins, T. C. 1993. Lipid metabolism in the rumen. Journal of Dairy Science 76:3851-3863. https://doi.org/10.3168/jds.S0022-0302(93)77727-9
https://doi.org/10.3168/jds.S0022-0302(9...
stated that rumen fermentation parameters can be modified by lipid supplementation, while other studies report no changes (Beauchemin et al., 2007Beauchemin, K. A.; McGinn, S. M. and Petit, H. V. 2007. Methane abatement strategies for cattle: lipid supplementation of diets. Canadian Journal of Animal Science 87:431-440. https://doi.org/10.4141/CJAS07011
https://doi.org/10.4141/CJAS07011...
). It is also known that the extent of lipid interference with ruminal parameters depends on the source and level of addition of the lipid source to the diet (Homem Junior et al., 2010).
Most studies involving lipid supplementation for ruminants use cattle as the animal model. As a consequence, little information is available on the effects of lipids on rumen fermentation characteristics in goats, a species with differentiated feeding behavior and metabolism compared with other ruminant species (Van Soest, 1994Van Soest, P. J. 1994. Nutritional ecology of the ruminant. 2nd ed. Cornell University Press, Ithaca.; Chilliard et al., 2003Chilliard, Y.; Ferlay, A.; Rouel, J. and Lambert, G. 2003. A review of nutritional and physiological factors affecting goat milk lipid synthesis and lipolysis. Journal of Dairy Science 86:1751-1770. https://doi.org/10.3168/jds.S0022-0302(03)73761-8
https://doi.org/10.3168/jds.S0022-0302(0...
). For these reasons, goats may have different responses to this feeding strategy.
Based on these considerations, the present study was conducted to evaluate the ruminal parameters of pH, short-chain fatty acids (SCFA), and ammonia nitrogen in goats fed diets supplemented with canola, sunflower, or soybean oils, and according to what was observed in this study, if the inclusion of oil in the goat diet does not alter ruminal health, this may mean that the inclusion of oil can be studied as an option to improve the quality of products of this species.
2. Material and Methods
The experiment was conducted in the experimental station, in São Paulo State, Brazil (22°53'08" S and 48°26'42" W, at 837 m asl), after approval by the local ethics committee (approval no. 29/2012 - CEUA).
We used four non-pregnant, non-lactating, rumen-cannulated Saanen goats with an initial average weight of 57 kg in the experiment. The animals were kept from 07.00 to 18.00 h in a 0.6-ha area established with Panicum maximum cv. Tobiatã, under rotational grazing with a fixed stocking rate. The field was divided into 11 paddocks of approximately 500 m2, which were equipped with automatic drinkers and a free-access rest area with artificial shade provided by a shade cloth. Each paddock had an occupation period of three days, with 30 days of rest. After the grazing period, the animals were moved to individual 3.5-m2 stalls with slatted floors, equipped with automatic drinkers and salt and feed troughs, where they received 0.8 kg of supplement/animal/day according to the treatment. In the stalls, water and mineral mixture were available ad libitum.
The experiment lasted 40 days, divided into four 10-day periods. The first nine days were used as a period of acclimation to the diets and the tenth day was used for data and sample collection to determine rumen parameters (SCFA, pH, and ammoniacal nitrogen).
Four treatments were tested, as follows: control diet or diets including 30 g/kg (diet dry matter [DM]) of canola (Brassica napus L.), sunflower (Helianthus annuus L.), or soybean (Glycyne max L.) oil.
The diets (Table 1) were formulated according to NRC (2007)NRC - National Research Council. 2007. Nutrient requirements of small ruminants: sheep, goat, cervids and new world camelids. The National Academies Press, Washington, DC. recommendations to meet the nutritional requirements of the goats. The Small Ruminant Nutrition System (SRNS) computer program based on the structure of the Cornell Net Carbohydrate and Protein System (Cannas et al., 2004Cannas, A.; Tedeschi, L. O.; Fox, D. G.; Pell, A. N. and Van Soest, P. J. 2004. A mechanistic model for predicting the nutrient requirements and feed biological value for sheep. Journal of Animal Science 82:149-169. https://doi.org/10.2527/2004.821149x
https://doi.org/10.2527/2004.821149x...
) for sheep was used to determine the nutritional composition of the diets, which is based on rumen simulation.
The chemical compositions of feed ingredients, supplement, and forage were determined (Table 1). For the analysis of the supplement, samples of approximately 200 g from each supply were obtained after mixing with the vegetable oil for each treatment. For the forage samples, the simulated grazing method was applied, which consists of manual harvesting so that the material is as similar as possible to that consumed by the animals (De Vries, 1995De Vries, M. F. W. 1995. Estimating forage intake and quality in grazing cattle: a reconsideration of the hand-plucking method. Journal Range Management 48:370-375.). For this step, the goats were accompanied upon entering the paddock and their grazing habit was observed so that representative samples of the consumed forage would be collected during the three days of stay in the paddock.
