Abstracts
Infection of Swiss/NIH mice with Leishmania major was compared with infection in isogenic resistant C57BL/6 and susceptible BALB/c mice. Swiss/NIH mice showed self-controlled lesions in the injected foot pad. The production of high levels of interferon-<FONT FACE="Symbol">g</font> (IFN-<FONT FACE="Symbol">g</font>) and low levels of interleukin-4 (IL-4) by cells from these animals suggests that they mount a Th1-type immune response. The importance of the indigenous microbiota on the development of murine leishmaniasis was investigated by infecting germfree Swiss/NIH in the hind footpad with L. major and conventionalizing after 3 weeks of infection. Lesions from conventionalized Swiss/NIH mice were significantly larger than conventional mice. Histopathological analysis of lesions from conventionalized animals showed abscesses of variable shapes and sizes and high numbers of parasitized macrophages. In the lesions from conventional mice, besides the absence of abscess formation, parasites were rarely observed. On the other hand, cells from conventional and conventionalized mice produced similar Th1-type response characterized by high levels of IFN-<FONT FACE="Symbol">g</font> and low levels of IL-4. In this study, we demonstrated that Swiss/NIH mice are resistant to L. major infection and that the absence of the normal microbiota at the beginning of infection significantly influenced the lesion size and the inflammatory response at the site of infection.
Leishmania; Swiss mice; Microbiota; Germfree; Th1; Leishmaniasis
Neste trabalho, estudamos a infecção experimental de camundongos Suíços/NIH com Leishmania major em comparação com camundongos isogênicos C57BL/6 e BALB/c que são resistentes e suceptíveis a esta infecção, respectivamente. Camundongos Suiços mostraram lesões que se curaram espontaneamente e se restringiram ao local de inoculação. Células linfóides derivadas destes animais desenvolveram uma resposta do tipo Th1, caracterizada pela produção de níveis altos de IFN-<FONT FACE="Symbol">g</font> e níveis baixos de IL-4. Com o objetivo de estudar a importância da microbiota no desenvolvimento da leishmaniose cutânea neste modelo, camundongos Suiços sem germes foram infectados na pata com promastigotas de L. major, convencionalizados após 3 semanas de infecção e as lesões comparadas com as observadas em camundongos convencionais. Os camundongos convencionalizados apresentaram lesões significativamente maiores do que as observadas nos camundongos convencionais. A análise histopatológica das lesões de todos os animais convencionalizados mostrou abcessos de tamanhos e formas variáveis e a presença de numerosos macrófagos parasitados. Nas lesões dos camundongos convencionais não houve a formação de abscessos e foram observados poucos macrófagos parasitados. Entretanto, células de ambos os grupos experimentais desenvolveram uma resposta similar do tipo Th1, caracterizada pela produção de altos níveis de IFN-<FONT FACE="Symbol">g</font> e baixos níveis de IL-4. Neste trabalho, demonstramos que os camundongos Suíços/NIH são resistentes à infecção por L. major, com o desenvolvimento de um fenótipo do tipo Th1 e que a ausência da microbiota no início da infecção influencia significativamente o tamanho da lesão e no processo inflamatório nestes animais.
