Abstracts
Epstein Barr Virus (EBV) is transmitted commonly by saliva, but it has been found in genital secretions, which suggests sexual transmission and led researchers to connect EBV and cervical neoplasia. People living with human immunodeficiency virus (HIV) are reported to be at high risk of acquiring genital infections and cervical lesions. To verify the presence of EBV in the genital tract and/or it could affect cervical changes, we analyzed cervical smears from 85 HIV seropositive women for EBV DNA determination. EBV was only detected in two (2.3%) samples. The present study provides neither evidence for EBV as sexually transmitted infection nor discards this possibility.
Epstein Barr Virus; Cervical smears; Human immunodeficiency virus
O vírus Epstein-Barr (EBV) é transmitido comumente pela saliva, mas pode ser encontrado também em secreções genitais, sugerindo transmissão sexual e levando pesquisadores a associar este vírus à neoplasia cervical. Pessoas infectadas pelo virus da imunodeficiência humana (HIV) são de alto risco para aquisição de infecções genitais e lesões de cérvice uterina. Com o objetivo de verificar a presença do DNA do EBV no trato genital e/ou se poderia ter efeito em alterações cervicais, analisamos esfregaços cervicais de 85 mulheres HIV soropositivas. O vírus foi detectado em apenas duas (2,3%) amostras. O presente estudo não fornece evidência da transmissão sexual do EBV, nem descarta esta possibilidade.
BRIEF COMMUNICATION
Epstein Barr virus detection in cervical samples of women living with human immunodeficiency virus
Detecção do vírus Epstein-Barr em amostras cervicais de mulheres infectadas pelo vírus da imunodeficiência humana
Ledy H.S. Oliveira; Larissa S. Santos; Fernanda G. Nogueira
Department of Microbiology and Parasitology, Universidade Federal Fluminense, Niterói, Rio de Janeiro, Brazil
Correspondence to Correspondence to: Ledy H.S. Oliveira Departamento de Microbiologia e Parasitologia Universidade Federal Fluminense Rua Prof. Ernani Melo 101 24210-130 Niterói, RJ, Brasil Phone 55 21 26292431 fax 55 21 26292433 E-mail: mipledy@centroin.com.br
SUMMARY
Epstein Barr Virus (EBV) is transmitted commonly by saliva, but it has been found in genital secretions, which suggests sexual transmission and led researchers to connect EBV and cervical neoplasia. People living with human immunodeficiency virus (HIV) are reported to be at high risk of acquiring genital infections and cervical lesions. To verify the presence of EBV in the genital tract and/or it could affect cervical changes, we analyzed cervical smears from 85 HIV seropositive women for EBV DNA determination. EBV was only detected in two (2.3%) samples. The present study provides neither evidence for EBV as sexually transmitted infection nor discards this possibility.
Keywords: Epstein Barr Virus; Cervical smears; Human immunodeficiency virus.
RESUMO
O vírus Epstein-Barr (EBV) é transmitido comumente pela saliva, mas pode ser encontrado também em secreções genitais, sugerindo transmissão sexual e levando pesquisadores a associar este vírus à neoplasia cervical. Pessoas infectadas pelo virus da imunodeficiência humana (HIV) são de alto risco para aquisição de infecções genitais e lesões de cérvice uterina. Com o objetivo de verificar a presença do DNA do EBV no trato genital e/ou se poderia ter efeito em alterações cervicais, analisamos esfregaços cervicais de 85 mulheres HIV soropositivas. O vírus foi detectado em apenas duas (2,3%) amostras. O presente estudo não fornece evidência da transmissão sexual do EBV, nem descarta esta possibilidade.
INTRODUCTION
Epstein Barr Virus (EBV) is a ubiquitous microorganism causing widespread infection worldwide. Most of EBV infected people (reaching 90% of humans) are lifelong asymptomatic carriers but the risk of diseases and malignancies linked to this virus increases in immune-compromised patients4. Human immunodeficiency virus (HIV) is commonly associated with activation and widespread of several pathogens including EBV16. The HIV, among other causes for immune-deficient status, enhances the deleterious potential of this infectious agent3.
