ABSTRACT
This review provides a general overview on the positivity and persistence of Zika virus (ZIKV) in female genital tract (FGT) of non-pregnant women and animals, as well as in cell cultures, and its influence on FGT health. We performed a systematic review based on the PRISMA statement to identify studies focused on “Zika virus” and “non-pregnant female” in PubMed, Embase, Scopus Scholar and Web of Knowledge databases of full-text papers and abstracts published in English, with no restrictions regarding the initial date of publication, up to August 2019. Our search terms yielded 625 records, that were 108 after removal of duplicates, leaving 517 items for title and abstract reviews. Of these, 475 did not meet the inclusion criteria, leaving 42 records for full-text review and resulting in the exclusion of 6 additional records. The remaining 36 met our inclusion criteria. Variations were observed regarding the presence and persistence of ZIKV in lower and upper genital samples. However, the FGT was the place in which ZIKV RNA has been detected, sometimes for relatively long periods, even after the clearance from blood and urine. In addition to the vagina and cervix, the endometrium, uterus and ovary (oocytes and follicles) could also be involved in persistent ZIKV infections. Further prospective studies are needed to assess the effect of ZIKV on FGT health.
Zika virus; Female; Non-pregnant; Genital tract
INTRODUCTION
Zika virus (ZIKV) is an arbovirus belonging to the genus flavivirus (family Flaviviridae) along with Japanese encephalitis virus (JEV), West Nile virus (WNV) and dengue virus (DENV), all of them are medically important viruses transmitted by mosquitoes or ticks 11. Kuno G, Chang GJ, Tsuchiya KR, Karabatsos N, Cropp CB. Phylogeny of the genus Flavivirus. J Virol. 1998;72:73-83.. The first human ZIKV infection was described in Nigeria, in 1954 22. MacNamara FN. Zika virus: a report on three cases of human infection during an epidemic of Jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48:139-45., after its isolation from a rhesus monkey in Uganda, in 1947 33. Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509-20.. Very few reports of infections were subsequently identified until the virus outbreak in 2007 in Yap Island Federal State of Micronesia 44. Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232-9., 55. Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. New Engl J Med. 2009;360:2536-43. and later in French Polynesia 66. Besnard M, Lastère S, Teissier A, Cao-Lormeau VM, Musso D. Evidence of perinatal transmission of zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19:20751., 77. Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect. 2014;20:O595-96.. Afterwards, ZIKV was introduced in Brazil in late 2013, early 2014 88. Metsky HC, Matranga CB, Wohl S, Schaffner SF, Freije CA, Winnicki SM, et al. Zika virus evolution and spread in the Americas. Nature. 2017;546:411-5., with an epidemic spread occurring throughout the Americas in 2015 99. Brasil P, Pereira Jr JP, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 2016;375:2321-34.. In January 2018, the Pan American Health Organization (PAHO) reported 223,477 confirmed cases of ZIKV infections that have increased worldwide between 2015 and 2018 1010. Pan American Health Organization. Zika cases and congenital syndrome associated with Zika virus reported by countries and territories in the Americas, 2015 - 2018: cumulative cases - Data as of 4 January 2018. [cited 2020 Feb 6]. Available from: https://www.paho.org/en/node/60231
https://www.paho.org/en/node/60231...
. Despite the global distribution of ZIKV, there are no clinically approved vaccines or specific treatments available 1111. Shankar A, Patil AA, Skariyachan S. Recent perspectives on genome, transmission, clinical manifestation, diagnosis, therapeutic strategies, vaccine developments, and challenges of Zika virus research. Front Microbiol. 2017;8: 1761..
It has been widely reported that approximately 80% of ZIKV-infected patients are asymptomatic 55. Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. New Engl J Med. 2009;360:2536-43.. However, symptoms including myalgia, headache, mild fever, rash, arthralgia, arthritis, conjunctival hyperemia and edema, with symptoms lasting for several days to weeks, have been reported 11. Kuno G, Chang GJ, Tsuchiya KR, Karabatsos N, Cropp CB. Phylogeny of the genus Flavivirus. J Virol. 1998;72:73-83.. Following the 2016 outbreak, a number of studies suggested that ZIKV infection in pregnant women was associated with congenital abnormalities, such as microcephaly, and may also be a trigger for Guillain-Barré syndrome 1212. Calvet G, Aguiar RS, Melo AS, Sampaio SA, de Filippis I, Fabri A, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016;16:653-60., 1313. Panchaud A, Stojanov M, Ammerdorffer A, Vouga M, Baud D. Emerging role of Zika virus in adverse fetal and neonatal outcomes. Clin Microbiol Rev. 2016;29:659-94..
The primary route of ZIKV transmission is a human-mosquito-human cycle in urban and peri-urban environments. The transmission occurs through the bite of infected Aedes species mosquitoes, mainly Aedes aegypti and Aedes albopictus, but the virus was isolated in many other Aedes species 1414. Grard G, Caron M, Mombo IM, Nkoghe D, Ondo SM, Jiolle D, et al. Zika virus in Gabon (Central Africa) - 2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8:e2681.. Other non-mosquito ZIKV transmission routes have been reported, such as maternal-fetal 1212. Calvet G, Aguiar RS, Melo AS, Sampaio SA, de Filippis I, Fabri A, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016;16:653-60., 1313. Panchaud A, Stojanov M, Ammerdorffer A, Vouga M, Baud D. Emerging role of Zika virus in adverse fetal and neonatal outcomes. Clin Microbiol Rev. 2016;29:659-94., by blood transfusion 1515. Petersen LR, Busch MP. Transfusion-transmitted arboviruses. Vox Sang. 2010;98: 495-503. and through sexual contact 1616. Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, et al. Probable non-vector-borne transmission of Zika virus, Colorado, US. Emerg Infect Dis. 2011;17:880-2., 1717. D’Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, et al. Evidence of sexual transmission of Zika virus. N Engl J Med. 2016;374:2195-8.. ZIKV ribonucleic acid (RNA) has been detected in the blood, saliva, urine, cerebrospinal fluid, semen, breast milk 1818. Sotelo JR, Sotelo AB, Sotelo FJ, Doi AM, Pinho JR, Oliveira RC, et al. Persistence of Zika virus in breast milk after infection in late stage of pregnancy. Emerg Infect Dis. 2017;23:856-7., 1919. Blohm GM, Lednicky JA, Márquez M, White SK, Loeb JC, Pacheco CA, et al. Evidence for mother-to-child transmission of Zika virus through breast milk. Clin Infect Dis. 2018;66:1120-1., vagina and cervix, but ZIKV dynamics in body fluids is not well understood 2020. World Health Organization. Prevention of sexual transmission of Zika virus: interim guidance update, 6 September 2016. [cited 2019 Feb 6]. Available from: http://apps.who.int/iris/bitstream/handle/10665/204421/WHO_ZIKV_MOC_16.1_eng.pdf;jsessionid=776D077B94EBA435074EF956F7DC2627?sequence=1
http://apps.who.int/iris/bitstream/handl...
, 2121. Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, Klein L, et al. Persistence of Zika virus in body fluids: final report. N Engl J Med. 2018;379:1234-43.. In the context of ZIKV sexual transmission, there are several reports of male-to-female 1616. Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, et al. Probable non-vector-borne transmission of Zika virus, Colorado, US. Emerg Infect Dis. 2011;17:880-2., 1717. D’Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, et al. Evidence of sexual transmission of Zika virus. N Engl J Med. 2016;374:2195-8., 2222. Arsuaga M, Bujalance SG, Díaz-Menéndez M, Vázquez A, Arribas JR. Probable sexual transmission of Zika virus from a vasectomised man. Lancet Infect Dis. 2016;16:1107.
23. Barzon L, Pacenti M, Franchin E, Lavezzo E, Trevisan M, Sgarabotto D, et al. Infection dynamics in a traveller with persistent shedding of Zika virus RNA in semen for six months after returning from Haiti to Italy, January 2016. Euro Surveill. 2016;21:30316.
24. Mansuy JM, Dutertre M, Mengelle C, Fourcade C, Marchou B, Delobel P, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16:405. - 2525. Sánchez-Montalvá A, Pou D, Sulleiro E, Salvador F, Bocanegra C, Treviño B, et al. Zika virus dynamics in body fluids and risk of sexual transmission in a non-endemic area. Trop Med Int Health. 2018;23:92-100. and male-to-male 2626. Deckard DT, Chung WM, Brooks JT, Smith JC, Woldai S, Hennessey M, et al. Male-to-male sexual transmission of Zika virus - Texas, January 2016. MMWR Morb Mortal Wkly Rep. 2016;65:372-4. transmission, but few studies showing female-to-male transmission 2727. Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of zika virus - New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:716-7..
Evidence of sexual transmission prompted the investigation of male and female reproductive tracts as ZIKV reservoirs 2828. Caine EA, Scheaffer SM, Arora N, Zaitsev K, Artyomov MN, Coyne CB, et al. Interferon lambda protects the female reproductive tract against Zika virus infection. Nat Commun. 2019;10:280.. Several groups have demonstrated that ZIKV exhibits tropism for the male reproductive tract and causes inflammation leading to structural damage and breakdown of the blood-testis barrier in mice 2929. Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, et al. Zika virus infection damages the testes in mice. Nature. 2016;540:438-42.
30. Ma W, Li S, Ma S, Zhang F, Zhang Y, Zhang J, et al. Zika virus causes testis damage and leads to male infertility in mice. Cell. 2016;167:1511-24. - 3131. Uraki R, Hwang J, Jurado KA, Householder S, Yockey LJ, Hastings AK, et al. Zika virus causes testicular atrophy. Sci Adv. 2017;3:e1602899.. The longest persistence of detectable ZIKV in semen was reported by Barzon and colleagues 3232. Barzon L, Percivalle E, Pacenti M, Rovida F, Zavattoni M, Del Bravo P, et al. Virus and antibody dynamics in travelers with acute zika virus infection. Clin Infect Dis. 2018;66:1173-80. who reported ZIKV-RNA detection up to 370 days post the onset of symptoms (dpo). Another studydetected the presence of viruses in semen up to 69 dpo 2222. Arsuaga M, Bujalance SG, Díaz-Menéndez M, Vázquez A, Arribas JR. Probable sexual transmission of Zika virus from a vasectomised man. Lancet Infect Dis. 2016;16:1107.. Identification of ZIKV RNA has been associated with genitourinary symptoms and oligospermia 2323. Barzon L, Pacenti M, Franchin E, Lavezzo E, Trevisan M, Sgarabotto D, et al. Infection dynamics in a traveller with persistent shedding of Zika virus RNA in semen for six months after returning from Haiti to Italy, January 2016. Euro Surveill. 2016;21:30316., 3333. Joguet G, Mansuy JM, Matusali G, Hamdi S, Walschaerts M, Pavili L, et al. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis. 2017;17:1200-8., 3434. Kurscheidt FA, Mesquita CS, Damke GM, Damke E, Carvalho AR, Suehiro TT, et al. Persistence and clinical relevance of Zika virus in the male genital tract. Nature Rev Urol. 2019;16:211-30.. In females, ZIKV RNA has been detected in cervical mucus and vaginal secretions at some time points after the clearance from blood 3535. Prisant N, Bujan L, Benichou H, Hayot PH, Pavili L, Lurel S, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1.
36. Murray KO, Gorchakov R, Carlson AR, Berry R, Lai L, Natrajan M, et al. Prolonged detection of Zika virus in vaginal secretions and whole blood. Emerg Infect Dis. 2017;23:99-101. - 3737. Cruz TE, Souza RP, Pelloso SM, Morelli F, Suehiro TT, Damke E, et al. Prolonged detection of Zika virus RNA in vaginal and endocervical samples from a Brazilian woman, 2018. Am J Trop Med Hyg. 2019;100:183-6.. ZIKV RNA has also been detected in the ovaries of female mice 3838. Dowall SD, Graham VA, Rayner E, Atkinson B, Hall G, Watson RJ, et al. A susceptible mouse model for Zika virus infection. PLoS Negl Trop Dis. 2016;10:e0004658.
