Abstract
Impatiens hawkeri is among the three important bedding plants in the world. There is no efficient protocol for fast micropropagation of Impatiens hawkeri cv. Sweeties Blue Star. Single nodes were germinated on Murashige and Skoog (MS) medium supplemented with 1-naphthaleneacetic acid (NAA; 0.00, 0.10 and 0.50 mg l-1), 6-benzyladenine (BA; 0.00, 0.10, 0.50 and 1.00 mg l-1) and kinetin (KIN; 0.00, 0.10, 0.50 and 1.00 mg l-1). The shoot length was highest in medium containing 0.10 mg l-1 NAA and control. The largest number of shoots (14.06 and 14.00 per explant) was produced in media supplemented with 1.00 mg l-1 BA, 0.50 mg l-1 NAA and 1.00 mg l-1 BA, 0.10 mg l-1 NAA, respectively. The 0.10 mg l-1 NAA along with 1.00 mg l-1 BA was found to be superior for production of leaf (57.13). Maximum root length (33.80 mm) and root number (29.13) were obtained on medium supplemented with 0.10 mg l-1 NAA without BA and KIN. Plantlets were transplanted to pots filled with perlite and peat moss (in ratio of 1:1) and transferred to the greenhouse for acclimatization. Regenerated plantlets were morphologically identical with mother plants. Upon ex vitro transfer, 100% of plants survived.
Key words:
Balsaminaceae; micropropagation; organogenesis; ornamentals; plant growth regulators
Resumo
Impatiens hawkeri está entre as três principais plantas para forragem do mundo. Não existe um protocolo eficiente para a micropropagação rápida de Impatiens hawkeri cv. Sweeties Blue Star. Nódulos individuais germinados em meio Murashige e Skoog (MS) suplementado com ácido 1-naftalenoacético (NAA; 0,00, 0,10 e 0,50 mg l- 1), 6-benziladenina (BA; 0,00, 0,10, 0,50 e 1,00 mg l - 1) e cinetina (KIN; 0,00, 0,10, 0,50 e 1,00mg l-1). O comprimento dos brotos foi maior no meio contendo 0,10 mg l-1 de ANA e controle. O maior número de brotações (14,06 e 14,00 por explante) ocorreu em meio suplementado com 1,00 mg l-1 BA, 0,50 mg l-1 NAA e 1,00 mg l-1 BA, 0,10 mg l-1 NAA, respectivamente. O 0,10 mg l-1 de ANA em conjunto com 1,00 mg l-1 de BA foi considerado superior para a produção de folhas (57,13). O comprimento máximo da raiz (33,80 mm) e o número de raízes (29,13) foram obtidos em meio suplementado com 0,10 mg l-1 de ANA sem BA e KIN. As mudas foram transplantadas para vasos com perlita e turfa (na proporção de 1: 1) e transferidas para casa de vegetação para aclimatação. As mudas regeneradas eram morfologicamente idênticas às plantas-mãe. Na transferência ex vitro, 100% das plantas sobreviveram.
