Abstract
Low-grade manganese ores that are composed of manganese oxides, wad, quartz, iron oxides, gibbsite, and especially of spessartine (Mn3Al2(SiO4)3) are known as gondite. Spessartine is a mineral with a high content of silica (SiO2) and alumina (Al2O3) that causes a decrease of the enrichment in manganese oxide concentrates by flotation, giving this ore more complexity for processing. Thus, by ensuring a higher selectivity between manganese oxides minerals and silicates, the use of gondite reserves becomes viable, and the correct reagent type can be useful for this objective. Moreover, there are few publications devoted to the study of spessartine in flotation. So, this study investigated the effects of Mn2+ ions on quartz and spessartine depression and showed positive results with the reduction of quartz floatability from 7.06% to 1.23% and the spessartine from 27.30% to 17.12% respectively when the Mn2+ (1000 mg/L) was added previously to the depressant. Zeta potential determinations showed that Mn2+ can act as a silicate activator, possibly by facilitating the SiO(OH)3 - adsorption. Infrared spectroscopy analysis revealed absorption bands at 3,450 cm-1 and 3,400 cm-1 frequency for quartz and spessartine and the adsorption of Mn(OH)2 was responsible for this result.
Keywords:
depressant reagents; Mn2+ ions; spessartine; quartz; gondite ore
Keywords:
depressant reagents; Mn2+ ions; spessartine; quartz; gondite ore
1. Introduction
The exploitation of manganese ores has become important for several industrial sectors; the metal is intensively used in steel productions, non-ferrous alloys, fertilizers, batteries, and other products (Sahoo et al., 2001SAHOO, R. N.; NAIK, P. K.; DAS, S. C. Leaching of manganese from low-grade manganese ore usingoxalic acid as reductant in sulphuric acid solution. Hydrometallurgy, v. 62, p. 157-163, 2001.). From the data made available by Mineral Commodity Summaries (USGS, 2019), the Brazil production of manganese in 2018 increased 3.45%, compared with the previous year. In the Brazilian crust, pyrolusite (a-MnO2), the main manganese mineral can be found in association with other minerals at ore deposits known as gondite, a type of protore. Protore is a mineral matrix with low metal content; that becomes an ore through secondary enrichment. Gondite (an example of protore) is a metasedimentary rock, composed of spessartine (Mn3Al2(SiO4)3), a manganese-aluminosilicate (Souza, 1979SOUZA, J. V. Geologia e gênese do protominério e do minério da província manganesífera de Aracoiaba-Pacajús, no Estado do Ceará. 1979. 188f. Tese (Doutorado em Geociências) - Instituto de Geociências, Universidade de São Paulo, São Paulo, 1979.), that decomposes into manganese oxide, silica, and alumina by weathering. This manganese oxide crystallizes and becomes pyrolusite in the rock (Faria, 2008FARIA, G. L. Estudo da intensidade de crepitação de minérios granulados de manganês do Brasil. 2008. 125f. Dissertação (Mestrado em Engenharia Mineral) - Escola de Minas, Universidade Federal de Ouro Preto, Ouro Preto, 2008.), and the high silica content, due to the presence of quartz and spessartine, makes this ore more complex for processing (Andrade, 1978ANDRADE, V. L. L. Estudo da concentração do colúvio. 1978. 66f. Dissertação (Mestrado em Engenharia Mineral) - Universidade Federal de Minas Gerais, Belo Horizonte, 1978.). Nevertheless, considering the probable decline of rich ores deposits, the gondite could be an important source of manganese and thus, more studies that explore the way to process this complex ore are essential for its future application.
