ABSTRACT
BACKGROUND AND OBJECTIVES:
Facial pain seems to be related to physiological responses to stress and sexual dimorphism. However, the relationship among facial pain, cortisol secretion and gender has been poorly investigated. This study aimed to investigate differences in morning salivary cortisol profile between males and females either with or without perceived facial pain symptoms.
METHODS:
Participated in the study 39 individuals reporting facial pain and 33 painless controls of both genders. Facial pain symptoms were evaluated with Axis II Research Diagnostic Criteria for Temporomandibular Disorders, which has supplied chronic pain scores. Saliva was collected in the morning to obtain cortisol peaks, being stored for further use. Salivary cortisol levels were evaluated by immunosorbent assay. Statistical analysis has included hypotheses tests and ANOVA with significance level of 5% and a binary logistic regression, which has tested the association between gender, cortisol and each facial pain symptom.
RESULTS:
There has been no association between facial pain and gender. Cortisol levels were similar among individuals with and without facial pain, regardless of gender. The adjusted model has shown that most symptoms were not associated to gender, regardless of cortisol levels.
CONCLUSION:
In individuals with and without facial pain symptoms, morning salivary cortisol levels regulation has been similar for both genders.
Keywords:
Chronic pain; Comorbidity; Cortisol; Facial pain; Gender; Psychological stress
RESUMO
JUSTIFICATIVA E OBJETIVOS:
A percepção da dor facial parece estar relacionada com respostas fisiológicas ao estresse e com o dimorfismo sexual. No entanto, a relação entre dor facial, secreção de cortisol e o sexo ainda foi pouco investigada. O objetivo deste estudo foi investigar as diferenças nos perfis de cortisol salivar matutino em homens e mulheres com ou sem sintomas de dor facial.
MÉTODOS:
Trinta e nove indivíduos que relataram dor facial e 33 controles sem dor, de ambos os sexos, participaram deste estudo. Os sintomas de dor facial foram avaliados utilizando o Eixo II do Critério de Diagnóstico para Pesquisa das Disfunções Temporomandibulares, que forneceu os escores de dor crônica. A saliva foi coletada dos participantes no turno matutino a fim de obter os picos de cortisol, sendo armazenada até utilização posterior. Os níveis salivares de cortisol foram avaliados por ensaio imunoenzimático. A análise estatística incluiu testes de hipóteses e ANOVA com nível de significância de 5%, e uma regressão logística binária que testou a associação entre o sexo, cortisol, e cada sintoma de dor facial.
RESULTADOS:
Não foi observada associação entre dor facial e o sexo. Os níveis de cortisol foram semelhantes entre indivíduos com ou sem dor facial, independentemente do sexo. O modelo ajustado mostrou que a maioria dos sintomas não teve associação com o sexo, independentemente dos níveis de cortisol. CONCLUSÃO: Nos indivíduos com e sem dor facial, a regulação dos níveis de cortisol salivar matutino ocorreu de forma semelhante em ambos os sexos.
Descritores:
Comorbidade; Cortisol; Dimorfismo sexual; Dor Crônica; Dor facial; Estresse psicológico
INTRODUCTION
Facial pain has been commonly associated with a multifactorial scenario that includes musculoskeletal disorders, biomechanical unbalances of the temporomandibular joint and the masticatory system, or pre-existing pain conditions triggering several orofacial symptoms11 Harper DE, Schrepf A, Clauw DJ. Pain mechanisms and centralized pain in temporomandibular disorders. J Dent Res. 2016;95(10):1102-8.
2 Sanders AE, Slade GD, Bair E, Fillingim RB, Knott C, Dubner R, et al. General health status and incidence of first-onset temporomandibular disorder: the OPPERA prospective cohort study. J Pain. 2013;14(Suppl 12):T51-62.
3 Bender SD. Temporomandibular disorders, facial pain, and headaches. Headache. 2012;52(Suppl 1):22-5.-44 da Silva Junior AA, Krymchantowski AV, Gomes JB, Leite FM, Alves BM, Lara RP, et al. Temporomandibular disorders and chronic daily headaches in the community and in specialty care. Headache. 2013;53(8):1350-5.. Neuropsychological comorbidities are often present, including neuroendocrine abnormalities, sleep disturbance, pain amplification, and psychological distress due to hormonal response to stress or to excitation of the sympathetic nervous system55 Yoshihara T, Shigeta K, Hasegawa H, Ishitani N, Masumoto Y, Yamasaki Y. Neuroendocrine responses to psychological stress in patients with myofascial pain. J Orofac Pain. 2005;19(3):202-8.
6 Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34(2):163-71.