After thawing, the samples were dried for 72 h at 55 °C in a forced-air oven and then processed in a knife mill with a 1-mm mesh sieve and packed for later analysis. The DM, mineral matter (MM), crude protein (CP), ether extract (EE), cellulose, and lignin contents of the samples were determined according to AOAC International (Cunniff, 1995Cunniff, P. 1995. Official methods of analysis of AOAC International. 16th ed. v.1. AOAC International, Arlington.), whereas the neutral (NDF) and acid detergent fiber (ADF) contents were measured following the methodology proposed by Van Soest et al. (1991)Van Soest, P. J.; Robertson, J. B. and Lewis, B. A. 1991. Methods for dietary fiber, neutral detergent, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science 74:3583-3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
https://doi.org/10.3168/jds.S0022-0302(9...
. The total digestible nutrients (TDN) content was determined according to Weiss (1999)Weiss, W. P. 1999. Energy prediction equations for ruminant feeds. p.76-185. In: Proceedings of the 61st Cornell Nutrition Conference for Feed Manufactures. Cornell University, Ithaca., as shown below (equation 1):
The concentration of the main fatty acids in the oils, forage, and supplements was determined by extraction, following the methodology of Rodrígues-Ruiz et al. (1998)Rodrígues-Ruiz, J.; Belarbi, E. H.; Sánches, J. L. G. and Alonso, D. L. 1998. Rapid simultaneous lipid extraction and transesterification for fatty acid analyses. Biotechnology Techniques 12:689-691. https://doi.org/10.1023/A:1008812904017
https://doi.org/10.1023/A:1008812904017...
(Table 2). Supplement intake was calculated as the difference between the amount supplied and orts, which were weighed daily. Forage intake was determined by using chromium oxide (Cr2O3) associated with the internal marker iNDF (indigestible neutral detergent fiber).
To estimate fecal output, chromium oxide (Cr2O3) capsules were administered orally in the amount of 2.5 g once daily (at 06.00 h), for 10 days, to 20 goats (five animals per treatment) (Detmann et al., 2001Detmann, E.; Paulino, M. F.; Zervoudakis, J. T.; Valadares Filho, S. C.; Euclydes, R. F.; Lana, R. P. and Queiroz, D. S. 2001. Cromo e indicadores internos na determinação do consumo de novilhos mestiços, suplementados, a pasto. Revista Brasileira de Zootecnia 30:1600-1609. https://doi.org/10.1590/S1516-35982001000600030
https://doi.org/10.1590/S1516-3598200100...
). The first five days were used to stabilize the concentration of Cr2O3 in the feces and the last five days for fecal collection, which was performed at 06.00 and 18.00 h. A composite sample of feces was made for each animal. These were dried for 72 h in a forced-air oven at 55 °C and ground through a knife mill with a 1-mm mesh sieve. The concentration of the marker in the feces was determined by colorimetry after nitric-perchloric acid digestion of the samples, in accordance with the methodology adapted from Bremer Neto et al. (2005).
Fecal DM output (FDMO) was estimated as the ratio between the supplied amount of the marker (AM supplied) and its concentration in the feces (AM feces) (equation 2):
Indigestible NDF (iNDF) was used as an internal marker to estimate voluntary DM intake from the forage, which was calculated by the equation proposed by Detmann et al. (2001)Detmann, E.; Paulino, M. F.; Zervoudakis, J. T.; Valadares Filho, S. C.; Euclydes, R. F.; Lana, R. P. and Queiroz, D. S. 2001. Cromo e indicadores internos na determinação do consumo de novilhos mestiços, suplementados, a pasto. Revista Brasileira de Zootecnia 30:1600-1609. https://doi.org/10.1590/S1516-35982001000600030
https://doi.org/10.1590/S1516-3598200100...
, as shown below (equation 3):
in which DMI (kg/day) = dry matter intake; FDMO = fecal dry matter output (kg/day); iNDFFe = iNDF concentration in the feces (kg/kg); iNDFIS = iNDF intake from supplement (kg/day); iNDFFo = iNDF concentration in the forage (kg/kg); and SDMI = supplement dry matter intake (kg/day).
Rumen fluid was collected on the 10th day of each period, at 2-h intervals, for 12 h. Collections started after the goats returned from the paddocks, at 18.00, and ended at 06.00 h, totaling seven collections per animal. The rumen content obtained from various parts of the rumen was filtered through gauze to separate the liquid from the solid part, which was returned to the rumen.