EBV is transmitted commonly by saliva, but it has been found in genital secretions suggesting that sexual transmission may occur9. WOODMAN et al.18 have reported that the acquisition of EBV infection is associated with the sexual behavior of young women. Other studies, however, indicate that the EBV sexual transmission is not sufficiently established. EBV could occasionally be transmitted by a sexual route, but the detection of the virus in genital secretions could reflect the infected stromal lymphocytes15. On the other hand, some reports show a connection between EBV and cervical changes7, but again, the role of this virus in the development of cervical neoplasia is also not clear. SHOJI et al.12 performed a in situ hybridization assay to show that the detection of EBV in cervical neoplasia was dependent on the degree of lymphocyte infiltration and therefore, the virus was not implicated in the etiology of cervical neoplasia.
Once HIV infected, people have more chance of acquiring pathogen agents as well as developing clinical symptoms, and in view of contradictory reports described above, we undertook a study to detect EBV infection in cervical smears from HIV positive women through conventional and nested polymerase chain reaction (PCR).
MATERIAL AND METHODS
A cross-sectional study included cervical samples from 85 HIV infected women collected between 2003 and 2005. Patients were referred to the Cervical Pathology Service of the Hospital dos Servidores do Estado (HSE), Rio de Janeiro, Brazil. They were screened according to the routine schedule among female patients from an AIDS reference hospital. Samples for routine Papanicolaou exam were collected after colposcopic examination. According to the Bethesda nomenclature cervical lesions were classified as Normal, atypical squamous cells of undetermined significance (ASCUS), low grade squamous intraepithelial lesions (LSIL), or high grade squamous intraepithelial lesions (HSIL)14. Demographic, behavior and data related to HIV infection were obtained through a structured questionnaire. The Ethics Committee of the College of Medicine at the University approved the protocols for collection and for informed consent.
DNA was extracted from samples either with phenol-chloroform method or by a commercial assay kit (Invisorb, Uniscience). EBV detection and typing were performed by generic and nested PCR respectively. For EBV DNA, the following primers were used: E2P1: 5'-AGG GAT GCC TGG ACA CAA GA-3' and E2P2: 5'-TGG TGC TGC TGG TGG TGG CAA T-3'. They amplify a 596 pb DNA sequence specific for EPB E2 ORF5. Amplification was developed in a 50 μL reaction mixture (1x PCR buffer, 200 μM dNTPs, 1.5 mM MgCl2, 50 pmol of each primer, 0.25 U unit of Taq polymerase, and 5 μL of sample) with 40 cycles of amplification. Each cycle included a denaturizing step at 94 °C for 30 seconds, an annealing step at 58 °C for seconds, and a chain elongation step at 72 °C for 60 seconds using DNA Thermal Cycler (Pekin Elmer, CETUS). Absence of DNA inhibitors was checked by β-actine primers (0.1 pmol each) for amplifying a 330 bp region of the human DNA as internal control. Polymerase chain reaction (PCR) products were analyzed on 1.5% agarose gel with ethidium bromide staining for visualization of DNA under ultraviolet light. The molecular weight of each sample was compared to 100 bp DNA ladder.
EBV-1 and EBV-2 typing - The nested-PCR was performed by inner amplification of 2 µL of the primary PCR products. The upstream and the downstream EBV-1 primers set were Ap1: 5'- TCT TGA TAG GGA TCC GCT AGG ATA -3' and Ap2: 5'- ACC GTG GTT CTG GAC TAT CTG GAT C -3' respectively, to amplify a 497 bp product. The EBV-2 primers set were Bp1: 5'- CAT GGT AGC CTT AGG ACA TA -3' and Bp2: 5'- AGA CTT AGT TGA TGC CCT AG -3' resulting in an amplicon of 150 bp (Life technologies, Brazil). It was performed with 25 cycles which consisted of steps at 94 °C for 30 seconds, 58 °C for 30 seconds, and 72 °C for 45 seconds, and 72 °C for the final elongation step5. Positive and negative controls were also performed. To avoid false positive results, besides the standard controls, a tube containing extracted DNA negative to EBV was amplified with EBV primers. Nested-PCR products were analyzed on 1.5% agarose gel with ethidium bromide staining for visualization of DNA under ultraviolet light and checked by comparison with a 100-bp DNA ladder.