7 Lavigne GJ, Sessle BJ. The neurobiology of orofacial pain and sleep and their interactions. J Dent Res. 2016;95(10):1109-16.-88 Bement MH, Weyer A, Keller M, Harkins AL, Hunter SK. Anxiety and stress can predict pain perception following a cognitive stress. Physiol Behav. 2010;101(1):87-92..
Moreover, neuroendocrine, psychosocial and psychological factors have been suggested as predisposing females to the development of facial pain, the severity of which has been shown to be greater in women than in men99 Slade GD, Ohrbach R, Greenspan JD, Fillingim RB, Bair E, Sanders AE, et al. Painful temporomandibular disorder: decade of discovery from OPPERA studies. J Dent Res. 2016;95(10):1084-92.
10 Turner JA, Mancl L, Huggins KH, Sherman JJ, Lentz G, LeResche L. Targeting temporomandibular disorder pain treatment to hormonal fluctuations: a randomized clinical trial. Pain. 2011;152(9):2074-84.-1111 Kou XX, Wu YW, Ding Y, HaoT, Bi RY, Gan YH, et al. Estradiol aggravates temporomandibular joint inflammation through the NF-κB pathway in ovariectomized rats. Arthritis Rheum. 2011;63(7):1888-97.. Although previous investigations have demonstrated higher prevalence of psychological comorbidities in females, data regarding sex differences in facial pain prevalence require further investigation44 da Silva Junior AA, Krymchantowski AV, Gomes JB, Leite FM, Alves BM, Lara RP, et al. Temporomandibular disorders and chronic daily headaches in the community and in specialty care. Headache. 2013;53(8):1350-5.,99 Slade GD, Ohrbach R, Greenspan JD, Fillingim RB, Bair E, Sanders AE, et al. Painful temporomandibular disorder: decade of discovery from OPPERA studies. J Dent Res. 2016;95(10):1084-92.,1212 Slade GD, Bair E, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, et al. Signs and symptoms of first-onset TMD and sociodemographic predictors of its development: the OPPERA prospective cohort study. J Pain. 2013;14(Suppl 12):T20-32.. Interestingly, females, either with or without chronic pain, exhibit higher responsiveness to stress1313 Weekes NY, Lewis RS, Gotoc SG, Garrison-Jakela J, Patelb F, Lupien S. The effect of an environmental stressor on gender differences on the awakening cortisol response. Psychoneuroendocrinology. 2008;33(6):766-72.,1414 Turner-Cobb JM, Osborn M, Silva L, Keogh E, Jessop DS. Sex differences in hypothalamic-pituitary-adrenal axis function in patients with chronic pain syndrome. Stress. 2010;13(4):292-300., and the gender was shown to contribute significantly to the relationship between distress, muscle tension and facial pain1515 Glaros AG, Marszalek JM, Williams KB. Longitudinal multilevel modeling of facial pain, muscle tension, and stress. J Dent Res. 2016;95(4):416-22.. However, the impact of facial pain on cortisol secretion in both genders remains unclear, deserving greater attention.
Cortisol is considered to be a stress biomarker66 Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34(2):163-71., and temporomandibular joint disorder (TMD) has been linked to increased levels of cortisol in women1616 Korszun A, Young EA, Singer K, Carlson NE, Brown MB, Crofford L. Basal circadian cortisol secretion in women with temporomandibular disorders. J Dent Res. 2002;81(4):279-83.. Nevertheless, recent but limited data found no association between distress, psychological factors and cortisol levels in women with TMD and facial pain1717 Nilsson AM, Dahlström L. Perceived symptoms of psychological distress and salivary cortisol levels in young women with muscular or disk-related temporomandibular disorders. Acta Odontol Scand. 2010;68(5):284-8.,1818 Jasim H, Louca S, Christidis N, Ernberg M. Salivary cortisol and psychological factors in women with chronic and acute oro-facial pain. J Oral Rehabil. 2014;41(2):122-32.. Accordingly, since most research that analyzed the connection between facial pain and cortisol levels included only female participants, evidences of the effects of both facial pain and gender on cortisol secretion are scarce.
Although psychoneuroendocrine factors involved in facial pain pathogenesis support its association with cortisol secretion and gender, a potential relationship remains controversial. Hence, this study was aimed at investigating whether morning salivary cortisol profiles would be affected by sexual dimorphism in individuals reporting facial pain symptomatology.