INFLUENCE OF MICROBIOTA IN EXPERIMENTAL CUTANEOUS LEISHMANIASIS IN SWISS MICE
Marcia Rosa de OLIVEIRA(2 (1 ) Departamento de Bioquímica e Imunologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. (2 ) Departamento de Parasitologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. (3 ) Departamento de Microbiologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. (4 ) Departamento de Patologia Geral, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil ), Wagner Luis TAFURI (4 (1 ) Departamento de Bioquímica e Imunologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. (2 ) Departamento de Parasitologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. (3 ) Departamento de Microbiologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. (4 ) Departamento de Patologia Geral, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil ), Jacques Robert NICOLI (3 (1 ) Departamento de Bioquímica e Imunologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. (2 ) Departamento de Parasitologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. (3 ) Departamento de Microbiologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. (4 ) Departamento de Patologia Geral, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil ), Enio Cardillo VIEIRA (1 (1 ) Departamento de Bioquímica e Imunologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. (2 ) Departamento de Parasitologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. (3 ) Departamento de Microbiologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. (4 ) Departamento de Patologia Geral, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil ), Maria Norma MELO (2 (1 ) Departamento de Bioquímica e Imunologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. (2 ) Departamento de Parasitologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. (3 ) Departamento de Microbiologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. (4 ) Departamento de Patologia Geral, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil ) & Leda Quercia VIEIRA (1 (1 ) Departamento de Bioquímica e Imunologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. (2 ) Departamento de Parasitologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. (3 ) Departamento de Microbiologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil. (4 ) Departamento de Patologia Geral, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brasil )
SUMMARY
Infection of Swiss/NIH mice with Leishmania major was compared with infection in isogenic resistant C57BL/6 and susceptible BALB/c mice. Swiss/NIH mice showed self-controlled lesions in the injected foot pad. The production of high levels of interferon-g (IFN-g) and low levels of interleukin-4 (IL-4) by cells from these animals suggests that they mount a Th1-type immune response. The importance of the indigenous microbiota on the development of murine leishmaniasis was investigated by infecting germfree Swiss/NIH in the hind footpad with L. major and conventionalizing after 3 weeks of infection. Lesions from conventionalized Swiss/NIH mice were significantly larger than conventional mice. Histopathological analysis of lesions from conventionalized animals showed abscesses of variable shapes and sizes and high numbers of parasitized macrophages. In the lesions from conventional mice, besides the absence of abscess formation, parasites were rarely observed. On the other hand, cells from conventional and conventionalized mice produced similar Th1-type response characterized by high levels of IFN-g and low levels of IL-4. In this study, we demonstrated that Swiss/NIH mice are resistant to L. major infection and that the absence of the normal microbiota at the beginning of infection significantly influenced the lesion size and the inflammatory response at the site of infection.
KEYWORDS: Leishmania; Swiss mice; Microbiota ; Germfree; Th1; Leishmaniasis.
INTRODUCTION
Leishmaniasis is a disease caused by parasites of the genus Leishmania that produce diversified clinical manifestation depending upon the characteristics of the parasite and on the immune response of the host2,5. The present understanding of the factors that lead to this diversity of clinical symptoms was obtained, in large part, through studies using isogenic mice experimentally infected with L. major. In these studies, the resistance to L. major is correlated with the development of Th1 CD4+ lymphocytes that produce high levels of IFN-g, a cytokine capable of activating macrophages to kill the intracellular forms of the parasite. On the other hand, susceptibility has been associated with the lack of IFN-g production and the presence of Th2 CD4+ lymphocytes that produce IL-4 which acts inhibiting the activation of macrophages12,18,23. There is, however, no report on infection of Swiss/NIH mice with L. major.
Many factors can influence the type of immune response mounted by the host. The effects of the intestinal microbiota on the outcome of infections are not well investigated. There are reports including ours showing that the indigenous microbiota can play an important role in the outcome of parasitic infections, making germfree animals either more susceptible or more resistant to infection when compared with the corresponding animals that are associated to the normal microbiota (conventional animals)19,26,28,29,30. Several investigators have reported that the indigenous microbiota affects the development of the host's immune response4. However, the mechanisms by which the microbiota exerts its effect on the host immune response are not well understood. Both the development of T cell responses (delayed-type hypersensitivity)13 and macrophage activity4,16 have been found impaired in the absence of the indigenous microbiota, whereas antibody responses seemingly is not affected13. Hence, it would be reasonable to suppose that the Th1 arm of the immune response could be deficient in the absence of the normal microbiota, while the Th2 arm could be intact.
In the present work, Swiss/NIH mice were characterized as to their response to infection with L. major by measuring the development of the primary lesion and cytokine production after subcutaneous injection with the parasite. We then investigated whether the absence of the microbiota at the onset of infection, and during the time period in which the development of a susceptible or resistant T cell response occurs3,12,23, would alter sizes of lesions and the cytokine profile when compared with animals bearing the microbiota from birth. We found that the absence of the normal microbiota early in infection renders Swiss/NIH mice more susceptible to L. major, and that this increased susceptibility is not due to an impaired Th1 response.
MATERIAL AND METHODS
Animals
C57BL/6 and BALB/c mice were obtained from CEBIO (UFMG, Belo Horizonte, Brazil). Germfree Swiss/NIH mice were derived from a germfree nucleus (Taconic Farms, Germantown, USA) and maintained in flexible plastic isolators (Standard Safety Equipment Co., Pallatine, USA) using classical gnotobiology techniques20. Conventionalized Swiss/NIH mice were obtained by transferring germfree animals from a sterile isolator environment to the experimental animal facility. Conventional Swiss/NIH mice were derived from germfree matrices, and considered conventional only after two generations in the conventional facility. All animals were females and 5-7 weeks old.
