Abstracts
The phlebotomine sand fly Lutzomyia longipalpis has been incriminated as a vector of American visceral leishmaniasis, caused by Leishmania chagasi. However, some evidence has been accumulated suggesting that it may exist in nature not as a single but as a species complex. Our goal was to compare four laboratory reference populations of L. longipalpis from distinct geographic regions at the molecular level by RAPD-PCR. We screened genomic DNA for polymorphic sites by PCR amplification with decamer single primers of arbitrary nucleotide sequences. One primer distinguished one population (Marajó Island, Pará State, Brazil) from the other three (Lapinha Cave, Minas Gerais State, Brazil; Melgar, Tolima Department, Colombia and Liberia, Guanacaste Province, Costa Rica). The population-specific and the conserved RAPD-PCR amplified fragments were cloned and shown to differ only in number of internal repeats.
RAPD-PCR; Fingerprintings; Sand fly; Lutzomyia; Genotyping
O flebotomíneo Lutzomyia longipalpis tem sido incriminado como vetor da leishmaniose visceral americana, causada pelo protozoário Leishmania chagasi. Entretanto, tem-se acumulado evidências que sugerem a existência de um complexo e não apenas uma espécie de L. longipalpis na natureza. Nosso trabalho teve como objetivo comparar, ao nível molecular, quatro populações de L. longipalpis de referência, utilizando especimens criados em laboratório, provenientes de regiões geograficamente distintas, através de RAPD-PCR (reação de polimerase em cadeia com amplificação por iniciadores ao acaso). Para isso, o DNA genômico de grupos de flebotomíneos foi amplificado com iniciadores decaméricos únicos com sequência de nucleotídeos arbitrária, na tentativa de se detectar sítios polimórficos. Apenas um dos iniciadores testados foi capaz de distinguir uma das populações (Ilha de Marajó, PA, Brasil) das outras três (Gruta da Lapinha, MG, Brasil; Melgar, Tolima, Colômbia e Libéria, Província de Guanacaste, Costa Rica). Os fragmentos população-específico e os conservados, amplificados por RAPD-PCR, foram clonados e sequenciados, mostrando que a diferença entre eles eles deve-se apenas à uma diferença no número de repetições internas.
Random amplified polymorphic DNA (RAPD) analysis of Lutzomyia longipalpis laboratory populations
Edelberto S. DiaS (1 Recebido para publicação em 01/09/1997 Aceito para publicação em 29/12/1997 ), Consuelo L. FORTES-DIAS (2 Recebido para publicação em 01/09/1997 Aceito para publicação em 29/12/1997 ), John M. StitEler (3 Recebido para publicação em 01/09/1997 Aceito para publicação em 29/12/1997 ),
Peter V. PERKINS(3 Recebido para publicação em 01/09/1997 Aceito para publicação em 29/12/1997 ) & Phillip G. Lawyer (3 Recebido para publicação em 01/09/1997 Aceito para publicação em 29/12/1997 )
SUMMARY
The phlebotomine sand fly Lutzomyia longipalpis has been incriminated as a vector of American visceral leishmaniasis, caused by Leishmania chagasi. However, some evidence has been accumulated suggesting that it may exist in nature not as a single but as a species complex. Our goal was to compare four laboratory reference populations of L. longipalpis from distinct geographic regions at the molecular level by RAPD-PCR. We screened genomic DNA for polymorphic sites by PCR amplification with decamer single primers of arbitrary nucleotide sequences. One primer distinguished one population (Marajó Island, Pará State, Brazil) from the other three (Lapinha Cave, Minas Gerais State, Brazil; Melgar, Tolima Department, Colombia and Liberia, Guanacaste Province, Costa Rica). The population-specific and the conserved RAPD-PCR amplified fragments were cloned and shown to differ only in number of internal repeats.
Keywords: RAPD-PCR; Fingerprintings; Sand fly; Lutzomyia; Genotyping
INTRODUCTION
Classical or alpha taxonomy of most organisms relies on detectable differences in morphologic characters between species. Among some vectors of disease, many "species" indistinguishable by currently used morphological characters are now recognized to contain reproductively isolated populations which may have very different biological characteristics.