METHODS
This study was advertised throughout a Brazilian University (São Luis, Brazil), and interested volunteers contacted the research team for further information. Those who agreed to participate in the study received no financial compensation. Volunteers undergoing exams or other stressful activities were not included. Eightyfive individuals were initially enrolled, including undergraduate or graduate students. However, 11 participants were identified with history of chronic daily headache or migraine in the past 12 months, being then excluded from this study for not meeting the selection criteria. Yet, 2 voluntary participants were excluded due to inadequate saliva samples. Finally, a total of 72 individuals (46 females and 26 males) aged 18-39 years were included in the study. The following exclusion criteria were applied: presence of diabetes mellitus; history of hyper- or hypocortisolism, mood disorders, sleep apnea or other neurologic disorders; pregnant or climacteric women; premenopausal women during the menstrual cycle; individuals taking antibiotics, nonsteroidal anti-inflammatory drugs, anxiolytics, antidepressants, contraceptives, and immunosuppressants; individuals with any musculoskeletal or craniofacial disorder, and those under treatment for such dysfunctions; history of neuropathic or dental pain, cluster or chronic daily headache, or migraine1919 Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808. in the past twelve months, and all participants signed an informed consent form after being informed about the aims, potential risks, and benefits of the study.
Individuals were firstly evaluated in terms of presence or absence of perceived facial pain by answering questions on the Axis II of the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD)2020 Manfredini D, Winocur E, Ahlberg J, Guarda-Nardini L, Lobbezoo F. Psychosocial impairment in temporomandibular disorders patients. RDC/TMD axis II findings from a multicentre study. J Dent. 2010;38(10):765-72.,2121 de Lucena LB, Kosminsky M, da Costa LJ, de Góes PS. Validation of the Portuguese version of the RDC/TMD Axis II questionnaire. Braz Oral Res. 2006;20(4):312-7.. The RDC/TMD Axis II was applied to investigate disabilities related to facial pain and psychological condition. Axis II main criteria included pain intensity, psychological stress, sleep quality, limitations related to mandibular function, among others2020 Manfredini D, Winocur E, Ahlberg J, Guarda-Nardini L, Lobbezoo F. Psychosocial impairment in temporomandibular disorders patients. RDC/TMD axis II findings from a multicentre study. J Dent. 2010;38(10):765-72..
These data allowed investigators to assign scores to determine each patient's chronic pain-related disability through the scores of the Graded Chronic Pain Scale (GCPS). GCPS scores ranged from 0 to IV. GCPS 0 indicates no disability in the past 6 months. GCPS I indicates low disability and low pain intensity. GCPS II indicates low disability and high pain intensity. GCPS III indicates high disability and moderately limiting. GPCS IV indicates high disability and severely limiting2020 Manfredini D, Winocur E, Ahlberg J, Guarda-Nardini L, Lobbezoo F. Psychosocial impairment in temporomandibular disorders patients. RDC/TMD axis II findings from a multicentre study. J Dent. 2010;38(10):765-72..
After data collection, study participants were separated into two groups: facial pain (n=39; 25 females and 14 males), which included patients with GCPS scores ranging I-IV, and control (n=33; 21 females and 12 males), including individuals with GCPS 0. To model the relationship between perceived facial pain symptomatology, cortisol and gender, the status of facial pain symptoms was labeled as present or absent. For the comparison of cortisol levels according to symptoms associated with sleep quality, these symptoms were classified according to their intensity as following: "no", indicating the absence of the symptom, and "mild", "moderate", "severe", or "very severe" symptomatology2020 Manfredini D, Winocur E, Ahlberg J, Guarda-Nardini L, Lobbezoo F. Psychosocial impairment in temporomandibular disorders patients. RDC/TMD axis II findings from a multicentre study. J Dent. 2010;38(10):765-72..
After the collection of clinical data, participants underwent saliva collection for the evaluation of salivary cortisol levels. Individuals of both groups (facial pain and control) had nonstimulated saliva collected in the morning shift between 9 and 10 am. Samples were stored in sterile graduated flasks and kept in ice to be used for the evaluation of cortisol levels by the immunoenzymatic test. These saliva samples were then centrifuged (11,900 rpm, 10 min). Then, supernatants received a protease inhibitor (phenylmethylsulfonylfluoride; final concentration of 1 mM), and were stored at -20ºC for posterior utilization. Salivary concentrations of cortisol were determined by Enzyme Linked Immunosorbent Assay (ELISA) using a commercially available specific kit (Cortisol Parameter Kit, R&D Systems, Inc., Minneapolis, MN, USA).
Sample size was calculated using the 11 PASS program (NCSS, LLC, Kaysville, UT, USA). A prevalence of 65.5% of at least one episode of facial pain as reported by Slade et al.2222 Slade GD, Sanders AE, Bair E, Brownstein N, Dampier D, Knott C, et al. Preclinical episodes of orofacial pain symptoms and their association with healthcare behaviors in the OPPERA prospective cohort study. Pain. 2013;154(5):750-60. was considered to produce a power of 80%.