RESULTS
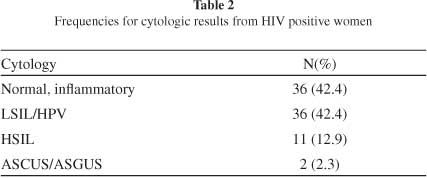
The features of the sample are shown in Table 1. The patients aged 14-59 years, mean 31 years and standard deviation 8.37. All the patients had an active sexual life. Most of them had their first sexual experience before 17 years and three had lifetime sexual partners. Herpetic genital lesions were found in 23.7% of the patients. More than half of the patients (57.6%) had cervical lesions (Table 2). Almost all the patients (97.7%) were under antiviral treatment.
EBV was only detected in two (2.3%) samples. In view of the small number of EBV positive samples, it was not possible to infer a statistical analysis. However, they shared some common features. Both samples were obtained from women carrying nearly 500 CD4 cells/mL with the length of time since HIV diagnosis being more than four years, under antiviral treatment, having had five sexual partners and their first sexual intercourse before 17 years. Their partners were also HIV positive. One of them presented low-grade squamous intraepithelial lesion and was infected with EBV-2. The other, showing normal cytology, harbored EBV 1 and 2 as well as clinical genital herpes.
DISCUSSION AND CONCLUSIONS
Although the evidence of EBV infection in cervical scrapings has been reported by several researches6,11, other studies do not agree with these findings. ANDERSSON-ELLSTRÖM et al.2, investigating EBV DNA in the cervix of virgin teenage girls or those with their first sexual experience, concluded that the non-sexual route of EBV was more plausible than the sexual route.
Despite having studied an HIV positive sample, we detected a very low EBV frequency. Interestingly, the EBV 2 type was seen in both samples. It was also related with HIV seropositivity and an increased number of sexual partners, suggesting sexual transmission for this type17. Even though we cannot show an association between EBV positivity and other factors due to the small number of EBV infected people, both EBV infected women had behavior that risked sexual infections. Thus, the possibility of sexual transmission is higher.
In two HIV positive women survey, the EBV prevalence in the genital tract was 10% and 23% respectively1,13. Both studies did not find any link between EBV and cervical lesions. Even though we detected 12.9% of HSIL cases, none of them had cervical EBV DNA. So, EBV could occasionally be transmitted by the sexual route without risk to cervical lesions. On the other hand, PAYNE et al.10, studying cervical cancers in HIV negative people, did not find any EBV infection in squamous epithelial cells.
Our results are similar to LANHAM et al.8. They found EBV DNA in only five out of 160 HIV negative women (3%). With regards to our findings (2.3%), HIV infection did not affect the frequency of EBV.
Finally, when we analyze the present study face to other reports, we can see that the spread of EBV by the sexual route remains inconclusive. Our findings do not provide evidence for EBV as a sexually transmitted infection but neither discard this possibility.
ACKNOWLEDGEMENTS
There is no conflict of interest with any of the authors. Financial support: FAPERJ (E-26/ 110.867/2009), CNPq (395075/2009-8) and PROPPi-UFF.
Received: 10 February 2011
Accepted: 31 May 2011
Financial support: FAPERJ, PROPPi-UFF, CNPq.
- 1. Ammatuna P, Giovanelli L, Giambelluca D, Mancuso S, Rubino E, Colletti P, et al. Presence of human papillomavirus and Epstein-Barr Virus in the cervix of women infected with the Human Immunodeficiency Virus. J Med Virol. 2000;62:410-5.
- 2. Andersson-Ellström A, Bergström T, Svennerholm B, Milsom I. Epstein-Barr virus DNA in the uterine cervix of teenage girls. Acta Obstet Gynecol Scand. 1997;76:779-83.