Parasite and antigen
A clone of Leishmania major (WHO MHOM/IL/80/Friedlin) was used for these studies. Parasites were cultured in Grace's insect medium (Life Technologies, Grand Island, USA) supplemented with 20% fetal bovine serum, 100 U/ml of penicillin and 100 µg/ml of streptomycin. Metacyclic promastigotes were obtained from stationary phase of growth using Arachis hypogae agglutinin (Sigma Chemical Co., St. Louis, MO) as described previously21. Antigen was prepared from L. major stationary phase cultures as described previously1. In short, cells were washed four times in PBS (0.9% NaCl buffered with 0.01M sodium phosphate, pH 7.2-7.4, the concentration adjusted to 1x108 cells/ml, freeze/thawed three times, the antigen was homogenized, aliquoted and maintained at -20 C until use.
Infection and monitoring of lesions
All mice were injected subcutaneously on the left hind footpad with 1x106 metacyclic promastigotes of L. major. For the infection of germfree Swiss/NIH mice, metacyclic promastigotes in Grace's medium (Life Technologies, Grand Island, USA) were asseptically transferred into sterile glass ampoules. The ampoules were sealed, the outer surface was chemically sterilized with 8% peracetic acid and introduced into the isolator. Another ampoule containing promastigotes and treated in the same way was used to infect conventional Swiss/NIH mice. Measurements of the footpad thickness were taken using a calliper (Starret S. A., Itu, SP, Brazil). The lesion size was calculated by subtracting the value of the uninfected contralateral footpad from that of the infected one. The lesions from conventionalized Swiss/NIH mice were monitored starting from the third week of infection when they were taken out of the isolator.
Conventionalization
Germfree Swiss/NIH mice were infected with L. major as described above. Three weeks after infection, these animals were placed in bedding which contained fresh faeces from conventional Swiss/NIH animals, in order to induce the colonization by the different species of microorganisms normally found in conventional mice. The conventionalized animals were kept in the experimental animal facility under the same conditions as the conventional mice.
Histopathology
Histopathological analysis of lesions were performed in conventional and conventionalized Swiss/NIH mice. Four animals from each group were sacrificed after seven or fifteen weeks of infection and footpad tissues were collected and fixed in 10% formalin in PBS. Later the material was dehydrated, cleared, embedded in paraffin, cut (3 to 5 µm thick) and stained with hematoxylin and eosin for microscopical examination.
Cell cultures and cytokine measurement
Spleen cells and a pool of popliteal lymph node cells were prepared as described previously24. Pools of popliteal lymph nodes were used since our Swiss/NIH mice did not give, in several experiments, a positive mixed lymphocyte reaction (our unpublished observations), which indicates that these animals are not genetically different as to their MHC antigens. The organs were homogenized in RPMI 1640 medium (Gibco Laboratories, Grand Island, NY, USA) and the concentraion of cells adjusted to 5x106 cells/ml RPMI 1640 supplemented with 10% fetal bovine serum (Nutricell, Campinas, SP, Brazil), 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 25 mM HEPES and 0.05 mM b-mercaptoethanol (Gibco Laboratories), and 1 ml/well plated in 24-well tissue culture plates either with or without ConA (5µg/ml, Sigma Chemical Co., St. Louis, MO, USA) or 50µl of L. major antigen. Supernatants were harvested at 72 h for IFN-g and IL-4 analyses. IFN-g and IL-4 were measured by capture ELISA as previously described1. IFN-g was measured using R46A2 rat monoclonal antibody as capture antibody (kind gift from Dr. P. Scott, University of Pennsylvania, Philadelphia, PA, USA) and a rabbit policlonal anti-IFN-g anti serum (kind gift from Dr. J. P. Farrell, University of Pennsylvania, Philadelphia, PA, USA) was used as a secondary antibody followed by donkey peroxidase conjugated anti-rabbit immunoglobulin (Jackson ImmunoResearch Laboratories, INC, West Grove, PA, USA). IL-4 was measured using 11B11 rat monoclonal antibody as capture antibody and biotinilated BVD6 rat monoclonal antibody as a secondary antibody (both kind gifts from Dr. J. P. Farrell, University of Pennsylvania, Philadelphia, PA, USA) followed by streptoavidin-peroxidase (Sigma Chemical Co. St. Louis, MO, USA). The levels of IFN-g and IL-4 were calculated by comparison with a standard curve using recombinant IFN-g and IL-4 (Genzyme, Cambridge, MA, USA). The sensitivity for the assays were as follows: IL-4, 0.2 U/ml and IFN-g, 30 pg/ml.