To determine the pH, rumen fluid obtained from each collection was placed in a 100-mL beaker and the pH was measured using a benchtop digital pH meter calibrated in buffer solutions of pH 4.0 and 7.0. For SCFA, rumen fluid aliquots were placed in 15-mL tubes and centrifuged at 3000 rpm for 15 min. Two milliliters of the supernatant were transferred to 5-mL test tubes containing 0.4 mL formic acid A.R. For N-NH3, the same procedure was adopted, except for the use of 1 mL of 1N sulfuric acid for preservation. Samples were kept frozen until laboratory analysis.
Acetic, propionic, and butyric acids were analyzed by gas chromatography, following the method described by Erwin et al. (1961)Erwin, E. S.; Marco, G. J. and Emery, E. M. 1961. Volatile fatty acids analyses of blood and rumen fluid by gas chromatography. Journal of Dairy Science 44:1768-1771. https://doi.org/10.3168/jds.S0022-0302(61)89956-6
https://doi.org/10.3168/jds.S0022-0302(6...
. For this evaluation, a gas chromatograph (Focus GC, Thermo Scientific®) was used with an automatic sample injector (AS-3000, Thermo Electron Corporation®) equipped with a 2-m-long glass column, 1/5” inner diameter, packed with a stationary phase (80/120, Carbopack®; B-DA/4%, Carbowax®; 20M, Supelco®) and a flame ionization detector kept at 270 °C. The gas chromatograph oven was kept at 190 °C during analysis, with an injector temperature of 220 °C. High-purity H2 was used as carrier gas, at a flow rate of 30 mL/min.
The N-NH3 content was determined by colorimetry, according to the method described by Kulasek (1972)Kulasek, G. A. 1972. A micromethod for determination of urea in plasma, whole blood cells using urease and phenol reagent. Polskie Archiwum Weterynaryjne 15:801-810. and adapted by Foldager (1977)Foldager, J. 1977. Protein requirement and non-protein nitrogen for high producing cow in early lactation. These (D.Sc.). Michigan State University, East Lasing, MI..
Body weight dynamics was monitored by weighing the animals at the start and end of each period.
The experiment was laid out in a balanced 4 × 4 Latin square design. Data were processed by analysis of variance with repeated measures over time and treatment means were compared using Tukey’s test. The effect of collection time was studied through polynomial regression by applying the “Sequential Regression” procedure, which evaluates the effect of each independent variable added to the analysis model. The chosen model was that which showed significance in regression analysis of variance (F test) and model coefficients (t test) and whose independent variable was responsible for most of the explanation (isolated effect) of the full model. For the analyses and tests, the significance level of 5% was adopted. Data were processed using SAEG statistical software (UFV, 2000UFV - Universidade Federal de Viçosa. 2000. Sistema de Análises Estatística e Genéticas – SAEG. Versão 9.0. Viçosa, MG.).
3. Results
Dry matter intake did not differ between the treatment groups (Table 3). This was also true for the intake of nutrients, except EE, inherent to the treatments, and ADF, whose lowest intake was observed in the control group, possibly due to the lower content of this nutrient in the supplement (Table 1). The diets including sunflower and soybean oils did not differ from the others, whereas the treatment including canola oil provided the highest average ADF intake. This result was likely due to the selection of particles in the feed, since the supplements used in treatments with oil inclusion were formulated to provide the same nutritional levels, only with different oil sources.
The evaluated treatments also did not influence average daily weight gain. Ammoniacal nitrogen (N-NH3) concentration was not influenced by lipid supplementation or by sampling time (Table 4). This variable averaged 35.82 mg/dL, which is higher than the 31.2 mg/dL obtained by Silva et al. (2007)Silva, M. M. C.; Rodrigues, M. T.; Rodrigues, C. A. F.; Branco, R. H.; Leão, M. I.; Magalhães, A. C. M. and Matos, R. S. 2007. Efeito da suplementação de lipídios sobre a digestibilidade e os parâmetros da fermentação ruminal em cabras leiteiras. Revista Brasileira de Zootecnia 36:246-256. https://doi.org/10.1590/S1516-35982007000100029
https://doi.org/10.1590/S1516-3598200700...
and the 22.9 mg/dL observed by Maia et al. (2006)Maia, F. J.; Branco, A. F.; Mouro, G. F.; Coneglian, S. M.; Santos, G. T.; Minella, T. F. and Macedo, F. A. F. 2006. Inclusão de fontes de óleo na dieta de cabras em lactação: digestibilidade dos nutrientes e parâmetros ruminais e sanguíneos. Revista Brasileira de Zootecnia 35:1496-1503. https://doi.org/10.1590/S1516-35982006000500032
https://doi.org/10.1590/S1516-3598200600...
, in an evaluation of lipid supplementation for dairy goats.
