Abstract
Adsorption of microorganisms and/or their different components onto a mineral surface would modify the surface characteristics of the mineral. Thus, this investigation evaluated the adsorption capacity of the Rhodococcus opacus strain onto an apatite surface. Zeta potential and contact angle measurements of the mineral showed dislocations of the values after interaction with the microorganism. The maximum adsorption density reached was 24.10 mg of bacterial cells per gram of mineral using a biomass concentration of 400 mg/L. The experimental data were linearly fitted by the Freundlich model and the adsorption density as a function of time was linearly fitted by the pseudo-second order kinetic equation. The results showed that the bacterial strain has affinity for the apatite surface and ability to make it hydrophobic.
Key words:
bioflotation; adhesion; adsorption, apatite;
R. opacus
; zeta potential
1. Introduction
Phosphorus is an essential element in the life of all living beings. In nature, phosphorus is used with other elements forming phosphates, which have different chemical and mineralogical characteristics, depending on the type of phosphoric rock deposit. Phosphate rock is an indispensable raw material for the manufacture of industrial products, and is widely used in agriculture, the chemical industry, food production, pharmacy and many others (Gu, 2007GU, Z. Dolomite flotation of high magnesium phosphate ores using fatty acid soap collectors. 2007. Dissertation (Doctor of Philosophy in Mineral Processing) - College of Engineering and Mineral Resources, West Virginia University, Morgantown, 2007. Available at: https://researchrepository.wvu.edu/etd/4303
https://researchrepository.wvu.edu/etd/4...
; Ruan et al., 2019RUAN, Y.; HE, D.; CHI, R. Review on beneficiation techniques and reagents used for phosphate ores. Minerals, [s. l.], v. 9, n. 4, 2019. Available at: https://doi.org/10.3390/MIN9040253
https://doi.org/10.3390/MIN9040253...
; Wang, 2004WANG, X. The surface chemistry of phosphate mineral flotation with alcohol solutions of octyl hydroxamic acid. 2004. Dissertation (Doctor of Philosophy) - Department of Metallurgical Engineering, University of Utah, Salt Lake City, 2004.).
The most common types of phosphate deposits are igneous, metamorphic, sedimentary and biogenic (guano accumulations). In the world, about 75% of phosphate resources are attributed to a sedimentary origin (Abouzeid, 2008ABOUZEID, A. Z. M. Physical and thermal treatment of phosphate ores: an overview. International Journal of Mineral Processing, v. 85, n.4, p. 59-84, 2008. Available at: https://doi.org/10.1016/j.minpro.2007.09.001
https://doi.org/10.1016/j.minpro.2007.09...
; Ruan et al., 2019RUAN, Y.; HE, D.; CHI, R. Review on beneficiation techniques and reagents used for phosphate ores. Minerals, [s. l.], v. 9, n. 4, 2019. Available at: https://doi.org/10.3390/MIN9040253
https://doi.org/10.3390/MIN9040253...
). The main mineral in phosphate rock is apatite and the importance in the processing of this mineral is the production of phosphoric acid, which is used as raw material to produce fertilizers. Population growth and high phosphate demand has depleted the supply of high-grade, low-impurity phosphate ores. Most phosphate minerals are composed of a low P2O5 content and typically contain several gangue minerals, such as feldspar, quartz, mica, dolomite, calcite and clays. Therefore, the phosphate processing industry faces a major challenge, which is how to economically and efficiently exploit those low-grade phosphate minerals (Ruan et al., 2019RUAN, Y.; HE, D.; CHI, R. Review on beneficiation techniques and reagents used for phosphate ores. Minerals, [s. l.], v. 9, n. 4, 2019. Available at: https://doi.org/10.3390/MIN9040253
https://doi.org/10.3390/MIN9040253...
; Zafar et al., 1996ZAFAR, I. Z.; ANWAR, M. M.; PRITCHARD, D. W. Innovations in beneficiation technology for low grade phosphate rocks. Nutrient Cycling in Agroecosystems, [s. l.], v. 46, n. 2, p. 135–151, 1996. Available at: https://doi.org/10.1007/bf00704313
https://doi.org/10.1007/bf00704313...
). Given the growing demand for the exploration of low-content phosphate deposits, the rigorous specifications of flotation concentrates, strict environmental laws and the need to reduce operational costs, encouraged several investigations with a view to finding better processing techniques and greater effectiveness of reagents in the selective separation of phosphate minerals. In this context, mineral biotechnology may be an attractive process, due to its ability for selective adhesion of microorganisms and their interactions with different mineral surfaces, low operating costs and lower environmental impact (Mesquita et al., 2003MESQUITA, L. M. S.; LINS, F. F.; TOREM, M. L. Interaction of a hydrophobic bacterium strain in a hematite-quartz flotation system. International Journal of Mineral Processing, [s. l.], v. 71, n. 1–4, p. 31–44, 2003. Available at: https://doi.org/10.1016/S0301-7516(03)00028-0
https://doi.org/10.1016/S0301-7516(03)00...
).
The chemical compounds produced in the microorganism surface cell may induce hydrophobic (for flotation processes) and/or hydrophilic (for flocculation processes) properties. Thus, in the flotation process, the adhesion/adsorption of the microorganism and/or metabolic products onto the mineral surface is a mandatory step. So, the attachment of hydrophobic mineral particles to the air bubbles can occur, as well as the flotation progression (Botero et al., 2007BOTERO, A. E. C.; TOREM, M. L.; MESQUITA, L. M. S. Fundamental studies of Rhodococcus opacus as a biocollector of calcite and magnesite. Minerals Engineering, [s. l.], v. 20, n. 10, p. 1026–1032, 2007. Available at: https://doi.org/10.1016/j.mineng.2007.03.017
https://doi.org/10.1016/j.mineng.2007.03...
; Mesquita et al., 2003MESQUITA, L. M. S.; LINS, F. F.; TOREM, M. L. Interaction of a hydrophobic bacterium strain in a hematite-quartz flotation system. International Journal of Mineral Processing, [s. l.], v. 71, n. 1–4, p. 31–44, 2003. Available at: https://doi.org/10.1016/S0301-7516(03)00028-0
https://doi.org/10.1016/S0301-7516(03)00...
).