Statistical analysis
The data obtained in each experiment were analysed by the Student's t test. Values of p £ 0.05 were considered statistically significant.
RESULTS
Development of the lesion and cytokine profile in Swiss/NIH mice after L. major infection
The outcome of infection with L. major in Swiss/NIH mice was compared with that in resistant C57BL/6 and susceptible BALB/c mice by measuring the development of primary lesions after subcutaneous infection with L. major metacyclic promastigotes into the left hind footpad. Footpad thickness in Swiss/NIH mice, like in C57BL/6 mice, peaked at 4-5 weeks and resolved to pre-infection size, whereas footpads of BALB/c mice continued to swell without any evidence of resolution (Figure 1A).
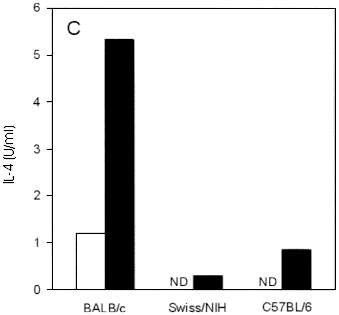
Fig. 1 - Course of infection and cytokine production in conventional Swiss/NIH, BALB/c and C57BL/6 mice infected in the hind footpad with L. major. (A) Each point represents the mean of lesion size of at least five mice per group, subtracted of the contralateral control foot. Vertical lines represent SD of means. IFN-g (B) and IL-4 (C) levels in supernatant of lymph node cell culture from mice at 2 weeks of infection. Cells were unstimulated (white bars) or stimulated (black bars) "in vitro" with L. major antigen for 72 hours, supernatants were harvested and the presence of cytokines was assayed by capture ELISA as described in Materials and Methods. ND: not detectable. Data are from one representative experiment of three performed.
IFN-g and IL-4 production by lymph node cells from Swiss/NIH mice after 2 weeks of infection was measured and compared with that of lymph node cells taken from C57BL/6 and BALB/c mice. Cells from both Swiss/NIH and C57BL/6 mice secrete similar amounts of IFN-g (Figure 1B). Clearly, these cells secreted substantially more IFN-g upon antigenic stimulation "in vitro" than cells from BALB/c mice. The IL-4 production occurred in lower levels in Swiss/NIH mice when compared with BALB/c mice (Figure 1C).
Infection of conventionalized Swiss/NIH mice with L. major
We had previously shown that germfree mice do not heal lesions when infected with L. major and that their higher susceptibility was not due to a Th2 type response30. In order to investigate whether we could revert this picture by associating germfree mice with the conventional microbiota after the T cell response was already stablished, we infected germfree mice with L. major and associated them with the normal microbiota three weeks after infection. The severity of infection in conventionalized and conventional Swiss/NIH mice with L. major was compared by measuring lesion were weekly after conventionalization (Figure 2). Like conventional mice, conventionalized animals showed cutaneous lesions restricted to the site of infection, presenting no evidence of metastasis. However, conventionalized mice had lesions significantly larger than those observed in conventional control mice (Figure 2A).
Fig. 2- Course of infection and IFN-g production in conventional or conventionalized Swiss/NIH mice infected in the hind footpad with L. major. (A) Each point represents the mean of lesion size of at least four mice per group, subtracted from the value obtained for the contralateral control foot. Vertical lines represent SD of means. Asterisks denote statistical difference between conventional and conventionalized mice at each time point, (p£ 0.05). (B) IFN-g production by spleen cells at 7 and 15 weeks of infection. Cells were unstimulated (white bars) or stimulated (black bars) "in vitro" with L. major antigen for 72 hours, supernatants were harvested and the presence of IFN-g was assayed by capture ELISA as described in Materials and Methods. CV: conventional mice, CVZ: conventionalized mice. Data are from one representative experiment of three performed.
Assays to analyze IFN-g and IL-4 production were carried out after seven and fifteen weeks of infection. Spleen cell cultures from conventional and conventionalized mice produced similar levels of IFN-g when stimulated "in vitro" with L. major antigen (Figure 2B). IL-4 production occurred at levels lower than 2 U/ml, and were similar in both groups (data not shown).