39. Tripathi S, Balasubramaniam VR, Brown JA, Mena I, Grant A, Bardina SV, et al. A novel Zika virus mouse model reveals strain specific differences in virus pathogenesis and host inflammatory immune responses. PLoS Pathog. 2017;13:e1006258. - 4040. Duggal NK, McDonald EM, Ritter JM, Brault AC. Sexual transmission of Zika virus enhances in utero transmission in a mouse model. Sci Rep. 2018;8:4510. and non-human primates 4141. Carroll T, Lo M, Lanteri M, Dutra J, Zarbock K, Silveira P, et al. Zika virus preferentially replicates in the female reproductive tract after vaginal inoculation of rhesus macaques. PLoS Pathog. 2017;13:e1006537.. A human case of in vitro fertilization (IVF) described ZIKV RNA-positive oocytes 4242. Filho EA, Fácio CL, Machado-Paula LA, Oliveira MA, Martinhago CD, Araújo LP, et al. Case report of Zika virus during controlled ovarian hyperstimulation: results from follicular fluid, cumulus cells and oocytes. JBRA Assist Reprod. 2019;23:172-4.. However, in comparison with the male genital tract, the consequences of ZIKV infection on the female genital tract (FGT) have not been extensively evaluated, so that little is known about the target cells of ZIKV in this site. Furthermore, most studies have evaluated ZIKV infections in pregnant womens’ FGT due to the relationship between ZIKV and congenital abnormalities. Thus, several important questions about ZIKV-RNA detection in the FGT of non-pregnant women and animals need to be answered, such as: does the virus have tropism for any specific FGT cell? What is the viral load and how long does the virus remain infectious? How long women need to wait to become pregnant after ZIKV infections? What is the long-term damage to women?
In this review, we summarized the current knowledge of ZIKV infections in the FGT of non-pregnant women and animals and the influence of ZIKV on the health and fertility of FGT, as well as the effects of ZIKV infections on assisted reproduction. This review provides a general overview of the positivity and persistence of ZIKV infections in the FGT of non-pregnant women and animals, as well as in cell culture, and its influence on FGT health.
METHODS
A review was conducted following a systematic search based on the PRISMA statement 4343. Beller EM, Glasziou PP, Altman DG, Hopewell S, Bastian H, Chalmers I, et al. PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10:e1001419. to identify studies focused on “Zika virus” and “non-pregnant female” in PubMed, Embase, Scopus Scholar and Web of Knowledge (WOK) databases of full-text papers and abstracts published in English, with no restrictions regarding the initial date of publication, up to August2019. The strategy searched for publications by using MeSH Terms grouped in two blocks. (1) “Zika virus” and “Zika virus infection” “arboviruses” “arbovirus infection” (2) “vagina”, “cervix uteri”, “uterus”, “ovary”, “peritoneum”, “fallopian tubes”, “cell culture techniques”, “animal experimentation”, “genitalia female”. The identification of relevant studies written in English was performed independently by all of the authors. In addition, the reference lists of selected papers were also searched for additional relevant publications. Epidemiology bulletins from the Center for Disease Control and Prevention (CDC) were also included.
Abstracts were carefully selected by all independent reviewers to ensure the publications’ originality, quantitative and qualitative consensus. The studies initially selected had to fit three criteria. The first criterium included original epidemiological, clinical and experimental studies involving humans, animals and cells and/or reconstructed epithelium. The second criteriumwas to exclude duplicate studies and review studies. The third criterium involved the screening of eligible publications on the genital tract. After a consensual analysis, the papers most closely related to the theme descriptors were selected. Then, the articles were randomly distributed to all the investigators who acted as independent evaluators in charge of the inclusion of papers in the final cohort, for data extraction. To increase the sensitivity of the search, the references of the original articles were carefully reviewed to retrieve articles that could be additionally included in this review. To ensure that all relevant data from each paper were included in the review, a final consensus was achieved following an additional examination of the full texts by two additional experts (MELC and VRSS).
RESULTS
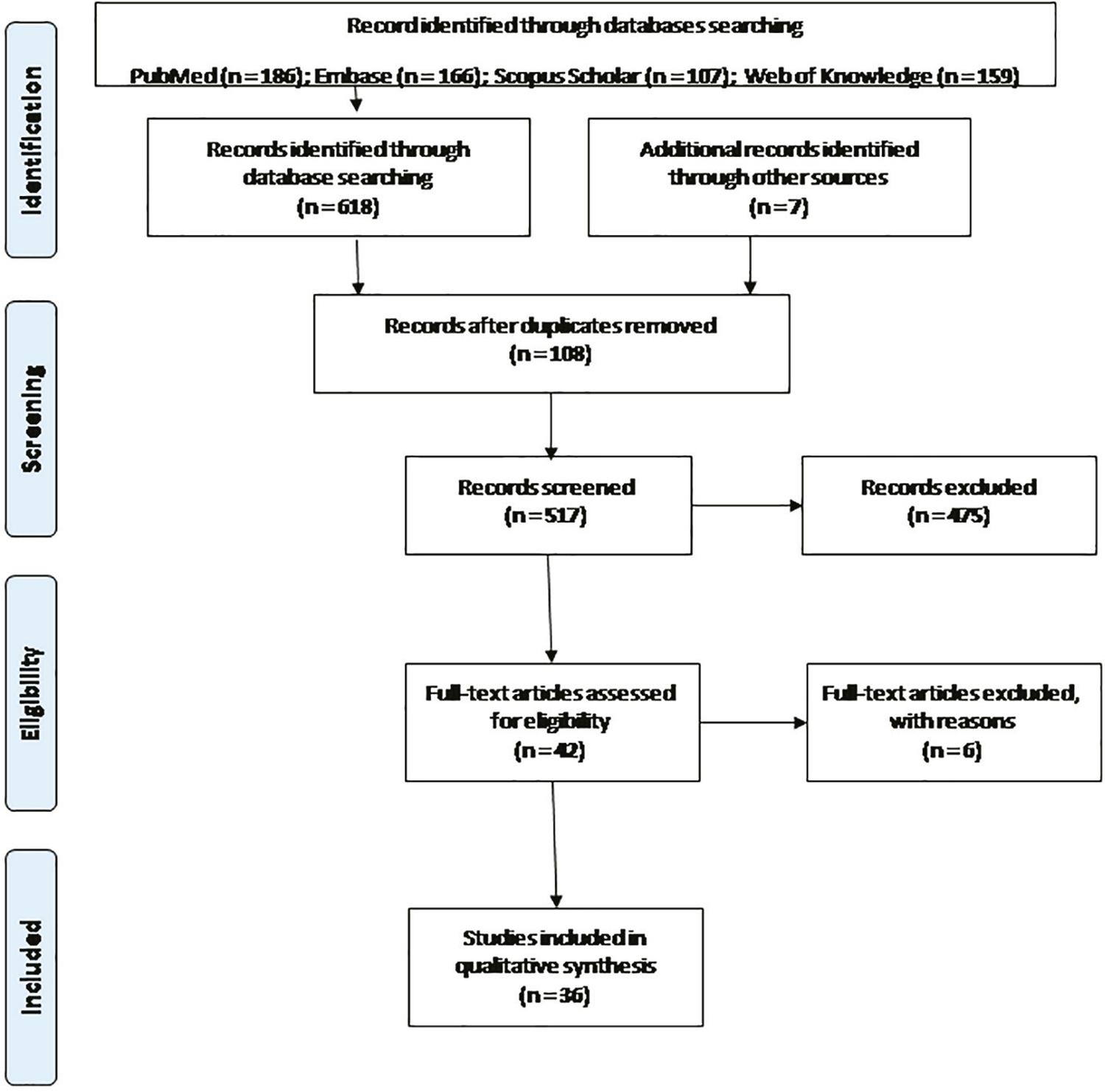
Our search terms yielded 625 records, 108 were removed due to duplication leaving 517 for title and abstract reviews. Of these, 475 did not meet the inclusion criteria, leaving 42 records for the full-text review, resulting in the exclusion of six additional records. The remaining 36 records met our inclusion criteria and were included in this review (Figure 1). These studies fell into three broad categories: ZIKV kinetics in FGT of non-pregnant women (n=12), in vivo ZIKV models in FGT of non-pregnant animals (n=14), and in vitro ZIKV cellular models in FGT (n=10) (Tables 1 and 2).
ZIKV kinetics in FGT of non-pregnant women
The majority of the studies evaluated ZIKV shedding in FGT of non-pregnant women using lower genital tract samples (LGT) 2121. Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, Klein L, et al. Persistence of Zika virus in body fluids: final report. N Engl J Med. 2018;379:1234-43., 2525. Sánchez-Montalvá A, Pou D, Sulleiro E, Salvador F, Bocanegra C, Treviño B, et al. Zika virus dynamics in body fluids and risk of sexual transmission in a non-endemic area. Trop Med Int Health. 2018;23:92-100., 3535. Prisant N, Bujan L, Benichou H, Hayot PH, Pavili L, Lurel S, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1.
36. Murray KO, Gorchakov R, Carlson AR, Berry R, Lai L, Natrajan M, et al. Prolonged detection of Zika virus in vaginal secretions and whole blood. Emerg Infect Dis. 2017;23:99-101. - 3737. Cruz TE, Souza RP, Pelloso SM, Morelli F, Suehiro TT, Damke E, et al. Prolonged detection of Zika virus RNA in vaginal and endocervical samples from a Brazilian woman, 2018. Am J Trop Med Hyg. 2019;100:183-6., 4444. Nicastri E, Castilletti C, Balestra P, Galgani S, Ippolito G. Zika virus infection in the central nervous system and female genital tract. Emerg Infect Dis. 2016;22:2228-30.
45. Penot P, Brichler S, Guilleminot J, Lascoux-Combe C, Taulera O, Gordien E, et al. Infectious Zika virus in vaginal secretions from HIV-infected woman. Euro Surveill. 2017;22:30444.
46. Tobar P, Vega M, Ordoñez C, Rivera L, Landivar J, Zambrano H. Detection of Zika virus and human papilloma virus in cervical cytology samples using two real time PCR based techniques in ecuadorian women diagnosed with ASCUS. P R Health Sci J. 2018;37 Spec Issue:S96-8.
47. Reyes Y, Bowman NM, Becker-Dreps S, Centeno E, Collins MH, Liou GA, et al. Prolonged shedding of Zika virus RNA in vaginal secretions, Nicaragua. Emerg Infect Dis. 2019;25:808-10. - 4848. Tozetto-Mendoza TR, Avelino-Silva VI, Fonseca S, Claro IM, Paula AV, Levin AS, et al. Zika virus infection among symptomatic patients from two healthcare centers in São Paulo State, Brazil: prevalence, clinical characteristics, viral detection in body fluids and serodynamics. Rev Inst Med Trop Sao Paulo. 2019;61:e19.. Very few samples from the upper genital tract (UGT) have been studied 4242. Filho EA, Fácio CL, Machado-Paula LA, Oliveira MA, Martinhago CD, Araújo LP, et al. Case report of Zika virus during controlled ovarian hyperstimulation: results from follicular fluid, cumulus cells and oocytes. JBRA Assist Reprod. 2019;23:172-4., 4949. Prisant N, Joguet G, Herrmann-Stock C, Moriniere C, Pavili L, Lurel S, et al. Upper and lower genital tract Zika virus screening in a large cohort of reproductive-age women during the Americas epidemic. Reprod Biomed Online. 2019;39:624-32. (Table 1).
Regarding the LGT studies, the first case of ZIKV RNA in vaginal and cervical samples of a non-pregnant woman was described by Prisant et al. 3535. Prisant N, Bujan L, Benichou H, Hayot PH, Pavili L, Lurel S, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1.. They presented the case of a 27-year-old woman from Guadeloupe (French territory in the Caribbean) during the 2016 outbreak of ZIKV in that region. The patient presented with clinical symptoms suggestive of an arbovirus infection, such as fever, maculopapular rash and conjunctivitis. The diagnosis of ZIKV infection was performed by real-time reverse transcription-polymerase chain reaction (RT-PCR) in a positive blood sample, while a urine sample was negative. Vaginal, cervical and endocervical samples were collected for ZIKV RNA analysis 3 dpo and they were positive. On 11 dpo, blood and urine samples were negative for ZIKV RNA, while the cervical mucus remained positive. According to the authors, the persistence of viral RNA in the vaginal and endocervical samples is of major importance in view of the risk of mother-to-fetus vertical transmission.
Subsequently, some studies have reported the presence and persistence of ZIKV RNA in the vaginal and/or cervical samples of non-pregnant women 2121. Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, Klein L, et al. Persistence of Zika virus in body fluids: final report. N Engl J Med. 2018;379:1234-43., 2525. Sánchez-Montalvá A, Pou D, Sulleiro E, Salvador F, Bocanegra C, Treviño B, et al. Zika virus dynamics in body fluids and risk of sexual transmission in a non-endemic area. Trop Med Int Health. 2018;23:92-100., 3636. Murray KO, Gorchakov R, Carlson AR, Berry R, Lai L, Natrajan M, et al. Prolonged detection of Zika virus in vaginal secretions and whole blood. Emerg Infect Dis. 2017;23:99-101., 3737. Cruz TE, Souza RP, Pelloso SM, Morelli F, Suehiro TT, Damke E, et al. Prolonged detection of Zika virus RNA in vaginal and endocervical samples from a Brazilian woman, 2018. Am J Trop Med Hyg. 2019;100:183-6., 4444. Nicastri E, Castilletti C, Balestra P, Galgani S, Ippolito G. Zika virus infection in the central nervous system and female genital tract. Emerg Infect Dis. 2016;22:2228-30.