Therefore, to obtain a better understanding of the promoted apatite bioflotation, the present study develops the basic principles of adhesion of the Rhodococcus opacus strain to an apatite surface.
2. Materials and methods
2.1 Mineral sample
The apatite mineral (Ca5 (PO4)3 (F, Cl, OH)) was supplied by CETEM - Brazil. The degree of purity of the mineral sample (40% P2O5 and 53% CaO) was confirmed using X-ray fluorescence. The mineral sample was crushed and ground dry, then screened wet to obtain the desired granulometric fractions and used in zeta potential tests (<38 µm), adhesion experiments (-74 +38 µm) and contact angle measurements (5 × 5 × 10 mm).
2.2 Microorganism, media and growth
The Rhodococcus opacus strain was supplied by the CBMAI (Brazilian Collection of Environmental and Industrial Microorganisms) in Brazil. The microorganism was inoculated in a YMG (yeast and malt extract with glucose) culture medium, composed of 1 g/dL glucose, 0.5 g/dL peptone, 0.3 g/d malt extract and 0.3 g/dL yeast extract. The growth of the microorganism was carried out in liquid medium using 500 mL Erlenmeyer bottles that were placed in a rotary shaker at 170 rpm for 70 h at a temperature of 28 °C. Then, the culture broth was centrifuged, and the biomass obtained was washed with deionized water and sterilized in an autoclave to avoid further development of the bacteria. In order to improve the affinity of the microorganisms for apatite surface, the adaptation procedure developed in Merma et al. (2017)MERMA, A. G.; HACHA, R. H.; TOREM, M. L. Cellular adaptation: culture conditions of R. opacus and bioflotation of apatite and quartz. REM - International Engineering Journal, Ouro Preto, v. 70, n.1, p. 67 – 76, 2017. Available at: https://doi.org/10.1590/0370-446720167000063.
https://doi.org/10.1590/0370-44672016700...
was followed.
2.3 Surface properties of the mineral
Measurements of the zeta potential for the mineral, microorganism and mineral-microorganism interaction were performed using a Zeta meter system 4.0+ micro-electrophoresis equipment. For this, mineral and microorganism, solutions were prepared with concentrations of 0.1 g/L in an indifferent electrolyte of 0.001 mol/L NaCl. The pH modification of the solutions was performed using aliquots of HCl and NaOH.
The hydrophobicity evaluation of the mineral surface before and after the interaction with the microorganism was carried out by means of a standard Ramé-Hart goniometer using the captive bubble method. The ore sample was cut (0.5 x 0.5 x 0.5 x 0.1 cm) and polished with a diamond paste with 3 µm and 1 µm particles. The samples were then cleaned with distilled water jets and ultrasonic water bath. Next, the sample was suspended in 0.15 g/L of the biomass for 5 min and washed lightly with NaCl solution (10-3 mol/L) to remove excess bacterial cells. Finally, the samples were dried under vacuum in a desiccator for 10 min and then contact angle measurements were performed. This procedure followed the methodology of Merma et al. (2013)MERMA, A. G.; TOREM, M. L.; MORÁN, J. J.; MONTE, M. B. On the fundamental aspects of apatite and quartz flotation using a Gram positive strain as a bioreagent. Minerals Engineering, [s. l.], v. 48, p. 61–67, 2013. Available at: https://doi.org/10.1016/j.mineng.2012.10.018
https://doi.org/10.1016/j.mineng.2012.10...
and was performed at different pH values. The measurements were carried out in triplicate to guarantee a consistent result.
2.4 Adhesion experiments
Adhesion experiments were performed in 0.25 L Erlenmeyer bottles containing 0.1 L of bacterial solution of known concentration (25, 50, 100, 200, 300 and 400 mg/L) and at different pH values (6, 7, 8, 9 and 10). To each Erlenmeyer flask, 1 g of mineral sample was added and placed on a rotary shaker at 170 rpm for 30 min at different temperature values (20, 30 and 40 °C). Once the contact time had elapsed, the suspension (bacterial cells and mineral) was centrifuged at 2,000 rpm for 5 minutes, whereby the mineral with the adsorbed bacteria sank to the bottom of the tube and the non-adsorbed bacteria remained in the aqueous solution. Then, an aliquot of aqueous solution was extracted to measure the absorbance of the solution by a Shimadzu UV-1800 spectrophotometer. The concentration of biomass adhered to the mineral surface was estimated by a calibration curve of absorbance versus cell concentration, more details of the procedure can be found somewhere else (Olivera et al., 2017OLIVERA, C. A. C.; MERMA, A. G.; PUELLES, J. G. S.; TOREM, M. L. On the fundamentals aspects of hematite bioflotation using a Gram positive strain. Minerals Engineering, [S. l.], v. 106, p. 55-63, 2017. Available at: https://doi.org/10.1016/j.mineng.2016.10.017.
https://doi.org/10.1016/j.mineng.2016.10...
). It is possible to observe the bacterial adhesion of the R. opacus onto apatite surface by using scanning electron microscopy (Carl Zeiss-DSM 960 SEM) (Morán, 2014; Olivera, 2018).
In addition, to determine the interaction between Rhodococcus opacus bacteria and the apatite surface, the Langmiur (Equation 1) and Freundlich isotherm (Equation 2) models were used.
Where: Cf, is equilibrium concentration (mg/L); q (mg/g), is the amount of bacterial cells adhered per mass of mineral at equilibrium; qmax (mg/g), is the Langmuir parameter related to the adsorption capacity; Kads (L/mg), is the Langmuir constant. In addition, kf and 1/n are the Freundlich constants. The constant kf, is a function of adsorption energy and temperature and is a measure of adsorption capacity, and 1/n determines the adsorption intensity (Kalavathy et al., 2005KALAVATHY, M. H.; KARTHIKEYAN, T.; RAJGOPAL, S.; MIRANDA, L. R. Kinetic and isotherm studies of Cu(II) adsorption onto H3PO4-activated rubber wood sawdust. Journal of Colloid and Interface Science, [s. l.], v. 292, n. 2, p. 354–362, 2005. Available at: https://doi.org/10.1016/j.jcis.2005.05.087
https://doi.org/10.1016/j.jcis.2005.05.0...