Statistical analyses
Chi-square test of independence was applied to investigate associations between facial pain symptomatology and gender. Sha-piro-Wilk test was used in the comparison of numerical variables between groups, followed by Student t test, which was used in the comparison of cortisol levels between genders. When comparing more than one group, one-way ANOVA was utilized, followed by Bonferroni test. A standard binary logistic regression approach was developed to test the association between gender, cortisol and each perceived symptom of facial pain. The level of significance considered for all statistical analyses was 5%. Data obtained were analyzed using the SAS 9.4 software (SAS Institute Inc., Cary, NC, USA). A Human Research Ethics Committee (Protocol #633/11, Brazil) approved this study that was conducted according to the W.M.A. Declaration of Helsinki.
RESULTS
In the male group, 46% of patients presented with GCPS 0, 27% with GCPS I, 19% with GCPS II, 4% with GCPS III, and 4% with GCPS IV. In the female group, 46% of patients presented with GCPS 0, 19.5% with GCPS I, 15% with GCPS II, 13% with GCPS III, and 6.5% with GCPS IV. Similar GCPS scores were observed in male and female participants with perceived facial pain (p=0.72). The risk of having some level of facial pain (GCPS scores > 0) was also found to be similar in both men and women (odds ratio [OR] = 1.02, 95% confidence interval [CI] = 0.38-2.67). Still, when the relationship between facial pain symptoms and gender was established, as shown in table 1, no association between facial pain and the gender was found (p>0.05). Figure 1A shows the values of salivary cortisol in male and female participants considering only the facial pain group. Statistically similar values were observed for men and women, regardless of the presence of pain. When considering both facial pain and the control group, although there has been a trend for higher cortisol means in female as compared to male participants, the difference did not reach significance (p=0.05). Figure 1B shows the comparison of cortisol values between participants with orofacial symptoms and controls, respectively, in men and women separately. For both situations, no statistically significant difference was obtained (p>0.05). Yet, the comparison of cortisol levels according to GCPS scores in each gender showed similar levels of morning salivary cortisol in both men (p=0.74) and women (p=0.65).
Association between perceived symptoms of facial pain reported by participants, gender and scores of the Graded Chronic Pain Scale obtained from the Research Diagnostic Criteria for Temporomandibular Disorders, Axis II
Comparison of values of salivary cortisol concentration according to gender or presence of perceived facial pain. A – Comparison of cortisol means ± standard deviation between male (801.8 ± 415.6) and female (863.3 ± 403.6) participants, considering the facial pain group isolated (p=0.69), and then males (949.1 ± 432.5) and females (873.3 ± 425.9), considering both facial pain and the control group (p=0.50); B – Comparison of cortisol means between controls and participants with facial pain in males (932.9 ± 443.2; 801.8 ± 415.7; p=0.48) and females (1058.6 ± 454.6; 863.3 ± 403.6; p=0.15); Student t test.
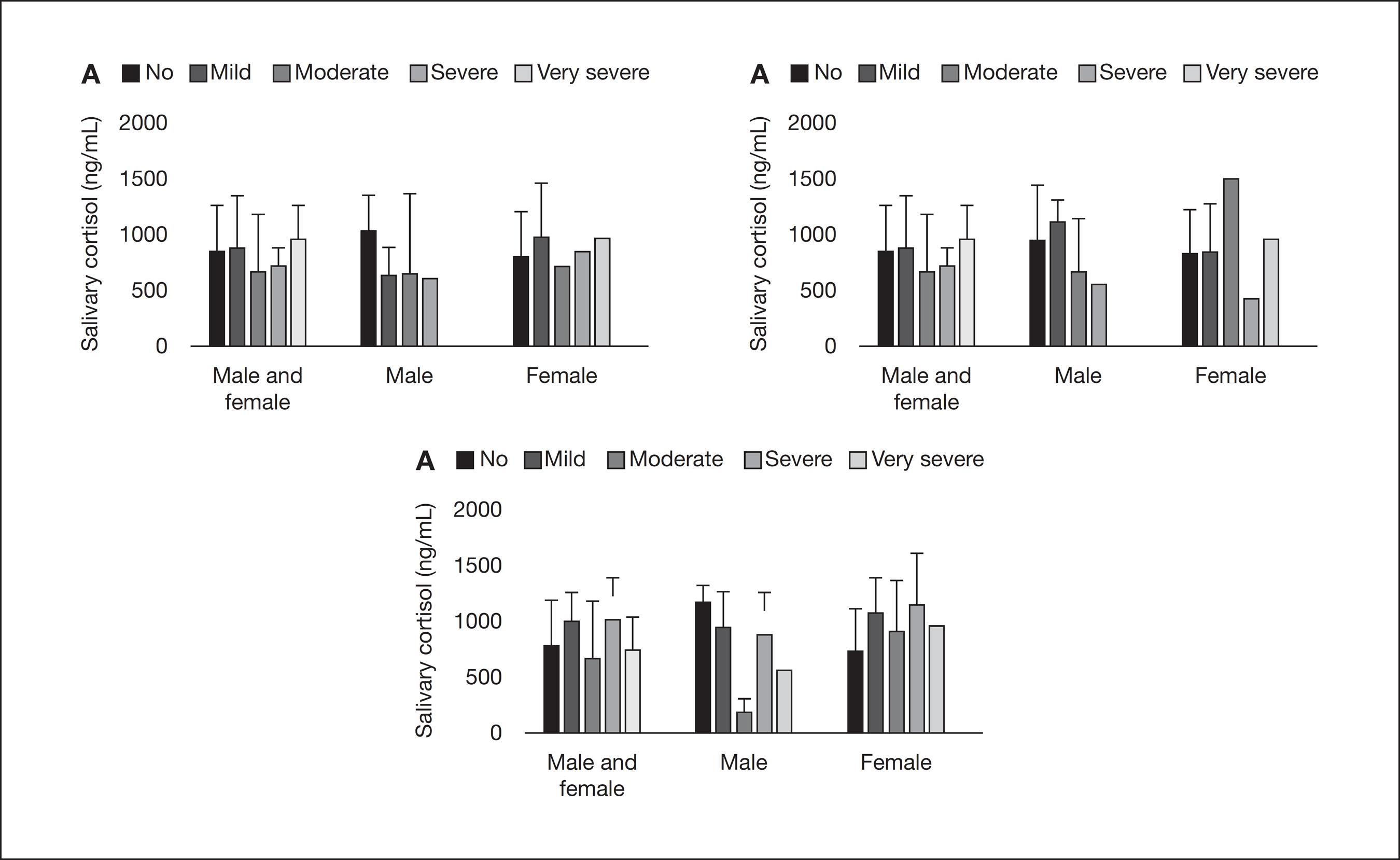