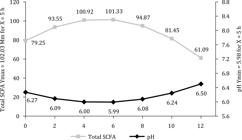
In terms of rumen pH, the lowest mean was obtained with control treatment, whereas supplementation with soybean oil provided the highest values. The observed variations in pH and total SCFA during the collection period suggest a strong relationship with feed intake. Both variables showed a quadratic response to collection time, but with an inverse behavior on the curve, with a minimum pH of 5.98 and maximum total SCFA content of 102.03 Mm at around 5 h after the supplement was supplied (Figure 1). This pH decline and increased total SCFA concentration characterize the peak of rumen fermentation due to the use of readily fermentable feedstuffs, which lead to a decrease in rumination and a consequent reduction in the production of saliva, which has a buffering action.
Total SCFA, acetic, propionic, and butyric acids, and acetic:propionic acid ratio were not influenced by lipid supplementation. However, there was a sampling time effect for all variables due to the fermentation of the ingested feed particles.
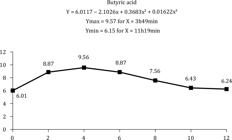
Acetic acid (Figure 2) responded quadratically to the collection times, with the highest concentration (73.10 mM) reached 5 h after the supplement was given. Collection time had a cubic effect on butyric and propionic acid contents (Figures 3 and 4), whose maximum concentrations of 9.75 and 21.43 mM, respectively, were attained approximately 4 h, and minimum concentrations of 6.15 and 12.79 mM, respectively, approximately 11 h after feeding.
Acetic:propionic acid ratio (Figure 5) showed a cubic response to sampling time, with very close minimum (3.73 mM) and maximum (4.01 mM) values at the 4.5 and 10 h, respectively.
4. Discussion
Changes in DM intake may be a consequence of the lipid source used, whether mono- or polyunsaturated (Benson et al., 2001Benson, J. A.; Reynolds, C. K.; Humphries, D. J.; Rutter, S. M. and Beever, D. E. 2001. Effects of abomasal infusion of long chain fatty acids on intake, feeding behaviour and milk production in dairy cows. Journal of Dairy Science 84:1182-1191. https://doi.org/10.3168/jds.S0022-0302(01)74579-1
https://doi.org/10.3168/jds.S0022-0302(0...
). Canola oil, one of the lipid sources used in this study, is mostly composed of oleic acid (Table 2), which, despite being a long-chain fatty acid, is monounsaturated and thus less toxic to rumen microorganisms. Sunflower and soybean oils are mostly composed of linoleic acid (Table 2), which is polyunsaturated, suggesting that the lack of effects on DM intake is related to the use of an insufficient level of EE to promote significant differences in this parameter, and not to the type of oil or level of unsaturation.
Average daily weight gain was possibly not influenced due to the similar DM intakes among the treatment groups and the very similar TDN levels of the diets (average of 803 g/kg for the supplement with oil inclusion vs. 773 g/kg for control supplement) (Table 1), which met the maintenance requirements of the animals homogeneously.
Ruminal ammonia originates from the degradation of amino acids and non-protein nitrogen in the diet and is indispensable for the development of rumen microflora (Russel et al., 1992Russel, J. B.; O´Connor, J. D.; Fox, D. G.; Van Soest, P. J. and Sniffen, C. J. 1992. A net carbohydrate and protein system for evaluating cattle diets: I Ruminal fermentation. Journal of Animal Science 70:3551-3561. https://doi.org/10.2527/1992.70113551x
https://doi.org/10.2527/1992.70113551x...
). For all treatment groups, the N-NH3 level was above 5 mg/dL, which is the minimum level necessary to maintain normal rumen functions and not limit microbial growth (Satter and Slyter, 1974Satter, L. D. and Slyter, L. L. 1974. Effect of ammonia concentration on rumen microbial protein production in vitro. British Journal of Nutrition 32:199-208. https://doi.org/10.1079/BJN19740073
https://doi.org/10.1079/BJN19740073...
).
The high N-NH3 value observed in this experiment may be related to the use of animals under maintenance, as the protein content of the diet was established to meet the requirements of lactating animals. Thus, according to Santos and Pedroso (2011)Santos, F. A. P. and Pedroso, A. M. 2011. Metabolismo de proteínas. p.265-292. In: Nutrição de ruminantes. Berchielli, T. T.; Pires, A. V.; Oliveira, S. G. D., eds. Funep, Jaboticabal., when the rate of ruminal degradation of protein exceeds the use of nitrogenous compounds for microbial synthesis, the excess ammonia produced is absorbed through the rumen wall and eliminated via urine or, according to Van Soest (1994)Van Soest, P. J. 1994. Nutritional ecology of the ruminant. 2nd ed. Cornell University Press, Ithaca., it can be recycled into the rumen via saliva, maintaining the values high.
Other researchers have reported a reduction in N-NH3 values following lipid supplementation (Lin et al., 1995Lin, H.; Boylston, D.; Chang, M. J.; Luedecke, L. O. and Shultz, T. D. 1995. Survey of the conjugated linoleic acid contents of dairy products. Journal of Dairy Science 78:2358-2365. https://doi.org/10.3168/jds.S0022-0302(95)76863-1
https://doi.org/10.3168/jds.S0022-0302(9...