; Okeola and Odebunmi, 2010OKEOLA, E. O.; ODEBUNMI, F. O. Freundlich and Langmuir isotherms parameters for adsorption of methylene blue by activated carbon derived from Agrowastes. Advances in Natural and Applied Sciences, [s. l.], v. 4, n. 3, p. 281–288, 2010.; Volesky and Holan, 1995VOLESKY, B.; HOLAN, Z. R. Biosorption of heavy metals. Biotechnology Progress, [s. l.], v. 11, n. 3, p. 235–250, 1995. Available at: https://doi.org/10.1021/bp00033a001
https://doi.org/10.1021/bp00033a001...
).
The adsorption kinetic was studied by the pseudo-first and pseudo-second order kinetic model, represented in Equation 3 and Equation 4.
Where: q and qe are the amount of bacterial cells adhered per mass of mineral (mg/g) at any time t and at equilibrium, respectively, and k is the pseudo first order rate constant of adsorption (min−1); h=k2 qe2 can be regarded as the initial adsorption rate and k2 is the pseudo second order rate constant of adsorption (g/mg.min) (Kowanga et al., 2016KOWANGA, K. D.; GATEBE, E.; MAUTI, G. O.; MAUTI, E. M. Kinetic, sorption isotherms, pseudo-first-order model and pseudo-second-order model studies of Cu ( II ) and Pb ( II ) using defatted Moringa oleifera seed powder. The journal of phytopharmacology, [s. l.], v. 5, n. 2, p. 71–78, 2016. Available at: http://www.phytopharmajournal.com
http://www.phytopharmajournal.com...
).
3. Results and discussion
3.1 Zeta potential measurements
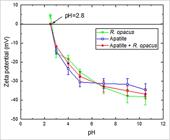
Figure 1 shows the results of zeta potential of the Rhodococcus opacus bacteria and the mineral before and after the interaction with the microorganism.
Zeta potential curves of the Rhodococcus opacus bacteria and the apatite mineral (indifferent electrolytic: NaCl 10-3 mol/L).
Figure 1 shows the zeta potential profiles of the mineral, the strain and the mineral/strain interaction. It is observed that the microorganism presented an isoelectric point (IEP) surrounding pH of 2.8 (Morán, 2014), a result that coincides with the study carried out by Vásquez et al. (2007)VÁSQUEZ, T. G. P.; BOTERO, A. E. C.; MESQUITA, L. M. S.; TOREM, M. L. Biosorptive removal of Cd and Zn from liquid streams with a Rhodococcus opacus strain. Minerals Engineering, [s. l.], v. 20, n. 9, p. 939–944, 2007. Available at: https://doi.org/10.1016/j.mineng.2007.03.014
https://doi.org/10.1016/j.mineng.2007.03...
. Other authors, such as Botero et al. (2007)BOTERO, A. E. C.; TOREM, M. L.; MESQUITA, L. M. S. Fundamental studies of Rhodococcus opacus as a biocollector of calcite and magnesite. Minerals Engineering, [s. l.], v. 20, n. 10, p. 1026–1032, 2007. Available at: https://doi.org/10.1016/j.mineng.2007.03.017
https://doi.org/10.1016/j.mineng.2007.03...
; Bueno et al. (2008)BUENO, B. Y. M.; TOREM, M. L.; MOLINA, F. A. L. M. S.; MESQUITA, L. M. S. Biosorption of lead(II), chromium(III) and copper(II) by R. opacus: equilibrium and kinetic studies. Minerals Engineering, [s. l.], v. 21, n. 1, p. 65–75, 2008. Available at: https://doi.org/10.1016/j.mineng.2007.08.013
https://doi.org/10.1016/j.mineng.2007.08...
and Cayllahua et al. (2009)CAYLLAHUA, J. E. B.; CARVALHO, R. J.; TOREM, M. L. Evaluation of equilibrium, kinetic and thermodynamic parameters for biosorption of nickel(II) ions onto bacteria strain, Rhodococcus opacus. Minerals Engineering, [s. l.], v. 22, n. 15, p. 1318–1325, 2009. Available at: https://doi.org/10.1016/j.mineng.2009.08.003
https://doi.org/10.1016/j.mineng.2009.08...
found pH values of around 3.2. This change could be attributed to the origin of the strain and to its growing conditions. On the other hand, the isoelectric point of the mineral was attained at a pH of around 2.6. After microorganism adhesion, no relevant change in the IEP of the mineral was identified, indicating little predominance of electrostatic interactions between the cell wall of the microorganism and the mineral surface. This effect is corroborated by Yang et al. (2013)YANG, H.; TANG, Q.; WANG, C.; ZHANG, J. Flocculation and flotation response of Rhodococcus erythropolis to pure minerals in hematite ores. Minerals Engineering, [s. l.], v. 45, p. 67–72, 2013. Available at: https://doi.org/10.1016/j.mineng.2013.01.005
https://doi.org/10.1016/j.mineng.2013.01...
, Yang et al. (2014)YANG, H. F.; LI, T.; CHANG, Y. H.; LUO, H.; TANG, Q. Y. Possibility of using strain F9 (Serratia marcescens) as a bio-collector for hematite flotation. International Journal of Minerals, Metallurgy and Materials, [s. l.], v. 21, n. 3, p. 210–215, 2014. Available at: https://doi.org/10.1007/s12613-014-0887-8. Accessed: 12 Dec. 2018.
https://doi.org/10.1007/s12613-014-0887-...
and Olivera et al. (2017)OLIVERA, C. A. C.; MERMA, A. G.; PUELLES, J. G. S.; TOREM, M. L. On the fundamentals aspects of hematite bioflotation using a Gram positive strain. Minerals Engineering, [S. l.], v. 106, p. 55-63, 2017. Available at: https://doi.org/10.1016/j.mineng.2016.10.017.
https://doi.org/10.1016/j.mineng.2016.10...
. In addition, at a pH value of 7 both curves approach each other, indicating very similar electrokinetic characteristics. This observation may suggest that the adhesion may be related to a combination of a specific and non-specific adsorption mechanisms of the bacterial cells.
