Abstract
The aim of this study is to assess the floatability of hematite using a crude biosurfactant (BS) extracted from Rhodococcus opacus. Throughout high-pressure ethanol extraction, unrefined surfactant was extracted from the bacteria with a yield of 0.3 g/L. An FTIR analysis on this extract, shows the presence of alcohol (–OH) and ketone (C=O) groups, as well as saturated and unsaturated carbon chains, which might originate from mycolates and trehalolipids moieties reported in literature. In addition, FTIR analysis on the modified mineral surface with the unrefined surfactant, confirms a strong interaction between hematite and the crude biosurfactant, showing similar vibrational peaks regarding the hydroxyl and carboxylate groups at 1631 and 3436 cm-1, respectively. Biosurfactant interaction with the mineral particles shifted the isoelectric point from 7.5 to 3.2, turning the hematite more hydrophobic in acidic environments. Surface tension measurements indicate that the unrefined surfactant is slightly more effective, decreasing the surface tension of water compared to the bacteria itself, with a variation of 4 mM/m. Finally, throughout microflotation tests in a Hallimond tube, it is shown that the surfactant doubles bacteria recovery efficiency, reaching a maximum recovery of around 95 % in acidic conditions, which is consistent with electrophoretic studies. The statistical models adjust well the experimental data, achieving R2 values of 93.6 and 91.1% for the hematite flotation using the biomass and the biosurfactant, respectively.
Key words:
bioflotation; biosurfactants; hematite flotation; Rhodococcus opacus
1. Introduction
Mineral resources are steadily diminishing and the search for new technology able to recover low grade deposits is becoming evident. Additionally, nowadays there is an increasing awareness of the environmental impact of the mining industry. Mineral flotation processes use surfactants that significantly contribute to water pollution when released in effluents (Zoller et al., 2000ZOLLER, U.; HUSHAN, M. The nonionic surfactant pollution profile of Israel Mediterranean Sea coastal water. Water science and technology, v. 42, n. 1-2, p. 429-435, 2000.). The use of microorganisms and their by-products to selectively recover minerals with a lower environmental impact could be a relevant alternative. Previous studies showed the potential of developing a new technology by the harnessing of microorganisms as bioreagents (Yang et al., 2013YANG, H.; LI, T.; TANG, Q.; WANG, C.; MA, W. Development of a bio-based collector by isolating a bacterial strain using flotation and culturing techniques. International Journal of Mineral Processing, v. 123, p. 145-151, 2013.; Mesquita et al., 2003MESQUITA, L. M. S.; LINS, F. A. F.; Torem, M. L. Interaction of a hydrophobic bacterium strain in a hematite–quartz flotation system. International Journal of Mineral Processing, vol. 57, p. 609-618, 2003.). However insufficient understanding of the mechanisms, kinetics, and thermodynamics of the process, and even physical factors (Puelles, 2016PUELLES, J. G. S. Bioflotation of hematite using the crude biosurfactant extracted from Rhodococcus opacus. 2016. Dissertação (Mestrado em Engenharia Química, de Materiais e Processos Ambientais) - Departamento de Engenharia Química e de Materiais, Pontifícia Universidade Católica do Rio de Janeiro, Rio de Janeiro, 2016.; Kim et al., 2017KIM, G.; CHOI, J.; SILVA, R. A.; SONG. Y.; KIM, H. Feasibility of bench-scale selective bioflotation of copper oxide minerals using Rhodococcus opacus. Hydrometallurgy, v. 168, p. 94-102, 2017.) hinders their successful upscale. Moreover, the potential uses of microbes in the flotation process was properly discussed by Kinnunen et al. (2020)KINNUNEN, P.; MIETTINEN, H.; BOMBERG, M. Review of potential microbial effects on flotation. Minerals, v. 10, n. 533, p. 1-14, 2020.. The authors suggested that certain categories of bacteria could be used as potential flotation collectors.