Palavras-chave:
Balsaminaceae; micropropagação; organogênese; ornamentais; reguladores de crescimento vegetal
Introduction
Impatiens W.Bull (from family Balsaminaceae) is an important genus of ornamental plants that contains 400–850 species and is cultivated as spring pot and bedding plants (Grey- Wilson 1980Grey-Wilson C (1980) Impatiens of Africa: morphology, pollination and pollinators, ecology, phytogeography, hybridisation, keys and a systematic treatment of all African species: with a note on collecting and cultivation. A.A. Balkema, Rotterdam. 248p.). Impatiens is the next most important bedding plant worldwide. The USA consumes more impatiens than any other country, with a wholesale crop valued at about US$ 157 million (Hamrick 2005Hamrick D (2005) Ornamental bedding plant industry and plug production. Flower seed: biology and technology. EE. UU, Cambridge. Pp. 27-38.). Propagation of Impatiens is done by seed and stem cutting. Propagation by seed often encounters with some limits and problems such as production of heterogeneous plants and time consuming with segregation of traits. Plant tissue culture is a potential alternative to overcome these limits and problems. Micropropagation of species belonging to the Impatiens genus has been reported by various researchers using explants of shoot tips, hypocotyl segments and cotyledons explants and BA, 6-benzylaminopurine (BAP), 1-phenyl-3-(1,2,3-thiadiazol-5-yl)-urea or thidiazuron (TDZ), 2-isopentenyl adenine (2-iP), KIN, NAA, indole-3-butyric acid (IBA) and indole-3-acetic acid (IAA) as plant growth regulators (PGRs) (Stephens et al. 1985Stephens LC, Krell SL & Weigle JL (1985) In vitro propagation of Java, New Guinea, and Java X New Guinea Impatiens. HortScience 20: 362-363.; Kyungkul & Stephens 1987Kyungkul H & Stephens LC (1987) Growth regulators affect in vitro propagation two interspecific Impatiens hybrids. Scientia Horticulturae 32: 307-313.; Kyungkul 1993Kyungkul H (1993) In vitro shoot regeneration from cotyledons of immature ovules of Impatiens pelatypelata Lindl. In vitro cellular and developmental biology. Plant 30: 108-112.; Taha et al. 2009; Dan et al. 2010Dan Y, Baxter A, Zhang S, Pantazis CJ & Veilleux RE (2010) Development of effcient plant regeneration and transformation system for Impatiens using Agrobacterium tumefaciens and multiple bud cultures as explants. BMC Plant Biology 10: 165-177.). In most studies, the combination of these PGRs was used especially for shoot multiplication. Dan et al. (2010)Dan Y, Baxter A, Zhang S, Pantazis CJ & Veilleux RE (2010) Development of effcient plant regeneration and transformation system for Impatiens using Agrobacterium tumefaciens and multiple bud cultures as explants. BMC Plant Biology 10: 165-177. used a combination of BA and TDZ for shoot regeneration of Impatiens walleriana cv. Accent Red and Salmon Picote. Results showed more than 95% of explants from both genotypes produced multiple shoots by using both BA and TDZ, whereas TDZ without BA promoted only 40% response for Accent Red and 50% response for Salmon Picote. In contrast, the greatest number of shoots in two interspecific Impatiens hybrids was obtained with BA and 2-iP used alone (Han & Stephens 1987Han K & Stephens LC (1987) Growth regulators affect in vitro propagation of two interspecifc Impatiens hybrids. Scientia Horticulturae 32: 307-313.). Sometime, the use of a cytokinin (e.g., KIN) along with an auxin (e.g., IAA) was found to be effective for the production of multiaxillary shoots in some genotypes of Impatiens (Nikolova et al. 1996Nikolova RV, Lall N & Bosa AJN (1996) An assessment of the conditions for the rapid propagation of Impatiens flanaganae in-vivo and in-vitro. Acta Horticulture 440: 633-638.). Therefore, this study was undertaken to evaluate the concentration of the different PGRs. Optimum concentrations of PGRs is different for maximum shoot multiplication in different genotypes of Impatiens. This is the first report on micropropagation of cultivar of Sweeties Blue Star. The current study describes a simple and efficient organogenesis protocol in Impatiens hawkeri Bull. cv. Sweeties Blue Star using BA, KIN and NAA as PGRs and single node as explant.
Materials and Methods
Plant material
Mother plants of impatiens (Impatiens hawkeri Bull. cv. Sweeties Blue Star) were prepared from an ornamentals garden in Varamin city, Tehran province, Iran on 2017. Pots containing mature plants were transferred to a greenhouse located in Chaboksar city, Guilan province, the northern part of Iran. The mature plants were treated with full fertilizers, regular irrigation, fungicide and insecticide. These treated plants were gradually transferred to the plant tissue culture laboratory after 2-3 weeks.