Depressing reagents are essential for flotation selectivity since they accentuate the existing hydrophilic feature or prevent the hydrophobization of gangue minerals (Bulatovic, 2007BULATOVIC, S. R. Handbook of flotation reagents: chemistry, theory and practice: volume 1 - flotation of sulfide ores. Amsterdam: Elsevier, 2007. 448p.). Sodium silicate, sodium hexametaphosphate, and sodium fluorosilicate are among the reagents with depressant action for silicates; all are intensively used in mineral processing plants. Sodium silicate is intensively used in flotation, consisting of metasilicate (Na2SiO3), dimetasilicate (Na2Si2O5), and orthosilicate (Na4SiO4) in its composition. Fundamental studies have already been carried out to investigate ion effects on mineral flotation, whereby these ions can help reagents to improve their performance, acting as activators, for example, and widening the selectivity gap between different mineral species. For example, Lelis (2014LELIS, D. F. Influência de cátions Ca2+, Mg2+ e Mn2+ na flotação reversa de minério de ferro. 2014. 89f. Dissertação (Mestrado em Engenharia Mineral) - Escola de Minas, Universidade Federal de Ouro Preto, Ouro Perto, 2014.) concluded that additions of Mn2+ ions were able to influence the quartz flotation in microflotation tests, in the presence of the collector Flotigam EDA (2.5 mg/L) and at pH 10.5. The values were reduced from approximately 100% of floatability to less than 5% with the use of 200 mg/L of Mn2+.
In this study, the evaluation of reagent schemes can offer greater selectivity in the direct flotation of oxidized manganese minerals, based on the depressant action for quartz and spessartine, as gangue minerals in gondite, representing more than 80% (De São José, 2019) of the ore mass. The spessartine is the mineral responsible for the low manganese content, high silica and alumina contents in the oxidized manganese concentrate by flotation (Abreu, 1973ABREU, S. F. Recursos minerais do Brasil. São Paulo: Editora Edgard Blucher, 1973.). Therefore, it is extremely important to reduce the amounts of silica to increase the product quality and to lower the costs for gondite processing. Thus, this study investigated the influence of Mn2+ ions (intentionally added) on some depressants for quartz and spessartine as an attempt to improve the beneficiation of gondite.
2. Material and methods
The minerals used at experiments were spessartine (a mineral that is difficult to find with purity greater than 95% w/w) and quartz. Spessartine was purchased from a mineral hoarder in Natal - RN and quartz pebbles were obtained in Ribeirão do Carmo located in the Mariana - MG. The samples were ground in a ceramic mortar and pestle, and after comminution, were classified between −106 +38 µm by wet screening (sieves of Tyler's series). The representative aliquots were subjected to semi-quantitative analysis using X-Ray Diffractometer (XRD), performed at PANalytical Empyrean diffractometer using radiation CuKa (λ = 1,54056 Å) and a graphite crystal monochromator to confirm the samples mineralogy and data analyzed by the Rietveld method. The chemical composition was determined by X-Ray Fluorescence (PANalytical, MagiX - PW 2540/2.4 kW).
Microflotation tests were carried out in duplicate in a modified Hallimond tube (volume = 310 cm3) to evaluate the performance of depressants (sodium silicate, sodium hexametaphosphate, and sodium fluorosilicate) added after the Mn2+ ions. For each test, 1 gram of minerals was used and the gas N2 was injected at a flow rate of 75 mL/min to produce the bubbles. The depressant performance was evaluated at three concentrations: 20; 40; and 80 mg/L and the conditioning time of 5 minutes. These experiments were performed at pH values close to 6, 8, and 10. The Mn2+ concentrations were: 1, 10, 100, and 1000 mg/L and the conditioning time of 5 minutes. The all solutions and the microflotation tests used distilled water. In this phase of the studies, no collector was used. Table 1 shows the conditions of the microflotation tests in a more accessible way.
An electrophoresis cell (Zeta Meter 4.0) determined the mineral zeta potentials for the reagent evaluation. Sodium silicate and manganese (II) chloride dehydrate (MnCl2×2H2O), Mn2+ ion source, with analytical purity (SYNTH) were used for zeta potential measurements. Using Smoluchowiski's equation, the Zeta determines the electrophoretic mobility and transforms into zeta potential (ζ). Moreover, the zeta potential for spessartine and quartz was determined in the pH range of 1 to 12. A 0.01% w/v suspension was prepared by the adding 1 g of the mineral (−38 µm) into a 1000 mL solution of KNO3 (10−3 mol/L), as an indifferent electrolyte. After 24 hours (an arbitrarily chosen period), the suspension was transferred to a 500 mL beaker, the reagents were added according to each test, and the pH was adjusted with HCl and NaOH under constant agitation. For each pH value, the zeta potential was determined as the average obtained from the duplicate values.