Amphiphilic substances located in the cellular wall or excreted by the microorganisms are responsible for their adhesion onto the mineral surface, and consequently, for their selective floatability (Kuyumcu et al., 2009KUYUMCU, H. Z.; BIELIG, T.; VILINSKA, A.; RAO, K. H. Biocoagulation and its application potentials for mineral bioprocessing. The Open Mineral Processing Journal, v. 2, n. 1, p. 1-11, 2009.). Such biosurfactants fulfil several functions, such as facilitating the growth of their producers by increasing substrate availability, transporting nutrients, or acting as biocide agents (Puelles, 2016PUELLES, J. G. S. Bioflotation of hematite using the crude biosurfactant extracted from Rhodococcus opacus. 2016. Dissertação (Mestrado em Engenharia Química, de Materiais e Processos Ambientais) - Departamento de Engenharia Química e de Materiais, Pontifícia Universidade Católica do Rio de Janeiro, Rio de Janeiro, 2016.; Rodrigues et al., 2006RODRIGUES, L.; BANAT, I. M.; TEIXEIRA, J.; OLIVEIRA, R. Biosurfactants: potential applications in medicine. Journal of Antimicrobial Chemotherapy, v. 57, n. 4, p. 609-618, 2006.). The isolation of these substances by a simple process and their harnessing in mineral flotation might be the next level in the research of mineral bioremediation. The complex molecular structure of biosurfactants grant them with unique properties such as resilience to extreme environments, selective adsorption, and even antimicrobial characteristics. The present study evaluates the effectiveness of the crude biosurfactant extracted from the gram-positive bacteria Rhodococcus Opacus, recovering hematite mineral at neutral and acid conditions. In addition, surface characterization studies involving FTIR and Zeta potential, aid us in proposing an interaction mechanism. Finally, a comparison performance between the biosurfactant and the biomass was done through statistical modelling.
2. Methodology
2.1 Microorganism and culture media
The growing of the Rhodococcus opacus strain was based on the research work of Puelles (2016)PUELLES, J. G. S. Bioflotation of hematite using the crude biosurfactant extracted from Rhodococcus opacus. 2016. Dissertação (Mestrado em Engenharia Química, de Materiais e Processos Ambientais) - Departamento de Engenharia Química e de Materiais, Pontifícia Universidade Católica do Rio de Janeiro, Rio de Janeiro, 2016.; where the details of all experimental procedures are fully described. The strain was obtained from the Chemical, Biological and Agricultural Pluridisciplinary Research Center (CPQBA - UNICAMP). The microorganism was grown in an YMG (yeast, malt and glucose) solid and liquid medium (Table 1). The liquid broth was kept three days at 28 °C and continuously stirred at 150 rpm. In addition, the initial pH was adjusted to 7.2.
2.2 Extraction of the crude biosurfactant
The extraction of the crude biosurfactant was based on the high-pressure ethanol extraction method (Moreau et al., 2003MOREAU, R.; POWELL, M. J.; SINGH, V. Pressurized liquid extraction of polar and nonpolar lipids in corn and oats with hexane, methylene chloride, isopropanol, and ethanol. Journal of the American Oil Chemists’ Society, v. 80, p. 1063-1067, 2003.; Puelles, 2016PUELLES, J. G. S. Bioflotation of hematite using the crude biosurfactant extracted from Rhodococcus opacus. 2016. Dissertação (Mestrado em Engenharia Química, de Materiais e Processos Ambientais) - Departamento de Engenharia Química e de Materiais, Pontifícia Universidade Católica do Rio de Janeiro, Rio de Janeiro, 2016.) (Figure 1). The protocol involves the separation of the planktonic cells from their broth through centrifugation, washing the biomass with deionized water and its resuspension in ethanol (98%) at 1 bar (relative to atmospheric pressure) and 121 °C. At these conditions, it was possible to keep the liquid phase of ethanol, effectively extracting all ethanol soluble compounds from the bacteria. The extract was dried and redissolved in deionized water and the liquid phase, obtained by centrifugation and filtration, was dried again and stored at 4 °C.
2.3 FTIR analysis
FTIR spectroscopy was carried out on the dried biosurfactant and on hematite particles before and after biosurfactant interaction, using a Nicolet FTIR 6700 instrument with a KBr matrix. Initially, the samples were dried at 40°C and the samples were mixed with KBr in a proportion of 1/200 (weight). The mineral particles (-20 µm) were conditioned with a known concentration of the biosurfactant (150 mg/L) for 3 minutes. After that time, the samples were rinsed with deionized water and dried up to 40 °C for 12 h.
2.4 Zeta potential measurements
Zeta potential was measured with a Zeta-Meter 4.0 instrument. Hematite particles (-20 µm) were suspended in different electrolyte concentrations to determine the isoelectric point. For the modified mineral, the particles were conditioned with the crude biosurfactant solution for 3 minutes. Table 2 shows details of the experiment.
2.5 Surface tension measurements
The surface tension of the biosurfactant solution was measured using a DC 200 Surface Electro-Optics tensiometer, by the Nöuy ring method at 22 ± 2 °C. The pH solution was neutral and the biosurfactant concentration was varied from 0 to 250 g/L to estimate the critical micelle concentration (CMC).