; Silva et al., 2007Silva, M. M. C.; Rodrigues, M. T.; Rodrigues, C. A. F.; Branco, R. H.; Leão, M. I.; Magalhães, A. C. M. and Matos, R. S. 2007. Efeito da suplementação de lipídios sobre a digestibilidade e os parâmetros da fermentação ruminal em cabras leiteiras. Revista Brasileira de Zootecnia 36:246-256. https://doi.org/10.1590/S1516-35982007000100029
https://doi.org/10.1590/S1516-3598200700...
; Shingfield et al., 2008Shingfield, K. J.; Ahvenjarvi, S.; Toivonen, V.; Vanhatalo, A.; Huhtanen, P. and Griinari, J. M. 2008. Effect of incremental levels of sunflower-seed oil in the diet on ruminal lipid metabolism in lactating cows. British Journal of Nutrition 99:971-983. https://doi.org/10.1017/S0007114507853323
https://doi.org/10.1017/S000711450785332...
), which, according to Tesfa et al. (1992)Tesfa, A. T.; Touri, M. R. and Syrjala-Quist, L. 1992. The effects of increasing levels of rapeseed oil in the diets of lactating milking cows on milk yield, milk composition, blood and rumen metabolites. World Review Animal Production 27:34-40., is due to the decreased protozoal population in the rumen environment caused by the addition of unsaturated oils. However, the data for this parameter are still contradictory, since other studies show that lipid supplementation commonly results in no variation for the rumen ammonia concentration (Doreau and Ferlay, 1995Doreau, M. and Ferlay, A. 1995. Effect of dietary lipids on nitrogen metabolism in the rumen: A review. Livestock Production Science 43:97-110. https://doi.org/10.1016/0301-6226(95)00041-I
https://doi.org/10.1016/0301-6226(95)000...
; Szumacher-Strabel et al., 2004Szumacher-Strabel, M.; Martin, S. A.; Potkański, A.; Cieślak, A. and Kowalczyk, J. 2004. Changes in fermentation processes as the effect of vegetable oil supplementation in in vitro studies. Journal of Animal Feed Sciences 13(suppl. 1):215-218. https://doi.org/10.22358/jafs/73843/2004
https://doi.org/10.22358/jafs/73843/2004...
), as was observed in the present study.
Unsaturated fatty acids are known to change the rumen metabolism (Gómez-Cortés et al., 2008Gómez-Cortés, P.; Frutos, P.; Mantecón, A. R.; Juárez, M.; de la Fuente, M. A. and Hervás, G. 2008. Milk production, conjugated linoleic acid content, and in vitro ruminal fermentation in response to high levels of soybean oil in dairy ewe diet. Journal of Dairy Science 91:1560-1569. https://doi.org/10.3168/jds.2007-0722
https://doi.org/10.3168/jds.2007-0722...
) by their toxic effect on microorganisms or by coating the fibers, thereby reducing their fermentation (Palmquist and Mattos, 2011Palmquist, D. L. and Mattos, W. R. S. 2011. Metabolismo de lipídios. p.299-321. In: Nutrição de ruminantes. Berchielli, T. T.; Pires, A. V.; Oliveira, S. G., eds. Funep, Jaboticabal.), which may have an effect on pH. Although canola and sunflower oils are rich in unsaturated fatty acids, like soybean oil, no effect of these two lipid sources was observed on the rumen pH in the present study.
The increased pH in the treatment with soybean oil compared with control diet can be attributed to feed intake, which, though not significant, tended to be lower in the former treatment, leading to a slight decrease in rumen fermentation. Another possible explanation is the presence of corn in control diet (Table 1), which provides a greater supply of fermentable carbohydrates and promotes pH decline, since there was an upward trend for pH in the groups fed diets with canola and sunflower oils (which do not have corn in their composition), despite the similar mean pH values between these treatments and control diet.
According to Toral et al. (2009)Toral, P. G.; Belenguer, A.; Frutos, P. and Hervás, G. 2009. Effect of the supplementation of a high-concentrate diet with sunflower and fish oils on ruminal fermentation in sheep. Small Ruminant Research 81:119-125. https://doi.org/10.1016/j.smallrumres.2008.12.009
https://doi.org/10.1016/j.smallrumres.20...