45. Penot P, Brichler S, Guilleminot J, Lascoux-Combe C, Taulera O, Gordien E, et al. Infectious Zika virus in vaginal secretions from HIV-infected woman. Euro Surveill. 2017;22:30444.
46. Tobar P, Vega M, Ordoñez C, Rivera L, Landivar J, Zambrano H. Detection of Zika virus and human papilloma virus in cervical cytology samples using two real time PCR based techniques in ecuadorian women diagnosed with ASCUS. P R Health Sci J. 2018;37 Spec Issue:S96-8.
47. Reyes Y, Bowman NM, Becker-Dreps S, Centeno E, Collins MH, Liou GA, et al. Prolonged shedding of Zika virus RNA in vaginal secretions, Nicaragua. Emerg Infect Dis. 2019;25:808-10. - 4848. Tozetto-Mendoza TR, Avelino-Silva VI, Fonseca S, Claro IM, Paula AV, Levin AS, et al. Zika virus infection among symptomatic patients from two healthcare centers in São Paulo State, Brazil: prevalence, clinical characteristics, viral detection in body fluids and serodynamics. Rev Inst Med Trop Sao Paulo. 2019;61:e19.. In these studies, the authors reported data on the symptoms, and the variability on the period of viral RNA detection and persistence in vaginal and/or cervical samples.
Penot et al. 4545. Penot P, Brichler S, Guilleminot J, Lascoux-Combe C, Taulera O, Gordien E, et al. Infectious Zika virus in vaginal secretions from HIV-infected woman. Euro Surveill. 2017;22:30444. described the case of a 40-year-old French woman with controlled HIV infection, who had recently returned from a trip to the Caribbean-French Islands. She reported asthenia, myalgia, and developed abdominal pain, diarrhea and a pruritic rash on the face, chest, back and arms two days later. On the physical examination, the patient had generalized maculopapular pruritus, conjunctival hyperemia, fever (38.1 °C), asthenia and intense diffuse myalgia. The diagnosis was performed by RT-PCR and was negative for dengue and chikungunya. ZIKV RNA was positive in the plasma, urineand vaginal secretions 3 dpo. The viral RNA load was 3.8 log copies/mL in plasma, 6.1 log copies/mL in urine, 5.3 log copies/mL in vaginal sample 1 (collected by swab) and 3.9 log copies/mL in vaginal sample 2 (collected after instilling 5 mL of saline solution between the cervix and the posterior vaginal wall). The two vaginal samples were inoculated into Vero cell cultures and C6/36 cells lineage derived from A. albopictus, and ZIKV was isolated from both cultures, demonstrating the presence of infective ZIKV. The patient consented to collect another vaginal swab and cervical mucus on 10 dpo, and both samples were negative for ZIKV RNA. The authors considered unlikely that the HIV-1 infection had influenced the evolution of ZIKV infection, as the initial symptoms were similar to the ones of HIV-negative patients. Moreover, the results suggested a short period of infectivity of women with acute ZIKV infections through their genital secretions. Murray et al. 3636. Murray KO, Gorchakov R, Carlson AR, Berry R, Lai L, Natrajan M, et al. Prolonged detection of Zika virus in vaginal secretions and whole blood. Emerg Infect Dis. 2017;23:99-101. have already reported the presence of ZIKV RNA in vaginal samples of a 26-year-old patient who visited Honduras, up to 14 dpo. She presented with rash, fever, headache and conjunctivitis. Diagnosis was performed by RT-PCR and ZIKV was detected in serum samples up to 8 dpo and in other body fluids up to 14 dpo, including vaginal secretions. In serum samples, ZIKV was detected until 81 dpo. According to the authors, the presence of ZIKV RNA in vaginal secretions is a risk for sexual or intrapartum transmission, during a relatively long period.
Nicastri et al. 4141. Carroll T, Lo M, Lanteri M, Dutra J, Zarbock K, Silveira P, et al. Zika virus preferentially replicates in the female reproductive tract after vaginal inoculation of rhesus macaques. PLoS Pathog. 2017;13:e1006537. reported the case of a nurse who worked as a volunteer and raveled from Italy to Santo Domingo in the Dominican Republic. After her return, she presented with symptoms compatible with ZIKV infection and tested positive for ZIKV RNA in a vaginal swab up to 13 dpo. Besides, serum (7 dpo), urine (up to 27 dpo), cerebrospinal fluid (6 dpo) and saliva (up to 13 dpo) were positive for ZIKV RNA. Sánchez-Montalvá et al. 2525. Sánchez-Montalvá A, Pou D, Sulleiro E, Salvador F, Bocanegra C, Treviño B, et al. Zika virus dynamics in body fluids and risk of sexual transmission in a non-endemic area. Trop Med Int Health. 2018;23:92-100. performed a prospective study in two Spanish centers in 2016. Among four non-pregnant women, one tested positive for ZIKV RNA in vaginal swabs collected during the first 45 dpo and the viral clearance occurred between 37 and 69 dpo. As this study involved the investigation of ZIKV in different biological fluids of men and women, few details were available regarding the positivity of ZIKV in vaginal secretions. However, the authors did not identify any sexual transmission among the investigated sexual partners by serological testing.
Tobar et al. 4646. Tobar P, Vega M, Ordoñez C, Rivera L, Landivar J, Zambrano H. Detection of Zika virus and human papilloma virus in cervical cytology samples using two real time PCR based techniques in ecuadorian women diagnosed with ASCUS. P R Health Sci J. 2018;37 Spec Issue:S96-8. performed RT-PCR, including ZIKV, on cervical samples from the gynecological service of a hospital in Guayaquil, Ecuador. This work was part of a larger study on the presence of ZIKV in different body fluids. From the total of 89 samples, 19 were diagnosed as atypical squamous cells of undetermined significance (ASCUS) in cytological exams. From these, five were positive for ZIKV and five were positive for HPV, and there were no co-infections. The authors concluded that, given the presence of ZIKV RNA and the absence of HPV DNA in cervical samples diagnosed as ASCUS, it is plausible that ZIKV could be a triggering factor for the induction of cellular damage observed in these cells.
The study of Reyes et al. 4747. Reyes Y, Bowman NM, Becker-Dreps S, Centeno E, Collins MH, Liou GA, et al. Prolonged shedding of Zika virus RNA in vaginal secretions, Nicaragua. Emerg Infect Dis. 2019;25:808-10., from October 2016 to November 2017, recruited women ≥18 years old with symptoms suggestive of ZIKV infections (any combination of rash, fever, and conjunctivitis for ≤7 days), who attended a public health center and an University Hospital in Nicaragua. Five women were enrolled in a prospective cohort to characterize the duration of viral shedding in FGT. Blood, urine, saliva and vaginal samples were collected. The five women with acute ZIKV infections provided at least one vaginal secretion. Four women were pregnant; gestational age at enrollment was 1–8 months. The infants of all four women were healthy at birth, with no obvious congenital anomalies. However, the authors did not describe the specific results of the participating non-pregnant women. Nevertheless, the study had unprecedented results, with ZIKV-RNA detection in vaginal secretions between 60 and 180 dpo.
A study conducted by Paz-Bailey et al. 2121. Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, Klein L, et al. Persistence of Zika virus in body fluids: final report. N Engl J Med. 2018;379:1234-43. prospectively assessed a cohort of newly infected people in Puerto Rico. Only two of 119 women (1.6%) had ZIKV RNA in vaginal secretions (one was an asymptomatic participant and one was positive on 3 dpo). In this study, half of the participants had detectable viral RNA in urine for at least one week, in serum for two weeks, and in semen for more than one and a half months, while 5% or less had detectable ZIKV RNA in urine for five weeks, in serum for six weeks, and in semen for four months. Conversely, ZIKV RNA was seldomly detected in saliva and vaginal secretions.
Cruz et al. 3737. Cruz TE, Souza RP, Pelloso SM, Morelli F, Suehiro TT, Damke E, et al. Prolonged detection of Zika virus RNA in vaginal and endocervical samples from a Brazilian woman, 2018. Am J Trop Med Hyg. 2019;100:183-6. detected ZIKV RNA in vaginal secretions and in endocervical samples for up to 31 dpo. Interestingly, in this case report, ZIKV RNA remained positive in the vaginal secretions and endocervical samples, but in urine, saliva, plasma, serum and whole blood, the viral detection was negative on 31 dpo. The authors raised the hypothesis of a possible association between ZIKV and vaginal and endocervical epithelial cells, evidencing that, like in semen, variations in positivity rates found in FGT secretions and the persistence of the virus may occur.
Finally, the study of Tozetto-Mendoza et al. 4848. Tozetto-Mendoza TR, Avelino-Silva VI, Fonseca S, Claro IM, Paula AV, Levin AS, et al. Zika virus infection among symptomatic patients from two healthcare centers in São Paulo State, Brazil: prevalence, clinical characteristics, viral detection in body fluids and serodynamics. Rev Inst Med Trop Sao Paulo. 2019;61:e19. evaluated symptomatic ZIKV-infected individuals identified in two hospitals in Sao Paulo State, Brazil. Patients were investigated regarding their clinical characteristics, viral shedding in body fluids and serodynamics. Ninety-four of 235 symptomatic patients had ZIKV infection confirmed by RT-PCR. Shedding in genital fluids and saliva was rare. Ten women were tested at least once for ZIKV in genital fluids, and only one had a positive RT-PCR result. This patient was a 26-year-old non-pregnant participant who was enrolled 3 dpo with rash, headache and arthralgia and had a cervicovaginal sample collected on 18 dpo. The vaginal fluid and a plasma sample were positive for ZIKV. Moreover, genital fluid samples were collected from 16 women with confirmed ZIKV infection, with a median of 12 months after enrollment, and none tested positive for ZIKV by RT-PCR.
As far as we know, only two studies detected ZIKV shedding in the upper genital tract (UGT) of non-pregnant women. Prisant et al. 4949. Prisant N, Joguet G, Herrmann-Stock C, Moriniere C, Pavili L, Lurel S, et al. Upper and lower genital tract Zika virus screening in a large cohort of reproductive-age women during the Americas epidemic. Reprod Biomed Online. 2019;39:624-32., in a controlled observational clinical study, followed up 179 female patients undergoing oocyte vitrification cycles in an university fertility center during the ZIKV epidemic in French Territories in America. At that time, the French Ministry of Health banned medically-induced pregnancies. Oocyte vitrification cycles were the only means to preserve fertility options and to ensure the freezing of ZIKV-free oocytes for currently exposed and/or recently infected patients. Samples of serum, urine, LGT, endometrium, follicular fluid and immature oocytes were tested for ZIKV RNA by RT-PCR. ZIKV RNA was not detected in various samples of exposed patients. Furthermore, no ZIKV RNA was detected in the genital tracts of women with a recent (3 months) history of acute infection. In this study, there was a lack of ZIKV RNA persistence in FGT of ZIKV-exposed and/or recently infected women in reproductive age.
The second study on UGT of non-pregnant women was performed by Filho et al. 4242. Filho EA, Fácio CL, Machado-Paula LA, Oliveira MA, Martinhago CD, Araújo LP, et al. Case report of Zika virus during controlled ovarian hyperstimulation: results from follicular fluid, cumulus cells and oocytes. JBRA Assist Reprod. 2019;23:172-4. and described a case of a 37-year-old female who underwent in vitro fertilization (IVF). She developed a skin rash on her trunk and limbs during the treatment. RT-PCR results were positive for ZIKV in a blood sample and negative in her husband’s blood and semen. Oocyte aspiration was performed, retrieving seven oocytes, follicular fluidand cumulus cells. ZIKV RT-PCR results for the follicular fluid and cumulus cells were negative, but results were positive for two oocytes. This was the first report in the literature analyzing ZIKV in oocytes, follicular fluid, and cumulus cells. For these authors, the presence of ZIKV RNA in two oocytes showed the importance of testing couples seeking assisted reproductive technology (ART) because of the real risk of the embryo contamination.