Explant preparation and disinfection
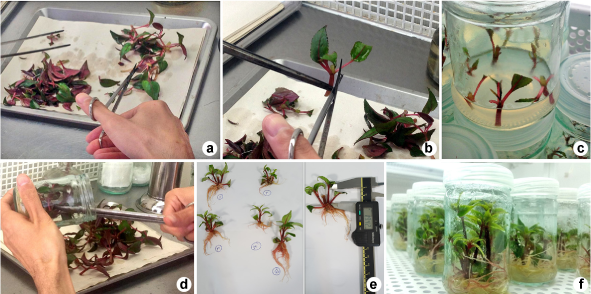
Stem segments with 7-12 cm long containing several nodes were excised from mother plants (Fig. 1a). These segments were washed thoroughly under tap water and a few drops of dish-washing liquid for 10 min then rinsed twice with distilled water. Cleaned segments were transferred to the laminar air flow cabinet and disinfected with 70% ethanol (v/v) for 30 sec followed by 20% Clorox (commercial bleach containing 1% w/v NaOCl) with a few drops of Tween® 20 for 4 min and then rinsed three times each for 5 min with sterilized distilled water. The nodal segments (20 mm) were excised from disinfected stem segments and used as explants for experimentation (Fig. 1b).
a-f. Micropropagation process of impatiens (Impatiens hawkeri cv. Sweeties Blue Star) – a. shoots of mother plants used for obtaining the explants; b. preparation of single nodes used as explants; c. explants cultured on MS medium containing PGRs for shoot multiplication; d. subculture of multiplied shoots through cultivation on fresh medium for more multiplication and root induction; e. measurement of shoot and root length; f. rooting of shoots cultivated in media fortified with PGRs for root induction.
Culture medium and treatments
Nodal explants were cultured on solidified Murashige and Skoog (MS, Murashige & Skoog 1962Murashige T & Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15: 473-497.) medium supplemented with 21 combinations of 1-Naphthaleneacetic acid (NAA; 0.00, 0.10 and 0.50 mg l–1) as auxin and 6-benzyladenine (BA; 0.00, 0.10, 0.50 and 1.00 mg l–1) and kinetin (KIN; 0.00, 0.10, 0.50 and 1.00 mg l–1) as cytokinins (CKs) (Fig. 1c). Sucrose (3%) was used as carbon source and media were solidified with Agar-agar (0.7%). The pH of the media was adjusted to 5.7 ± 0.10 using 1 M HCl or 1 M NaOH and autoclaved for 20 min at 15 psi, 121 °C. The cultures were incubated in a growth chamber at 24 ± 2 °C under a 16-h photoperiod, with photosynthetic flux density (PFD) of approximately 50 µmolm–2 s–1 provided by cool white fluorescent lamps and 75-80% relative humidity (RH).
Shoot induction and multiplication and rooting
Single nodes were cultured on MS medium containing different concentrations of NAA, BA and KIN. Multiplied shoot were removed from bottles for measurement of shoot parameters (Fig. 1d). The shoot height, number of shoots, number of leaves and fresh weight of leaves per nodal segment were calculated a month of culture (Fig. 1e). Single nodes produced from first step of shoot multiplication were aseptically excised and used as explants for second step of multiplication.
This process was done for third and fourth steps. Therefore, single nodes explants were sub-cultured several times every 30 days in fresh medium (Fig. 1d). Afterwards, plantlets were rooted in the same medium. The roots height, number of roots and fresh weight of roots were measured a month of culture (Fig. 1e).
Acclimatization
The regenerated plantlets from organogenesis media were harvested and transferred to pots containing a 1:1 (v/v) perlite: peat moss and irrigated with tap water every third day. The plantlets were maintained in a greenhouse (with the temperature of 24–26 °C, RH 70%, under natural photoperiod conditions) for acclimatization.
Experimental design and statistical analysis
A factorial experiment in a completely randomized design was carried out. First factor was three levels of auxin (NAA) and the second one was four levels of cytokinins (BA and KIN). Experiments were conducted three times with three replicates per treatment for organogenesis (shoot and root regeneration), and three specimens per replicate. Data were analyzed using a one-way analysis of variance (ANOVA). Treatment means were separated using Duncan’s multiple range tests (DMRT) at the 5% probability level and analyzed using SAS software.