2.6 Microflotation tests
The microflotation assays were carried out in a modified Hallimond tube configured with a ceramic frit that diffuses air inside the tube. The mineral sample was conditioned with the biosurfactant inside the tube for 3 minutes and subsequently, a constant airflow rate of 35 ml/min was supplied for 2 minutes. Then, the floated and non-floated fractions were collected, dried, and weighed and floatability was calculated. We studied two scenarios, hematite flotation with the bacteria itself and with the biosurfactant solution. Table 3 presents the experimental design for both cases; note that the variables ranges were the same for the bacteria as well as the biosurfactant.
3. Results and discussion
3.1 Production and characterization of Rhodococcus opacus and its biosurfactant
This study did not evaluate the bacterial growth, however, Botero et al. (2007)BOTERO, A. E. C.; TOREM, M. L.; MESQUITA, L. M. S. Fundamental studies of Rhodococcus opacus as a biocollector of calcite and magnesite. Minerals Engineering, v. 20, n. 10, p. 1026-1032, 2007. reported a cell density of 3.5 x 1010 cells/L, using the same strain. The biosurfactant extracted from Rhodococcus opacus bacteria presented a production of around 0.3 g of biosurfactant per litre of broth. According to Lang and Philp (1998)LANG, S.; PHILP, J. C. Surface-active lipids in Rhodococci. Antonie van Leeuwenhoek, v. 74, n. 1-3, p. 59-70, 1998., the Rhodoccoci species produce surfactants of predominantly cell-associated trehalolipids, which justifies the broth removal in the extraction process.
Figure 2 illustrates the FTIR spectra of R. opacus and its extracted biosurfactant. In the fingerprint region (below 1500 cm−1), a large number of absorption peaks were identified because of the variety of C–C, C–O, and C–N single-bond vibrations that might occur. In the spectra of R. opacus, intense peaks between 1700 and 1620 and medium peaks between 2924 and 2850 and between 1540 and 1080 cm−1 were detected. These peaks are characteristics of the functional groups presented in aldehydes, ketones, aromatic compounds, and alkanes. Additionally, the peak at 1237.15 cm−1 is typical of a phosphate group that may come from nucleic acids; a more detailed analysis can be found in Puelles (2016)PUELLES, J. G. S. Bioflotation of hematite using the crude biosurfactant extracted from Rhodococcus opacus. 2016. Dissertação (Mestrado em Engenharia Química, de Materiais e Processos Ambientais) - Departamento de Engenharia Química e de Materiais, Pontifícia Universidade Católica do Rio de Janeiro, Rio de Janeiro, 2016.. These functional groups are characteristic of mycolate substances that form part of the bacterial cell wall, and it is believed that they are responsible for the hydrophobicity of the bacteria. The FTIR spectra of the crude surfactant suggest the presence of alkane (2929 cm-1, 1047 cm-1), alkene (1629 cm-1), alcohol (3397 cm-1) and ketone groups (1629 cm-1). These functional groups could be related to mycolic acids, which are produced by the Rhodococci species (Nishiuchi et al., 2000; Puelles, 2016PUELLES, J. G. S. Bioflotation of hematite using the crude biosurfactant extracted from Rhodococcus opacus. 2016. Dissertação (Mestrado em Engenharia Química, de Materiais e Processos Ambientais) - Departamento de Engenharia Química e de Materiais, Pontifícia Universidade Católica do Rio de Janeiro, Rio de Janeiro, 2016.). Although, it was reported that bacterial proteins play a determinant role in flocculation and flotation processes because of its amphiphilic character (Patra and Natarajan, 2008PATRA, P.; NATARAJAN, K. Role of mineral specific bacterial proteins in selective flocculation and flotation. International Journal of Mineral Processing, v. 88, n. 1-2, p. 53-58, 2008.). In the present study, the presence of proteins was discarded due to the high temperatures and pressures used in the extraction process that would denaturize them, irreversibly. However, the possible presence of amino and aromatic compounds may indicate soluble amino acids contained in the crude biosurfactant solution.
3.2 Infrared analysis of hematite by FTIR
Figure 3 shows the FTIR spectra of the hematite surface before and after crude biosurfactant interaction at pH 3. The new peak formed at 3436 cm−1, after biosurfactant interaction, might correspond to the vibrations of the –OH groups; a similar behaviour was observed during the FTIR analysis of magnesite and calcite after interacting with R. opacus (Botero et al., 2008BOTERO, A. E. C.; TOREM, M. L.; MESQUITA, L. M. S. Surface chemistry fundamentals of biosorption of Rhodococcus opacus and its effect in calcite and magnesite flotation. Minerals Engineering, v. 21, n. 1, p. 83–92, 2008.). The adsorption of the BS onto the hematite surface could be proved by the presence of the resonance peaks at 2923 and 1631 cm−1, which correspond to the vibrations of alkane and carboxyl groups, respectively.