The vectors of the leishmaniases in humans and other mammalian hosts are the phlebotomine sand flies. As observed for other vectors, difficulties in the taxonomy of these insects are due mainly to similar morphology and the existence of sibling species20. These difficulties in identification associated with a wide range of causative parasites, pose problems in deciding with certainty which sand fly is responsible for the transmission of a particular species of Leishmania in a given locality7. Differentiation and characterization of those populations are fundamental for epidemiological studies as some may act as important vectors of the Leishmania parasite, while others may be unable to transmit the parasite due to differences in their behavior or physiology.
In the last few years, several modern techniques have been introduced to complement the classical taxonomy data. The use of DNA probes is also becoming established as one important taxonomy tool, mainly for the identification of vector sibling species3,13, although requering a prior knowledge of specific region in their genomic DNAs. More recently, another molecular technique, random-amplified polymorphic DNA polymerase chain reaction (RAPD-PCR) was developed19. This approach is independent of previous DNA sequence information, involving the amplification via PCR of random segments of genomic DNA using a series of single primers of arbitrary nucleotide sequences. RAPD-PCR fingerprintings are generated by analysing the amplification products by electrophoresis on agarose gels. Polymorphic RAPD fragments can be used as diagnostic markers or, after cloning and sequencing, they can generate specific primers to be used in diagnostic PCR6.
The phlebotomine sand fly Lutzomyia longipalpis has been incriminated as the vector of American visceral leishmaniasis4 caused by Leishmania chagasi7,8,10. The geographic distribution of L. longipalpis, extending from Mexico to Southern Brazil, coincides in general with the geographic distribution of the disease. L. longipalpis was found in all known foci of visceral leishmaniasis and is a proven vector in some of them5. There is strong evidence that this phlebotomine sand fly may exist in nature as a complex of species9,16. The aim of our work was to study four geographic laboratory populations of L. longipalpis for the existence of DNA polymorphisms by RAPD-PCR.
MATERIAL AND METHODS
Source of genomic DNA Genomic DNA was isolated by standard procedures11 from four well characterized sand fly reference populations of L. longipalpis from established colonies maintained at the Walter Reed Army Institute of Research (WRAIR). Two populations originated from distinct geographic regions in Brazil, Lapinha Cave, Minas Gerais State (approximate coordinates 20o0S, 44o0W) and Marajó Island, Pará State (approximate coordinates 1o0S, 49o0W), one from Colombia, near Melgar, Tolima Department (4o11N, 74o18W) and one from Costa Rica, near Liberia, Guanacaste Province (10o37N,85o26W). Briefly, male adult sand flies in groups of ten were disrupted together with a mortar and pestil and lysed in 50 mM Tris-HCl, 100 mM NaCl, 50 mM EDTA, 0.5% SDS pH 8.0. The extracts were digested with 1 mg/ml of RNase A for 30 min at 37oC, followed by 50 mg/ml proteinase K for 3 hr at 55oC. The DNAs were phenol/chloroform extracted and concentrated by ethanol precipitation. After resuspension in 50 ml TE buffer pH 8.0, the DNA concentrations were estimated by comparison with known standards by agarose-ethidium bromide method (data not shown).
Amplification conditions PCR amplifications were performed under conditions previously established in a 100 ul reaction volume of 10 mM TRIS-HCl pH 8.3, 50 mM KCl, 1.5 mM MgCl2, 0.001% gelatin containing 10 ng of sand fly genomic DNA, 2.5 units of AmpliTaq DNA polymerase (Perkin-Elmer/Cetus), 0.2 mM of a single RAPD-PCR primer and 200 mM each of dATP, dCTP, dGTP and dTTP. Control reactions were run omitting genomic DNA. Decamer primers were arbitrarily selected for this study (Kit#G, Operon Technologies, Alameda, CA). Reactions were overlaid with 50 ml of mineral oil and amplified by cycling in a Perkin Elmer Cetus DNA Thermal Cycler 480 for 35 cycles of 30 sec at 94oC, 1 min at 34oC, and 2 min at 72oC, prior to soaking at 4oC.