Infrared Fourier Transform Spectroscopy of MnCl2 salt dissolution species adsorbed on the surface of the spessartine and quartz was also performed. Thermo Nicolet model 6700 infrared spectrometer and FTIR-OMNIC software were used.
3. Results
3.1 Samples characterization
Semi-quantitative analysis refined XRD data by the Rietveld method showed the sample mineral proportions (Table 2): the spessartine sample was comprised of 94.70% of spessartine, considered a rare mineral, so the proportion was satisfactory for the proposed study. Quartz represented less than 5.0% of the spessartine proportion, and it was an acceptable impurity. The purification of spessartine sample by leaching was discarded, since such a procedure would not be controlled uni-directionally for the iron element and due to the great difficulties of finding pure spessartine, the use of the available sample was decided. The quartz sample was pure, free of others crystalline phases. Table 2 shows the chemical compositions of each mineral sample, useful for confirming its quality.
3.2 Microflotation tests
Fig. 1 summarizes the results of recovery for quartz and spessartine by flotation. Quartz showed 20% recovery at natural pH (~7.07) for the three depressants tested. The pH adjustment to 6 and the using of sodium silicate, for example, reduced the quartz and spessartine floatability at more than 12% and 15% respectively. Despite some oscillation, increased pH further reduced quartz and spessartine recoveries, but the exception was observed with sodium hexametaphosphate, which acted as a dispersant for quartz at pH 10. As such, the elevation in the dispersion role favored the quartz recovery by drag. As pH was increased, the reagents depressant role was intensified (except sodium hexametaphosphate), and with pH 10 the best result was reached (Fig. 1). At pH 10, the sodium silicate and sodium fluorosilicate provide the SiO(OH)3- species, which could be acting as a depressant for the minerals. However, the assumption needs to be confirmed through future research.
Minerals recovery effect of sodium silicate (SS), sodium hexametaphosphate (SH), sodium fluorosilicate (SF) as a function of pH values. N = pH natural and without depressant (point X and Z for quartz and spessartine, respectively).
Initially, the microflotation tests were carried out using three depressants reagents and Mn2+ dosages to investigate the depression for quartz and spessartine. The result pointed out that sodium silicate was the best depressant for quartz. The depressant concentration and pH value were found to be 80 mg/L at a pH of 10.00 ± 0.15. In these conditions, the sodium silicate reduces the quartz floatability by 13.24% (X-Y, Fig. 1). For spessartine, the reduction was more expressive and equal to 33.85% (Z-K, Fig. 1).When analyzing Fig. 3, it can show that Mn2+ additions can assist the depression of both quartz and spessartine. The effects of Mn2+ ions on quartz and spessartine depression showed positive results, such as reduction of quartz floatability from 7.06% (point A, Fig. 2) to 1.23% (point C, Fig. 3) and spessartine floatability of 27.30% (point B, Fig. 2) to 17.12% (point D, Fig. 3).
3.3. Zeta potential
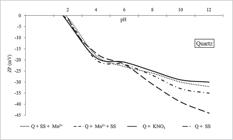
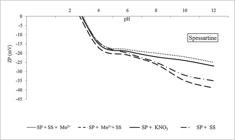
As shown in Fig. 4, the quartz sample presented an isoelectric point close to 1.8, using only KNO3 (10−3 mol/L) and sodium silicate (80 mg/L because it produced a satisfactory depression). Similar to quartz, the spessartine zeta potential was negative in a wide range of pH values (Fig. 5).
Quartz zeta potential. Q + SS + Mn2+: (1st sodium silicate; 2nd MnCl2); Q + Mn2+ + SS: (1st MnCl2; 2nd sodium silicate); Q + KNO3: quartz conditioned with KNO3 (10−3 mol/L); Q + SS: quartz; conditioned with sodium silicate.
Spessartine zeta potential. SP: spessartine conditioned with KNO3 (10−3 mol/L); SP+SS: spessartine conditioned with sodium silicate (80 mg/L); SP+SS+Mn2+: (1st sodium silicate; 2nd MnCl2); SP+Mn2++SS: (1st MnCl2; 2nd sodium silicate).