FTIR spectra of mineral before interacting (blue) and after interacting (red) with the BS extracted from R. opacus.
Even though, the spectra evidence adhesion of the BS onto the surface mineral, not all the resonance peaks of the biosurfactant appeared. Decreasing in the spectra resolution due to scattering interference of the hematite particles may hide some other active groups attached to the mineral surface. Similar peaks between the biosurfactant and modified hematite spectra may suggest a specific adsorption driven force. Before the study, we rinsed the samples with deionized water to remove weakly attached substances.
3.3 Zeta potential measurements of hematite before and after interacting with BS
Figure 4 illustrates the zeta potential profile of hematite as a function of pH. Three electrolyte concentrations were used in order to confirm the isoelectric point value (IEP). It is possible to observe that the zeta potential value decreased as the NaCl concentration increased. According to Hiemenz et al. (1997)HIEMENZ, P. C.; RAJAGOPALAN, R. Principles of colloid and surface chemistry. 3rd ed. Boca Raton: CRC Press, 1997. this is due to the electric double layer compression effect. Additionally, it is observed that the resultant IEP was 7.4. In a previous study, the hematite presented an IEP of 5.1 (Mesquita et al., 2003MESQUITA, L. M. S.; LINS, F. A. F.; Torem, M. L. Interaction of a hydrophobic bacterium strain in a hematite–quartz flotation system. International Journal of Mineral Processing, vol. 57, p. 609-618, 2003.); this difference with the present study might be a consequence of a different elemental composition of the mineral. It is suggested that when no other ions except the potential determining ions (PDI) and indifferent ions were present, then the point of zero charge and the isoelectric point could be approximately the same. Nevertheless, these points can be different, if surface-active ions were present (Leja and Rao, 2004LEJA, J.; RAO, R. Surface chemistry of froth flotation. 2nd ed. New York: Springer, 2004.).
The mechanism of charge acquisition and complexation of hematite surface is described in Equations (1) and (2). For this, it is suggested that the hydrolysed species (≡ XOH) might be the surface groups of the mineral. According to the surface complexation models of metal oxides, it is assumed that the hematite surface group could undergo two protonating steps (Gunnarsson, 2002GUNNARSSON, M. Surface complexation at the iron oxide/water interface: experimental investigations and theoretical developments. 2002. Akademisk avhandling (Filosofie doktor i kemi med inriktning mot oorganisk kemi) - Institutionen för kemi, Göteborgs universitet Göteborg, 2002.).
Hence, for pH values lower than 7.6 (IEP), the ≡ FeOH2+ species may be the predominant species, and the hematite surface acquires a positive electric charge. Around its IEP value, the neutral ≡ FeOH species might predominate, and finally, at pH values higher than its IEP, a negative surface charge would be expected due to the predominance of the species ≡ FeO-.
Figure 5 shows the zeta potential profile of hematite after interacting with the crude biosurfactant, using an electrolyte concentration of 10−3 mol/L. The error bars represent the maximum and minimum measurement, the experimental procedure had two replicas, and the middle point was the average. Previous studies (Puelles, 2016PUELLES, J. G. S. Bioflotation of hematite using the crude biosurfactant extracted from Rhodococcus opacus. 2016. Dissertação (Mestrado em Engenharia Química, de Materiais e Processos Ambientais) - Departamento de Engenharia Química e de Materiais, Pontifícia Universidade Católica do Rio de Janeiro, Rio de Janeiro, 2016.; Merma et al., 2013MERMA, A. G.; TOREM, M. L.; MORÁN, J. J. V.; MONTE, M. B. M. On the fundamental aspects of apatite and quartz flotation using a Gram positive strain as a bioreagents. Minerals Engineering, v. 48, p. 61-67, 2013.; Botero et al., 2007BOTERO, A. E. C.; TOREM, M. L.; MESQUITA, L. M. S. Fundamental studies of Rhodococcus opacus as a biocollector of calcite and magnesite. Minerals Engineering, v. 20, n. 10, p. 1026-1032, 2007., Mesquita et al., 2003MESQUITA, L. M. S.; LINS, F. A. F.; Torem, M. L. Interaction of a hydrophobic bacterium strain in a hematite–quartz flotation system. International Journal of Mineral Processing, vol. 57, p. 609-618, 2003.) showed an acidic shift of the IEP using the bacteria as bioreagent. The similarity between the biosurfactant and the bacteria may suggest that the same active component of the bacteria wall was present in the biosurfactant.