Table 2 shows the results of a standard binary logistic regression developed for modeling the relationship between facial pain symptomatology and gender. Adjusted ORs (95% CI) were obtained taking cortisol levels into consideration. Gender was found to be associated with "difficulty falling asleep" (p<0.05). Figure 2 shows the distribution of means and standard deviation of salivary cortisol, according to scores of disturbed sleep symptoms in both men and women, men only, and women only. In all these situations, no statistically significant difference was demonstrated between groups (p>0.05), similarly to the comparison according to other evaluated symptoms (data not shown).
Standard binary logistic regression testing the effect of gender on each symptom reported. The adjusted model included the level of morning salivary cortisol as a covariate. The presence or absence of facial pain symptoms was obtained from the Research Diagnostic Criteria for Temporomandibular Disorders, Axis II
Comparison of means and standard deviation of salivary cortisol in both genders, in the male group only and, lastly, in the female group, according to the scores of symptoms related to disturbed sleep ("no" indicates absence, and the intensity of the symptom ranged from "mild" to "very severe"). A – Comparison according to the scores of "night-awake" (p>0.05); B – Comparison according to the scores of "restless sleep" (p>0.05). C – Comparison according to the scores of "difficulty falling asleep" (p>0.05); one-way ANOVA, followed by Bonferroni test.
DISCUSSION
Facial pain, as most chronic conditions, is unlikely to be characterized by a homogenous and unmixed disease picture. In the present study, no perceived symptom of facial pain was significantly associated with gender, and no relationship was found between facial pain, gender and morning salivary cortisol levels. This suggests that the regulation of morning salivary cortisol might occur similarly in both genders in individuals with or without perceived facial pain.
The severity of facial pain has been reported to be elevated in women, mainly due to psychoneuroendocrine factors, as well as the role of estrogen in temporomandibular joint inflammation. Estrogen replacement therapy was associated with high prevalence of TMD, and serum estradiol levels during the luteal phase or in synovial fluid are higher in TMD women than in controls1010 Turner JA, Mancl L, Huggins KH, Sherman JJ, Lentz G, LeResche L. Targeting temporomandibular disorder pain treatment to hormonal fluctuations: a randomized clinical trial. Pain. 2011;152(9):2074-84.,1111 Kou XX, Wu YW, Ding Y, HaoT, Bi RY, Gan YH, et al. Estradiol aggravates temporomandibular joint inflammation through the NF-κB pathway in ovariectomized rats. Arthritis Rheum. 2011;63(7):1888-97.. This indicates that sex hormones, particularly estrogens, may be involved in facial pain, reason by which women during menstrual cycle, pregnancy and climacteric phase were excluded from our sample population. Conversely, the involvement of psychological distress in pain perception is becoming clearer as higher stress levels were found to enhance hyperalgesia, regardless of gender88 Bement MH, Weyer A, Keller M, Harkins AL, Hunter SK. Anxiety and stress can predict pain perception following a cognitive stress. Physiol Behav. 2010;101(1):87-92..