RAPD-PCR fingerprintings 40 ml of each of the RAPD-PCR products were loaded on a 1.5% low-melting agarose gel in TAE buffer (0.04 M TRIS-acetate and 0.001 M EDTA) and electrophoresis was run for 3 hr at 80 V. Hae III restriction fragments of fX174RFI DNA were used as molecular weight markers. DNA bands were stained with ethidium bromide and visualized using a U.V. transilluminator.
Cloning of RAPD-PCR bands RAPD-PCR bands of interest were extracted from a 1.5% low-melting agarose gel using the Magic PCRä kit (Promega, Madison, WI) and reamplified by PCR with the same random primer used for the primary amplification. The secondary PCR products were cloned with the TA Cloningä kit version 1.0 (Invitrogen, San Diego, CA). Ligation to pCR1000ä vector and transformation of E. coli-competent cells were performed according to the manufacturers instructions.
Screening of recombinants and plasmid DNA isolation The presence and size of inserts in the recombinants were checked by direct PCR amplification of the DNA from bacterial colonies using M13 reverse (-48) 24-mer (5'-AGCGGATAACAATTTCACACAGGA-3') and M13 forward (-40) 17-mer (5'-GTTTCCCAGTCACGAC-3') primers (New England BioLabs, Beverly, MA). The amplification products were analysed by electrophoresis in a 1.0 % agarose gel in TAE buffer. Selected recombinants were propagated by growing individual clones in LB media containing kanamycin (50 ug/ml) until log phase. Plasmid DNA was isolated by cesium chloride gradient or by the Wizard DNA purification kit (Promega, Madison, WI).
Sequencing of recombinant DNA DNA was sequenced according to the dideoxy-chain termination method14 using sequenaseâ T7 DNA polymerase version 2.0 (United States Biochemical Co.) and appropriate internal and external primers. DNA sequencing runs were analyzed using the software MacVector from IBI, Kodak Co. (New Heaven, CT). Homology comparison was performed in the database at NCBI (National Center for Biotechnology Information, NIH, Bethesda, MD) using the basic local alignment search tool (BLASTN) developed by Altschul et al.2.
RESULTS
Among all the primers tested for the generation of RAPD-PCR fingerprintings from the genomic sand fly DNA, OPG-01 (5'-CTACGGAGGA-3') was the only one to generate a polymorphic fragment (about 620bp) unique for population B, besides a non-polymorphic band for all four populations of about 560bp. All the other decamer tested produced only non-polymorphic bands. Figure 1 shows the typical RAPD-PCR fingerprinting obtained with male DNA amplified with the primer OPG-01. Two different samples from each of the four populations of L. longipalpis were loaded in a single run to exemplify the profiles obtained. The same profiles were obtained on several independent occasions. No detectable band was seen in control reactions missing genomic DNA (data not shown).
Fig. 1 - RAPD-PCR fingerprintings on 1.5% agarose gel after amplification of genomic DNA. Two different samples from the four populations of L. longipalpis were loaded onto the same gel to exemplify the profile generated by amplification of L. longipalpis DNA with OPG-01. Origin of populations: A - Lapinha Cave, Minas Gerais State - Brazil; B - Marajó Island, Pará State - Brazil; C - Melgar, Tolima Department - Colombia; D - Liberia, Guanacaste Province - Costa Rica. Lane M represents the molecular weight markers.
The 620bp fragment was isolated and cloned into a suitable vector as described. After PCR screening for the presence of the insert, four recombinant clones were obtained (Fig. 2). Their sizes were estimated as 760bp for clones 1 and 3, and 820bp for clones 2 and 4, respectively. These sizes roughly correspond to the sum of the lengths of the non-polymorphic (560bp) and polymorphic (620bp) bands plus the internal vector sequence flanking the insert site (194bp).
- Agarose gel analysis of the amplification products obtained by PCR of selected recombinant colonies with M13 reverse (-48) and forward (-40) primers. Lanes M and 1-4 represent molecular markers and clones 1 to 4, respectively.