- 3. Cesarman E. Gammaherpesvirus and lymphoproliferative disorders in immunocompromised patients. Cancer Lett. 2011;305:163-74.
- 4. Dehee A, Asselot C, Piolot T, Jacomet C, Rozenbaum W, Vidaud M, et al. Quantification of Epstein-Barr virus load in peripheral blood of human immunodeficiency virus-infected patients using real-time PCR. J Med Virol. 2001;65:543-52.
- 5. Durmaz R, Aydin A, Köroglu M, Aker H, Özercan IH, Atik E, et al. Detection and genotyping of Epstein-Barr virus by polymerase chain reaction in tissues obtained from cases with Hodgkin´s Disease in Turkey. Acta Virologica. 1998;42:375-81.
- 6. Kantakamalakul W, Naksawat P, Kanyok R, Puthavathana P. Prevalence of type specific Epstein-Barr virus in the genital tract of genital herpes suspected patients. J Med Assoc Thai. 1999;82:263-7.
- 7. Landers RJ, O'Leary JJ, Crowley M, Healy I, Annis P, Burke L, et al. Epstein-Barr virus in normal, pre-malignant, and malignant lesions of the uterine cervix. J Clin Pathol. 1993;46:931-5.
- 8. Lanham S, Herbert A, Basarab A, Watt P. Detection of cervical infections in colposcopy clinic patients. J Clin Microbiol. 2001;39:2946-50.
- 9. Näher H, Gissmann L, Freese UK, Petzoldt D, Helfrich S. Subclinical Epstein-Barr virus infection of both the male and female genital tract-indication for sexual transmission. J Invest Dermatol. 1992;98:791-3.
- 10. Payne S, Kernohan NM, Walker F. Absence of in situ hybridization evidence for latent- or lytic-phase Epstein-Barr virus infection of preinvasive squamous lesions of the cervix. J Pathol. 1995;176:221-6.
- 11. Santos NB, Villanova FE, Andrade PM, Ribalta J, Focchi J, Otsuka AY, et al. Epstein-Barr virus detection in invasive and pre-invasive lesions of the uterine cervix. Oncol Rep. 2009;21:403-5.
- 12. Shoji Y, Saegusa M, Takano Y, Hashimura M, Okayasu I. Detection of the Epstein-Barr virus genome in cervical neoplasia is closely related to the degree of infiltrating lymphoid cells: a polymerase chain reaction and in situ hybridization approach. Pathol Int. 1997;47:507-11.
- 13. Smith JR, Kitchen VS, Botcherby M, Hepburn M, Wells C, Gor D, et al. Is HIV infection associated with an increase in the prevalence of cervical neoplasia? Br J Obstet Gynaecol. 1993;100:149-53.
- 14. Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114-9.
- 15. Thomas R, MacSween KF, McAulay K, Clutterbuck D, Anderson R, Reid S, et al. Evidence of shared Epstein-Barr virus isolates between sexual partners, and low level EBV in genital secretions. J Med Virol. 2006;78:1204-9.
- 16. Tugizov SM, Webster-Cyriaque JY, Syrianen S, Chattopadyay A, Sroussi H, Zhang L, et al. Mechanisms of viral infections associated with HIV: Workshop 2B. Adv Dent Res. 2011;23(1):130-6.
- 17. van Baarle D, Hovenkamp E, Dukers NH, Renwick N, Kersten MJ, Goudsmit J, et al. High prevalence of Epstein-Barr virus type 2 among homosexual men is caused by sexual transmission. J Infect Dis. 2000;181:2045-9.
- 18. Woodman CB, Collins SI, Vavrusova N, Rao A, Middeldorp JM, Kolar Z, et al. Role of sexual behavior in the acquisition of asymptomatic Epstein-Barr virus infection: a longitudinal study. Pediatr Infect Dis J. 2005;24:498-502.
Correspondence to:
Publication Dates
-
Publication in this collection
05 Sept 2011 -
Date of issue
Aug 2011
History
-
Received
10 Feb 2011 -
Accepted
31 May 2011