Histopathological analysis
To determine if the absence of the microbiota at three weeks of infection would interfere with the inflammatory process associated with L. major infection, cutaneous lesions from conventional and conventionalized mice were histologically examined. At the seventh week after infection, histological sections from both groups showed a diffuse chronic inflammatory cellular exudate (Figure 3A and 3B). However, the footpad skin fragments of all the conventionalized animals showed a chronic focal inflammation with abscesses of variable sizes constituted of collagen fibers associated with the cellular exudate (pyogenic membrane), vacuolated macrophages with the presence of several amastigotes (sometimes found outside the cells); and finally, in the central region, the presence of cellular acidophilic residue (necrotic cells) and a large quantity of neutrophils in fatty degeneration (Figure 3C) was noted. In conventional animals, besides the absence of abscess formation, amastigotes inside the macrophages or even in the interstitial space were rarely observed.
Fig. 3 - Histologic sections of lesions from conventional (A) and conventionalized (B, C) mice after 7 weeks of infection with L. major. (A) Typical lesion from a conventional mouse, showing chronic and diffused inflammation reaching the superficial and deep dermis (H.E. 4x). (B) Lesion from conventionalized mouse, showing chronic diffuse and focal inflammation with abscess formation (arrow head) (H.E. 4x). (C) Detail of structure of abscess. From right to left: pyogenic membrane (fibrosis and leukocytic exudate), vacuolated macrophages with several intracellular amastigotes (arrow), central area with neutrophils in fatty granule degeneration and acidophilic residue (H.E. 40x).
At the fifteenth week of infection the footpad skin fragments from both groups showed the presence of a chronic productive diffuse inflammation, mainly observed in the deep dermis. However, in all of the conventionalized animals, the reactions were more intense (Figure 4 A and B ).
- Histologic sections of lesions from conventional and conventionalized mice after 15 weeks of L. major infection. (A) Lesion from a conventional mouse showing chronic productive and diffused moderate inflammatory process, preferentially localized in the deep dermis (H.E. 4x). (B) Lesion from a conventionalized mouse, showing greater intensity of the inflammatory reactions qualitatively similar to the conventional shown in (A) (H.E. 10x).
DISCUSSION
There are well established isogenic mouse models for leishmaniasis, specially concerning infections with L. major. Upon infection with this intracellular parasite, most isogenic mouse strains, such as C57BL/6 and C3H, will develop an ultimately self-healing lesion around the inoculation site. Thereafter, these mouse strains will remain refractory to other infections by the same parasite. On the other hand, BALB/c mice are extremely susceptible to this infection and develop a non-healing lesion and subsequent visceral infection7,8.
In this work, our interest was to study the effect of the association of Swiss/NIH non-isogenic germfree mice with the normal microbiota on the outcome of infection with L. major. Thus, we found it necessary to characterize the course of infection and cytokine production in conventional Swiss/NIH mice. In order to achieve this goal, we compared infection with L. major in Swiss/NIH mice with the infection in the isogenic resistant C57BL/6 and susceptible BALB/c mice. We observed that conventional Swiss/NIH mice presented a pattern of resistance to experimental L. major infection, showing a self-controlled lesion in the injected footpad. This pattern was similar to that observed in C57BL/6 mice, characterized as resistant to this infection.
Several evidences support the idea that the ability to control infection with L. major is due to the development of T CD4+ helper 1 (Th1) lymphocytes, which secrete high levels of the potent macrophage activator IFN-g, thus stimulating effective killing of the parasite. Mice genetically resistant to infection by L. major become susceptible after the neutralization of IFN-g3, moreover, the deletion of the gene that codes for this cytokine also renders resistant mice susceptible31. On the other hand, the susceptibility to this infection is correlated to the development of T CD4+ helper 2 (Th2) lymphocytes, which inhibit macrophage activation by producing mainly IL-4 and IL-1012,14. Despite conflicting results about the exact role of IL-4 in vivo9,17, there are significant correlations between Th2 type response and disease progression14, 22. In order to determine the cytokine profile induced by L. major in Swiss/NIH mice, IFN-g and IL-4 production were analyzed as markers of Th1- and Th2-type responses, respectively. We observed that conventional Swiss/NIH mice produced high levels of IFN-g and low levels of IL-4, comparable to the cytokine production by C57BL/6 mice. Our results show that resistance in Swiss/NIH mice is associated with the development of a Th1-type immune response against L. major .