Zeta potential profile of hematite after interacting with BS. [NaCl]: 10-3 mol/L, [BS]: 120 mg/L.
According to Fuerstenau et al. (2005)FUERSTENAU, D. W.; PRADIP. Zeta potentials in the flotation of oxide and silicate minerals. Advances in Colloid and Interface Science, v. 114-115, p. 9-26, 2005., the adsorption mechanisms of oleate onto the hematite surface might be evidenced by zeta potential analysis. The authors suggested that oleate behaves like an anionic collector and electrostatic repulsion hinders its adhesion at basic pH. In the present study, the whole zeta potential profile was altered after biosurfactant interaction, which may be evidence of amphoteric substances contained in the crude biosurfactant. Lang and Philp (1998)LANG, S.; PHILP, J. C. Surface-active lipids in Rhodococci. Antonie van Leeuwenhoek, v. 74, n. 1-3, p. 59-70, 1998. proposed that the trehalolipids, located on the cellular wall of Rhodococcus opacus, develop a non-ionic form as trehalose-dimycolates or an anionic form as trehalose-tetraester, due to their ionizable carboxyl group. It is important to mention that the crude BS consisted of a mixture of different organic substances, such as polysaccharides, fatty acids, phospholipids, and perhaps even soluble amino acids, which may interfere in the zeta potential analysis.
3.4 Surface tension measurements
Figure 6 illustrates the surface tension of crude biosurfactant solutions and bacteria suspensions at various concentrations. It is observed that the crude biosurfactant is more effective decreasing the surface tension at the liquid-air interface, compared to the bacteria itself with a variation of 4 mN/m. In both cases the surface tension stabilizes around 150 mg/L. Merma et al (2013)MERMA, A. G.; TOREM, M. L.; MORÁN, J. J. V.; MONTE, M. B. M. On the fundamental aspects of apatite and quartz flotation using a Gram positive strain as a bioreagents. Minerals Engineering, v. 48, p. 61-67, 2013. proposed that the bacteria adsorbs at the water/solution interface, promoting the formation of a stable froth. Christova et al. (2014)CHRISTOVA, N.; STOINEVA, I. Trehalose biosurfactants. In: MULLIGAN, C. N.; SHARMAN, S. K.; MUDHOO, A. (ed.). Biosurfactants research trends and applicactions. Boca Raton: CRC Press, 2014. p. 183-190. indicated that most of the biosurfactants identified in the Rhodococcus genus reduce the surface tension of water between 19 and 43 mN/m, which is consistent with our results.
Surface tension in function of the concentration for crude biosurfactant solution and bacteria suspensions at 23 °C and pH 7.
Thus, it is possible to suggest that both, the biosurfactant and the biomass may act as frothers in flotation processes. Moreover, the BS may form a more stable froth.
3.5 Hematite flotation using Rhodococcus opacus
Figure 7 depicts the floatability of hematite using Rhodococcus opacus as bioreagent varying pH and concentration; the maximum floatability was between 45 and 47% at pH 7 for concentrations greater than 75 mg/L. The cellular wall of the bacteria has mycolic acids, whose unsaturated chains are perpendicular to the exterior medium (Kuyukina et al., 2010KUYUKINA, M. S.; IVSHINA, I. B. Rhodococcus biosurfactants: biosynthesis, properties, and potential applications. In: ALVAREZ, H. M. (ed.). Biology of Rhodococcus. Heidelberg: Springer-Verlag, 2010.) and are responsible for its hydrophobic nature. When the microorganisms adhere onto the mineral surface, it may form a hydrophobic layer, which has a strong affinity by the gas phase. Merma et al. (2013)MERMA, A. G.; TOREM, M. L.; MORÁN, J. J. V.; MONTE, M. B. M. On the fundamental aspects of apatite and quartz flotation using a Gram positive strain as a bioreagents. Minerals Engineering, v. 48, p. 61-67, 2013. showed that the hematite presented high contact angles near the IEP. Zeta potential measurements showed that the IEP of hematite is close to neutral pH, therefore the mycolates of the bacteria cellular wall and the mineral surface may have hydrophobic interaction.
Hematite floatability as a function of pH, using R. opacus bacteria as collector. [NaCl]: 10-3 mol/L; particle size (+75-150) µm; flotation time: 90 seconds; airflow rate: 35 L/min.
At pH 5 and 7, there was still significant floatability of hematite which may indicate other adhesion mechanisms. Such results may indicate that different substances from the bacteria, for example extra polymeric saccharides, also adhere to the mineral surface, making the hematite surface partially hydrophobic.