3.4. Infrared spectroscopy
Fig. 6 and 7 show the results of infrared spectroscopy analysis to the spessartine and quartz samples conditioned with MnCl2. In Fig. 6, the letter A refers to the test in which the quartz was conditioned with Mn2+ and the letter B without this ion.
In Fig. 7, the letter A refers to the test in which the spessartine was conditioned with Mn2+ and the letter B without this ion.
4. Discussion
4.1 Microflotation tests
It can be noted that when the Mn2+ ion was previously added to the depressant reagents, it assisted in the depressant action of quartz and spessartine minerals. A suggested mechanism to explain the sodium hexametaphosphate undesirable effect, as dispersant, was based on the stable hydrophilic complex owing to the complexation between sodium hexametaphosphate and metal ions present in a mineral surface (Yang et al., 2014YANG, Z.; FENG, Y.; LI, H.; WANG, W.; TENG, Q.; ZHANG, X. Effect of Mn(II) on quartz flotation using dodecylamine as collector. Journal of Central South University, v. 21, p. 3603-3609, 2014.). The sodium silicate can act in the same way and interact with the metal ions solution, forming nearly insoluble silicates precipitates on the mineral surface (Radley, 1968RADLEY, J. A. Starches and their derivatives. 4th ed. London: Chapman & Hall, 1968.). The sodium silicate is hydrophilic hydroxyl and increasing the mineral's depression. Mishra (1982MISHRA, S. K. Electrokinetic properties and flotation behaviour of apatite and calcite in the presence of sodium oleate and sodium metasilicate. International Journal of Mineral Processing, v. 9, p. 59-73, 1982.) concluded that sodium silicate molecules can adsorb on apatite and form a hydrophilized layer on their surface, thereby promoting mineral depression.
Yang et al., (2014YANG, Z.; FENG, Y.; LI, H.; WANG, W.; TENG, Q.; ZHANG, X. Effect of Mn(II) on quartz flotation using dodecylamine as collector. Journal of Central South University, v. 21, p. 3603-3609, 2014.) show by Fourier Transform Infrared Spectroscopy and Scanning Electron Microscopy-Energy Dispersive Spectroscopy analysis that the Mn2+ ion can form precipitates and adsorb on the quartz surface negatively charged. This induces the quartz recovery drops in alkaline pH systems. Duarte et al., (2015DUARTE, R. S.; LIMA, R. M. F.; LEÃO, V. A. Effect of inorganic and organic depressants on the cationic flotation and surface charge of rhodonite-rhodochrosite. REM - Revista da Escola de Minas, v. 68, n. 4, p. 463-469, 2015. ) used a manganese calcium silicate mineral and provided results about the MnCl2 depression effects on it. In agreement with the Mn speciation diagram, the manganese species in solution (at pH 10) are Mn2+ (0.0870 mg/L) and MnOH+ (0.0692 mg/L), and such species in solution enable the formation of precipitates like Mn(OH)2 that adsorb on the mineral surface, causing depression. The speciation diagram highlighted in Figure 8 reinforces that pH 10 can favor the existence of Mn2+ as the ionic species predominant at this pH. However, due to possible slow dissolution kinetics of manganese oxide minerals, which implies insufficient ions useful for the activation process, it is necessary to study mechanisms that can intensify the dissolution of minerals containing manganese.
Diagram of the log concentration of the Mn++ cation with a concentration of 10-4 M. (Fuerstenau et al., 1985FUERSTENAU, M. C.; MILLER, J. D.; KUHN, M. C. Chemistry of flotation. New York: Society of Mining Engineers of the AIME, 1985. 177p.).