Moreover, this study failed to observe changes in morning salivary cortisol levels when comparing male versus female participants, with and without perceived facial pain. Recent data have also found no difference in cortisol levels between women with facial pain and controls; however, sex differences were not taken into account as men were excluded from such studies1717 Nilsson AM, Dahlström L. Perceived symptoms of psychological distress and salivary cortisol levels in young women with muscular or disk-related temporomandibular disorders. Acta Odontol Scand. 2010;68(5):284-8.,1818 Jasim H, Louca S, Christidis N, Ernberg M. Salivary cortisol and psychological factors in women with chronic and acute oro-facial pain. J Oral Rehabil. 2014;41(2):122-32.. High correlation between salivary and blood levels of cortisol have been reported, and differences between measures during the circadian cycle with peaks occurring soon after awakening were shown66 Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34(2):163-71.. Moreover, about 30% of free cortisol is enzymatically converted to cortisone and about 14% is bound to corticosteroid-binding globulin in saliva. Thus, one can imply that saliva contains lower levels of free cortisol when compared to plasma2323 Levine A, Zagoory-Sharon O, Feldman R, Lewis JG, Weller A. Measuring cortisol in human psychobiological studies. Physiol Behav. 2007;90(1):43-53.. Nevertheless, the salivary measure includes important advantages, such as stress-free collection and processing66 Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34(2):163-71..
Importantly, the differences between each gender in cortisol and distress levels might be impacted by social interactions and by the scenario where data were collected2424 Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Biological and psychological markers of stress in humans: focus on the Trier Social Stress test. Neurosci Biobehav Rev. 2014;38:94-124.. Strini et al.2525 Strini PJ, Strini PJ, Barbosa TS, Gavião MB. Assessment of orofacial dysfunctions, salivary cortisol levels and oral health related quality of life (ORHQoL) in young adults. Arch Oral Biol. 2011;56(12):1521-7. demonstrated that patients of both genders with different scores of orofacial dysfunction, including pain, were found to experience similar cortisol responses. Still, while most of the studies have reported women to be more susceptible to changes in HPA-axis function related to gender-oriented pain sensitivity1414 Turner-Cobb JM, Osborn M, Silva L, Keogh E, Jessop DS. Sex differences in hypothalamic-pituitary-adrenal axis function in patients with chronic pain syndrome. Stress. 2010;13(4):292-300.,2626 Godfrey KM, Strachan E, Dansie E, Crofford LJ, Buchwald D, Goldberg J, et al. Salivary cortisol and cold pain sensitivity in female twins. Ann Behav Med. 2014;47(2):180-8.,2727 Allen LB, Lu Q, Tsao JCI, Worthman CM, Zeltzer LK. Sex differences in the association between cortisol concentrations and laboratory pain responses in healthy children. Gender Med. 2009;6(Suppl 2):193-207., the relationship between facial pain and HPA-axis activity requires further exploration. In addition, the present study had some limitations, as it was not possible to develop a case-matched control design.
Persistent facial pain shows several aspects associated with a multisystem deregulation in sensory, autonomic, inflammatory, and psychological domains2828 Chen H, Nackley A, Miller V, Diatchenko L, Maixner W. Multisystem dysregulation in painful temporomandibular disorders. J Pain. 2013;14(9):983-96.. In the present investigation, frequent headache was not found to be associated with variations in morning salivary cortisol levels between male and female participants. This is in agreement with previous data, which showed that sexual dimorphism do not influence salivary cortisol levels in patients with headache2929 Fernández-de-Las-Peñas C, Fernández-Mayoralas DM, Arroyo-Morales M, Ambite-Quesada S, Rivas-Martínez I, Ortega-Santiago R, et al. Lower immunglobulin A levels but not lower cortisol or a-amylase activity in children with chronic tension-type headache. Cephalalgia. 2011;31(4):481-7..
It is important not to confuse "frequent headache" with "chronic daily headache". One major criteria to define chronic headache is the frequency of headache symptoms, which must appear at least fifteen days per month1919 Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808.. Hence, we considered frequent headache as that experienced less than fifteen days per month, without characteristics of migraine or cluster headache syndromes. Furthermore, both sleep and awake bruxism were not found to be associated with either gender or morning salivary cortisol levels in the group of individuals with facial pain evaluated here. A previous report observed reduced levels of morning cortisol in patients with sleep bruxism in comparison to controls; however, no effect of gender on sleep bruxism was detected3030 Castelo PM, Barbosa T de S, Pereira LJ, Fonseca FL, Gavião MB. Awakening salivary cortisol levels of children with sleep bruxism. Clin Biochem. 2012;45(9):651-4.. It is important to emphasize that their population sample was composed by children, whereas we focused on young adults. Thus, despite the relevance of such preliminary findings, it can be difficult to draw conclusions based solely on them, particularly due to the impact of age on psychoneuroendocrine activity2323 Levine A, Zagoory-Sharon O, Feldman R, Lewis JG, Weller A. Measuring cortisol in human psychobiological studies. Physiol Behav. 2007;90(1):43-53..