Polynomial models are empirical statistical modelling techniques that integrate statistical experimental designs and empirical model building by regression for process description, development or improvement. These models are commonly used to map a response surface over a delimited region of interest, optimize the responses, or manage the parameter conditions to reach a desirable specification (Myers et al., 2016MYERS, R. H.; MONTGOMERY, D. C.; ANDERSON-COOK, C. M. Response surface methodology: process and product optimization using designed experiments. 4th ed. New Jersey: John Wiley & Sons, 2016.).
The adjusted model to predict mineral floatability was a polynomial function of 4th and 3th order of pH (X1) and biomass concentration (X2), respectively. Table 4 shows the estimate coefficients of the statistical model, with their respective p-value. The terms in red do not significantly affect the hematite floatability and can be discarded from the model. Thus, according to the statistical analysis the polynomial model that better fits the effect of the biomass concentration and pH on the hematite floatability is presented in Eq. 3. The criteria for finding the best correlation relays on the statistics p and the square correlation coefficient R2 (Walpole et al., 2012WALPOLE, R. E.; MYERS, R.; MYERS, S.; KEYING, Y. E. Probability and statistics for engineers and scientists. 9th ed. Boston: Prentice Hall, 2012. ), for this case the R2 was of 93.6%.
Figure 8a shows the surface response contrasted with the real data. Additionally, Figure 8b presents the corresponding contour graphic (isolines). The blue lines at pH 3 and 9 indicate lower floatability, under 10%; the effect of biomass concentration at these conditions were not significant. Similar behaviour was observed during the flotation of hematite and apatite using the R. erythropolis and R. opacus bacteria, respectively. In both cases the mineral floatability was no significant for pH values higher than 9 (Olivera et al., 2017OLIVERA, C. A. C; MERMA, A. G.; PUELLES, J. G. S.; TOREM, M. L. On the fundamental aspects of hematite bioflotation using a Gram positive strain. Minerals Engineering, 106, 55-63, 2017.; Merma et al., 2013MERMA, A. G.; TOREM, M. L.; MORÁN, J. J. V.; MONTE, M. B. M. On the fundamental aspects of apatite and quartz flotation using a Gram positive strain as a bioreagents. Minerals Engineering, v. 48, p. 61-67, 2013.). At neutral pH, there was observed a positive effect of bacteria concentration on mineral floatability. The maximum value (between 40 and 45 %) was found in the centre of the contour plot, at pH 7 and a bacterial concentration of 125 mg/L. A similar value was found by Olivera et al. (2017)OLIVERA, C. A. C; MERMA, A. G.; PUELLES, J. G. S.; TOREM, M. L. On the fundamental aspects of hematite bioflotation using a Gram positive strain. Minerals Engineering, 106, 55-63, 2017., but at pH 6, the difference could be related to the different kind of strains.
Floatability of hematite using the biomass as collector in a surface response graphic (a) and a contour plot (b).
3.6 Hematite flotation using the crude biosurfactant
Figure 9 illustrates the floatability of hematite using the crude biosurfactant. There is a difference in mineral floatability between the biosurfactant and the bacteria. In the former case, the floatability is greater at acidic pH while in the last scenario, the bacteria floated the mineral in neutral pH. In addition, the floatability of hematite using the biosurfactant was approximately 96%, whereas the maximum, using the bacteria, was between 45 and 47%. The behaviour of the crude biosurfactant was similar to sodium dodecyl sulfate, reported to reach 95% floatability of hematite at acidic pH values (Vidyadhar et al., 2014VIDYADHAR, A.; KUMARI, N.; BHAGAT, R., P. Adsorption mechanism of mixed cationic/anionic collectors in quartz–hematite flotation system. Mineral Processing and Extractive Metallurgy Review, v. 35, n. 2, p. 117-125, 2014.), suggesting the biosurfactant may act as an anionic collector; this is supported by the fact that most of the non-pathogenic biosurfactants have an anionic nature (Christova et al., 2014CHRISTOVA, N.; STOINEVA, I. Trehalose biosurfactants. In: MULLIGAN, C. N.; SHARMAN, S. K.; MUDHOO, A. (ed.). Biosurfactants research trends and applicactions. Boca Raton: CRC Press, 2014. p. 183-190.). Based on electrophoretic studies, the IEP of hematite was 7.1; at acidic pH levels, electrostatic attractions may occur between the mineral surface and the anionic biosurfactant, resulting in maximum adhesion and therefore maximum hematite floatability. On the other hand, at basic pH levels the adhesion may be minimal because of electrostatic repulsion. Both, FTIR as well as zeta potential results showed that non-specific and specific (predominantly, chemisorption) adsorption might take place on the interaction between the biosurfactant and the mineral surface.