According to the results achieved in this study, the electrostatic interaction can be a trifling one between bacteria and surface apatite, which may suggest the predominance of a kind of specific adsorption between the different functional groups present in the cell wall and the apatite surface (Yang et al., 2013YANG, H.; TANG, Q.; WANG, C.; ZHANG, J. Flocculation and flotation response of Rhodococcus erythropolis to pure minerals in hematite ores. Minerals Engineering, [s. l.], v. 45, p. 67–72, 2013. Available at: https://doi.org/10.1016/j.mineng.2013.01.005
https://doi.org/10.1016/j.mineng.2013.01...
, 2014YANG, H. F.; LI, T.; CHANG, Y. H.; LUO, H.; TANG, Q. Y. Possibility of using strain F9 (Serratia marcescens) as a bio-collector for hematite flotation. International Journal of Minerals, Metallurgy and Materials, [s. l.], v. 21, n. 3, p. 210–215, 2014. Available at: https://doi.org/10.1007/s12613-014-0887-8. Accessed: 12 Dec. 2018.
https://doi.org/10.1007/s12613-014-0887-...
).
3.2 Contact angle measurements
The results of the apatite contact angle measurements before the interaction showed values around zero, indicating its hydrophilic character. After the interaction with bacterial cells (Figure 2), an increase in contact angle values of the apatite was observed. This is directly related to the adhesion of the R. opacus cells, which shared their hydrophobic properties to the apatite surface increasing its hydrophobic degree (Botero et al., 2008BOTERO, A. E. C.; TOREM, M. L.; MESQUITA, L. M. S. Surface chemistry fundamentals of biosorption of Rhodococcus opacus and its effect in calcite and magnesite flotation. Minerals Engineering, [s. l.], v. 21, n. 1, p. 83–92, 2008. Available at: https://doi.org/10.1016/j.mineng.2007.08.019
https://doi.org/10.1016/j.mineng.2007.08...
). This effect is clearly showed at a pH of 7, where the highest contact angle value (36°) is achieved. With respect to the other pH values, a lesser interaction between Rhodococcus opacus bacteria and apatite surface is shown. Considering the highest contact angle and corroborating the studies of Mesquita et al. (2003)MESQUITA, L. M. S.; LINS, F. F.; TOREM, M. L. Interaction of a hydrophobic bacterium strain in a hematite-quartz flotation system. International Journal of Mineral Processing, [s. l.], v. 71, n. 1–4, p. 31–44, 2003. Available at: https://doi.org/10.1016/S0301-7516(03)00028-0
https://doi.org/10.1016/S0301-7516(03)00...
and Merma et al., (2013)MERMA, A. G.; TOREM, M. L.; MORÁN, J. J.; MONTE, M. B. On the fundamental aspects of apatite and quartz flotation using a Gram positive strain as a bioreagent. Minerals Engineering, [s. l.], v. 48, p. 61–67, 2013. Available at: https://doi.org/10.1016/j.mineng.2012.10.018
https://doi.org/10.1016/j.mineng.2012.10...
, the results are in accordance. According to these studies, the maximum contact angle values were reached in the pH range between 3 and 5, demonstrating a larger interaction between the bacterial cells and mineral surfaces.
Values of contact angle of the apatite mineral after interaction with the R. opacus bacteria. Cell concentration 100 mg/L and contact time of 5 minutes.
The bacterial cell surface structure is composed of various components. These substances and biomolecules on the bacterial cell surface control the physicochemical properties of a bacterial cell and alter the properties of the mineral surface. For example, outer membrane lipopolysaccharides (LPS) are highly hydrophilic and the presence of proteins outside the LPS layer results in a hydrophobic surface, while the negative charge is provided by phosphate, carboxylate and sulfate groups (Rao and Subramanian, 2007RAO, K. H.; SUBRAMANIAN, S. Bioflotation and bioflocculation of relevance to minerals bioprocessing. In: DONATI, E. R., SAND, W. (ed.). Microbial processing of metal sulfides. Dordrecht: Springer, 2007. p. 267–286. Available at: https://doi.org/10.1007/1-4020-5589-7_14
https://doi.org/10.1007/1-4020-5589-7_14...
).
Adhesion experiments
Figure 3-a and Figure 3-b show SEM images of Rhodococcus opacus cells and cells adhered onto apatite surface, respectively.
SEM Images of the bacterial cells: a) Rhodococcus opacus cells and b) Rhodococcus opacus cells onto the apatite surface.
Figure 3 shows that the bacterial cells have a rod and spherical shape, which indicates a mixture of growth phases, mainly in the exponential and stationary stage, because there was no homogeneity of growth at the time that they were removed from the culture broth. Scanning electron micrographs show the attachment of bacterial cells on the apatite surface (Figure 3-b). It can be seen that the bacterial cells have a low surface affinity for the apatite surface, and consequently low adhesion. Other authors also studied the adhesion of microorganisms to the surfaces of various minerals and their adsorption capacity varied from one mineral to another (Chandraprabha and Natarajan, 2006CHANDRAPRABHA, M. N.; NATARAJAN, K. A. Surface chemical and flotation behaviour of chalcopyrite and pyrite in the presence of Acidithiobacillus thiooxidans. Hydrometallurgy, [s. l.], v. 83, n. 1–4, p. 146–152, 2006. Available at: https://doi.org/10.1016/j.hydromet.2006.03.021
https://doi.org/10.1016/j.hydromet.2006....
; Farahat et al., 2008FARAHAT, M.; HIRAJIMA, T.; SASAKI, K.; AIBA, Y.; DOI, K. Adsorption of SIP E. coli onto quartz and its applications in froth flotation. Minerals Engineering, [s. l.], v. 21, n. 5, p. 389–395, 2008. Available at: https://doi.org/10.1016/j.mineng.2007.10.019
https://doi.org/10.1016/j.mineng.2007.10...
; Olivera et al., 2017OLIVERA, C. A. C.; MERMA, A. G.; PUELLES, J. G. S.; TOREM, M. L. On the fundamentals aspects of hematite bioflotation using a Gram positive strain. Minerals Engineering, [S. l.], v. 106, p. 55-63, 2017. Available at: https://doi.org/10.1016/j.mineng.2016.10.017.
https://doi.org/10.1016/j.mineng.2016.10...
; Patra and Natarajan, 2008PATRA, P.; NATARAJAN, K. A. Role of mineral specific bacterial proteins in selective flocculation and flotation. International Journal of Mineral Processing, [s. l.], v. 88, n. 1–2, p. 53–58, 2008. Available at: https://doi.org/10.1016/j.minpro.2008.06.005. Accessed: 12 Dec. 2018.
https://doi.org/10.1016/j.minpro.2008.06...