, polyunsaturated fatty acids in the diet alter ruminal fermentation and induce a reduction in the total SCFA due to the inhibition of microbial activity. Nonetheless, studies such as those conducted by de Fievez et al. (2003)Fievez, V.; Dohme, F.; Danneels, M.; Raes, K. and Demeyer, D. 2003. Fish oils as potent rumen methane inhibitors and associated effects on rumen fermentation in vitro and in vivo. Animal Feed Science and Technology 104:41-58. https://doi.org/10.1016/S0377-8401(02)00330-9
https://doi.org/10.1016/S0377-8401(02)00...
and Shingfield et al. (2008)Shingfield, K. J.; Ahvenjarvi, S.; Toivonen, V.; Vanhatalo, A.; Huhtanen, P. and Griinari, J. M. 2008. Effect of incremental levels of sunflower-seed oil in the diet on ruminal lipid metabolism in lactating cows. British Journal of Nutrition 99:971-983. https://doi.org/10.1017/S0007114507853323
https://doi.org/10.1017/S000711450785332...
describe no effect for this parameter following lipid supplementation, which makes research results still contradictory.
A restricted number of studies have examined the effect of lipid supplementation on the rumen metabolism of goats, and the few existing ones, such as those developed by Maia et al. (2006)Maia, F. J.; Branco, A. F.; Mouro, G. F.; Coneglian, S. M.; Santos, G. T.; Minella, T. F. and Macedo, F. A. F. 2006. Inclusão de fontes de óleo na dieta de cabras em lactação: digestibilidade dos nutrientes e parâmetros ruminais e sanguíneos. Revista Brasileira de Zootecnia 35:1496-1503. https://doi.org/10.1590/S1516-35982006000500032
https://doi.org/10.1590/S1516-3598200600...
and Silva et al. (2007)Silva, M. M. C.; Rodrigues, M. T.; Rodrigues, C. A. F.; Branco, R. H.; Leão, M. I.; Magalhães, A. C. M. and Matos, R. S. 2007. Efeito da suplementação de lipídios sobre a digestibilidade e os parâmetros da fermentação ruminal em cabras leiteiras. Revista Brasileira de Zootecnia 36:246-256. https://doi.org/10.1590/S1516-35982007000100029
https://doi.org/10.1590/S1516-3598200700...
, did not evaluate the rumen SCFA concentrations.
Shingfield et al. (2008)Shingfield, K. J.; Ahvenjarvi, S.; Toivonen, V.; Vanhatalo, A.; Huhtanen, P. and Griinari, J. M. 2008. Effect of incremental levels of sunflower-seed oil in the diet on ruminal lipid metabolism in lactating cows. British Journal of Nutrition 99:971-983. https://doi.org/10.1017/S0007114507853323
https://doi.org/10.1017/S000711450785332...
examined the dietary inclusion of sunflower oil on the rumen metabolism of lactating cows and observed changes in rumen fermentation only at the highest inclusion level, 750 g/day (vs. 0, 250, and 500 g/day). These authors suggest that, in addition to the basal composition of the diet, the supplementary amount of lipid is also important for determining changes in rumen fermentation patterns. It is thus inferred that the vegetable oil level of 30 g/kg of diet DM used in the present study was not sufficient to induce changes in the N-NH3 or SCFA concentrations in goats.
5. Conclusions
Lipid supplementation in goat diets through the addition of canola, sunflower, or soybean oils at the level of 30 g/kg (diet dry matter) does not influence dry matter intake or the concentrations of ammoniacal nitrogen or short-chain fatty acids. Therefore, this strategy can be applied to increase the energy density of animal feed without compromising the rumen metabolism.
Acknowledgments
Thanks to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for the financial support to this project.
References
- Beauchemin, K. A.; McGinn, S. M. and Petit, H. V. 2007. Methane abatement strategies for cattle: lipid supplementation of diets. Canadian Journal of Animal Science 87:431-440. https://doi.org/10.4141/CJAS07011
» https://doi.org/10.4141/CJAS07011 - Benson, J. A.; Reynolds, C. K.; Humphries, D. J.; Rutter, S. M. and Beever, D. E. 2001. Effects of abomasal infusion of long chain fatty acids on intake, feeding behaviour and milk production in dairy cows. Journal of Dairy Science 84:1182-1191. https://doi.org/10.3168/jds.S0022-0302(01)74579-1
» https://doi.org/10.3168/jds.S0022-0302(01)74579-1 - Bremer Neto, H.; Graner, C. A. F.; Pezzato, L. E. and Padovani, C. R. 2005. Determinação da rotina do crômio em fezes, como marcador biológico, pelo método espectrofotométrico ajustado da 1,5-difenilcarbazida. Ciência Rural 35:691-697. https://doi.org/10.1590/S0103-84782005000300033
» https://doi.org/10.1590/S0103-84782005000300033 - Cannas, A.; Tedeschi, L. O.; Fox, D. G.; Pell, A. N. and Van Soest, P. J. 2004. A mechanistic model for predicting the nutrient requirements and feed biological value for sheep. Journal of Animal Science 82:149-169. https://doi.org/10.2527/2004.821149x
» https://doi.org/10.2527/2004.821149x - Chilliard, Y.; Ferlay, A.; Rouel, J. and Lambert, G. 2003. A review of nutritional and physiological factors affecting goat milk lipid synthesis and lipolysis. Journal of Dairy Science 86:1751-1770. https://doi.org/10.3168/jds.S0022-0302(03)73761-8
» https://doi.org/10.3168/jds.S0022-0302(03)73761-8 - Costa, R. G.; Queiroga, R. C. R. E. and Pereira, R. A. G. 2009. Influência do alimento na produção e qualidade do leite de cabra. Revista Brasileira de Zootecnia 38(supl. especial):307-321. https://doi.org/10.1590/S1516-35982009001300031
» https://doi.org/10.1590/S1516-35982009001300031 - Cunniff, P. 1995. Official methods of analysis of AOAC International. 16th ed. v.1. AOAC International, Arlington.