Overall, among the cases and studies described, there are variations regarding the presence and persistence of ZIKV in LGT samples of non-pregnant women. However, it is unanimous that FGT is the place in which ZIKV RNA has been mostly detected, and in some cases for relatively long periods, even after the clearance from blood and urine. Similarly, there is extensive literature on the persistence of ZIKV in semen, with reports of viral positivity up to 370 days 3232. Barzon L, Percivalle E, Pacenti M, Rovida F, Zavattoni M, Del Bravo P, et al. Virus and antibody dynamics in travelers with acute zika virus infection. Clin Infect Dis. 2018;66:1173-80.. Thus, it is possible that FGT can also act as a sanctuary for ZIKV survival, allowing and extending the risk of sexual transmission. However, regarding UGT, further studies are needed to determine the current infection rate, as well as the kinetics of ZIKV infection. However, these reports evidenced that ZIKV shedding in FGT remains remains poorly studied but is a serious question due to its potential for sexual transmission from female-to-male, damaging the reproductive health of non-pregnant women, as well as favoring ascending fetal infections in pregnant women. This evidence supports the recommendation of safe sex practices for women returning from areas with ongoing ZIKV transmission. Nevertheless, the reasons for ZIKV persistence in FGT remain unknown and need clarification, but factors such as the magnitude of viral load, previous flavivirus infections and host-virus interactions should be taken into account.
In vivo ZIKV models in the FGT of non-pregnant animals
Several animal studies 2828. Caine EA, Scheaffer SM, Arora N, Zaitsev K, Artyomov MN, Coyne CB, et al. Interferon lambda protects the female reproductive tract against Zika virus infection. Nat Commun. 2019;10:280., 4141. Carroll T, Lo M, Lanteri M, Dutra J, Zarbock K, Silveira P, et al. Zika virus preferentially replicates in the female reproductive tract after vaginal inoculation of rhesus macaques. PLoS Pathog. 2017;13:e1006537., 5050. Mandl AM, Zuckerman S. Numbers of normal and atretic oocytes in unilaterally spayed rats. J Endocrinol. 1951;7:112-9.
51. Grant A, Ponia SS, Tripathi S, Balasubramaniam V, Miorin L, Sourisseau M, et al. Zika virus targets human STAT2 to inhibit type I interferon signaling. Cell Host Microbe. 2016;19:882-90.
52. Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med. 2016;22:1448-55.
53. Khan S, Woodruff EM, Trapecar M, Fontaine KA, Ezaki A, Borbet T, et al. Dampened antiviral immunity to intravaginal exposure to RNA viral pathogens allows enhanced viral replication. J Exp Med. 2016;213:2913-29.
54. Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, et al. A mouse model of Zika virus pathogenesis. Cell Host Microbe. 2016;19:720-30.
55. Tang WW, Young MP, Mamidi A, Regla-Nava JA, Kim K, Shresta S. A mouse model of Zika Virus sexual transmission and vaginal viral replication. Cell Rep. 2016;17:3091-8.
56. Broughton DE, Scheaffer S, Skaznik-Wikie ME, Halabi J, Govero J, Caine E, et al. Zika virus exhibits tropism to the ovary and increases follicular apoptosis in a mouse model. Fertil Steril. 2017;108 Suppl:e35.
57. Haddow AD, Nalca A, Rossi FD, Miller LJ, Wiley MR, Perez-Sautu U, et al. High infection rates for adult macaques after intravaginal or intrarectal inoculation with Zika virus. Emerg Infect Dis. 2017;23:1274-81.
58. Hastings AK, Yockey LJ, Jagger BW, Hwang J, Uraki R, Gaitsch HF, et al. TAM receptors are not required for Zika virus infection in mice. Cell Rep. 2017;19:558-68.
59. Hirsch AJ, Smith JL, Haese NN, Broeckel RM, Parkins CJ, Kreklywich C, et al. Zika virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog. 2017;13:e1006219.
60. Winkler CW, Woods TA, Rosenke R, Scott DP, Best SM, Peterson KE. Sexual and vertical transmission of Zika virus in anti-interferon receptor-treated Rag1-deficient mice. Sci Rep. 2017;7:7176.
61. Scott JM, Lebratti TJ, Richner JM, Jiang X, Fernandez E, Zhao H, et al. Cellular and humoral immunity protect against vaginal Zika virus infection in mice. J Virol. 2018;92:e00038-18. - 6262. Caine EA, Scheaffer SM, Broughton DE, Salazar V, Govero J, Poddar S, et al. Zika virus causes acute infection and inflammation in the ovary of mice without apparent defects in fertility. J Infect Dis. 2019;220:1904-14. tried to elucidate ZIKV viral dynamics aiming at observing the distribution of the virus in tissues and the triggered immune response, as well as the damage to tissues, including FGT (Table 2). The majority of animal studies were performed in small animals, such as mice 2828. Caine EA, Scheaffer SM, Arora N, Zaitsev K, Artyomov MN, Coyne CB, et al. Interferon lambda protects the female reproductive tract against Zika virus infection. Nat Commun. 2019;10:280., 5050. Mandl AM, Zuckerman S. Numbers of normal and atretic oocytes in unilaterally spayed rats. J Endocrinol. 1951;7:112-9., 5353. Khan S, Woodruff EM, Trapecar M, Fontaine KA, Ezaki A, Borbet T, et al. Dampened antiviral immunity to intravaginal exposure to RNA viral pathogens allows enhanced viral replication. J Exp Med. 2016;213:2913-29., 5555. Tang WW, Young MP, Mamidi A, Regla-Nava JA, Kim K, Shresta S. A mouse model of Zika Virus sexual transmission and vaginal viral replication. Cell Rep. 2016;17:3091-8., 5656. Broughton DE, Scheaffer S, Skaznik-Wikie ME, Halabi J, Govero J, Caine E, et al. Zika virus exhibits tropism to the ovary and increases follicular apoptosis in a mouse model. Fertil Steril. 2017;108 Suppl:e35., 5858. Hastings AK, Yockey LJ, Jagger BW, Hwang J, Uraki R, Gaitsch HF, et al. TAM receptors are not required for Zika virus infection in mice. Cell Rep. 2017;19:558-68., 6060. Winkler CW, Woods TA, Rosenke R, Scott DP, Best SM, Peterson KE. Sexual and vertical transmission of Zika virus in anti-interferon receptor-treated Rag1-deficient mice. Sci Rep. 2017;7:7176.
61. Scott JM, Lebratti TJ, Richner JM, Jiang X, Fernandez E, Zhao H, et al. Cellular and humoral immunity protect against vaginal Zika virus infection in mice. J Virol. 2018;92:e00038-18. - 6262. Caine EA, Scheaffer SM, Broughton DE, Salazar V, Govero J, Poddar S, et al. Zika virus causes acute infection and inflammation in the ovary of mice without apparent defects in fertility. J Infect Dis. 2019;220:1904-14., but there are studies in non-human primates (NHPs) 4141. Carroll T, Lo M, Lanteri M, Dutra J, Zarbock K, Silveira P, et al. Zika virus preferentially replicates in the female reproductive tract after vaginal inoculation of rhesus macaques. PLoS Pathog. 2017;13:e1006537., 5252. Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med. 2016;22:1448-55., 5757. Haddow AD, Nalca A, Rossi FD, Miller LJ, Wiley MR, Perez-Sautu U, et al. High infection rates for adult macaques after intravaginal or intrarectal inoculation with Zika virus. Emerg Infect Dis. 2017;23:1274-81., 5959. Hirsch AJ, Smith JL, Haese NN, Broeckel RM, Parkins CJ, Kreklywich C, et al. Zika virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog. 2017;13:e1006219..
In vivo ZIKV models in FGT of non-pregnant NHPs
The physiology of NHPs is close to that of humans, and a human scenario of ZIKV infection can be simulated in these models, despite the difficulties of acquisition and maintenance of NHPs. These models are of great relevance to increase the knowledge on pathogenesis and immunity, as well as on strategies to develop vaccines and therapies against ZIKV 5252. Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med. 2016;22:1448-55..
In these NHPs models, ZIKV subcutaneously inoculated were designed to mimic mosquitoes transmission. Osuna et al. 5252. Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med. 2016;22:1448-55., using male and female rhesus (RM) and cynomolgus (CM) monkeys evaluated the dynamics of ZIKV in blood, cerebrospinal fluid and mucosal secretions. They have also evaluated the ZIKV distribution in tissues in parallel with immune responses during the acute infection monitored for 4 weeks post-infection. Low levels of ZIKV RNA were detected in vaginal swab samples and cervicovaginal lavage contrasting with high levels of ZIKV in the uterus and ovaries. Hirsch et al. 5959. Hirsch AJ, Smith JL, Haese NN, Broeckel RM, Parkins CJ, Kreklywich C, et al. Zika virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog. 2017;13:e1006219. have also developed a RMs model inoculating ZIKV subcutaneously.They were divided into three cohorts: cohort 1 was infected with three different subcutaneously doses; cohorts 2 and 3 were infected with the same dose but euthanized animals in different times to analyze tissues. All the infected animals presented with symptoms compatible with ZIKV infection. In cohort 2, viral RNA was found in the uterusand in cohort 1, viral RNA was detected in the uterus and vagina, even though at different times and inoculation doses. Both results suggested potential female-to-male sexual transmission and have also contributed to evidence the possibility of viral infection in both, LGT and UGT. However, it is not possible to predict the required viral dose and time of infection involved in sexual transmission, as well as infection of different FGT parts.
Haddow et al. 5757. Haddow AD, Nalca A, Rossi FD, Miller LJ, Wiley MR, Perez-Sautu U, et al. High infection rates for adult macaques after intravaginal or intrarectal inoculation with Zika virus. Emerg Infect Dis. 2017;23:1274-81. developed a sexual transmission risk model to study infection after sexual intercourse byinoculating ZIKV into the vaginal and rectal canal of 16 adult macaques. For the intravaginal inoculation, four female RMs and four female CMs were used. All macaques were monitored and daily evaluatedfor symptomatology during 28 days post-infection (dpi). After intravaginal inoculation, 50% of the RMs and 50% of the CMs had viremia, and after 15 dpi, they had seroconverted. On the other hand, 75% of the RMs and 100% of the CMs had viremia after intrarectal ZIKV inoculation. Although intravaginal and intrarectal exposure to ZIKV in both NHP species caused infection, the signs and symptoms of clinical disease were not observed. Thisstudy demonstrated that ZIKV sexual transmission through intravaginal and intrarectal routes is possible, indicating sexual intercourse as a mechanism of viral transmission in the absence of mosquitoes can possibly introduce the disease in non-epidemic areas. However, this study did not contribute to the understanding of ZIKV infection in FGT, since the presence of the virus in both, LGT and UGT, was not evaluated.
Another model of vaginal transmission in NHPs was developed by Carroll et al. 4141. Carroll T, Lo M, Lanteri M, Dutra J, Zarbock K, Silveira P, et al. Zika virus preferentially replicates in the female reproductive tract after vaginal inoculation of rhesus macaques. PLoS Pathog. 2017;13:e1006537. using only RMs and intravaginal inoculations. ZIKV was readily transmitted to mature cycling female RMs by intravaginal inoculation. However, susceptibility varied between individual RMs, with 1 to 8 intravaginal inoculations required to establish infection. After treatment with Depo-Provera, a widely used progestin contraceptive, two RMs that had initially resisted eight intravaginal ZIKV inoculations, became infected after one inoculation. Thus, Depo-Provera seemed to enhance the susceptibility to ZIKV vaginal transmission. Unexpectedly, the kinetics of virus replication and dissemination after intravaginal ZIKV inoculation were markedly different from RMs infected with ZIKV by subcutaneousvirus inoculation. In intravaginally inoculated RMs, plasma viral RNA was only detected after several days and viral RNA shedding from FGT (cervicovaginal lavages) was found in all six animals. Furthermore, after intravaginal transmission, ZIKV RNA shedding in FGT secretions was detected before or simultaneously with plasma viral RNA and persisted for the same period of time. Additionally, to better understand ZIKV tissue tropism, ZIKV RNA levels in tissues of all six RMs infected with ZIKV by intravaginal inoculation were determined. ZIKV RNA was detected in the vagina, cervix, uterus, ovaries and genital lymph nodes. These results corroborate the conclusion that ZIKV preferentially replicates in FGT after vaginal transmission, but not after subcutaneous inoculation transmission, raising the possibility that there is enhanced fetal infection and tissue damage after ZIKV vaginal transmission as compared to viral transmission by mosquitoes.
The results of NHPs studies suggest that ZIKV can replicate in FGT after intravaginal inoculation more than after subcutaneous inoculation. Therefore, it seems that depending on ZIKV transmission mode, the infection has a different behavior. In models of mosquitoes transmission, there were low levels of ZIKV RNA in the cervical and vaginal secretions, which were opposed to models of sexual transmission. However, in tissues such as the uterus, ZIKV RNA levels were high until 28 dpi in RMs after subcutaneous viral inoculation, suggesting an additional potential risk to pregnancy. Finally, it was evidenced that the use of contraceptives represent an additional risk factor for ZIKV vaginal transmission. However, it is not known whether this can actually happen in humans.