; Santhiya et al., 2001SANTHIYA, D.; SUBRAMANIAN, S.; NATARAJAN, K. A.; RAO, K. H.; FORSSBERG, K. S. E. Bio-modulation of galena and sphalerite surfaces using Thiobacillus thiooxidans. International Journal of Mineral Processing, [s. l.], v. 62, n. 1–4, p. 121–141, 2001. Available at: https://doi.org/10.1016/S0301-7516(00)00048-X
https://doi.org/10.1016/S0301-7516(00)00...
; Zheng et al., 2001ZHENG, X.; ARPS, P. J.; SMITH, R. W. Adhesion of two bacteria onto dolomite and apatite: their effect on dolomite depression in anianic flotation. International Journal of Mineral Processing, [s. l.], v. 62, n. 1–4, p. 159–172, 2001. Available at: https://doi.org/10.1016/S0301-7516(00)00050-8.
https://doi.org/10.1016/S0301-7516(00)00...
).
The adhesion results of Rhodococcus opacus bacteria on the apatite surface as a function of cell concentration, and at temperature values of 20, 30 and 40 °C are shown in Figure 4-a, Figure 4-b and Figure 4-c, respectively. It is observed that there was an increase in adsorption density as the concentration of bacterial cells increases. This phenomenon occurred because there was a greater amount of bacterial cells in the solution and therefore, a higher probability of collision between the bacterial cell and the mineral surface. Schilling et al. (1994)SCHILLING, K. M.; CARSON, R. G.; BOSKO, C. A.; GOLIKERI, G. D.; BRUINOOGE, A.; HOYBERG, K.; WALLER, A. M.; HUGHES, N. P. A microassay for bacterial adherence to hydroxyapatite. Colloids and Surfaces B: Biointerfaces, [s. l.], v. 3, n. 1–2, p. 31–38, 1994. Available at: https://doi.org/10.1016/0927-7765(93)01120-G
https://doi.org/10.1016/0927-7765(93)011...
studied the adhesion of the Actinomyces naeslundii bacteria to the hydroxyapatite surface and verified an increase in adsorption density as the concentration of bacterial cells in the solution increases. The same phenomenon was presented by Botero et al. (2007)BOTERO, A. E. C.; TOREM, M. L.; MESQUITA, L. M. S. Fundamental studies of Rhodococcus opacus as a biocollector of calcite and magnesite. Minerals Engineering, [s. l.], v. 20, n. 10, p. 1026–1032, 2007. Available at: https://doi.org/10.1016/j.mineng.2007.03.017
https://doi.org/10.1016/j.mineng.2007.03...
, who studied the adhesion of R. opacus bacteria on the calcite and magnesite surfaces.
Influence of bacterial concentration and solution pH on adhesion density at different temperature values: a) 20°C, b) 30 °C and c) 40 °C.
The pH value of the bacterial-mineral suspension plays an important role in the adhesion process. For the present study, the authors considered pH values higher than 6. The last was due to the high solubility of apatite in the acidic medium. From the results obtained, it is possible to observe the influence of the concentration of H+ and OH– ions in the cell adhesion onto an apatite surface. These ions interact with the functional groups of different molecules present in the cell wall of the bacteria, activating them and thus, allowing their interaction with the mineral surface. The adhesion results showed that at the pH value of 7 there was a greater affinity between the bacteria and the mineral, and therefore, a greater quantity of bacterial cells adhered to the mineral surface. The adsorption density at that pH was of 22.50, 23.30 and 24.10 mg of bacterial cells per gram of mineral, at temperature values of 20, 30 and 40 °C, respectively, using a cell concentration of 400 mg/L. For pH values different from 7, the adsorption capacity of bacterial cells to the mineral surface tends to decrease due to lower affinity. The same effect was found by Rong et al. (2010)RONG, X.; CHEN, W.; HUANG, Q.; CAI, P.; LIANG, W. Pseudomonas putida adhesion to goethite: Studied by equilibrium adsorption, SEM, FTIR and ITC. Colloids and Surfaces B: Biointerfaces, [s. l.], v. 80, n. 1, p. 79–85, 2010. Available at: https://doi.org/10.1016/j.colsurfb.2010.05.037
https://doi.org/10.1016/j.colsurfb.2010....
in the study of the adhesion of Pseudomonas putida bacteria to the goethite surface. On the other hand, Jiang et al. (2007)JIANG, D.; HUANG, Q.; CAI, P.; RONG, X.; CHEN, W. Adsorption of Pseudomonas putida on clay minerals and iron oxide. Colloids and Surfaces B: Biointerfaces, [s. l.], v. 54, n. 2, p. 217–221, 2007. Available at: https://doi.org/10.1016/j.colsurfb.2006.10.030
https://doi.org/10.1016/j.colsurfb.2006....
showed variations in the adhesion values of Pseudomonas putida bacteria to the mineral surfaces of kaolinite, goethite and montmorillonite due to the influence of the solution pH. The adsorption capacity of P. putida on the mineral surfaces increased with a pH of 2 to 3 and decreased with a pH of 3 to 10.
Adsorption capacity is usually described through by isotherms. The most common types of adsorption isotherms used in biosorption processes are the Langmuir and Freundlich models. Both models were used to adjust the experimental results of adsorption density (highest results at pH = 7). It was observed that the adsorption data did not fit well (r2 = 0.25) to the Langmiur isotherm because of the heterogeneity of the mineral surface (see Figure 3), and also because the model assumes that the adsorbed bacterial cells interact with an active site on the mineral surface and not with each other. Meanwhile, the Freundlich isotherm adequately described the absorption process by assuming interactions on heterogeneous surfaces, as well as linkages between bacterial cells. The graphic representation of the linearized plot is shown in Figure 5 and the Freundlich constants obtained at different conditions are summarized in Table 1.
Adsorption isotherm of R. opacus onto apatite surface. The data were fitted with Freundlich model (Temperature: 20°, 30°, 40° C).
