Abstract
The objective of this work was to evaluate the possibility of using a clay from the city of Silva Jardim, State of Rio de Janeiro, Brazil, in the composition of porcelain tile body. The clay investigated was a plastic kaolinitic type with white color. Initially, a typical porcelain body composed of the mixture of this clay with feldspar, quartz, kaolin and talc was prepared. Specimens were then obtained from this porcelain mixture by uniaxially pressing samples until a dry density of 2.0 g/cm3 was achieved. These specimens were then fired at 1180°C in a laboratory furnace. Physical and mechanical properties related to linear shrinkage, water absorption and flexural rupture strength, determined by Weibull statistical method, were evaluated. Microstructural analysis for the experimental body was performed by scanning electron microscopy, X-ray diffraction and mercury porosimetry. The technological properties of the elaborated composition satisfied the porcelain tile standard specifications. The microstructural analysis displayed small pores as well as a glassy matrix. The presence of quartz, mullite and plagioclase were also detected in the fired porcelain tile. These results indicated that the clay presents satisfactory characteristics to be used in porcelain tile body.
Clay; microstructure; porcelain tile; properties
Evaluation of a plastic clay from the state of Rio de Janeiro as a component of porcelain tile body
C. M. F. Vieira; S. N. Monteiro
State University of the North Fluminense Darcy Ribeiro - UENF Advanced Materials Laboratory - LAMAV Av. Alberto Lamego 2000, 28013-602, Campos dos Goytacazes, Brazil e-mail: vieira@uenf.br; sergio.neves@ig.com.br
ABSTRACT
The objective of this work was to evaluate the possibility of using a clay from the city of Silva Jardim, State of Rio de Janeiro, Brazil, in the composition of porcelain tile body. The clay investigated was a plastic kaolinitic type with white color. Initially, a typical porcelain body composed of the mixture of this clay with feldspar, quartz, kaolin and talc was prepared. Specimens were then obtained from this porcelain mixture by uniaxially pressing samples until a dry density of 2.0 g/cm3 was achieved. These specimens were then fired at 1180°C in a laboratory furnace. Physical and mechanical properties related to linear shrinkage, water absorption and flexural rupture strength, determined by Weibull statistical method, were evaluated. Microstructural analysis for the experimental body was performed by scanning electron microscopy, X-ray diffraction and mercury porosimetry. The technological properties of the elaborated composition satisfied the porcelain tile standard specifications. The microstructural analysis displayed small pores as well as a glassy matrix. The presence of quartz, mullite and plagioclase were also detected in the fired porcelain tile. These results indicated that the clay presents satisfactory characteristics to be used in porcelain tile body.
Keywords: Clay, microstructure, porcelain tile, properties.
1 INTRODUCTION
Among the different types of ceramic tiles, the porcelain tile is the one that presents the best technical performance associated with high mechanical strength, high chemical inertness, high abrasion strength and low water absorption [1-7]. According to the technical code [8], the required values for flexural rupture strength and water absorption are higher than 35 MPa and lower than 0.5%, respectively. In term of processing, higher compacting pressing and firing temperatures are used in comparison with other types of ceramic tiles. The final microstructure of a porcelain tile is generally formed by 7-12 vol.% of total porosity, 20-25% of quartz and 12-16% of mullite dispersed in a vitreous matrix [2].
Ceramic bodies for porcelain tile fabrication are characteristically composed of a specific mixture of plastic and non-plastic raw materials. This assures, after firing, an adequate vitrification and the proper technological performance, which includes imprevious condition associated with very small amount of open porosity.
The plastic materials used in porcelain tiles are clays and kaolin that supply plasticity and provide the desirable mechanical strength for both, the green and dried body pieces. In addition, the plastic materials must present favorable rheological properties for an efficient wet milling stage.
The non-plastic raw materials are inerts, fluxes and fluxing modifier. The inerts, mainly, quartz, quartzite and feldspar sand, increase the refractoriness, decrease the linear shrinkage during the firing stage and also regulate the SiO2/Al2O3 ratio, which is an important parameter for mullite formation. Quartz, with small particle size, in the range of 10-30 mm, improves the mechanical strength. By contrast, larger-sized quartz particles tend to decrease the mechanical strength due to the induced susceptibility to micro-cracks formation. This is a consequence of the allotropic transformation, which results in volumetric change at temperatures around 573oC [3, 9, 10]. The fluxes, mainly feldspar and feldspathoids, make possible the liquid phase formation, through the suppliment of alkaline oxides (K2O + Na2O) [3, 9, 11]. The fluxing modifier, such as talc, wollastonite, calcite, dolomite, are introduced in low amounts to decrease the melting point of the body through eutectic formation with alkaline feldspars [3, 9, 12].