4.2 Zeta potential
The isoelectric point (IEP) value for quartz conditioned with KNO3 is consistent with other previous studies, such as Parks (1967PARKS, G. A. Aqueous surface chemistry of oxides and complex oxide minerals. In: STUMM, W. (ed.). Equilibrium concepts in natural water systems. [S. l.]: American Chemical Society, 1967. cap. 6, p. 121-160. (Advances in Chemistry Series, v. 67).) and Ney (1973NEY, P. Zeta-potentiale und flotierbarkeit von mineralen. Berlim: Springer, 1973.) apud Rao (2004RAO, S. R. Surface chemistry of froth flotation: fundamentals. 2nd ed. Montreal: Kluwer Academic/Plenum Publishers, 2004.) who registered the quartz IEP equal to 1.9. Using the sodium silicate, the zeta potential was lowered to negative levels during the determinations and this behavior was intensified from pH 8.00. The sodium silicate dissociation results in species, such as Si(OH)4 and Si2O(OH)6, give rise to the anionic species SiO(OH)3- and SiO2(OH)22- in alkaline systems. The quartz surface has a negative charge net balance for all pH ranges below its IEP, showing that it has the same charge signal of the products dissociated from sodium silicate. Thus, chemical adsorption is responsible for reducing the zeta potential of the mineral in this case. Araújo and Lima (2017ARAÚJO, A. C. A.; LIMA, R. M. F. Influence of cations Ca2+, Mg2+ and Zn2+ on the flotation and surface charge of smithsonite and dolomite with sodium oleate and sodium silicate. International Journal of Mineral Processing, v. 167, p. 35-41, 2017.) showed similar results for smithsonite depression with sodium silicate and in the pH alkaline range, and the authors assumed that the SiO(OH)3- and SiO2(OH)22- species should adsorb and be responsible for intensifying the negative charge on the mineral surface (−20 mV to −50 mV). In the microflotation tests involving Mn2+ additions, there was observed a decline in quartz recovery until reaching the lowest value of 2.03% (point C, Fig. 3) at pH 10. And this result is in accordance with zeta potential determinations when the Mn2+ additions previous to the sodium silicate intensified the displacement of the quartz zeta potential to more negative values. The Mn2+ added previously to sodium silicate (Q + Mn2+ + SS curve, Fig. 4), can activate the adsorption of silicate species and therefore lead to this more negative potential on the quartz surface.
About spessartine, few studies have investigated the electrokinetic behavior of this mineral because it is a grenade and its industrial applications in the comminuted form have not been reported. Cases (1968CASES, J. M. Les phénoménes physico-chimiques à I’interface: application au procedé de la flotation. 1968. Thèse Dr. État - Faculté des Sciences de L’Université de Nancy, Nancy, 1968.) and Andrade (1978ANDRADE, V. L. L. Estudo da concentração do colúvio. 1978. 66f. Dissertação (Mestrado em Engenharia Mineral) - Universidade Federal de Minas Gerais, Belo Horizonte, 1978.) showed a spessartine's IEP equal to 4.45, more than the value found for the available spessartine. Variation in composition and the presence of contaminants can change the minerals electrokinetic parameters, as revealed in this study.
It should be emphasized that spessartine is a manganese aluminosilicate mineral, consisting of aluminum, manganese, and contaminant elements, with the power to raise the surface energy of the mineral and to reflect its electrokinetic behavior on flotation. The inclusion of the sodium silicate at the system not changed the spessartine's IEP. However, the sodium silicate showed to increase the density of charge at the mineral's double electric layer. At pH 10 (point of the greatest depression in microflotation), it was possible to generate a potential difference of 8 mV in the module. Regarding the species from the sodium silicate dissociation at pH 10, i.e., SiO(OH)3- and SiO2(OH)22-, these are responsible for the intensification of the negative charge at the electrical interface of the spessartine, for driving a negative charge balance.
Comparing the zeta potential for quartz and spessartine after conditioning with sodium silicate, higher effect on spessartine than the quartz is observed. The superficial Al3+ and Mn2+ cations present on spessartine act as preferential anchoring points to negative species and intensify the superficial negative net charge. For example, the adsorption of SiO(OH)3- on the kaolinite (Al4(Si4O10)(OH)8) surface occurs on the surfaces terminated in planes (001) with exposed aluminum atoms. As a result of SiO(OH)3- adsorption, the kaolinite surface becomes more hydrophilic and more negative (Jia et al., 2017).
The order of addition Mn2+ (first) and sodium silicate (second) showed an equal phenomenon for quartz and spessartine when the zeta potential changed to more negative values.