The results show a parameter "n" greater than 1, which represents a favourable adhesion process, demonstrating the affinity that exists between the mineral surface and the compounds present in the bacterial cell wall. It is observed that with the increase in temperature, there is an increase in the parameter "n", and consequently, an increase in adsorption capacity.
The adsorption density as a function of time is shown in Figure 6. The maximum contact time was 30 min. because in greater times aggregation of bacterial cells can be generated and these cell flocs would negatively influence the adhesion values (Jiang et al., 2007JIANG, D.; HUANG, Q.; CAI, P.; RONG, X.; CHEN, W. Adsorption of Pseudomonas putida on clay minerals and iron oxide. Colloids and Surfaces B: Biointerfaces, [s. l.], v. 54, n. 2, p. 217–221, 2007. Available at: https://doi.org/10.1016/j.colsurfb.2006.10.030
https://doi.org/10.1016/j.colsurfb.2006....
).
Effects of time and temperature on the adsorption density: pH 7 and cell concentration of 100 mg/L (Temperature: 20, 30 and 40° C).
Figure 6 shows that the adsorption density is higher with increasing temperature, indicating that increasing temperature accelerates the adhesion process of bacterial cells to the apatite surface by increasing their randomness, which facilitates the adsorption process. This increase in temperature may also provide more active sites on the mineral surface and consequently increase adsorption. The maximum adhesion reached at 20, 30 and 40 °C was 4, 5 and 6 mg of biomass per g of mineral.
The adsorption kinetics showed that the adsorption density is ascending and is influenced by the concentration of bacterial cells, contact time and temperature of the medium. The experimental data was fitted to the pseudo-second order kinetic model in the linearized form (Figure 7) to obtain the parameters of the equation (Table 2).
Kinetics of Rhodococcus opacus adsorption onto apatite surface. The data were fitted using the pseudo-second order kinetic model.
From Table 2, it was observed that the adsorption rate constants decrease as the temperature increases from 20 to 40 °C, and their correlation coefficient values were greater than 0.98, indicating an appropriate fit and good correlation. That suitable fit showed that the interaction of each bacterial cell with the mineral surface occurs through the occupation of active surface sites (Kumar et al., 2010KUMAR, P. S.; VINCENT, C.; KIRTHIKA, K.; KUMAR, K. S. Kinetics and equilibrium studies of Pb2+ ion removal from aqueous solutions by use of nano-silversol-coated activated carbon. Brazilian Journal of Chemical Engineering, [s. l.], v. 27, n. 2, p. 339–346, 2010. Available at: https://doi.org/10.1590/s0104-66322010000200012
https://doi.org/10.1590/s0104-6632201000...
).
Similarly, Tan and Chen (2012)TAN, S. N.; CHEN, M. Early stage adsorption behaviour of Acidithiobacillus Ferrooxidans on minerals I: an experimental approach. Hydrometallurgy, [s. l.], v. 119, p. 87–94, 2012. Available at: https://doi.org/10.1016/j.hydromet.2012.02.001
https://doi.org/10.1016/j.hydromet.2012....
evaluated the adsorption kinetics of the Acidothiobacillus ferrooxidans bacteria to the bornite surface using pseudo-second order kinetic model, and observed that the adsorption capacity increases with time and levels off at around 60 min. Other authors concluded that kinetic parameters are important, but these depend on several process variables that influence their efficiency. Therefore, the kinetic models are relative and are restricted to determinant factors of the adsorption process (Olivera et al., 2019OLIVERA, C. A. C.; MERMA, A. G.; TOREM, M. L. Evaluation of hematite and quartz flotation kinetics using surfactant produced by rhodococcus erythropolis as bioreagent. REM - International Engineering Journal, Ouro Preto, v. 72, n. 4, p. 655–659, 2019. Available at: https://doi.org/10.1590/0370-44672018720162.
https://doi.org/10.1590/0370-44672018720...
, 2017; Wills and Finch, 2015WILLS, B. A.; FINCH, J. Wills’ mineral processing technology: an introduction to the practical aspects of ore treatment and mineral recovery. Oxford: Butterworth-Heinemann, 2015. E-book.).
4. Conclusions
The study showed the affinity of Rhodococcus opacus strain for the apatite surface and its ability to make it hydrophobic. This aspect was shown in the displacements of the zeta potential and contact angle curves of the apatite mineral after interaction with the microorganism. These displacements occurred due to the adsorption of different cellular compounds on the mineral surface. On the other hand, the maximum adsorption density reached was 24.10 mg of bacterial cells per gram of mineral, at a temperature of 40 °C and using a cell concentration of 400 mg/L. In addition, the experimental data were linearly fitted by the Freundlich isotherm due to the heterogeneity of the mineral surface and cellular interactions. Meanwhile, the adsorption density as a function of time was linearly adjusted by the pseudo-second order kinetic model, and was observed that the adsorption rate constants decrease as the temperature increases from 20 to 40 °C.
Acknowledgements
The authors thank to the Pontifical Catholic University of Rio de Janeiro, CNPq, Faperj and CAPES for the financial support.