In vivo ZIKV models in FGT of non-pregnant small animals
Several studies have shown that immune competent mice are refractory to ZIKV infection 5151. Grant A, Ponia SS, Tripathi S, Balasubramaniam V, Miorin L, Sourisseau M, et al. Zika virus targets human STAT2 to inhibit type I interferon signaling. Cell Host Microbe. 2016;19:882-90., 5454. Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, et al. A mouse model of Zika virus pathogenesis. Cell Host Microbe. 2016;19:720-30.. Therefore, researchers have used immune compromised animals to study ZIKV infection and dissemination. In this way, some researchers have used isogenic mice strains such as C57BL/6 mice 2828. Caine EA, Scheaffer SM, Arora N, Zaitsev K, Artyomov MN, Coyne CB, et al. Interferon lambda protects the female reproductive tract against Zika virus infection. Nat Commun. 2019;10:280., 5353. Khan S, Woodruff EM, Trapecar M, Fontaine KA, Ezaki A, Borbet T, et al. Dampened antiviral immunity to intravaginal exposure to RNA viral pathogens allows enhanced viral replication. J Exp Med. 2016;213:2913-29., 5555. Tang WW, Young MP, Mamidi A, Regla-Nava JA, Kim K, Shresta S. A mouse model of Zika Virus sexual transmission and vaginal viral replication. Cell Rep. 2016;17:3091-8., 5656. Broughton DE, Scheaffer S, Skaznik-Wikie ME, Halabi J, Govero J, Caine E, et al. Zika virus exhibits tropism to the ovary and increases follicular apoptosis in a mouse model. Fertil Steril. 2017;108 Suppl:e35., 6161. Scott JM, Lebratti TJ, Richner JM, Jiang X, Fernandez E, Zhao H, et al. Cellular and humoral immunity protect against vaginal Zika virus infection in mice. J Virol. 2018;92:e00038-18., 6262. Caine EA, Scheaffer SM, Broughton DE, Salazar V, Govero J, Poddar S, et al. Zika virus causes acute infection and inflammation in the ovary of mice without apparent defects in fertility. J Infect Dis. 2019;220:1904-14. ; Axl-/-, Mertk-/-, Axl-/- Mertk-/-, and Axl-/- Tyro3-/- mice 5858. Hastings AK, Yockey LJ, Jagger BW, Hwang J, Uraki R, Gaitsch HF, et al. TAM receptors are not required for Zika virus infection in mice. Cell Rep. 2017;19:558-68. ; Rag-/- and Ifnar1-/- mice 6060. Winkler CW, Woods TA, Rosenke R, Scott DP, Best SM, Peterson KE. Sexual and vertical transmission of Zika virus in anti-interferon receptor-treated Rag1-deficient mice. Sci Rep. 2017;7:7176. or the immunodeficient AG129 (deficiency in the type-I IFNAR and type-II IFNGR interferon receptors) and the A129 (deficiency in the type-I interferon receptor) mice 3838. Dowall SD, Graham VA, Rayner E, Atkinson B, Hall G, Watson RJ, et al. A susceptible mouse model for Zika virus infection. PLoS Negl Trop Dis. 2016;10:e0004658., 4040. Duggal NK, McDonald EM, Ritter JM, Brault AC. Sexual transmission of Zika virus enhances in utero transmission in a mouse model. Sci Rep. 2018;8:4510., 5555. Tang WW, Young MP, Mamidi A, Regla-Nava JA, Kim K, Shresta S. A mouse model of Zika Virus sexual transmission and vaginal viral replication. Cell Rep. 2016;17:3091-8. to facilitate ZIKV infection, dissemination and study the presence of ZIKV in FGT and other tissues/fluids.
Four studies were performed in mice based on their hormonal status. Tang et al. 5555. Tang WW, Young MP, Mamidi A, Regla-Nava JA, Kim K, Shresta S. A mouse model of Zika Virus sexual transmission and vaginal viral replication. Cell Rep. 2016;17:3091-8. developed a model of ZIKV administration into the vaginas of AG129 and LysMCre+IFNARfl/flC57BL/6 mice after induction of diestrus and estrus-like phases, respectively. Mice infected during the estrus-like phase were resistant to vaginal infection. In contrast, when infected during the diestrus-like phase, AG129 mice succumbed to infection, whereas LysMCre+IFNARfl/fl mice experienced transient illness. Patency of transgenital transmission (TGT) in diestrus-like mice was evidenced by detecting viremia and ZIKV replication in the spleen and the brain, and viral RNA persistence in vaginal washes as late as 10 dpi. This study indicated that intravaginal ZIKV administration can cause TGT, hormonal changes in FGT-dependent transmission and ZIKV replication and persistence in FGT for several days. Scott et al. 6161. Scott JM, Lebratti TJ, Richner JM, Jiang X, Fernandez E, Zhao H, et al. Cellular and humoral immunity protect against vaginal Zika virus infection in mice. J Virol. 2018;92:e00038-18. evaluated the immune protection against an intravaginal ZIKV challenge after previous subcutaneous infection to evaluate the protection against reinfection with a homologous virus, considering that most ZIKV-infected mice are at risk of re-exposure to the same or to a similar ZIKV strain. WT C57BL/6 mice were first injected with depot medroxyprogesterone acetate (DMPA) to force females into the diestrus phase of the estrous cycle. One day prior to infection, mice were intraperitoneally treated with an anti-IFNAR1 monoclonal antibody or with an isotype control monoclonal antibody (mAb). DMPA-treated mice were intravaginally inoculated with ZIKV. In animals treated with anti-IFNAR1 mAb, infectious ZIKV was detected in the vaginal lumen beginning on 1 dpi until 9 dpi. Infectious ZIKV was also detected in the vaginal lumen of mice treated with the isotype control antibody, although the amount was less than and lasted for a shorter period of time in comparison to animals treated with the anti-IFNAR1 mAb. Remarkably, infectious ZIKV was detected in the vaginal tissue at relatively similar levels in the isotype control and anti-IFNAR1 mAb-treated animals, which is consistent with the observation of IFN type I responses dampened in this tissue. In contrast to the vaginal results, differences were observed in the spread of ZIKV throughout FGT in isotype controls and in anti-IFNAR1 mAb-treated mice. ZIKV was detected in both, lower (vagina and cervix) and upper (uterine horns and ovaries) FGT of most mice treated with anti-IFNAR1 mAb, whereas only a small subset of animals treated with the isotype control mAb had ZIKV in the cervix, with none in the uterine horn or ovaries. In all FGT tissues, infectious viruses were cleared almost completely by 10 dpi, regardless of anti-IFNAR1 mAb treatment. The distribution of virally infected cells by in situ hybridization (ISH) with ZIKV-specific probes in the lower (vagina) and upper (uterine horns) FGT from uninfected or ZIKV-infected animals treated with anti-IFNAR1 Ab was examined. The majority of ZIKV RNA in the vagina was found in the luminal edge of the epithelium, suggesting the sloughing of dead cells. In the uterine horns, ZIKV RNA was detected in patches of cells throughout the tissue parenchyma. Thus, these data show that, while immunocompetent C57BL/6 mice treated with isotype control antibodies supported the viral replication invagina after genital infection, dissemination to UGT required suppression of type I IFN response.
The third study that evaluated ZIKV infection and host responses in tissues of FGT was conducted by Caine et al. 2828. Caine EA, Scheaffer SM, Arora N, Zaitsev K, Artyomov MN, Coyne CB, et al. Interferon lambda protects the female reproductive tract against Zika virus infection. Nat Commun. 2019;10:280.. Initial experiments in primary human vaginal and cervical epithelial cells demonstrated that IFN-β or IFN-λ treatment induce transcriptional programs that inhibit ZIKV infection. By performing studies in hormone-synchronized, non-pregnant, ovariectomized (OVX) female mice, the resistance to intravaginal ZIKV infection during the estradiol-high estrous phase and susceptibility during the progesterone-high, diestrus phase 4141. Carroll T, Lo M, Lanteri M, Dutra J, Zarbock K, Silveira P, et al. Zika virus preferentially replicates in the female reproductive tract after vaginal inoculation of rhesus macaques. PLoS Pathog. 2017;13:e1006537., 5555. Tang WW, Young MP, Mamidi A, Regla-Nava JA, Kim K, Shresta S. A mouse model of Zika Virus sexual transmission and vaginal viral replication. Cell Rep. 2016;17:3091-8., 6161. Scott JM, Lebratti TJ, Richner JM, Jiang X, Fernandez E, Zhao H, et al. Cellular and humoral immunity protect against vaginal Zika virus infection in mice. J Virol. 2018;92:e00038-18. were confirmed. The study observed that estradiol-treated mice were protected against intravaginal ZIKV infection, independently of IFN-α/β or IFN-λ signaling. In contrast, mice lacking IFN-λ signaling sustained greater FGT infection after progesteroneadministration. Exogenous IFN-λ treatment confers an antiviral effect when mice received both, estradiol and progesterone, but not progesterone alone. According to the authors, these results identified a hormonal stage-dependent role of IFN-λ in controlling FGT ZIKV infection suggesting a way to minimize ZIKV sexual transmission in women. Khan et al. 5353. Khan S, Woodruff EM, Trapecar M, Fontaine KA, Ezaki A, Borbet T, et al. Dampened antiviral immunity to intravaginal exposure to RNA viral pathogens allows enhanced viral replication. J Exp Med. 2016;213:2913-29., using the lymphocytic choriomeningitis virus (LCMV) and ZIKV in C57BL/6NCr CD45.2+ WT mice, showed that these viruses replicate in the vaginal mucosa with minimal induction of antiviral interferon and inflammatory response, causing dampened innate-mediated control of viral replication and failure of local antigen-presenting cells (APCs) to reach maturity. All the female mice in all the infection groups or the uninfected group were injected with Depo-Provera. Enhancement of innate-mediated inflammation in the vaginal mucosa rescued this phenotype and completely inhibited ZIKV replication. To better understand how this dampened innate immune activation in lower FGT may also affect the adaptive immunity, CD8 T cell responses using vaginal LCMV infection were modeled. They showed that the lack of APC maturation in the vaginal mucosa leads to a delay in CD8 T cell activation in the draining lymph nodes, hindering the timely appearance of effector CD8 T cells in the vaginal mucosa, further delaying the viral control in this tissue. The study demonstrated that the vaginal tissue is exceptionally vulnerable to infection by RNA viruses, providing a conceptual framework for the male-to-female sexual transmission observed in ZIKV infections. Taken together, the results of these four studies could indicate that hormones can influence the natural history of ZIKV infection in women, which is in agreement with the results of Carroll et al. 4141. Carroll T, Lo M, Lanteri M, Dutra J, Zarbock K, Silveira P, et al. Zika virus preferentially replicates in the female reproductive tract after vaginal inoculation of rhesus macaques. PLoS Pathog. 2017;13:e1006537. in NHPs discussed above. This is interesting considering that women of reproductive age are usually under the influence of endogenous and exogenous hormones and could consequently present with pronounced ZIKV infections symptoms. In addition, according to these results concerning the importance of progesterone in the development of LGT ZIKV infection, one can hypothesize that both, women in the second phase of the menstrual cycle (luteal phase) and pregnant women, who are under the action of high progesterone levels, could be more susceptible to ZIKV genital infection due to rodents’ epithelial and cervical vaginal stroma responses to sexual steroids, which are similar to those in humans 5050. Mandl AM, Zuckerman S. Numbers of normal and atretic oocytes in unilaterally spayed rats. J Endocrinol. 1951;7:112-9.. Progesterone induces the diestrus phase of the estrous cycle in rats, which is similar to the luteal phase of the menstrual cycle and pregnancy, in which intermediate epithelial squamous cells (ICs) predominate. Thus, ZIKV could have a higher affinity for vaginal and ectocervical ICs, or the vaginal environment with the predominance of ICs could favor ZIKV infection (Figure 2). However, this hypothesis can only be clarified by studies on women with LGT ZIKV infections.
Schematic representation of the possible role of progesterone in the development of ZIKV infection in the lower genital tract, according to animal studies. The responses of rodent epithelium and cervical vaginal stroma to sex steroids are similar to those of humans. Progesterone induces the diestrus phase of the estrous cycle in rats, which is similar to the luteal phase of the menstrual cycle and pregnancy, in which intermediate epithelial squamous cells (IC) predominate. ZIKV could have a higher affinity for vaginal and ectocervical IC, or the vaginal environment with predominance of IC could favor infection with this virus. BC: basal cells; PC: parabasal cells; SC: superficial cells.