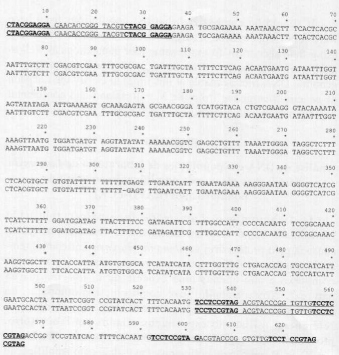
The inserts in clones 1 and 4 were subjected to DNA sequencing. Figure 3 presents both nucleotide sequences for comparison. The non-polymorphic band, with estimated size of 560bp, is composed of 565 nucleotide pairs. It displays a 35bp repeat at the beginning of its sequence that starts (nucleotides 1-10) and ends (nucleotides 26-35) with the OPG-01 sequence. The 35bp repeat is present in inverted position at the end of the DNA sequence. The polymorphic band is composed of 626 nucleotides and contains additional 26bp plus another 35bp inverted repeat when compared to the non-polymorphic fragment.
Fig. 3 - Nucleotide sequence of the polymorphic (626bp) and non-polymorphic (565bp) RAPD-PCR bands generated with L. longipalpis DNA and OPG-01. OPG-01 nucleotide sequence is shown in bold type and the 35bp repeat is underlined.
DISCUSSION
RAPD-PCR analysis has been previously used for genetic mapping and population studies6,17. Recently, it was successfully applied in the distinction of two sympatric species of sand fly in Venezuela1, as well as in the identification of unknown specimens of Anopheles (Nyssorhynchus) albitarsis, an important vector of malaria parasites constituted by a complex of cryptic species18.
The use of RAPD-PCR analysis presents the advantages of a simple, fast, effective and powerful technology. However, recent reports have shown that the amplification conditions have to be optimized and very well defined to allow for reproducible fingerprintings12,15,21. In our study, the RAPD-PCR fingerprintings were consistently reproducible for several pools of male DNA from the four populations.
Although resulting in a simple band pattern, amplification of L. longipalpis DNA by OPG-01 was able to discriminate population B (Marajó Island, Brazil) from A (Lapinha Cave, Brazil), C (Tolima, Colombia) and D (Guanacaste, Costa Rica) as the 626bp fragment is amplified only in specimens from population B.
The screening of recombinants demonstrated the insertion in the vector of not only the 620bp band, but also of the lowest 540bp band, common to all four Lutzomyia populations. The cloning technique used here comprises an initial PCR step in the presence of OPG-01, using the 620bp as template. As shown by the nucleotide sequence determined for the 620bp and 560bp fragments (Fig. 3), the latter is contained in the 620bp band. This would explain the presence of both bands inserted into the vector later (Fig. 2).
The real sizes of the 620bp and 560bp fragments, based on their nucleotide sequence, are 626bp and 565bp, respectively. Both sequences contain a 35bp repeat at the 5end that is inversely repeated at the 3end of the 565bp clone. As this 35bp repeat starts and ends with motifs that are complementary to the OPG-01, one would expect hybridization of OPG-01 at both sites. However, this event did not happen during the experiments carried out so far. No fragments have been obtained corresponding to priming at the end of the 35bp repeat. The 626bp clone corresponds to the 565bp sequence followed by 26bp plus another 35bp at the 3end. We expected the 626bp clone to contain a short specific region not present in the 565bp band, making it possible the development of a specific probe for population B. Unfortunately, the extra 26bp in the 620bp clone occurs also in the 506-530 region of both clones, thus excluding this possibility.
Structural data base comparison with the 626bp nucleotide sequence showed 68% identity with "gb[U28944]CELC18A3" from Caenorhabditis elegans cosmid in a fragment 60bp long. The significance of this finding, if any, is unknown.