To determine if the absence of the microbiota at the early weeks of infection could influence the response to L. major, germfree animals were infected with the parasite and later conventionalized. We observed that the conventionalized Swiss/NIH mice presented lesions significantly larger than those observed in conventional animals, suggesting that the absence of the microbiota in the first three weeks of infection induces an impaired resistance to L. major. However, the higher susceptibility was not due to a lower production of IFN-g or the presence of IL-4, since cells from conventional and conventionalized mice produced similar levels of these cytokines when cultured in vitro. Besides the lesion sizes, the histopathological picture observed in the footpad lesions from conventionalized Swiss/NIH mice was qualitative and quantitatively different from conventional animals. At the seventh week of infection, when lesions were at their peak, multiple abscesses constituted by neutrophils and macrophages heavily loaded with amastigotes were observed only in conventionalized mice. At the fifteenth week of infection, when both conventional and conventionalized mice were healing, there was regression of the inflammatory process in both experimental groups, in agreement with the lesion size at this time. In conventionalized mice the abscesses present at seven weeks of infection were replaced by a chronic productive diffuse inflammation. Besides, this inflammatory reaction was more intense than in the conventional animals.
The reason for the delay in healing of lesions from conventionalized mice is unknown, but certainly is independent of IFN-g and IL-4 production since the levels of these cytokines were similar in both groups. However, several evidences suggest that macrophage function is impaired in germfree animals30, which could explain the slower healing. One candidate cytokine to play a role in resistance in conventional animals is TNF-a, which production is impaired in the absence of the normal microbiota16. TNF-a, endogenously produced by activated infected macrophages, was shown by several authors to be essential for NO production and consequent killing of parasites6,11. Anti-TNF-a treatment during infection with L. major resulted in larger lesions that healed, similarly to our results10,27. It is possible that a lower capacity of TNF-a production in conventionalized mice is responsible for an impaired capacity to kill parasites. This issue is currently under investigation.
The association with the microbiota has diverse effects on virulence and pathogenicity of parasites. For example, the pathogenicity of both Giardia lamblia28 and Entamoeba histolytica19 in mice is dependent on the presence of the microbiota. In contrast, infection with the intracellular parasite Trypanosoma cruzi is more severe in germfree mice, resulting in a higher mortality of these animals, when compared to their conventional counterparts26. Moreover, in contrast to the results described here, germfree mice infected with another species of Leishmania, L. amazonensis, were more resistant than conventional controls, showing no lesions although parasites could be seen at the site of infection29. Nevertheless, we should point out that the species of Leishmania, the lineage of the mice and the site of infection were distinct in the two experiments and these are factors that have been described to influence the outcome of infection15,25.
In summary, Swiss/NIH mice were resistant to infection with L. major and this resistance was accompanied by an increase in IFN-g production by antigen-specific cells. The absence of the microbiota during the first few weeks of infection significantly influenced the lesion size and the inflammatory process in Swiss/NIH mice infected with L. major. Conventional mice were more resistant to this infection when compared with conventionalized animals, as revealed by significantly smaller lesions and by histopathological analysis showing scarce parasitized macrophages. Differences in production of other cytokines between conventional and conventionalized Swiss/NIH mice may explain our results.
RESUMO
Influência da microbiota normal na leishmaniose cutânea experimental em camundongos suíços
Neste trabalho, estudamos a infecção experimental de camundongos Suíços/NIH com Leishmania major em comparação com camundongos isogênicos C57BL/6 e BALB/c que são resistentes e suceptíveis a esta infecção, respectivamente. Camundongos Suiços mostraram lesões que se curaram espontaneamente e se restringiram ao local de inoculação. Células linfóides derivadas destes animais desenvolveram uma resposta do tipo Th1, caracterizada pela produção de níveis altos de IFN-g e níveis baixos de IL-4. Com o objetivo de estudar a importância da microbiota no desenvolvimento da leishmaniose cutânea neste modelo, camundongos Suiços sem germes foram infectados na pata com promastigotas de L. major, convencionalizados após 3 semanas de infecção e as lesões comparadas com as observadas em camundongos convencionais. Os camundongos convencionalizados apresentaram lesões significativamente maiores do que as observadas nos camundongos convencionais. A análise histopatológica das lesões de todos os animais convencionalizados mostrou abcessos de tamanhos e formas variáveis e a presença de numerosos macrófagos parasitados. Nas lesões dos camundongos convencionais não houve a formação de abscessos e foram observados poucos macrófagos parasitados. Entretanto, células de ambos os grupos experimentais desenvolveram uma resposta similar do tipo Th1, caracterizada pela produção de altos níveis de IFN-g e baixos níveis de IL-4. Neste trabalho, demonstramos que os camundongos Suíços/NIH são resistentes à infecção por L. major, com o desenvolvimento de um fenótipo do tipo Th1 e que a ausência da microbiota no início da infecção influencia significativamente o tamanho da lesão e no processo inflamatório nestes animais.