In addition to the study of Scott et al. 6161. Scott JM, Lebratti TJ, Richner JM, Jiang X, Fernandez E, Zhao H, et al. Cellular and humoral immunity protect against vaginal Zika virus infection in mice. J Virol. 2018;92:e00038-18. described earlier, four other studies detected ZIKV in mice ovaries. Dowall et al. 3838. Dowall SD, Graham VA, Rayner E, Atkinson B, Hall G, Watson RJ, et al. A susceptible mouse model for Zika virus infection. PLoS Negl Trop Dis. 2016;10:e0004658. studied the effect of ZIKV infection in type I IFN receptor-deficient A129 female mice. They were subcutaneously injected with a ZIKV strain isolated from A. africanus mosquitoes to develop an adult model simulating a natural route of infection. They compared the A129 and 129Sv/Ev (WT strain) and could observe that A129 mice were susceptible to ZIKV infection, while all WT 129Sv/Ev survived the entire period of study, as did the group who received only PBS injections (control group). A129 mice developed severe symptoms with widespread viral RNA detection in the blood, brain, spleen, liver and ovaries. Histological changes were also striking in these animals. 129Sv/Ev mice developed no clinical symptoms or histological changes, despite viral RNA being detected in the blood, spleen and ovaries, albeit at lower levels than those seen in A129 mice. Duggal et al.
4040. Duggal NK, McDonald EM, Ritter JM, Brault AC. Sexual transmission of Zika virus enhances in utero transmission in a mouse model. Sci Rep. 2018;8:4510., studied viral dissemination to FGT that was assessed in IFN-α/β and -γ receptor knockout AG129 female mice exposed to ZIKV by subcutaneous inoculation, intravaginal inoculation, or sexual transmission from AG129-male infected mice. Sexual transmission resulted in significantly higher morbidity and mortality in females and higher ZIKV titers in FGT as compared to subcutaneous or intravaginal inoculation. The greater contribution of this study with respect to ZIKV infection in FGT of non-pregnant females was that ovaries from sexually-infected females contained ZIKV RNA within the ovarian follicles. Broughton et al. 5656. Broughton DE, Scheaffer S, Skaznik-Wikie ME, Halabi J, Govero J, Caine E, et al. Zika virus exhibits tropism to the ovary and increases follicular apoptosis in a mouse model. Fertil Steril. 2017;108 Suppl:e35. inoculated C57BL/6 female mice subcutaneously with ZIKV and collected ovaries from infected and control mice on 7, 14 and 21 dpi. They performed RT-PCR to determine the viral load and detected ZIKV RNA at all time points in infected mice. Ovarian titers were higher than the ones found in serum samples. Caine et al. 6262. Caine EA, Scheaffer SM, Broughton DE, Salazar V, Govero J, Poddar S, et al. Zika virus causes acute infection and inflammation in the ovary of mice without apparent defects in fertility. J Infect Dis. 2019;220:1904-14. characterized ZIKV infection and the ensuing immune response in the ovary using a murine model (WT and congenic CD8−/− and TCRβδ−/− C57BL/6) and found that animals can be acutely infected. On 7 dpi, CD4+ and CD8+ T cells had infiltrated the ovary causing oophoritis, with detectable ZIKV-specific CD8+ T cells. Infection and inflammation were associated to dying cells during the acute stage of infection. In contrast to results in the testis 2727. Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of zika virus - New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:716-7.
28. Caine EA, Scheaffer SM, Arora N, Zaitsev K, Artyomov MN, Coyne CB, et al. Interferon lambda protects the female reproductive tract against Zika virus infection. Nat Commun. 2019;10:280. - 2929. Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, et al. Zika virus infection damages the testes in mice. Nature. 2016;540:438-42., excessive and persistent tissue damage was not seen in ZIKV-infected mice ovaries, and persistent viral RNA was not detected in most animals on 90 dpi. The resolution of infection and tissue injury were associated to the lack of detrimental long-term impact on fertility or ovarian reserve. Thus, even though the ovary is vulnerable to acute ZIKV infection and inflammation, long-term effects on fertility were not observed, at least in mice.
Hastings et al. 5858. Hastings AK, Yockey LJ, Jagger BW, Hwang J, Uraki R, Gaitsch HF, et al. TAM receptors are not required for Zika virus infection in mice. Cell Rep. 2017;19:558-68. investigated the requirement of Tyro 3, Axl and Mer (TAM) receptors for ZIKV infection by inoculating the virus through different routes and comparing viral replication in WT mice versus Axl-/-, Mertk-/-, Axl-/- Mertk-/-, and Axl-/- Tyro3-/- mice, in different organs. TAM receptors are candidates of ZIKV entry receptors. The researchers demonstrated that TAM receptors are not required for ZIKV infection throughsubcutaneous, transplacental, vaginal or intracranial routes. ZIKV replication was not affected by the absence of TAM receptors in the spleen, placenta, vagina and the brain. These results indicate that, in mice, TAM receptors are not required for ZIKV infection.
Finally, Winkler et al. 6060. Winkler CW, Woods TA, Rosenke R, Scott DP, Best SM, Peterson KE. Sexual and vertical transmission of Zika virus in anti-interferon receptor-treated Rag1-deficient mice. Sci Rep. 2017;7:7176. studied both, ZIKV sexual transmission (STx) and vertical transmission (VTx) using anti-IFNAR1-treatment of Rag1 -/- (AIR) mice. These mice have suppressed type I IFN responses and lack adaptive immune responses, leading to a prolonged infection prior to clinical disease. The STx of ZIKV from infected AIR males to naive Ifnar1 -/- females was observed with a higher than 50% incidence and infection observed in the vaginal tract at early time points. By mating Ifnar1-/-, they have also observed that ZIKV infection of the vaginal tract correlates with development of clinical disease.
Studies presented in this section showed that small animals, mainly mice, are good models for ZIKV infection and that these viruses have tropism for FGT cells, tissues and organs. The results have also indicated that the natural history of ZIKV infection in females is directly influenced by hormones, components of the innate and the adaptive immune system. It is interesting to observe that the antiviral IFN response and inflammation are minimally induced in LGT. This provides an opportunity for ZIKV replication in the vaginal mucosa and dissemination to other FGT parts, damaging tissues and the fetus.
In vitro ZIKV cellular models in FGT studies
Chen et al. 6363. Chen JC, Wang Z, Huang H, Weitz SH, Wang A, Qiu X, et al. Infection of human uterine fibroblastos by Zika virus in vitro: implications for viral transmission in women. Int J Infect Dis. 2016;51:139-40. cultured uterine fibroblasts inoculated with two different ZIKV strains (VR-84 or VR-1843). Their results indicated that uterine fibroblasts are susceptible to ZIKV infection and the uterus may act as a viral pathway during heterosexual intercourse. In addition, the study of Pagani et al. 6464. Pagani I, Ghezzi S, Ulisse A, Rubio A, Turrini F, Garavaglia E, et al. Human endometrial stromal cells are highly permissive to productive infection by Zika virus. Sci Rep. 2017;7:44286. showed that primary human endometrial stromal cells (HESCs) are highly permissive to ZIKV infection and support in vitro viral replication. ZIKV productive infection has also occurred in a human endometrial stromal cell line (T-HESC) together with the induction of IFN-β and of IFN-stimulated genes.
Chan et al. 6565. Chan JF, Yip CC, Tsang JO, Tee KM, Cai JP, Chik KK, et al. Differential cell line susceptibility to the emerging Zika virus: implications for disease pathogenesis, non-vector-borne human transmission and animal reservoirs. Emerg Microbes Infect. 2016;5:e93. characterized differential cell lines susceptibility using 18 human and 15 non-human cell lines and two ZIKV isolates (human and primate), as well as DENV type 2 (DENV-2). Considering FGT, the cell lines were HeLa (cervical adenocarcinoma) and HOSE6-3 (ovarian surface epithelium). A ≥ 2 log increase in the mean viral load (P <0.01) within 5 dpi was observed in both cell lines. The highest mean viral load (≥10 log copies/mL) was observed in the HeLa cell line that has also shown a clear ZIKV-NS1 protein expression in addition to a high viral load. Although HOSE6-3 cells showed high mean viral loads in RT-PCR, ZIKV-NS1 protein expression was consistently found in ≤ 5% of infected cells. Comparatively, none of the placental and genital tract cell lines allowed efficient DENV-2 replication.
Fink et al. 6666. Fink SL, Vojtech L, Wagoner J, Slivinski NS, Jackson KJ, Wang R, et al. The antiviral drug Arbidol inhibits Zika virus. Sci Rep. 2018;8:8989. studied if Arbidol (ARB, umifenovir) could inhibit different ZIKV isolates. The efficacy of ARB in primary cell lines was evaluated by infecting primary human vaginal (HVE2) and primary endocervical (ENDO) and ectocervical (ECTO) epithelial cells with ZIKV in the presence and absence of ARB pretreatment. In relation to FGT cells, HVE2, ENDO and ECTO cells were markedly infected by ZIKV. ARB treatment (20 μM) resulted in significant suppression of ZIKV protein synthesis. Finally, ZIKV RNA expression in primary vaginal and cervical epithelial cells from different ZIKV strains-infected donors has also been suppressed by ARB.
To begin studies addressing the impact of selected human vaginal microbiome (VMB) communities on ZIKV infection and HSV-2 infection, Amerson-Brown et al. 6767. Amerson-Brown MH, Miller AL, Maxwell CA, White MM, Vincent KL, Bourne N, et al. Cultivated human vaginal microbiome communities’ impact Zika and herpes simplex virus replication in ex vivo vaginal mucosal cultures. Front Microbiol. 2019;9:3340. employed their polarized vaginal epithelial cell (VEC) multilayer culture model. Taken together, results confirmed that, after ZIKV infection, VECs supported viral replication before being systemically released into the basal chamber. In addition, ZIKV infection did not cause significant changes in established VMBs.
Cagno et al. 6868. Cagno V, Tseligka ED, Bettex Q, Huang S, Constant S, Tapparel C. Growth of Zika virus in human reconstituted respiratory, intestinal, vaginal and neural tissues. Clin Microbiol Infect. 2019;25:1042.e1-4. investigated the tropism of Asian and African ZIKV strains using human-derived neural, vaginal, intestinal and respiratory tissues. Both ZIKV strains were able to grow in all the tissues tested, although with different efficiencies (7.3 log RNA copies released apically in vaginal tissues versus 9.8 log RNA copies released in intestinal tissues) and without the need of major adaptation. The results underlined that ZIKV tropism may be broader than expected in humans and highlighted the need to better explore all possible virus shedding sites and transmission routes.
Müller et al. 6969. Müller JA, Harms M, Krüger F, Groß R, Joas S, Hayn M, et al. Semen inhibits Zika virus infection of cells and tissues from the anogenital region. Nat Commun. 2018;9:2207. analyzed the effect of semen on ZIKV infection of anogenital cells and tissues. ZIKV replicated in all the analyzed cell lines, primary cells and endometrial or vaginal tissues. However, in the presence of semen, ZIKV infection and other flaviviruses were potently inhibited. More specifically, to clarify whether anogenital cells support the productive infection, primary endometrial stromal fibroblasts (eSFs) and human foreskin fibroblasts (HFFs), as well as cell lines derived from the cervix (HeLa), endometrium (TZM-bl), colon (SW480 and T-84) and ovaries (OVCAR-3 and SKOV3) were inoculated with ZIKV. Confocal microscopy demonstrated that the analyzed cell types were infected as assessed by E protein expression. All the infected cells released viral RNA and infectious viruses. Among the tested cell lines, viral replication was less efficient in SKOV3 and T-84 cells and the most efficient in SW480, HeLa and OVCAR-3 cells; eSFs and HFFs supported efficient levels of ZIKV replication. Moreover, to analyze whether ZIKV can also replicate in FGT intact tissues, surgically removed vaginal (VT) or endometrial (ET) tissues were cut into blocks and infected with ZIKV, and a productive viral infection was established in the majority of VTs and ETs analyzed. The absolute titers and kinetics of replication, however, varied between experiments and donors.
Two other studies showed ZIKV tropism for HeLa cells. Among them, Silva et al. 7070. Silva SR, Cheng F, Huang C, Jung JU, Gao SJ. Efficiencies and kinetics of infection in different cell types/lines by African and Asian strains of Zika virus. J Med Virol. 2019;91:179-89. infected 10 human and 4 non-human cell lines with two African (IbH30656 and MR766) and two Asian (PRVABC59 and H/FP/2013) ZIKV strains. In the context of FGT, only HeLa cells were evaluated. Among the human cell lines, all were permissive to ZIKV infection. However, 293T and HeLa cells showed differential susceptibilities to African strains. On the other hand, considering that previous studies have shown that mosquito-transmitted flaviviruses, including yellow fever, JEV, and WNV, could be attenuated by serial HeLa cells passages, Li et al. 7171. Li L, Collins ND, Widen SG, Davis EH, Kaiser JA, White MM, et al. Attenuation of Zika virus by passage in human HeLa cells. Vaccines (Basel). 2019;7:93. hypothesized that ZIKV could also be attenuated after passages in HeLa cells. A human isolate from a recent ZIKV epidemic was subjected to serial passages in HeLa cells resulting in attenuated in vitro replication in both, Vero and A549 cells. These data showed that, similarly to other mosquito-borne flaviviruses, ZIKV is attenuated after passages in HeLa cells.