The existence of high level of genetic divergence among three out of the four laboratory populations of L. longipalpis used here (A, C and D), has been described elsewhere9. Population from Marajó Island (B) has not been used in their study. Those authors have found a number of diagnostic loci that discriminates populations A, C and D through isozyme analysis. Besides, sterility has been observed in male progeny from all intercolony crosses upon experimental hybridization. Those data provided strong evidence that L. longipalpis is not a single species, but exists in nature as a complex of at least three distinct species. Unfortunately, no polymorphisms were detected with the RAPD primers used in our study that would distinguish between populations A, C and D. However, there is a difference between the population from the Marajó Island (B) and the other three. This finding is not surprising considering the geographic isolation of Marajó Island (population B) from the American continental area (populations A, C and D). The epidemiological and/or vetorial significance of this finding remain to be determined when more data is available.
It should be emphasized that the populations used here were laboratory-reared. Further studies would be necessary using field specimens from the same four populations as well as testing of other series of random primers and use of female genomic DNA (not available at this time) to clarify the question of genetic divergence from the Marajó Island and continental populations of L. longipalpis.
RESUMO
Análise de DNA em populações de laboratório de Lutzomyia longipalpis através de amplificação ao acaso de polimorfismos (RAPD-PCR)
O flebotomíneo Lutzomyia longipalpis tem sido incriminado como vetor da leishmaniose visceral americana, causada pelo protozoário Leishmania chagasi. Entretanto, tem-se acumulado evidências que sugerem a existência de um complexo e não apenas uma espécie de L. longipalpis na natureza. Nosso trabalho teve como objetivo comparar, ao nível molecular, quatro populações de L. longipalpis de referência, utilizando especimens criados em laboratório, provenientes de regiões geograficamente distintas, através de RAPD-PCR (reação de polimerase em cadeia com amplificação por iniciadores ao acaso). Para isso, o DNA genômico de grupos de flebotomíneos foi amplificado com iniciadores decaméricos únicos com sequência de nucleotídeos arbitrária, na tentativa de se detectar sítios polimórficos. Apenas um dos iniciadores testados foi capaz de distinguir uma das populações (Ilha de Marajó, PA, Brasil) das outras três (Gruta da Lapinha, MG, Brasil; Melgar, Tolima, Colômbia e Libéria, Província de Guanacaste, Costa Rica). Os fragmentos população-específico e os conservados, amplificados por RAPD-PCR, foram clonados e sequenciados, mostrando que a diferença entre eles eles deve-se apenas à uma diferença no número de repetições internas.
ACKNOELEDGMENTS
This work was partially supported by CNPq (PDE# 201125/90, PDE# 201124/90) and FAPEMIG (CBS 305/95), Brazil. We gratefully thank Dr. Yuan Lin from the Laboratory of Allergenic Products of the Food and Drug Administration (Bethesda, MD, USA) for the DNA sequencing facilities.
(1) Laboratório de Leishmanioses, Centro de Pesquisas "René Rachou", FIOCRUZ, Belo Horizonte, MG, Brasil.
(2) Centro de Pesquisa e Desenvolvimento, Fundação Ezequiel Dias, Belo Horizonte, MG, Brasil.
(3) Department of Entomology, Walter Reed Army Institute of Research, Walter Reed Army Medical Center, Washington, D.C., USA.
Correspondence to: Dr. Edelberto Santos Dias, Laboratório de Leishmanioses, Centro de Pesquisas "René Rachou", Avenida Augusto de Lima 1715, 30190-002 Belo Horizonte, MG, Brazil.
- 1. Adamson, R.E.; Ward, R.D.; Feliciangeli, M.D. & Maingon, R. - The application of random amplified polymorphic DNA for sand fly species identification. Med. vet. Entomol., 7: 203-207, 1993.
- 2. ALTSCHUL, S.F.; GISH, W.; MILLER, W.;MYERS, E.W. & LIPMAN, D.J. - Basic local alignement search tool. J. Molec. Biol., 215: 403-410, 1990.
- 3. Crampton, J.; Knapp, T. & Ward, R. - DNA probes for vector taxonomy. In: HART, D.T Leishmaniasis: the current status and new strategies for control. New York, Plenum Press, 1989. p. 957-964. (NATO ASI Series A, Life Sciences, v. 163).