ACKNOWLEDGMENTS
This work was supported by FAPEMIG grant number CBS 117895. J.R.N., E.C.V., M.N.M. and L.Q.V. are recipients of CNPq fellowships, M.R.O. is a fellow of CAPES and was on leave from the Universidade Federal da Paraíba, PB, Brazil. The authors are indebted to Ronilda Maria de Paula, Maria Helena Alves de Oliveira and Antonio Mesquita Vaz for technical assistance and animal care. Varig Brazilian Airlines is acknowledged for helpful assistance and excellent service during the several occasions in which animals were imported from Taconic Farms.
Correspondence to: Leda Quercia Vieira, Departamento de Bioquímica e Imunologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, C.P. 486, 31270-901 Belo Horizonte, MG, Brazil. E-mail: lqvieira@icb.ufmg.br
Received: 13 July 1998
Accepted: 20 January 1999
- 1. AFONSO, L.C.C. & SCOTT, P. - Immune response associated with susceptibility of C57BL/6 mice to Leishmania amazonensis. Infect. Immun., 61: 2952-2959, 1993.
- 2. BEEBE, A.M.; MAUZE, S.; SCHORK, N.J. & COFFMAN, L. - Serial backcross mapping of multiple loci associated with resistance to Leishmania major Immunity, 6: 551-557, 1997.
- 3. BELOSEVIC, M.; FINBLOOM, D.S.; MEIDE, P.H.V.D.; SLAYTER, M.V. & NACY, C.A. - Administration of monoclonal anti-IFN antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major J. Immunol., 143: 266-274, 1989.
- 4. BOCCI, V. - The neglected organ: bacterial flora has a crucial immunostimulatory role. Perspect. Biol. Med., 35: 251-260, 1992.
- 5. BOGDAN, C.; GESSNER, A.; SOLBACH, W. & ROLLINGHOFF, M. - Invasion, control and persistence of Leishmania parasites. Curr. Opin. Immunol., 8: 517-525, 1996.
- 6. GREEN, S.J.; CRAWFORD, R.M.; HOCKMEYER, J.T.; MELTZER, M.S. & NACY, C.A. - Leishmania major amastigotes initiates the l-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J. Immunol., 145: 4290-4297, 1990.
- 7. HANDMAN, E.; CEREDIG, R. & MITCHELL, G.F. - Murine cutaneous leishmaniasis: disease patterns in intact and nude mice of various genotypes and examination of some differences between normal and infected macrophages. Aust. J. exp. Biol. med. Sci, 57: 9-29, 1979.
- 8. HOWARD, J.G.; HALE, C. & CHAN-LIEW, W.L. - Immunological regulation of experimental cutaneous leishmaniasis. 1. Immunogenetic aspects of susceptibility to Leishmania tropica in mice. Paras. Immunol., 2: 303-314, 1980.
- 9. KOPF, M.; BROMBACHER, F.; KOHLER, G. et al. - IL-4-deficient Balb/c mice resist infection with Leishmania major J. exp. Med, 184: 1127-1136, 1996.
- 10. KOSSODO, S.; GRAU, G.; LOUIS, J.L. & MULLER, I. - Tumor necrosis factor alpha (TNF-a) and TNF-b and their receptors in experimental cutaneous leishmaniasis. Infect. Immun., 62: 1414-1428, 1994.
- 11. LIEW, F.Y.; LI, Y. & MILLOTT, S. - Tumor necrosis factor-a synergizes with IFN-g in mediating killing of Leishmania major through the induction of nitric oxide. J. Immunol., 145: 4306-4310, 1990.
- 12. LOCKSLEY, R.M.; HEINZEL, F.P.; HOLADAY, B.J. et al. - Induction of TH1 and TH2 CD4+ subsets during murine Leishmania major infection. Res. Immunol., 142: 28-32, 1991.
- 13. McDONALD, T.T. & CARTER, P.B. - Requirement for a bacterial flora before mice generate cells capable of mediating the delayed hypersensitivity reaction to sheep red blood cells. J. Immunol., 122: 2624-2629, 1979.