Finally, to characterize the innate immune response of FGT tissues to ZIKV infections, Caine et al. 2828. Caine EA, Scheaffer SM, Arora N, Zaitsev K, Artyomov MN, Coyne CB, et al. Interferon lambda protects the female reproductive tract against Zika virus infection. Nat Commun. 2019;10:280. first evaluated the effect of recombinant IFN-β and IFN-λ on ZIKV infection in primary human vaginal epithelial cells (HVECs) obtained from four different donors and primary human cervical epithelial cells (HCECs) obtained from three donors. The results showed that HVECs and HCECs treatments with IFN-α/β or IFN-λ induced host defense transcriptional signatures and inhibited ZIKV infection. The study has also assessed the effects of IFNs on intravaginal FGT infections using ovariectomized mice treated with reproductive hormones.
Although few in vitro studies have been performed, they have provided important evidence regarding tropism of ZIKV for LGT and UGT. However, considering that few in vitro studies have evaluated the interaction between ZIKV and FGT cells, new studies with organ-technology and organ-specific chip models are still urgently needed.
Assisted reproductive technologies (ART) recommendations
Due to the persistence of ZIKV RNA in female and male genital tracts, specific and essential care are required for couples who have performed or intend to perform ART, especially in view of ZIKV’s association to congenital microcephaly in newborns. There is a potential risk for disease transmission at several ART stages 7272. Cordeiro CN, Bano R, Washington Cross CI, Segars JH. Zika virus and assisted reproduction. Curr Opin Obstet Gynecol. 2017;29:175-9.. However, there are no validated molecular biology-based commercial tests for ZIKV detection in FGT samples, including oocytes. There is a limited understanding on how these test results should be interpreted, especially ZIKV RNA detection in FGT that does not necessarily indicate the presence of a replication-competent or infectious virus. The detection of viable infectious ZIKV is only possible using cell culture techniques 3636. Murray KO, Gorchakov R, Carlson AR, Berry R, Lai L, Natrajan M, et al. Prolonged detection of Zika virus in vaginal secretions and whole blood. Emerg Infect Dis. 2017;23:99-101.. Additionally, serological tests do not provide furtherinformation, as a negative serological test does not exclude the possibility of ZIKV in FGT. Finally, ZIKV Immunoglobulin (Ig) M and IgG detection by serological tests do have cross-reaction problems with other circulating flaviviruses, including dengue 7373. Simmons CP, Farrar JJ, Nguyen V, Wills B. Dengue. N Engl J Med. 2012;366:1423-32., 7474. Clapham HE, Cummings DA, Johansson MA. Immune status alters the probability of apparent illness due to dengue virus infection: evidence from a pooled analysis across multiple cohort and cluster studies. PLoS Negl Trop Dis. 2017;11:e0005926.. As a result, testing on FGT samples is not currently recommend by CDC 7575. Sharp TM, Fischer M, Muñoz-Jordán JL, Paz-Bailey G, Staples JE, Gregory CJ, et al. Dengue and Zika Virus diagnostic testing for patients with a clinically compatible illness and risk for infection with both viruses. MMWR Recomm Rep. 2019;68:1-10. and WHO 2020. World Health Organization. Prevention of sexual transmission of Zika virus: interim guidance update, 6 September 2016. [cited 2019 Feb 6]. Available from: http://apps.who.int/iris/bitstream/handle/10665/204421/WHO_ZIKV_MOC_16.1_eng.pdf;jsessionid=776D077B94EBA435074EF956F7DC2627?sequence=1
http://apps.who.int/iris/bitstream/handl...
. Therefore, CDC, the European Centers for Disease Prevention and Control (ECDC), Food and Drug Administration (FDA) and WHO recommendations do not directly address non-pregnant women regarding ZIKV outbreaks, but recommendations provided for women of childbearing age or planning pregnancy apply to the ART sector 7676. Epelboin S, Dulioust E, Epelboin L, Benachi A, Merlet F, Patrat C. Zika virus and reproduction: facts, questions and current management. Hum Reprod Update. 2017;23:629-45..
The American Society for Reproductive Medicine (ASRM) guidelines 7777. American Society for Reproductive Medicine. Guidance for providers caring for women and men of reproductive age with possible Zika virus exposure. [cited 2020 Feb 6]. Available from: https://www.asrm.org/news-and-publications/news-and-research/announcements/guidance-for-providers-caring-for-women-and-men-of-reproductive-age-with-possible-zika-virus-exposure/
https://www.asrm.org/news-and-publicatio...
, recommend that males or females with a positive viral RT-PCR test result, should deffer the treatment of infertility until (i) a subsequent RT-PCR test is negative in the couple and (ii) at least 6 months have passed from the time of the last positive result. For males or females without testing or with a negative RT-PCR test result, gamete or embryo cryopreservation have to be considered and quarantined until (i) a subsequent RT-PCR is negative in the couple and (ii) at least 8 weeks have passed from the time of the gamete collection.
In Brazil, technical criteria were defined for the health risk management of cells, germ tissues and human embryos intended for therapeutic use in the face of cases of ZIKV infection in the country. In summary, ART recommendations related to non-pregnant women are 7878. Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução de Diretoria Colegiada – RDC N° 72, de 30 de março de 2016. Altera a Resolução da Diretoria Colegiada - RDC Nº 23, de 27 de maio de 2011, que dispõe sobre o regulamento técnico para o funcionamento dos bancos de células e tecidos germinativos e dá outras providências. Diário Oficial da União, Brasília, 30 maio 2011. [cited 2020 Feb 6]. Available from: http://portal.anvisa.gov.br/documents/10181/2718376/RDC_72_2016_.pdf/7283a105-bb94-4a6c-a3d6-ca69f8563dff
http://portal.anvisa.gov.br/documents/10...
: (i) For donors’ and patients’ selection, laboratory tests for ZIKV (detection of antibody against ZIKV-IgM) should be performed; (ii) For female patients with reactive or inconclusive results in serological screening tests to ZIKV, the following is recommended: I - Repeat the serological test (IgM) after 30 days; or II - Perform a molecular biology test for ZIKV markers at any time; (iii) Gametes or germ tissues may only be collected for proper use in ART after obtaining non-reactive or negative results for ZIKV.
Therefore, understanding the mechanisms that underlie persistent ZIKV infections in non-pregnant women is crucial to develop guidelines, effective vaccinesand therapies. Furthermore, the development and validation of commercial tests based on molecular technology for ZIKV detection in FGT samples, including oocytes, are also essential in new ARTs’ guidelines.
CONCLUSION
This review summarized the current information on the impact of ZIKV infections in FGT of non-pregnant ZIKV-infected females. There are variations regarding the presence and persistence of the virus in lower and upper genital samples. However, it is consensual that FGT is the place in which ZIKV RNA has been detected, sometimes for relatively long periods, even after clearance from blood and urine. Studies evaluating the kinetics of the ZIKV in FGT of ZIKV-infected non-pregnant women, as well as in animal and cellular infection models have shown that, in addition to the vagina and cervix, the endometrium, uterus and ovaries (oocytes and follicles) can also be involved in persistent ZIKV infection. However, little is known about the effect of ZIKV on female reproductive health. FGT may possibly act as a sanctuary for ZIKV survival, allowing and extending the risk of sexual transmission. This evidence supports the recommendation of safe sex practices for women returning from areas with ongoing ZIKV transmission. Due to the scarce and non consensual literature, new studies are needed to establish the natural history of ZIKV transmission, pathogenesis and methods to detect ZIKV in FGT. In addition, future studies may clarify the impact of ZIKV infection on female reproductive health.
ACKNOWLEDGMENTS
This work was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/CAPES, Brazilian Government (Grant Nº 88881.130822/2016-01), Conselho Nacional de Desenvolvimento Científico e Tecnológico/CNPq, Brazilian Government (Grant Nº 440521/2016-5) and Departamento de Ciência e Tecnologia da Secretaria de Ciência, Tecnologia e Insumos Estratégicos do Ministério da Saúde/DECIT/SCTIE/MS.
REFERENCES
-
1Kuno G, Chang GJ, Tsuchiya KR, Karabatsos N, Cropp CB. Phylogeny of the genus Flavivirus. J Virol. 1998;72:73-83.
-
2MacNamara FN. Zika virus: a report on three cases of human infection during an epidemic of Jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48:139-45.
-
3Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509-20.
-
4Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232-9.
-
5Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. New Engl J Med. 2009;360:2536-43.
-
6Besnard M, Lastère S, Teissier A, Cao-Lormeau VM, Musso D. Evidence of perinatal transmission of zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19:20751.
-
7Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect. 2014;20:O595-96.
-
8Metsky HC, Matranga CB, Wohl S, Schaffner SF, Freije CA, Winnicki SM, et al. Zika virus evolution and spread in the Americas. Nature. 2017;546:411-5.
-
9Brasil P, Pereira Jr JP, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 2016;375:2321-34.
-
10Pan American Health Organization. Zika cases and congenital syndrome associated with Zika virus reported by countries and territories in the Americas, 2015 - 2018: cumulative cases - Data as of 4 January 2018. [cited 2020 Feb 6]. Available from: https://www.paho.org/en/node/60231
» https://www.paho.org/en/node/60231 -
11Shankar A, Patil AA, Skariyachan S. Recent perspectives on genome, transmission, clinical manifestation, diagnosis, therapeutic strategies, vaccine developments, and challenges of Zika virus research. Front Microbiol. 2017;8: 1761.
-
12Calvet G, Aguiar RS, Melo AS, Sampaio SA, de Filippis I, Fabri A, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016;16:653-60.
-
13Panchaud A, Stojanov M, Ammerdorffer A, Vouga M, Baud D. Emerging role of Zika virus in adverse fetal and neonatal outcomes. Clin Microbiol Rev. 2016;29:659-94.
-
14Grard G, Caron M, Mombo IM, Nkoghe D, Ondo SM, Jiolle D, et al. Zika virus in Gabon (Central Africa) - 2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8:e2681.
-
15Petersen LR, Busch MP. Transfusion-transmitted arboviruses. Vox Sang. 2010;98: 495-503.
-
16Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, et al. Probable non-vector-borne transmission of Zika virus, Colorado, US. Emerg Infect Dis. 2011;17:880-2.
-
17D’Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, et al. Evidence of sexual transmission of Zika virus. N Engl J Med. 2016;374:2195-8.
-
18Sotelo JR, Sotelo AB, Sotelo FJ, Doi AM, Pinho JR, Oliveira RC, et al. Persistence of Zika virus in breast milk after infection in late stage of pregnancy. Emerg Infect Dis. 2017;23:856-7.
-
19Blohm GM, Lednicky JA, Márquez M, White SK, Loeb JC, Pacheco CA, et al. Evidence for mother-to-child transmission of Zika virus through breast milk. Clin Infect Dis. 2018;66:1120-1.
-
20World Health Organization. Prevention of sexual transmission of Zika virus: interim guidance update, 6 September 2016. [cited 2019 Feb 6]. Available from: http://apps.who.int/iris/bitstream/handle/10665/204421/WHO_ZIKV_MOC_16.1_eng.pdf;jsessionid=776D077B94EBA435074EF956F7DC2627?sequence=1
» http://apps.who.int/iris/bitstream/handle/10665/204421/WHO_ZIKV_MOC_16.1_eng.pdf;jsessionid=776D077B94EBA435074EF956F7DC2627?sequence=1 -
21Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, Klein L, et al. Persistence of Zika virus in body fluids: final report. N Engl J Med. 2018;379:1234-43.
-
22Arsuaga M, Bujalance SG, Díaz-Menéndez M, Vázquez A, Arribas JR. Probable sexual transmission of Zika virus from a vasectomised man. Lancet Infect Dis. 2016;16:1107.
-
23Barzon L, Pacenti M, Franchin E, Lavezzo E, Trevisan M, Sgarabotto D, et al. Infection dynamics in a traveller with persistent shedding of Zika virus RNA in semen for six months after returning from Haiti to Italy, January 2016. Euro Surveill. 2016;21:30316.
-
24Mansuy JM, Dutertre M, Mengelle C, Fourcade C, Marchou B, Delobel P, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16:405.