4.3 Infrared spectroscopy
For quartz (Fig. 6), a more marked absorption band was observed at the 3,450 cm-1 frequency band. The result is correlated with Lelis (2014LELIS, D. F. Influência de cátions Ca2+, Mg2+ e Mn2+ na flotação reversa de minério de ferro. 2014. 89f. Dissertação (Mestrado em Engenharia Mineral) - Escola de Minas, Universidade Federal de Ouro Preto, Ouro Perto, 2014.) determinations. The compound Mn(OH)2 exhibits absorption in the band near this frequency, which induces its presence as a precipitate on the surface of the quartz. For the spessartine (Fig. 7), absorption peak was identified at approximately 3,400 cm-1. The adsorption of Mn(OH)2 was responsible for the result. Duarte (2012DUARTE, R. S. Estudo de flotação de finos de minério de manganês sílico-carbonatado com amina. 2012. 101f. Dissertação (Mestrado em Engenharia Mineral) - Escola de Minas, Universidade Federal de Ouro Preto, Ouro Preto, 2012.) registered a reduction in the quartz recovery due to Mn2+ ions action because at pH 10, the Mn(OH)+ hydroxyl complex was formed, which adsorbed on the mineral surface and decreased the mineral floatability.
5. Conclusions
As a low-grade manganese ore, gondite can be a viable source of metal manganese after adequate industrial beneficiation. The demand for manganese is growing, rich manganese ores are being overexploited (Liu et al., 2014LIU, Y.; LIN, Q.; LI, L.; FU, J.; ZHU, Z.; WANG, C.; QIAN, D. Study on hydrometallurgical process and kinetics of manganese extraction from low-grade manganese carbonate ores. International Journal of Mining Science and Technology, v. 24, n.4, p.567-571, 2014.), and the poorer ones being set aside. Microflotation studies and zeta potential determinations show that Mn2+ acts as an activator and favors the adsorption of the depressants. The activation mechanisms are responsible for increasing the hydrophilization of sodium silicate capacity, related to the creation of anchorage points for species such as SiO(OH)3-. Moreover, the precipitation of manganese Mn(OH)+, favored by pH 10, had a depressant role for quartz and spessartine minerals. Zeta potential studies proved that the order of addition of Mn2+ and sodium silicate plays a key role in increasing mineral depression. The microflotation tests showed that pH 10 was crucial to the silicates' depression process because at this pH, the depressant reagents were hydrolyzed to negative species, with a stronger power of hydrophilization, and the precipitation phenomenon also helped mineral hydrophilization.
Acknowledgments
The authors thank the company Oratórios Engenharia Mineral LTDA for their support in the design and execution of this research, Gorceix Foundation, UFOP, CEFET-MG, CAPES.
References
- ABREU, S. F. Recursos minerais do Brasil São Paulo: Editora Edgard Blucher, 1973.
- ANDRADE, V. L. L. Estudo da concentração do colúvio 1978. 66f. Dissertação (Mestrado em Engenharia Mineral) - Universidade Federal de Minas Gerais, Belo Horizonte, 1978.
- ARAÚJO, A. C. A.; LIMA, R. M. F. Influence of cations Ca2+, Mg2+ and Zn2+ on the flotation and surface charge of smithsonite and dolomite with sodium oleate and sodium silicate. International Journal of Mineral Processing, v. 167, p. 35-41, 2017.
- BULATOVIC, S. R. Handbook of flotation reagents: chemistry, theory and practice: volume 1 - flotation of sulfide ores. Amsterdam: Elsevier, 2007. 448p.
- CASES, J. M. Les phénoménes physico-chimiques à I’interface: application au procedé de la flotation. 1968. Thèse Dr. État - Faculté des Sciences de L’Université de Nancy, Nancy, 1968.
- DE SÃO JOSÉ, F. Influência de íons Mn2+ na depressão de quartzo e espessartita em gondito de manganês 2019. 150f. Tese (Doutorado em Engenharia Mineral) - Escola de Minas, Universidade Federal de Ouro Preto, Ouro Preto, 2019.