Notably, in the population studied, disturbed sleep was found to be frequent in patients of both genders with perceived facial pain. The relationship between pain and sleep has been a topic of wide discussion since sleep fragmentation was significantly associated with decreased levels of conditioned pain modulation in patients with facial pain3131 Smith MT, Wickwire EM, Grace EG. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep. 2009;32(6):779-90.,3232 Edwards RR, Grace E, Peterson S, Klick B, Haythornthwaite JA, Smith MT. Sleep continuity and architecture: associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain. 2009;13(10):1043-7.. Additionally, sleep quality is also thought to be involved in HPA-axis activity, probably acting through distinct pathways in men and women in terms of capability to recover from sleep deprivation3333 Eek F, Karlson B, Garde AH, Hansen AM, Ørbæk P. Cortisol, sleep, and recovery - some gender differences but no straight associations. Psychoneuroendocrinology. 2012;37(1):56-64.. Therefore, efforts to treat sleep disturbance early in the course of a pain condition should be kept in mind towards reducing the severity and impact of clinical pain and psychological stress77 Lavigne GJ, Sessle BJ. The neurobiology of orofacial pain and sleep and their interactions. J Dent Res. 2016;95(10):1109-16.,3232 Edwards RR, Grace E, Peterson S, Klick B, Haythornthwaite JA, Smith MT. Sleep continuity and architecture: associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain. 2009;13(10):1043-7.,3333 Eek F, Karlson B, Garde AH, Hansen AM, Ørbæk P. Cortisol, sleep, and recovery - some gender differences but no straight associations. Psychoneuroendocrinology. 2012;37(1):56-64..
CONCLUSION
Considering the data obtained, our results suggest that the regulation of morning salivary cortisol levels may occur similarly in individuals of both genders, either with or without facial pain symptoms. Such preliminary findings definitely require further investigation, considering that the exposition to facial pain symptomatology and comorbidity can dramatically impair patients' quality of life.
-
Sponsoring sources: Funding for this study was provided by FAPEMA, the State Government Agency for Support to Research and Scientific and Technological Development (Maranhão, Brazil; grant numbers 366/12, 2209/12, 588/13, and 877/16).
ACKNOWLEDGMENTS
The authors would like to thank FAPEMA for the research support (grant numbers 366/12, 2209/12, 588/13, and 877/16).
REFERENCES
-
1Harper DE, Schrepf A, Clauw DJ. Pain mechanisms and centralized pain in temporomandibular disorders. J Dent Res. 2016;95(10):1102-8.
-
2Sanders AE, Slade GD, Bair E, Fillingim RB, Knott C, Dubner R, et al. General health status and incidence of first-onset temporomandibular disorder: the OPPERA prospective cohort study. J Pain. 2013;14(Suppl 12):T51-62.
-
3Bender SD. Temporomandibular disorders, facial pain, and headaches. Headache. 2012;52(Suppl 1):22-5.
-
4da Silva Junior AA, Krymchantowski AV, Gomes JB, Leite FM, Alves BM, Lara RP, et al. Temporomandibular disorders and chronic daily headaches in the community and in specialty care. Headache. 2013;53(8):1350-5.
-
5Yoshihara T, Shigeta K, Hasegawa H, Ishitani N, Masumoto Y, Yamasaki Y. Neuroendocrine responses to psychological stress in patients with myofascial pain. J Orofac Pain. 2005;19(3):202-8.
-
6Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34(2):163-71.
-
7Lavigne GJ, Sessle BJ. The neurobiology of orofacial pain and sleep and their interactions. J Dent Res. 2016;95(10):1109-16.
-
8Bement MH, Weyer A, Keller M, Harkins AL, Hunter SK. Anxiety and stress can predict pain perception following a cognitive stress. Physiol Behav. 2010;101(1):87-92.
-
9Slade GD, Ohrbach R, Greenspan JD, Fillingim RB, Bair E, Sanders AE, et al. Painful temporomandibular disorder: decade of discovery from OPPERA studies. J Dent Res. 2016;95(10):1084-92.
-
10Turner JA, Mancl L, Huggins KH, Sherman JJ, Lentz G, LeResche L. Targeting temporomandibular disorder pain treatment to hormonal fluctuations: a randomized clinical trial. Pain. 2011;152(9):2074-84.
-
11Kou XX, Wu YW, Ding Y, HaoT, Bi RY, Gan YH, et al. Estradiol aggravates temporomandibular joint inflammation through the NF-κB pathway in ovariectomized rats. Arthritis Rheum. 2011;63(7):1888-97.