- Detmann, E.; Paulino, M. F.; Zervoudakis, J. T.; Valadares Filho, S. C.; Euclydes, R. F.; Lana, R. P. and Queiroz, D. S. 2001. Cromo e indicadores internos na determinação do consumo de novilhos mestiços, suplementados, a pasto. Revista Brasileira de Zootecnia 30:1600-1609. https://doi.org/10.1590/S1516-35982001000600030
» https://doi.org/10.1590/S1516-35982001000600030 - De Vries, M. F. W. 1995. Estimating forage intake and quality in grazing cattle: a reconsideration of the hand-plucking method. Journal Range Management 48:370-375.
- Doreau, M. and Ferlay, A. 1995. Effect of dietary lipids on nitrogen metabolism in the rumen: A review. Livestock Production Science 43:97-110. https://doi.org/10.1016/0301-6226(95)00041-I
» https://doi.org/10.1016/0301-6226(95)00041-I - Erwin, E. S.; Marco, G. J. and Emery, E. M. 1961. Volatile fatty acids analyses of blood and rumen fluid by gas chromatography. Journal of Dairy Science 44:1768-1771. https://doi.org/10.3168/jds.S0022-0302(61)89956-6
» https://doi.org/10.3168/jds.S0022-0302(61)89956-6 - Fievez, V.; Dohme, F.; Danneels, M.; Raes, K. and Demeyer, D. 2003. Fish oils as potent rumen methane inhibitors and associated effects on rumen fermentation in vitro and in vivo Animal Feed Science and Technology 104:41-58. https://doi.org/10.1016/S0377-8401(02)00330-9
» https://doi.org/10.1016/S0377-8401(02)00330-9 - Foldager, J. 1977. Protein requirement and non-protein nitrogen for high producing cow in early lactation. These (D.Sc.). Michigan State University, East Lasing, MI.
- Gómez-Cortés, P.; Frutos, P.; Mantecón, A. R.; Juárez, M.; de la Fuente, M. A. and Hervás, G. 2008. Milk production, conjugated linoleic acid content, and in vitro ruminal fermentation in response to high levels of soybean oil in dairy ewe diet. Journal of Dairy Science 91:1560-1569. https://doi.org/10.3168/jds.2007-0722
» https://doi.org/10.3168/jds.2007-0722 - Homem Junior, A. C.; Ezequiel, J. M. B.; Fávaro, V. R.; Oliveira, P. S. N.; D’Aurea, A. P.; Santos, V. C. and Gonçalves, J. S. 2010. Fermentação ruminal de ovinos alimentados com alto concentrado e grãos de girassol ou gordura protegida. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 62:144-153. https://doi.org/10.1590/S0102-09352010000100020
» https://doi.org/10.1590/S0102-09352010000100020 - Jenkins, T. C. 1993. Lipid metabolism in the rumen. Journal of Dairy Science 76:3851-3863. https://doi.org/10.3168/jds.S0022-0302(93)77727-9
» https://doi.org/10.3168/jds.S0022-0302(93)77727-9 - Kulasek, G. A. 1972. A micromethod for determination of urea in plasma, whole blood cells using urease and phenol reagent. Polskie Archiwum Weterynaryjne 15:801-810.
- Lin, H.; Boylston, D.; Chang, M. J.; Luedecke, L. O. and Shultz, T. D. 1995. Survey of the conjugated linoleic acid contents of dairy products. Journal of Dairy Science 78:2358-2365. https://doi.org/10.3168/jds.S0022-0302(95)76863-1
» https://doi.org/10.3168/jds.S0022-0302(95)76863-1 - Maia, F. J.; Branco, A. F.; Mouro, G. F.; Coneglian, S. M.; Santos, G. T.; Minella, T. F. and Macedo, F. A. F. 2006. Inclusão de fontes de óleo na dieta de cabras em lactação: digestibilidade dos nutrientes e parâmetros ruminais e sanguíneos. Revista Brasileira de Zootecnia 35:1496-1503. https://doi.org/10.1590/S1516-35982006000500032
» https://doi.org/10.1590/S1516-35982006000500032 - Nagaraja, T. G.; Newbold, C. J.; van Nevel, C. J. and Demeyer, D. I. 1997. Manipulation of ruminal fermentation. p.523-632. In: The rumen microbial ecosystem. 2nd ed. Hobson, P. N. and Stewart, C. S., eds. Blackie Academic & Professional, London.