-
25Sánchez-Montalvá A, Pou D, Sulleiro E, Salvador F, Bocanegra C, Treviño B, et al. Zika virus dynamics in body fluids and risk of sexual transmission in a non-endemic area. Trop Med Int Health. 2018;23:92-100.
-
26Deckard DT, Chung WM, Brooks JT, Smith JC, Woldai S, Hennessey M, et al. Male-to-male sexual transmission of Zika virus - Texas, January 2016. MMWR Morb Mortal Wkly Rep. 2016;65:372-4.
-
27Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of zika virus - New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:716-7.
-
28Caine EA, Scheaffer SM, Arora N, Zaitsev K, Artyomov MN, Coyne CB, et al. Interferon lambda protects the female reproductive tract against Zika virus infection. Nat Commun. 2019;10:280.
-
29Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, et al. Zika virus infection damages the testes in mice. Nature. 2016;540:438-42.
-
30Ma W, Li S, Ma S, Zhang F, Zhang Y, Zhang J, et al. Zika virus causes testis damage and leads to male infertility in mice. Cell. 2016;167:1511-24.
-
31Uraki R, Hwang J, Jurado KA, Householder S, Yockey LJ, Hastings AK, et al. Zika virus causes testicular atrophy. Sci Adv. 2017;3:e1602899.
-
32Barzon L, Percivalle E, Pacenti M, Rovida F, Zavattoni M, Del Bravo P, et al. Virus and antibody dynamics in travelers with acute zika virus infection. Clin Infect Dis. 2018;66:1173-80.
-
33Joguet G, Mansuy JM, Matusali G, Hamdi S, Walschaerts M, Pavili L, et al. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis. 2017;17:1200-8.
-
34Kurscheidt FA, Mesquita CS, Damke GM, Damke E, Carvalho AR, Suehiro TT, et al. Persistence and clinical relevance of Zika virus in the male genital tract. Nature Rev Urol. 2019;16:211-30.
-
35Prisant N, Bujan L, Benichou H, Hayot PH, Pavili L, Lurel S, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1.
-
36Murray KO, Gorchakov R, Carlson AR, Berry R, Lai L, Natrajan M, et al. Prolonged detection of Zika virus in vaginal secretions and whole blood. Emerg Infect Dis. 2017;23:99-101.
-
37Cruz TE, Souza RP, Pelloso SM, Morelli F, Suehiro TT, Damke E, et al. Prolonged detection of Zika virus RNA in vaginal and endocervical samples from a Brazilian woman, 2018. Am J Trop Med Hyg. 2019;100:183-6.
-
38Dowall SD, Graham VA, Rayner E, Atkinson B, Hall G, Watson RJ, et al. A susceptible mouse model for Zika virus infection. PLoS Negl Trop Dis. 2016;10:e0004658.
-
39Tripathi S, Balasubramaniam VR, Brown JA, Mena I, Grant A, Bardina SV, et al. A novel Zika virus mouse model reveals strain specific differences in virus pathogenesis and host inflammatory immune responses. PLoS Pathog. 2017;13:e1006258.
-
40Duggal NK, McDonald EM, Ritter JM, Brault AC. Sexual transmission of Zika virus enhances in utero transmission in a mouse model. Sci Rep. 2018;8:4510.
-
41Carroll T, Lo M, Lanteri M, Dutra J, Zarbock K, Silveira P, et al. Zika virus preferentially replicates in the female reproductive tract after vaginal inoculation of rhesus macaques. PLoS Pathog. 2017;13:e1006537.
-
42Filho EA, Fácio CL, Machado-Paula LA, Oliveira MA, Martinhago CD, Araújo LP, et al. Case report of Zika virus during controlled ovarian hyperstimulation: results from follicular fluid, cumulus cells and oocytes. JBRA Assist Reprod. 2019;23:172-4.
-
43Beller EM, Glasziou PP, Altman DG, Hopewell S, Bastian H, Chalmers I, et al. PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10:e1001419.
-
44Nicastri E, Castilletti C, Balestra P, Galgani S, Ippolito G. Zika virus infection in the central nervous system and female genital tract. Emerg Infect Dis. 2016;22:2228-30.
-
45Penot P, Brichler S, Guilleminot J, Lascoux-Combe C, Taulera O, Gordien E, et al. Infectious Zika virus in vaginal secretions from HIV-infected woman. Euro Surveill. 2017;22:30444.
-
46Tobar P, Vega M, Ordoñez C, Rivera L, Landivar J, Zambrano H. Detection of Zika virus and human papilloma virus in cervical cytology samples using two real time PCR based techniques in ecuadorian women diagnosed with ASCUS. P R Health Sci J. 2018;37 Spec Issue:S96-8.
-
47Reyes Y, Bowman NM, Becker-Dreps S, Centeno E, Collins MH, Liou GA, et al. Prolonged shedding of Zika virus RNA in vaginal secretions, Nicaragua. Emerg Infect Dis. 2019;25:808-10.
-
48Tozetto-Mendoza TR, Avelino-Silva VI, Fonseca S, Claro IM, Paula AV, Levin AS, et al. Zika virus infection among symptomatic patients from two healthcare centers in São Paulo State, Brazil: prevalence, clinical characteristics, viral detection in body fluids and serodynamics. Rev Inst Med Trop Sao Paulo. 2019;61:e19.
-
49Prisant N, Joguet G, Herrmann-Stock C, Moriniere C, Pavili L, Lurel S, et al. Upper and lower genital tract Zika virus screening in a large cohort of reproductive-age women during the Americas epidemic. Reprod Biomed Online. 2019;39:624-32.
-
50Mandl AM, Zuckerman S. Numbers of normal and atretic oocytes in unilaterally spayed rats. J Endocrinol. 1951;7:112-9.
-
51Grant A, Ponia SS, Tripathi S, Balasubramaniam V, Miorin L, Sourisseau M, et al. Zika virus targets human STAT2 to inhibit type I interferon signaling. Cell Host Microbe. 2016;19:882-90.
-
52Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med. 2016;22:1448-55.
-
53Khan S, Woodruff EM, Trapecar M, Fontaine KA, Ezaki A, Borbet T, et al. Dampened antiviral immunity to intravaginal exposure to RNA viral pathogens allows enhanced viral replication. J Exp Med. 2016;213:2913-29.
-
54Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, et al. A mouse model of Zika virus pathogenesis. Cell Host Microbe. 2016;19:720-30.
-
55Tang WW, Young MP, Mamidi A, Regla-Nava JA, Kim K, Shresta S. A mouse model of Zika Virus sexual transmission and vaginal viral replication. Cell Rep. 2016;17:3091-8.
-
56Broughton DE, Scheaffer S, Skaznik-Wikie ME, Halabi J, Govero J, Caine E, et al. Zika virus exhibits tropism to the ovary and increases follicular apoptosis in a mouse model. Fertil Steril. 2017;108 Suppl:e35.
-
57Haddow AD, Nalca A, Rossi FD, Miller LJ, Wiley MR, Perez-Sautu U, et al. High infection rates for adult macaques after intravaginal or intrarectal inoculation with Zika virus. Emerg Infect Dis. 2017;23:1274-81.
-
58Hastings AK, Yockey LJ, Jagger BW, Hwang J, Uraki R, Gaitsch HF, et al. TAM receptors are not required for Zika virus infection in mice. Cell Rep. 2017;19:558-68.
-
59Hirsch AJ, Smith JL, Haese NN, Broeckel RM, Parkins CJ, Kreklywich C, et al. Zika virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog. 2017;13:e1006219.
-
60Winkler CW, Woods TA, Rosenke R, Scott DP, Best SM, Peterson KE. Sexual and vertical transmission of Zika virus in anti-interferon receptor-treated Rag1-deficient mice. Sci Rep. 2017;7:7176.
-
61Scott JM, Lebratti TJ, Richner JM, Jiang X, Fernandez E, Zhao H, et al. Cellular and humoral immunity protect against vaginal Zika virus infection in mice. J Virol. 2018;92:e00038-18.
-
62Caine EA, Scheaffer SM, Broughton DE, Salazar V, Govero J, Poddar S, et al. Zika virus causes acute infection and inflammation in the ovary of mice without apparent defects in fertility. J Infect Dis. 2019;220:1904-14.
-
63Chen JC, Wang Z, Huang H, Weitz SH, Wang A, Qiu X, et al. Infection of human uterine fibroblastos by Zika virus in vitro: implications for viral transmission in women. Int J Infect Dis. 2016;51:139-40.
-
64Pagani I, Ghezzi S, Ulisse A, Rubio A, Turrini F, Garavaglia E, et al. Human endometrial stromal cells are highly permissive to productive infection by Zika virus. Sci Rep. 2017;7:44286.
-
65Chan JF, Yip CC, Tsang JO, Tee KM, Cai JP, Chik KK, et al. Differential cell line susceptibility to the emerging Zika virus: implications for disease pathogenesis, non-vector-borne human transmission and animal reservoirs. Emerg Microbes Infect. 2016;5:e93.
-
66Fink SL, Vojtech L, Wagoner J, Slivinski NS, Jackson KJ, Wang R, et al. The antiviral drug Arbidol inhibits Zika virus. Sci Rep. 2018;8:8989.
-
67Amerson-Brown MH, Miller AL, Maxwell CA, White MM, Vincent KL, Bourne N, et al. Cultivated human vaginal microbiome communities’ impact Zika and herpes simplex virus replication in ex vivo vaginal mucosal cultures. Front Microbiol. 2019;9:3340.
-
68Cagno V, Tseligka ED, Bettex Q, Huang S, Constant S, Tapparel C. Growth of Zika virus in human reconstituted respiratory, intestinal, vaginal and neural tissues. Clin Microbiol Infect. 2019;25:1042.e1-4.
-
69Müller JA, Harms M, Krüger F, Groß R, Joas S, Hayn M, et al. Semen inhibits Zika virus infection of cells and tissues from the anogenital region. Nat Commun. 2018;9:2207.
-
70Silva SR, Cheng F, Huang C, Jung JU, Gao SJ. Efficiencies and kinetics of infection in different cell types/lines by African and Asian strains of Zika virus. J Med Virol. 2019;91:179-89.
-
71Li L, Collins ND, Widen SG, Davis EH, Kaiser JA, White MM, et al. Attenuation of Zika virus by passage in human HeLa cells. Vaccines (Basel). 2019;7:93.
-
72Cordeiro CN, Bano R, Washington Cross CI, Segars JH. Zika virus and assisted reproduction. Curr Opin Obstet Gynecol. 2017;29:175-9.
-
73Simmons CP, Farrar JJ, Nguyen V, Wills B. Dengue. N Engl J Med. 2012;366:1423-32.
-
74Clapham HE, Cummings DA, Johansson MA. Immune status alters the probability of apparent illness due to dengue virus infection: evidence from a pooled analysis across multiple cohort and cluster studies. PLoS Negl Trop Dis. 2017;11:e0005926.
-
75Sharp TM, Fischer M, Muñoz-Jordán JL, Paz-Bailey G, Staples JE, Gregory CJ, et al. Dengue and Zika Virus diagnostic testing for patients with a clinically compatible illness and risk for infection with both viruses. MMWR Recomm Rep. 2019;68:1-10.
-
76Epelboin S, Dulioust E, Epelboin L, Benachi A, Merlet F, Patrat C. Zika virus and reproduction: facts, questions and current management. Hum Reprod Update. 2017;23:629-45.
-
77American Society for Reproductive Medicine. Guidance for providers caring for women and men of reproductive age with possible Zika virus exposure. [cited 2020 Feb 6]. Available from: https://www.asrm.org/news-and-publications/news-and-research/announcements/guidance-for-providers-caring-for-women-and-men-of-reproductive-age-with-possible-zika-virus-exposure/
» https://www.asrm.org/news-and-publications/news-and-research/announcements/guidance-for-providers-caring-for-women-and-men-of-reproductive-age-with-possible-zika-virus-exposure/ -
78Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução de Diretoria Colegiada – RDC N° 72, de 30 de março de 2016. Altera a Resolução da Diretoria Colegiada - RDC Nº 23, de 27 de maio de 2011, que dispõe sobre o regulamento técnico para o funcionamento dos bancos de células e tecidos germinativos e dá outras providências. Diário Oficial da União, Brasília, 30 maio 2011. [cited 2020 Feb 6]. Available from: http://portal.anvisa.gov.br/documents/10181/2718376/RDC_72_2016_.pdf/7283a105-bb94-4a6c-a3d6-ca69f8563dff
» http://portal.anvisa.gov.br/documents/10181/2718376/RDC_72_2016_.pdf/7283a105-bb94-4a6c-a3d6-ca69f8563dff
Publication Dates
-
Publication in this collection
02 Mar 2020 -
Date of issue
2020
History
-
Received
6 Dec 2019 -
Accepted
3 Feb 2020