References
- ABOUZEID, A. Z. M. Physical and thermal treatment of phosphate ores: an overview. International Journal of Mineral Processing, v. 85, n.4, p. 59-84, 2008. Available at: https://doi.org/10.1016/j.minpro.2007.09.001
» https://doi.org/10.1016/j.minpro.2007.09.001 - BOTERO, A. E. C.; TOREM, M. L.; MESQUITA, L. M. S. Fundamental studies of Rhodococcus opacus as a biocollector of calcite and magnesite. Minerals Engineering, [s. l], v. 20, n. 10, p. 1026–1032, 2007. Available at: https://doi.org/10.1016/j.mineng.2007.03.017
» https://doi.org/10.1016/j.mineng.2007.03.017 - BOTERO, A. E. C.; TOREM, M. L.; MESQUITA, L. M. S. Surface chemistry fundamentals of biosorption of Rhodococcus opacus and its effect in calcite and magnesite flotation. Minerals Engineering, [s. l.], v. 21, n. 1, p. 83–92, 2008. Available at: https://doi.org/10.1016/j.mineng.2007.08.019
» https://doi.org/10.1016/j.mineng.2007.08.019 - BUENO, B. Y. M.; TOREM, M. L.; MOLINA, F. A. L. M. S.; MESQUITA, L. M. S. Biosorption of lead(II), chromium(III) and copper(II) by R. opacus: equilibrium and kinetic studies. Minerals Engineering, [s. l], v. 21, n. 1, p. 65–75, 2008. Available at: https://doi.org/10.1016/j.mineng.2007.08.013
» https://doi.org/10.1016/j.mineng.2007.08.013 - CAYLLAHUA, J. E. B.; CARVALHO, R. J.; TOREM, M. L. Evaluation of equilibrium, kinetic and thermodynamic parameters for biosorption of nickel(II) ions onto bacteria strain, Rhodococcus opacus. Minerals Engineering, [s. l.], v. 22, n. 15, p. 1318–1325, 2009. Available at: https://doi.org/10.1016/j.mineng.2009.08.003
» https://doi.org/10.1016/j.mineng.2009.08.003 - CHANDRAPRABHA, M. N.; NATARAJAN, K. A. Surface chemical and flotation behaviour of chalcopyrite and pyrite in the presence of Acidithiobacillus thiooxidans. Hydrometallurgy, [s. l.], v. 83, n. 1–4, p. 146–152, 2006. Available at: https://doi.org/10.1016/j.hydromet.2006.03.021
» https://doi.org/10.1016/j.hydromet.2006.03.021 - FARAHAT, M.; HIRAJIMA, T.; SASAKI, K.; AIBA, Y.; DOI, K. Adsorption of SIP E. coli onto quartz and its applications in froth flotation. Minerals Engineering, [s. l.], v. 21, n. 5, p. 389–395, 2008. Available at: https://doi.org/10.1016/j.mineng.2007.10.019
» https://doi.org/10.1016/j.mineng.2007.10.019 - GU, Z. Dolomite flotation of high magnesium phosphate ores using fatty acid soap collectors 2007. Dissertation (Doctor of Philosophy in Mineral Processing) - College of Engineering and Mineral Resources, West Virginia University, Morgantown, 2007. Available at: https://researchrepository.wvu.edu/etd/4303
» https://researchrepository.wvu.edu/etd/4303 - JIANG, D.; HUANG, Q.; CAI, P.; RONG, X.; CHEN, W. Adsorption of Pseudomonas putida on clay minerals and iron oxide. Colloids and Surfaces B: Biointerfaces, [s. l.], v. 54, n. 2, p. 217–221, 2007. Available at: https://doi.org/10.1016/j.colsurfb.2006.10.030
» https://doi.org/10.1016/j.colsurfb.2006.10.030 - KALAVATHY, M. H.; KARTHIKEYAN, T.; RAJGOPAL, S.; MIRANDA, L. R. Kinetic and isotherm studies of Cu(II) adsorption onto H3PO4-activated rubber wood sawdust. Journal of Colloid and Interface Science, [s. l.], v. 292, n. 2, p. 354–362, 2005. Available at: https://doi.org/10.1016/j.jcis.2005.05.087
» https://doi.org/10.1016/j.jcis.2005.05.087 - KOWANGA, K. D.; GATEBE, E.; MAUTI, G. O.; MAUTI, E. M. Kinetic, sorption isotherms, pseudo-first-order model and pseudo-second-order model studies of Cu ( II ) and Pb ( II ) using defatted Moringa oleifera seed powder. The journal of phytopharmacology, [s. l.], v. 5, n. 2, p. 71–78, 2016. Available at: http://www.phytopharmajournal.com
» http://www.phytopharmajournal.com - KUMAR, P. S.; VINCENT, C.; KIRTHIKA, K.; KUMAR, K. S. Kinetics and equilibrium studies of Pb2+ ion removal from aqueous solutions by use of nano-silversol-coated activated carbon. Brazilian Journal of Chemical Engineering, [s. l.], v. 27, n. 2, p. 339–346, 2010. Available at: https://doi.org/10.1590/s0104-66322010000200012
» https://doi.org/10.1590/s0104-66322010000200012 - MERMA, A. G.; HACHA, R. H.; TOREM, M. L. Cellular adaptation: culture conditions of R. opacus and bioflotation of apatite and quartz. REM - International Engineering Journal, Ouro Preto, v. 70, n.1, p. 67 – 76, 2017. Available at: https://doi.org/10.1590/0370-446720167000063
» https://doi.org/10.1590/0370-446720167000063 - MERMA, A. G.; TOREM, M. L.; MORÁN, J. J.; MONTE, M. B. On the fundamental aspects of apatite and quartz flotation using a Gram positive strain as a bioreagent. Minerals Engineering, [s. l.], v. 48, p. 61–67, 2013. Available at: https://doi.org/10.1016/j.mineng.2012.10.018
» https://doi.org/10.1016/j.mineng.2012.10.018 - MESQUITA, L. M. S.; LINS, F. F.; TOREM, M. L. Interaction of a hydrophobic bacterium strain in a hematite-quartz flotation system. International Journal of Mineral Processing, [s. l.], v. 71, n. 1–4, p. 31–44, 2003. Available at: https://doi.org/10.1016/S0301-7516(03)00028-0
» https://doi.org/10.1016/S0301-7516(03)00028-0 - OKEOLA, E. O.; ODEBUNMI, F. O. Freundlich and Langmuir isotherms parameters for adsorption of methylene blue by activated carbon derived from Agrowastes. Advances in Natural and Applied Sciences, [s. l.], v. 4, n. 3, p. 281–288, 2010.
- OLIVERA, C. A. C.; MERMA, A. G.; PUELLES, J. G. S.; TOREM, M. L. On the fundamentals aspects of hematite bioflotation using a Gram positive strain. Minerals Engineering, [S. l.], v. 106, p. 55-63, 2017. Available at: https://doi.org/10.1016/j.mineng.2016.10.017
» https://doi.org/10.1016/j.mineng.2016.10.017 - OLIVERA, C. A. C.; MERMA, A. G.; TOREM, M. L. Evaluation of hematite and quartz flotation kinetics using surfactant produced by rhodococcus erythropolis as bioreagent. REM - International Engineering Journal, Ouro Preto, v. 72, n. 4, p. 655–659, 2019. Available at: https://doi.org/10.1590/0370-44672018720162
» https://doi.org/10.1590/0370-44672018720162 - PATRA, P.; NATARAJAN, K. A. Role of mineral specific bacterial proteins in selective flocculation and flotation. International Journal of Mineral Processing, [s. l.], v. 88, n. 1–2, p. 53–58, 2008. Available at: https://doi.org/10.1016/j.minpro.2008.06.005 Accessed: 12 Dec. 2018.