Figure 1 shows a typical mineralogical composition used for porcelain tile fabrication [1]. In this pie chart, the major constituent is the feldspar, followed by plastic materials such as clay and kaolin. Quartz and talc are in relatively low amounts.
Table 1 shows the range of variation for the main chemical compounds of typical raw materials used in the composition of porcelain tile bodies as well as a typical industrial porcelain tile body. It is observed that in the industrial body, SiO2 and Al2O3 are the most abundant oxides. Generally, the SiO2 amount is higher than 60 wt.%. The Fe2O3 + TiO2 must be maintained at low amount to avoid undesired color in the ceramic product. The alkaline oxides, K2O + Na2O, are essential to liquid phase formation. The earth alkaline oxides, CaO + MgO, must be maintained in the low amounts showed in Figure 1, due to the possibility of excessive fluxing as well as undesired variation on the ceramic color. Finally, the loss on ignition (LoI) is mainly associated with the dehydratation of clay minerals. Organic matter oxidation as well as carbonates decomposition also contributes to this parameter. The LoI must be maintained in low amounts, normally lower than 6%, because excessive weight loss during the firing stage contributes to increase the porosity and the linear shrinkage. The gas released can also generate defects in the glaze, which is a surface vitreous covering.
So, the objective of this work was to evaluate the use of a kaolinitic plastic clay from the State of Rio de Janeiro as a potential raw material for porcelain tile composition. This was performed by determining the fired technological properties and observing the microstructure of the elaborated porcelain tile body.
2 MATERIALS AND METHODS
The clay used in the present investigation was collected from the municipal area of Silva Jardim, located in the State of Rio de Janeiro. This clay, denoted in this work as SJ clay, presents kaolinitic predominance and elevated plasticity [13]. The other raw materials, such as kaolin, quartz, potash feldspar and talc were obtained from mining facilities outside the State of Rio de Janeiro. Table 2 shows the elaborated composition for the experimental porcelain tile body. This composition was analytically formulated based on the typical mineralogical distribution for porcelain tiles shown in Figure 1. In practice, this composition corresponds to the optimum amounts of fluxing, inert and plastic agents, which provides the required technical performance after firing. Table 3 shows the chemical composition of the raw materials and the elaborated composition. The chemical analysis was performed by X-ray fluorescence in a Philips equipment, model PW 2400.
The prepared experimental composition was initially wet-milled in porcelain pots during two hours. The resulting slurry was then sieved through 230 mesh (63 mm) and allowed to dry at 110ºC before a final sieving through 20 mesh (820 mm).
Twenty rectangular specimens measuring 114.3 mm x 25.4 mm x 10 mm were molded under uniaxial pressure at 30 MPa. Specimens initially containing 8% of moisture were dried at 110ºC until a constant weight was achieved and then fired at 1180ºC. A controlled heating rate of 10ºC/min was applied up to the patamar temperature, at which each specimen was left for 6 minutes. Cooling occurred by natural convection after turning the furnace off and leaving the specimen inside. These chosen values for the technological parameter are commonly applied in laboratory procedures to simulate industrial conditions. The evaluated properties in the fired state were: bulk density, total porosity, water absorption, linear shrinkage and flexural strength. The bulk density was determined according to the Archimedes Principle. The absolute porosity (PT) was obtained using the equation: PT = 1 (rB/rT), where rB is the bulk density and rT is the absolute density obtained by helium picnometry, using a Ultrapycnometer 1000-Quantachrome equipment. The water absorption was determined according to standard procedure [14]. The linear shrinkage was obtained by the measurement of the length of the specimens before and after the firing stage using a caliper with precision of ± 0.01 mm. The flexural rupture strength was determined by the three point bending test in a universal Instron 5582 machine, following the code [15]. Weibull method [16] was applied to statistically analysis the mechanical strength results.