Hematite floatability as a function of pH, using BS as collector. [NaCl] 10-3 mol/L; particle size: (+75-150) µm; flotation time: 60 seconds; airflow rate: 35 L/min.
Another difference between using the biomass and the biosurfactant for the flotation of hematite was the amount and structure of the created froth. The latter produced a stable froth, even though the amount was smaller compared to the bacteria. Therefore, the production of froth did not relate to the efficiency of the collector.
The polynomial regression for the bioflotation using the biosurfactant is of 2nd order on the pH (X1) and 3rd order on the BS concentration (X2). Table 5 shows the p-value criteria and the significant factors. It is interesting to highlight that qualitatively it seems that the pH has a significant effect on the hematite floatability. However, it is statistically demonstrated that the real effect is a combination between the pH and the quadratic term of BS concentration.
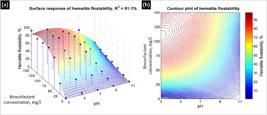
Equation (4) represents the polynomial function, fitted though multilinear regression with a correlation coefficient of 91.1%. The statistical model of the biosurfactant is simpler than the bacteria, Eq. (3), which may evidence an easy control of the process.
The surface response of the model of the hematite recovery using the biosurfactant is shown in Figure 10. The red isolines represent a hematite floatability greater than 75%. The maximum floatability region (red zone) corresponds to a pH between 3 and 7 and a biosurfactant concentration greater than 75 mg/L.
Floatability of hematite using the crude BS as collector in a surface response graphic (a) and a contour plot (b).
It is possible to observe that both models well adjust the experimental data, presenting correlation coefficients higher than 90%. The models showed that pH of solution and bioreagents concentration presented statistical significance, and the model for the biosurfactant was simpler.
It is important to point out that the flotation with the biosurfactant presented a higher effectiveness in comparison with the bacteria itself. Moreover, the flotation using the biosurfactant presented a fast kinetic process, achieving the maximum hematite floatability in about 45 seconds, while for the biomass, there was required at least 2 minutes. In this context, it is suggested that the biosurfactant presents a higher potential as a chemical bioreagent to be used in mineral processing, especially for the flotation processing of hematite ores.
4. Conclusions
-
The floatability of hematite was greater using the crude biosurfactant than the bacteria, with a maximum mineral recovery of 95 % at a slightly acid pH 5 and a concentration of 100 mg/L.
-
FTIR test shows modification on the mineral surface after interaction with the biosurfactant, which may be attributed predominantly to specific adsorption. However, due to scattering losses in the sample because of the hematite particles, other surface analytical techniques should help in the understanding of the surface chemistry of the modified mineral.
-
The zeta potential profile of the hematite suggested an anionic biosurfactant, where maximum mineral recovery exists at positive surface potentials, below the IEP, and electrostatic repulsion may hinder the hematite floatability at basic pH.
-
Both zeta potential and FTIR tests showed that a combined adsorption process (specific and non-specific adsorption), take place in the interaction between the biosurfactant and hematite surface.
Acknowledgements
The authors acknowledge CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), ITV-VALE (Vale Institute of Technology), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and FAPERJ (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro) for their financial support.
References
- BOTERO, A. E. C.; TOREM, M. L.; MESQUITA, L. M. S. Surface chemistry fundamentals of biosorption of Rhodococcus opacus and its effect in calcite and magnesite flotation. Minerals Engineering, v. 21, n. 1, p. 83–92, 2008.
- BOTERO, A. E. C.; TOREM, M. L.; MESQUITA, L. M. S. Fundamental studies of Rhodococcus opacus as a biocollector of calcite and magnesite. Minerals Engineering, v. 20, n. 10, p. 1026-1032, 2007.
- CHRISTOVA, N.; STOINEVA, I. Trehalose biosurfactants. In: MULLIGAN, C. N.; SHARMAN, S. K.; MUDHOO, A. (ed.). Biosurfactants research trends and applicactions Boca Raton: CRC Press, 2014. p. 183-190.
- FUERSTENAU, D. W.; PRADIP. Zeta potentials in the flotation of oxide and silicate minerals. Advances in Colloid and Interface Science, v. 114-115, p. 9-26, 2005.
- GUNNARSSON, M. Surface complexation at the iron oxide/water interface: experimental investigations and theoretical developments. 2002. Akademisk avhandling (Filosofie doktor i kemi med inriktning mot oorganisk kemi) - Institutionen för kemi, Göteborgs universitet Göteborg, 2002.