» https://doi.org/10.1016/j.minpro.2008.06.005 - RAO, K. H.; SUBRAMANIAN, S. Bioflotation and bioflocculation of relevance to minerals bioprocessing. In: DONATI, E. R., SAND, W. (ed.). Microbial processing of metal sulfides Dordrecht: Springer, 2007. p. 267–286. Available at: https://doi.org/10.1007/1-4020-5589-7_14
» https://doi.org/10.1007/1-4020-5589-7_14 - RONG, X.; CHEN, W.; HUANG, Q.; CAI, P.; LIANG, W. Pseudomonas putida adhesion to goethite: Studied by equilibrium adsorption, SEM, FTIR and ITC. Colloids and Surfaces B: Biointerfaces, [s. l.], v. 80, n. 1, p. 79–85, 2010. Available at: https://doi.org/10.1016/j.colsurfb.2010.05.037
» https://doi.org/10.1016/j.colsurfb.2010.05.037 - RUAN, Y.; HE, D.; CHI, R. Review on beneficiation techniques and reagents used for phosphate ores. Minerals, [s. l.], v. 9, n. 4, 2019. Available at: https://doi.org/10.3390/MIN9040253
» https://doi.org/10.3390/MIN9040253 - SANTHIYA, D.; SUBRAMANIAN, S.; NATARAJAN, K. A.; RAO, K. H.; FORSSBERG, K. S. E. Bio-modulation of galena and sphalerite surfaces using Thiobacillus thiooxidans. International Journal of Mineral Processing, [s. l.], v. 62, n. 1–4, p. 121–141, 2001. Available at: https://doi.org/10.1016/S0301-7516(00)00048-X
» https://doi.org/10.1016/S0301-7516(00)00048-X - SCHILLING, K. M.; CARSON, R. G.; BOSKO, C. A.; GOLIKERI, G. D.; BRUINOOGE, A.; HOYBERG, K.; WALLER, A. M.; HUGHES, N. P. A microassay for bacterial adherence to hydroxyapatite. Colloids and Surfaces B: Biointerfaces, [s. l.], v. 3, n. 1–2, p. 31–38, 1994. Available at: https://doi.org/10.1016/0927-7765(93)01120-G
» https://doi.org/10.1016/0927-7765(93)01120-G - TAN, S. N.; CHEN, M. Early stage adsorption behaviour of Acidithiobacillus Ferrooxidans on minerals I: an experimental approach. Hydrometallurgy, [s. l.], v. 119, p. 87–94, 2012. Available at: https://doi.org/10.1016/j.hydromet.2012.02.001
» https://doi.org/10.1016/j.hydromet.2012.02.001 - VÁSQUEZ, T. G. P.; BOTERO, A. E. C.; MESQUITA, L. M. S.; TOREM, M. L. Biosorptive removal of Cd and Zn from liquid streams with a Rhodococcus opacus strain. Minerals Engineering, [s. l.], v. 20, n. 9, p. 939–944, 2007. Available at: https://doi.org/10.1016/j.mineng.2007.03.014
» https://doi.org/10.1016/j.mineng.2007.03.014 - VOLESKY, B.; HOLAN, Z. R. Biosorption of heavy metals. Biotechnology Progress, [s. l.], v. 11, n. 3, p. 235–250, 1995. Available at: https://doi.org/10.1021/bp00033a001
» https://doi.org/10.1021/bp00033a001 - WANG, X. The surface chemistry of phosphate mineral flotation with alcohol solutions of octyl hydroxamic acid. 2004. Dissertation (Doctor of Philosophy) - Department of Metallurgical Engineering, University of Utah, Salt Lake City, 2004.
- WILLS, B. A.; FINCH, J. Wills’ mineral processing technology: an introduction to the practical aspects of ore treatment and mineral recovery. Oxford: Butterworth-Heinemann, 2015. E-book.
- WYPYCH, G. Mechanisms of adhesion. In: WYPYCH, G. Handbook of adhesion promoters. [S. l.]: ChemTec Pub, 2018. p. 5–44. Available at:https://doi.org/10.1016/b978-1-927885-29-1.50004-3
» https://doi.org/10.1016/b978-1-927885-29-1.50004-3 - YANG, H.; TANG, Q.; WANG, C.; ZHANG, J. Flocculation and flotation response of Rhodococcus erythropolis to pure minerals in hematite ores. Minerals Engineering, [s. l.], v. 45, p. 67–72, 2013. Available at: https://doi.org/10.1016/j.mineng.2013.01.005
» https://doi.org/10.1016/j.mineng.2013.01.005 - YANG, H. F.; LI, T.; CHANG, Y. H.; LUO, H.; TANG, Q. Y. Possibility of using strain F9 (Serratia marcescens) as a bio-collector for hematite flotation. International Journal of Minerals, Metallurgy and Materials, [s. l.], v. 21, n. 3, p. 210–215, 2014. Available at: https://doi.org/10.1007/s12613-014-0887-8 Accessed: 12 Dec. 2018.
» https://doi.org/10.1007/s12613-014-0887-8 - ZAFAR, I. Z.; ANWAR, M. M.; PRITCHARD, D. W. Innovations in beneficiation technology for low grade phosphate rocks. Nutrient Cycling in Agroecosystems, [s. l.], v. 46, n. 2, p. 135–151, 1996. Available at: https://doi.org/10.1007/bf00704313
» https://doi.org/10.1007/bf00704313 - ZHENG, X.; ARPS, P. J.; SMITH, R. W. Adhesion of two bacteria onto dolomite and apatite: their effect on dolomite depression in anianic flotation. International Journal of Mineral Processing, [s. l.], v. 62, n. 1–4, p. 159–172, 2001. Available at: https://doi.org/10.1016/S0301-7516(00)00050-8
» https://doi.org/10.1016/S0301-7516(00)00050-8
Publication Dates
-
Publication in this collection
21 July 2021 -
Date of issue
Jul-Sep 2021
History
-
Received
13 Oct 2020 -
Accepted
17 Apr 2021