- NRC - National Research Council. 2007. Nutrient requirements of small ruminants: sheep, goat, cervids and new world camelids. The National Academies Press, Washington, DC.
- Palmquist, D. L. and Mattos, W. R. S. 2011. Metabolismo de lipídios. p.299-321. In: Nutrição de ruminantes. Berchielli, T. T.; Pires, A. V.; Oliveira, S. G., eds. Funep, Jaboticabal.
- Rodrígues-Ruiz, J.; Belarbi, E. H.; Sánches, J. L. G. and Alonso, D. L. 1998. Rapid simultaneous lipid extraction and transesterification for fatty acid analyses. Biotechnology Techniques 12:689-691. https://doi.org/10.1023/A:1008812904017
» https://doi.org/10.1023/A:1008812904017 - Russel, J. B.; O´Connor, J. D.; Fox, D. G.; Van Soest, P. J. and Sniffen, C. J. 1992. A net carbohydrate and protein system for evaluating cattle diets: I Ruminal fermentation. Journal of Animal Science 70:3551-3561. https://doi.org/10.2527/1992.70113551x
» https://doi.org/10.2527/1992.70113551x - Santos, F. A. P. and Pedroso, A. M. 2011. Metabolismo de proteínas. p.265-292. In: Nutrição de ruminantes. Berchielli, T. T.; Pires, A. V.; Oliveira, S. G. D., eds. Funep, Jaboticabal.
- Satter, L. D. and Slyter, L. L. 1974. Effect of ammonia concentration on rumen microbial protein production in vitro British Journal of Nutrition 32:199-208. https://doi.org/10.1079/BJN19740073
» https://doi.org/10.1079/BJN19740073 - Shingfield, K. J.; Ahvenjarvi, S.; Toivonen, V.; Vanhatalo, A.; Huhtanen, P. and Griinari, J. M. 2008. Effect of incremental levels of sunflower-seed oil in the diet on ruminal lipid metabolism in lactating cows. British Journal of Nutrition 99:971-983. https://doi.org/10.1017/S0007114507853323
» https://doi.org/10.1017/S0007114507853323 - Silva, M. M. C.; Rodrigues, M. T.; Rodrigues, C. A. F.; Branco, R. H.; Leão, M. I.; Magalhães, A. C. M. and Matos, R. S. 2007. Efeito da suplementação de lipídios sobre a digestibilidade e os parâmetros da fermentação ruminal em cabras leiteiras. Revista Brasileira de Zootecnia 36:246-256. https://doi.org/10.1590/S1516-35982007000100029
» https://doi.org/10.1590/S1516-35982007000100029 - Szumacher-Strabel, M.; Martin, S. A.; Potkański, A.; Cieślak, A. and Kowalczyk, J. 2004. Changes in fermentation processes as the effect of vegetable oil supplementation in in vitro studies. Journal of Animal Feed Sciences 13(suppl. 1):215-218. https://doi.org/10.22358/jafs/73843/2004
» https://doi.org/10.22358/jafs/73843/2004 - Tesfa, A. T.; Touri, M. R. and Syrjala-Quist, L. 1992. The effects of increasing levels of rapeseed oil in the diets of lactating milking cows on milk yield, milk composition, blood and rumen metabolites. World Review Animal Production 27:34-40.
- Toral, P. G.; Belenguer, A.; Frutos, P. and Hervás, G. 2009. Effect of the supplementation of a high-concentrate diet with sunflower and fish oils on ruminal fermentation in sheep. Small Ruminant Research 81:119-125. https://doi.org/10.1016/j.smallrumres.2008.12.009
» https://doi.org/10.1016/j.smallrumres.2008.12.009 - UFV - Universidade Federal de Viçosa. 2000. Sistema de Análises Estatística e Genéticas – SAEG. Versão 9.0. Viçosa, MG.
- Van Soest, P. J.; Robertson, J. B. and Lewis, B. A. 1991. Methods for dietary fiber, neutral detergent, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science 74:3583-3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
» https://doi.org/10.3168/jds.S0022-0302(91)78551-2 - Van Soest, P. J. 1994. Nutritional ecology of the ruminant. 2nd ed. Cornell University Press, Ithaca.
- Weiss, W. P. 1999. Energy prediction equations for ruminant feeds. p.76-185. In: Proceedings of the 61st Cornell Nutrition Conference for Feed Manufactures. Cornell University, Ithaca.
Publication Dates
-
Publication in this collection
26 Apr 2021 -
Date of issue
2021
History
-
Received
6 June 2020 -
Accepted
18 Feb 2021