- DUARTE, R. S. Estudo de flotação de finos de minério de manganês sílico-carbonatado com amina 2012. 101f. Dissertação (Mestrado em Engenharia Mineral) - Escola de Minas, Universidade Federal de Ouro Preto, Ouro Preto, 2012.
- DUARTE, R. S.; LIMA, R. M. F.; LEÃO, V. A. Effect of inorganic and organic depressants on the cationic flotation and surface charge of rhodonite-rhodochrosite. REM - Revista da Escola de Minas, v. 68, n. 4, p. 463-469, 2015.
- FARIA, G. L. Estudo da intensidade de crepitação de minérios granulados de manganês do Brasil 2008. 125f. Dissertação (Mestrado em Engenharia Mineral) - Escola de Minas, Universidade Federal de Ouro Preto, Ouro Preto, 2008.
- FUERSTENAU, M. C.; MILLER, J. D.; KUHN, M. C. Chemistry of flotation New York: Society of Mining Engineers of the AIME, 1985. 177p.
- JIA, Z. et al. Adsorption of ions at the interface of clay minerals and aqueous solutions. In: RAHMAN, M. M.; ASIRI, A. M. (ed.). Advances in colloid science. [S. l.]: IntechOpen, 2016. DOI 10.5772/65529. Available at: https://www.intechopen.com/books/advances-in-colloid-science/adsorption-of-ions-at-the-interface-of-clay-minerals-and-aqueous-solutions
» https://doi.org/10.5772/65529» https://www.intechopen.com/books/advances-in-colloid-science/adsorption-of-ions-at-the-interface-of-clay-minerals-and-aqueous-solutions - LELIS, D. F. Influência de cátions Ca2+, Mg2+ e Mn2+ na flotação reversa de minério de ferro 2014. 89f. Dissertação (Mestrado em Engenharia Mineral) - Escola de Minas, Universidade Federal de Ouro Preto, Ouro Perto, 2014.
- LIU, Y.; LIN, Q.; LI, L.; FU, J.; ZHU, Z.; WANG, C.; QIAN, D. Study on hydrometallurgical process and kinetics of manganese extraction from low-grade manganese carbonate ores. International Journal of Mining Science and Technology, v. 24, n.4, p.567-571, 2014.
- MISHRA, S. K. Electrokinetic properties and flotation behaviour of apatite and calcite in the presence of sodium oleate and sodium metasilicate. International Journal of Mineral Processing, v. 9, p. 59-73, 1982.
- NEY, P. Zeta-potentiale und flotierbarkeit von mineralen Berlim: Springer, 1973.
- PARKS, G. A. Aqueous surface chemistry of oxides and complex oxide minerals. In: STUMM, W. (ed.). Equilibrium concepts in natural water systems. [S. l.]: American Chemical Society, 1967. cap. 6, p. 121-160. (Advances in Chemistry Series, v. 67).
- RADLEY, J. A. Starches and their derivatives 4th ed. London: Chapman & Hall, 1968.
- RAO, S. R. Surface chemistry of froth flotation: fundamentals. 2nd ed. Montreal: Kluwer Academic/Plenum Publishers, 2004.
- SAHOO, R. N.; NAIK, P. K.; DAS, S. C. Leaching of manganese from low-grade manganese ore usingoxalic acid as reductant in sulphuric acid solution. Hydrometallurgy, v. 62, p. 157-163, 2001.
- SOUZA, J. V. Geologia e gênese do protominério e do minério da província manganesífera de Aracoiaba-Pacajús, no Estado do Ceará 1979. 188f. Tese (Doutorado em Geociências) - Instituto de Geociências, Universidade de São Paulo, São Paulo, 1979.
- UNITED STATES GEOLOGICAL SURVEY. Mineral commodity summaries 2019 Reston, Virginia: U.S. Geological Survey, 2019. 204 p.
- YANG, Z.; FENG, Y.; LI, H.; WANG, W.; TENG, Q.; ZHANG, X. Effect of Mn(II) on quartz flotation using dodecylamine as collector. Journal of Central South University, v. 21, p. 3603-3609, 2014.
Publication Dates
-
Publication in this collection
13 Jan 2021 -
Date of issue
Jan-Mar 2021
History
-
Received
01 Jan 2020 -
Accepted
30 July 2020