The microstructural analysis of the experimental and industrial ceramic bodies was performed in the fired specimens by X-ray diffraction (XRD), mercury intrusion porosimetry (MIP) and scanning electron microscopy (SEM). The fracture surface of a selected specimen was studied by scanning electron microscopy (SEM) using a Zeiss model DSM 962 equipment. The pore size distribution between 0.00648 to 8.8884 mm was obtained by MIP, using a contact angle of 140º, in an Autoscan 33 Quantachrome Porosimeter. Major crystalline phases were determined by XRD in sectioned specimens, collected from the center of fired specimens. The XRD of randomly-oriented powder was carried out in a Seifert model URD 65, diffractometer equipped with a graphite monochromator operating with Cu-Ka radiation for a 2q range from 5º to 65º.
3 RESULTS AND DISCUSSION
The values of bulk density, total porosity, water absorption and linear shrinkage of the tested composition fired at 1180ºC are shown in Table 4. The results indicated that the experimental fired ceramic of the present work displays an excessive absolute porosity and low bulk density in comparison with others fired porcelain tile bodies [2]. Although the water absorption (WA) attend the code, WA < 0.5%, the value of 0.48% is considered elevated. The linear shrinkage is lower than the values normally adopted for industrial porcelain bodies. These results are associated with the low silica and high alumina and loss on ignition contents of the elaborated composition. Both, dry bulk density and the firing temperature must be elevated for a better densification of the investigated composition.
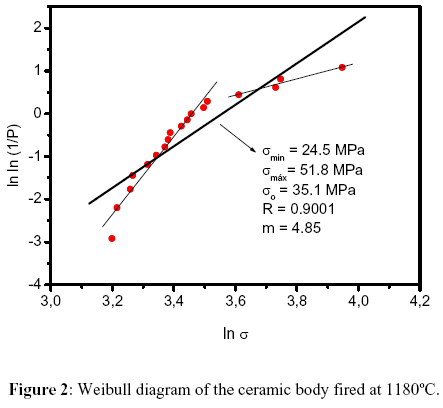
Figure 2 shows the Weibull diagram for the analysis of the mechanical strength results obtained for the investigated body composition. In principle two groups of points can be interpolated by straight lines with different slopes. Therefore, it is suggested that a bimodal distribution, with relatively low value for m, is associated with the mechanical behavior. The average parameters related to this bimodal distribution were calculated from the bolder single straight line also presented in Figure 2. It is also observed that the characteristic strength, so, of 35.1 MPa attends the specification for porcelain tile [8].
Figure 3 shows the XRD pattern of the experimental ceramic body fired at 1180ºC. In this figure it is observed that the identified crystalline phases are quartz, mullite and plagioclase. The quartz and plagioclase are residual phases that were already present in the raw materials. By contrast, the mullite was probably formed at temperatures around 1075ºC from the unstable aluminosilicate spinel phase [10].
Figure 4 shows that the main distribution of pore peaks occurs around 0.16 mm. In this figure it is also important to note that there are two others pore population peaks at 0.02 and 0.014 mm. These pore sizes are relatively small, indicating that the experimental body displayed elevated densification mainly through liquid phase. It was not possible to identified others population pores due to the detection limit of the equipment.
Figure 5 shows the micrographs corresponding to the fractured surface of the specimen fired at 1180ºC. Spherical and isolated pores in a vitreous matrix can be seen in this figure. These characteristics correspond to typical porcelain tile bodies [17], being associated with high densification through liquid phase sintering. The coarse pores can be attributed to the O2 release from the reduction of Fe2O3 [18]. These pores present radius of 10-20 mm, which were not detected by the MIP in Figure 3.
4 CONCLUSIONS
· The investigated clay shows chemical characteristics that make possible its use in porcelain tile composition. However, the elaborated porcelain tile body shows low silica and high alumina contents and loss on ignition. The kaolin must be partially substituted by the investigated clay.
· The elaborated porcelain tile body showed low bulk density, which impairs the sintering process. The investigated body showed a microstructure and technological properties compatible with porcelain tile bodies. However, the porosity must be decreased. This can be obtained changing the elaborated composition as well as the process parameters. The silica content must be increased, the alumina and loss on ignition must be decreased. The changing in the process parameter includes the increase in the dry bulk density and the firing temperature.
5 ACKNOWLEDGEMENTS
The authors would like to express their thanks for the financial support provided by FAPERJ, CNPq, Capes and Tecnorte/Fenorte.
6 REFERENCES
Data de envio: 21/01/06 Data de aceite: 23/10/06
- [1] BIFFI, G., Porcelain Stoneware Production Manual and Methods of use, Faenza, Gruppo Editoriale Faenza Editrice, 1999.