-
12Slade GD, Bair E, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, et al. Signs and symptoms of first-onset TMD and sociodemographic predictors of its development: the OPPERA prospective cohort study. J Pain. 2013;14(Suppl 12):T20-32.
-
13Weekes NY, Lewis RS, Gotoc SG, Garrison-Jakela J, Patelb F, Lupien S. The effect of an environmental stressor on gender differences on the awakening cortisol response. Psychoneuroendocrinology. 2008;33(6):766-72.
-
14Turner-Cobb JM, Osborn M, Silva L, Keogh E, Jessop DS. Sex differences in hypothalamic-pituitary-adrenal axis function in patients with chronic pain syndrome. Stress. 2010;13(4):292-300.
-
15Glaros AG, Marszalek JM, Williams KB. Longitudinal multilevel modeling of facial pain, muscle tension, and stress. J Dent Res. 2016;95(4):416-22.
-
16Korszun A, Young EA, Singer K, Carlson NE, Brown MB, Crofford L. Basal circadian cortisol secretion in women with temporomandibular disorders. J Dent Res. 2002;81(4):279-83.
-
17Nilsson AM, Dahlström L. Perceived symptoms of psychological distress and salivary cortisol levels in young women with muscular or disk-related temporomandibular disorders. Acta Odontol Scand. 2010;68(5):284-8.
-
18Jasim H, Louca S, Christidis N, Ernberg M. Salivary cortisol and psychological factors in women with chronic and acute oro-facial pain. J Oral Rehabil. 2014;41(2):122-32.
-
19Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808.
-
20Manfredini D, Winocur E, Ahlberg J, Guarda-Nardini L, Lobbezoo F. Psychosocial impairment in temporomandibular disorders patients. RDC/TMD axis II findings from a multicentre study. J Dent. 2010;38(10):765-72.
-
21de Lucena LB, Kosminsky M, da Costa LJ, de Góes PS. Validation of the Portuguese version of the RDC/TMD Axis II questionnaire. Braz Oral Res. 2006;20(4):312-7.
-
22Slade GD, Sanders AE, Bair E, Brownstein N, Dampier D, Knott C, et al. Preclinical episodes of orofacial pain symptoms and their association with healthcare behaviors in the OPPERA prospective cohort study. Pain. 2013;154(5):750-60.
-
23Levine A, Zagoory-Sharon O, Feldman R, Lewis JG, Weller A. Measuring cortisol in human psychobiological studies. Physiol Behav. 2007;90(1):43-53.
-
24Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Biological and psychological markers of stress in humans: focus on the Trier Social Stress test. Neurosci Biobehav Rev. 2014;38:94-124.
-
25Strini PJ, Strini PJ, Barbosa TS, Gavião MB. Assessment of orofacial dysfunctions, salivary cortisol levels and oral health related quality of life (ORHQoL) in young adults. Arch Oral Biol. 2011;56(12):1521-7.
-
26Godfrey KM, Strachan E, Dansie E, Crofford LJ, Buchwald D, Goldberg J, et al. Salivary cortisol and cold pain sensitivity in female twins. Ann Behav Med. 2014;47(2):180-8.
-
27Allen LB, Lu Q, Tsao JCI, Worthman CM, Zeltzer LK. Sex differences in the association between cortisol concentrations and laboratory pain responses in healthy children. Gender Med. 2009;6(Suppl 2):193-207.
-
28Chen H, Nackley A, Miller V, Diatchenko L, Maixner W. Multisystem dysregulation in painful temporomandibular disorders. J Pain. 2013;14(9):983-96.
-
29Fernández-de-Las-Peñas C, Fernández-Mayoralas DM, Arroyo-Morales M, Ambite-Quesada S, Rivas-Martínez I, Ortega-Santiago R, et al. Lower immunglobulin A levels but not lower cortisol or a-amylase activity in children with chronic tension-type headache. Cephalalgia. 2011;31(4):481-7.
-
30Castelo PM, Barbosa T de S, Pereira LJ, Fonseca FL, Gavião MB. Awakening salivary cortisol levels of children with sleep bruxism. Clin Biochem. 2012;45(9):651-4.
-
31Smith MT, Wickwire EM, Grace EG. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep. 2009;32(6):779-90.
-
32Edwards RR, Grace E, Peterson S, Klick B, Haythornthwaite JA, Smith MT. Sleep continuity and architecture: associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain. 2009;13(10):1043-7.
-
33Eek F, Karlson B, Garde AH, Hansen AM, Ørbæk P. Cortisol, sleep, and recovery - some gender differences but no straight associations. Psychoneuroendocrinology. 2012;37(1):56-64.
Publication Dates
-
Publication in this collection
Oct-Dec 2016
History
-
Received
20 July 2016 -
Accepted
28 Oct 2016