- 4. Deane, L.M. - Leishmaniose visceral no Brasil. Estudos sobre reservatórios e transmissores realizados no Estado do Ceará. Rio de Janeiro. Serviço Nacional de Educaçăo, 1956.
- 5. Grimaldi, G.; Tesh, R.B. & McMahon-Pratt, D. - A review of geographic distribution and epidemiology of leishmaniasis in the World. Amer J. trop. Med. Hyg., 40: 687-725, 1989.
- 6. Hadrys, H.; Balick, M. & Schierwater, B. - Applications of random amplified polymorphic DNA (RAPD) in molecular biology. Molec. Ecol., 1: 55-63, 1992.
- 7. Killick-Kendrick, R. - Phlebotominae vectors of the leishmaniasis: a review. Med. vet. Entomol., 4: 1-24, 1990.
- 8. Lainson, R. & Shaw, J.J. - Evolution, classification and geographical distribution. In: PETERS, W. & KILLICK-KENDRICK, R., ed. The leishmaniases in biology and medicine London, Academic Press, 1987. v.1, p. 1-120.
- 9. Lanzaro, G.C.; Ostrovska, K.; Herrero, M.V.; Lawyer, P.G. & Warburg, A. - Lutzomyia longipalpis is a species complex: genetic divergence and interspecific hybrid sterility among three populations. Amer. J. trop. Med. Hyg., 48: 839-847, 1993.
- 10. Lewis, D.J. & Ward, R.D. - Transmission and vectors. In: PETERS, W. & KILLICK-KENDRICK, R., ed. The leishmaniases in biology and medicine London, Academic Press, 1987. v.1, p. 235-262.
- 11. Maniatis, T.; Fritsch, E.F. & Sambrook, J. - Molecular cloning: a laboratory manual. New York, Cold Spring Harbor Laboratory, 1982.
- 12. Muralidharan, K. & Wakeland, E.K. - Concentration of primer and template qualitatively affects products in random-amplified polymorphic DNA PCR. Biotechniques, 14: 362-363, 1993.
- 13. Post, R.J. - DNA probes for vector identification. Parasit. today, 1: 89- 90, 1985.
- 14. Sanger, F.; Nicklen, F.S. & Coulson, A.R. - DNA sequencing with chain termination inhibition. Proc. nat. Acad. Sci. (Wash.), 74: 5463-5467, 1977.
- 15. Schierwater, B. &. Ender, A. - Different thermostable DNA polymerases may amplify different RAPD PCR products. Nucleic Acids Res., 21, 4647-4649, 1993.
- 16. Ward, R.D.; Phillips, A.; Burnet, B. & Marcondes, C.B. - The Lutzomyia longipalpis complex: reproduction and distribution. In: SERVICE, M.W. Biosystematics and haematophagous insects. Oxford, Clarendon Press, 1988. p. 257-269. (Systematics Association Special, no. 37).
- 17. Welsh, J.; Peterson, C. & McClelland, M. - Polymorphisms generated by arbitrarily primed PCR in the mouse: application to strain identification and genetic mapping. Nucleic Acids Res., 19: 303-306, 1991.
- 18. Wilkerson, R.C.; Gaffigan, T.V. & Bento Lima, J. - Identification of species related to Anopheles (Nyssorhynchus) albitarsis by random amplified DNA polymerase chain reaction (Diptera: Culicidae). Mem. Inst. Oswaldo Cruz, 90: 721-732, 1995.
- 19. Williams, J.G.K.; Kubelik, A.R.; Livak, K.J.; Rafalski, J.A. & Tingey, S.V. - DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res., 18: 6531-6535, 1990.
- 20. Young, D.G. & Lawyer, P.G. - New world vectors of the leishmaniases. Recent Top. Vector Res., 4: 29-71, 1987.
- 21. Yu, K. & Pauls, K.P. - Optimization of the PCR program for RAPD analysis. Nucleic Acids Res., 20: 2606, 1992.
Publication Dates
-
Publication in this collection
16 June 1999 -
Date of issue
Jan 1998
History
-
Received
01 Sept 1997 -
Accepted
29 Dec 1997