- 14. MCSORLEY, S.; PROUDFOOT, L.; O'DONNEL, C.A. & LIEW, F-Y. - Immunology of murine leishmaniasis. Clin. Derm., 14: 451-464, 1996.
- 15. NABORS, G.S.; NOLAN, T.; CROOP, W.; LI, J. & FARRELL, J. - The influence of the site of parasite inoculation on the development of Th1 and Th2 type immune response in (BALB/c x C57BL/6) F1 mice infected with Leishmania major Paras. Immunol., 17: 569-579, 1995.
- 16. NICAISE, P.; GLEIZES, A.; FORESTIER, F. et al. - The influence of E. coli implantation in axenic mice on cytokine production by peritoneal and bone marrow-derived macrophages. Cytokine, 7: 713-719, 1995.
- 17. NOBEN-TRAUTH, N.; KROPF, P. & MULLER, I. - Susceptibility to Leishmania major infection in interleukin-4-deficient mice. Science, 271: 987-990, 1996.
- 18. O'GARRA, A. & MURPHY, K. - Role of cytokines in determining T-lymphocyte function. Curr. Opin. Immunol., 6: 458-466, 1994.
- 19. PHILLIPS, B.P. & WOLFE, P.A. - The use of germfree guinea pigs in studies on the microbial interrelationships in amoebiasis. Ann. N. Y. Acad. Sci., 78: 308-314, 1959.
- 20. PLEASANTS, J.R. - Gnotobiotics. In: MELBY E. C.; ALTMAN Jr., N. H. & ALTMAN, N.H. Handbook of Laboratory Animal Science Cleveland, CRC Press, 1974. p.114-174.
- 21. SACKS, D.L.; HIENY, S. & SHER, A. - Identification of cell surface carbohydrate and antigenic changes between noninfective and infective developmental stages of Leishmania major promastigotes. J. Immunol., 135: 564-569, 1985.
- 22. SADICK, M.D.; HEINZEL, F.P.; HOLANDAY, B.J. et al. - Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J. exp. Med., 171: 115-127, 1990.
- 23. SCOTT, P. & KAUFMANN, H.E. - The role of T-cell subsets and cytokines in the regulation of infection. Immunol. today, 12: 346-348, 1991.
- 24. SCOTT, P.; NATOVITZ, P.; COFFMAN, R.L.; PEARCE, E. & SHER, A. - Immunoregulation of cutaneous leishmaniasis - T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J. exp. Med., 168: 1675-1684, 1988.
- 25. SCOTT, P. & SHER, A. - A spectrum in the susceptibility of leishmanial strains to intracellular killing by murine macrophages. J. Immunol., 136: 1461-1466, 1986.
- 26. SILVA, M.E.; EVANGELISTA, E.A.; NICOLI, J.R.; BAMBIRRA, E.A. & VIEIRA, E.C. - American trypanosomiasis (Chagas' disease) in conventional and germfree rats and mice. Rev. Inst. Med. trop. S. Paulo, 29: 284-288, 1987.
- 27. TITUS, R.G.; SHERRY, B. & CERAMI, A. - Tumor necrosis factor plays a protective role in experimental murine cutaneous leishmaniasis. J. exp. Med., 170: 2097-2103, 1989.
- 28. TORRES, M.R.F.; SILVA, M.E.C.; VIEIRA, E.C. et al. - Intragastric infection of conventional and germfree mice with Giardia lamblia Braz. J. med. biol. Res., 25: 349-352, 1992.
- 29. VIEIRA, E.C.; NICOLI, J.R.; MORAES-SANTOS, T. et al.- Cutaneous leishmaniasis in germfree, gnotobiotic, and conventional mice. Rev. Inst. Med. trop. S. Paulo, 29: 385-387, 1987.
- 30. VIEIRA, L.Q.; OLIVEIRA, M.R.; NEUMANN, E.; NICOLI, J.R. & VIEIRA, E.C. - Parasitic infections in germfree animals. Braz. J. med. biol. Res., 31: 105-110, 1998.
- 31. WANG, Z -E.; REINER, S.L.; ZHENG, S.; DALTON, D.K. & LOCKSLEY, R.M. - CD4+ effector cells default to the Th2 pathway in interferon-g deficient mice infected with Leishmania major J. exp. Med., 179: 1367-1371, 1994.
Publication Dates
-
Publication in this collection
02 July 1999 -
Date of issue
Mar 1999
History
-
Received
13 July 1998 -
Accepted
20 Jan 1999