- [2] DONDI, M., ERCOLANI, G., MELANDRI, C., MINGAZZINI, C., MARSIGLI, M., The Chemical Composition of Porcelain Stoneware tiles and its Influence on Microstructural and Mechanical Properties, Interceram, v. 48, pp. 75-83, 1999.
- [3] BARBA, A., BELTRÁN, V., FELIU, C., GARCÍA, J., GINÉS, F., SÁNCHEZ, E., SANZ, V., Materias Primas para la Fabricación de Soportes de Baldosas Cerámicas, Castellón, Instituto de Tecnologia Cerámica-AICE, 1997.
- [4] OLIVEIRA, A.P.N., Gręs Porcelanato: Aspectos Mercadológicos e Tecnológicos, Cerâmica Industrial, v. 3, n.3, pp. 34-41, Mai/Jun 1998.
- [5] LLORENS, F.G., Matérias-primas para a Fabricaçăo de Gręs Porcelanato, Cerâmica Informaçăo, n. 9, pp. 51-55, 2000.
- [6] MENEGAZZO, A.P., LEMOS, F.N., PASCHOAL, J.O.A., GOUVĘA, D., CARVALHO, J.C., NÓBREGA, R.S., Gręs Porcelanato: Uma Abordagem Técnica e Mercadológica, Cerâmica Informaçăo, n. 14, pp. 53-60, 2001.
- [7] SANCHEZ, E., ORTZ, M.J., GARCÍA-TEM, J., CANTAVELLA, V., Efeito da Composiçăo das matérias-primas Empregadas na Fabricaçăo de Gręs Porcelanato sobre as Fases Formadas Durante a Queima e as Propriedades do Produto Final, Cerâmica Industrial, v. l6, n. 5, pp. 15-22, Set/Out 2001.
- [8] ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS, NBR 13818: Placas Cerâmicas para Revestimento Especificação e Métodos de Ensaio, Rio de Janeiro, 1997.
- [9] EMILIANI, G.P., CORBARA, F., Tecnología Cerámica La Lavorazione, Faenza, Gruppo Editoriale Faenza Editrice, 1999.
- [10] CARTY, W.M., SENAPATI, U., Porcelain-raw Materials, Processing, Phase Evolution, and Mechanical Behavior, Journal of the American Ceramic Society, v. 81, pp. 3-19, 1998.
- [11] DONDI, M., Compositional Parameters to Evaluate Feldspathic Fluxes for Ceramic Tiles, Tile & Brick International, v. 10, pp. 77-84, 1992.
- [12] SALAM, E.H., NAGA, S.M., IBRAHIM, D.M., Mold of Talc Addition and its Effect on the Properties of Ceramic Bodies, Ceramics International, v. 10, pp. 87-92, 1981.
- [13] CAPITANEO, J.L., VIEIRA, C.M.F., MONTEIRO, S.N., SILVA, F.T., Caracterizaçăo Tecnológica de Argila Branca do Município de Silva Jardim - RJ, In: Anais do XV Congresso Brasileiro de Engenharia e Cięncia dos Materiais-CBECIMAT, pp. 173-179, Natal, Nov. 2002.
- [14] AMERICAN SOCIETY FOR TESTING AND MATERIALS, C 373-72: Water Absorption, Bulk Density, Apparent Porosity, and Apparent Specific Gravity of fired Whiteware Products, USA, 1972.
- [15] AMERICAN SOCIETY FOR TESTING AND MATERIALS, C 674-7: Flexural Properties of Ceramic Whiteware Materials, USA, 1977.
- [16] WEIBULL, W.A., A Statistical Theory of Strength of Materials, Ingeniorsvetenskapskademies, Handlingar, 1939, 151, pp. 4549, 1939.
- [17] ESPOSITO, L., TUCCI, A., ALBERTAZZI, A., RASTELLI, E., Physical-mechanical Characterization of a Porcelain Stoneware Mix, Ceramic Acta, v. 8, n. 1, pp. 11-19, 1996.
- [18] ORTZ, M.J., ESCARDINO, A., AMORÓS, J.L., NEGRE, F., Microstructural Changes During the Firing of Stoneware Floor Tiles, Applied Clay Science, v. 8, n. 2-3, pp. 193-205, Aug. 1993.
Publication Dates
-
Publication in this collection
26 June 2007 -
Date of issue
2007
History
-
Accepted
23 Oct 2006 -
Received
21 Jan 2006