- HIEMENZ, P. C.; RAJAGOPALAN, R. Principles of colloid and surface chemistry 3rd ed. Boca Raton: CRC Press, 1997.
- KIM, G.; CHOI, J.; SILVA, R. A.; SONG. Y.; KIM, H. Feasibility of bench-scale selective bioflotation of copper oxide minerals using Rhodococcus opacus. Hydrometallurgy, v. 168, p. 94-102, 2017.
- KINNUNEN, P.; MIETTINEN, H.; BOMBERG, M. Review of potential microbial effects on flotation. Minerals, v. 10, n. 533, p. 1-14, 2020.
- KUYUKINA, M. S.; IVSHINA, I. B. Rhodococcus biosurfactants: biosynthesis, properties, and potential applications. In: ALVAREZ, H. M. (ed.). Biology of Rhodococcus Heidelberg: Springer-Verlag, 2010.
- KUYUMCU, H. Z.; BIELIG, T.; VILINSKA, A.; RAO, K. H. Biocoagulation and its application potentials for mineral bioprocessing. The Open Mineral Processing Journal, v. 2, n. 1, p. 1-11, 2009.
- LANG, S.; PHILP, J. C. Surface-active lipids in Rhodococci. Antonie van Leeuwenhoek, v. 74, n. 1-3, p. 59-70, 1998.
- LEJA, J.; RAO, R. Surface chemistry of froth flotation 2nd ed. New York: Springer, 2004.
- MERMA, A. G.; TOREM, M. L.; MORÁN, J. J. V.; MONTE, M. B. M. On the fundamental aspects of apatite and quartz flotation using a Gram positive strain as a bioreagents. Minerals Engineering, v. 48, p. 61-67, 2013.
- MESQUITA, L. M. S.; LINS, F. A. F.; Torem, M. L. Interaction of a hydrophobic bacterium strain in a hematite–quartz flotation system. International Journal of Mineral Processing, vol. 57, p. 609-618, 2003.
- MOREAU, R.; POWELL, M. J.; SINGH, V. Pressurized liquid extraction of polar and nonpolar lipids in corn and oats with hexane, methylene chloride, isopropanol, and ethanol. Journal of the American Oil Chemists’ Society, v. 80, p. 1063-1067, 2003.
- MYERS, R. H.; MONTGOMERY, D. C.; ANDERSON-COOK, C. M. Response surface methodology: process and product optimization using designed experiments. 4th ed. New Jersey: John Wiley & Sons, 2016.
- OLIVERA, C. A. C; MERMA, A. G.; PUELLES, J. G. S.; TOREM, M. L. On the fundamental aspects of hematite bioflotation using a Gram positive strain. Minerals Engineering, 106, 55-63, 2017.
- PATRA, P.; NATARAJAN, K. Role of mineral specific bacterial proteins in selective flocculation and flotation. International Journal of Mineral Processing, v. 88, n. 1-2, p. 53-58, 2008.
- PUELLES, J. G. S. Bioflotation of hematite using the crude biosurfactant extracted from Rhodococcus opacus 2016. Dissertação (Mestrado em Engenharia Química, de Materiais e Processos Ambientais) - Departamento de Engenharia Química e de Materiais, Pontifícia Universidade Católica do Rio de Janeiro, Rio de Janeiro, 2016.
- RODRIGUES, L.; BANAT, I. M.; TEIXEIRA, J.; OLIVEIRA, R. Biosurfactants: potential applications in medicine. Journal of Antimicrobial Chemotherapy, v. 57, n. 4, p. 609-618, 2006.
- VIDYADHAR, A.; KUMARI, N.; BHAGAT, R., P. Adsorption mechanism of mixed cationic/anionic collectors in quartz–hematite flotation system. Mineral Processing and Extractive Metallurgy Review, v. 35, n. 2, p. 117-125, 2014.
- WALPOLE, R. E.; MYERS, R.; MYERS, S.; KEYING, Y. E. Probability and statistics for engineers and scientists 9th ed. Boston: Prentice Hall, 2012.
- YANG, H.; LI, T.; TANG, Q.; WANG, C.; MA, W. Development of a bio-based collector by isolating a bacterial strain using flotation and culturing techniques. International Journal of Mineral Processing, v. 123, p. 145-151, 2013.
- ZOLLER, U.; HUSHAN, M. The nonionic surfactant pollution profile of Israel Mediterranean Sea coastal water. Water science and technology, v. 42, n. 1-2, p. 429-435, 2000.
Publication Dates
-
Publication in this collection
21 July 2021 -
Date of issue
Jul-Sep 2021
History
-
Received
09 Sept 2020 -
Accepted
10 Feb 2021