Results and Discussion
Shoot induction and multiplication
Significant differences were found for all measured characteristics treated with different concentrations of PGRs (Tab. 1). Results showed the combined treatments of PGRs (especially NAA and BA) were more effective for shoot regeneration. The combination of 1.00 mg l–1 BA and 0.50 mg l–1 NAA as well as 1.00 mg l–1 BA and 0.10 mg l–1 NAA produced a significantly higher number of shoots with 14.06 and 14.00 per plantlet, respectively (Tab. 2). This number of shoots is about five times more than the control and two to three times more than when explants were cultured on medium supplemented with CKs without NAA. However, a high number of shoots (higher than 13.00 per plantlet) was produced in combinations of 1.00 mg l–1 KIN and 0.50 mg l–1 NAA as well as 0.50 mg l–1 BA and 0.10 mg l–1 NAA (Tab. 2; Fig. 1f).
Analysis of variance of the effect of different concentrations of NAA, BA and KIN on measured characters of impatiens (Impatiens hawkeri cv. Sweeties Blue Star) grown in vitro condition.
Mean comparison of the effect of different concentrations of NAA, BA and KIN on measured characters of impatiens (Impatiens hawkeri cv. Sweeties Blue Star) grown in vitro condition.
Current study revealed that effective shoot multiplication of Impatiens hawkeri Bull. cv.
Sweeties Blue Star is strongly dependent on the correct selection of concentrations of CKs and NAA in combination with each other. Similar findings have been reported with auxin-cytokinin influence on shoot multiplication for Impatiens flanaganae (Nikolova et al. 1996Nikolova RV, Lall N & Bosa AJN (1996) An assessment of the conditions for the rapid propagation of Impatiens flanaganae in-vivo and in-vitro. Acta Horticulture 440: 633-638.) and other plant species (Jain & Ochatt 2010Jain SM & Ochatt SJ (2010) Protocols for in vitro propagation of ornamental plants. Springer Protocols, Humana Press, New York. 400p.; Kaviani 2015Kaviani B (2015) Some useful information about micropropagation. Journal of Ornamental Plants 5: 29-40.; Kaviani & Negahdar 2017Kaviani B & Negahdar N (2017) Propagation, micropropagation and cryopreservation of Buxus hyrcana Pojark., an endangered ornamental shrub. South African Journal of Botany 111: 326-335.). Medium supplemented with 2.00 mg l–1 indoleacetic acid (IAA) and 2.00 mg l–1 KIN were found to be effective for the production of multi-axillary shoots (10 to 12 per explant) when apical and lateral shoot tips were used as explants (Nikolova et al. 1996Nikolova RV, Lall N & Bosa AJN (1996) An assessment of the conditions for the rapid propagation of Impatiens flanaganae in-vivo and in-vitro. Acta Horticulture 440: 633-638.). Contrary to our findings, Taha et al. (2009)Taha AM, Wagiran A, Ghazali H, Huyop F & Parveez GKA (2009) Optimization and transformation of garden balsam, Impatiens balsamina, mediated by microprojectile bombardment. Biotechnology 8: 1-12. demonstrated that maximum shoot regeneration (93%) in Impatiens balsamina L. was obtained with 1.00 mg l–1 BAP. Impatiens hybrids (T63-1 and Star Fire) and Impatiens balsamina does not dependent on NAA for shoot multiplication (Stephens et al. 1985Stephens LC, Krell SL & Weigle JL (1985) In vitro propagation of Java, New Guinea, and Java X New Guinea Impatiens. HortScience 20: 362-363.; Taha et al. 2009Taha AM, Wagiran A, Ghazali H, Huyop F & Parveez GKA (2009) Optimization and transformation of garden balsam, Impatiens balsamina, mediated by microprojectile bombardment. Biotechnology 8: 1-12.). Presence of various concentrations of endogenous auxins in different species and cultivars can be the cause of these alterations.
In some other species and genotypes of Impatiens genus, most shoot regeneration was induced in medium containing a combination of two CKs. In Impatiens walleriana Hook.f. cv. AccentRed and Salmon Picote, 95% of explants produced multiple shoots on media containing a combination of TDZ and BA at different concentrations, whereas TDZ without BA promoted only 40–50% response, respectively (Dan et al. 2010Dan Y, Baxter A, Zhang S, Pantazis CJ & Veilleux RE (2010) Development of effcient plant regeneration and transformation system for Impatiens using Agrobacterium tumefaciens and multiple bud cultures as explants. BMC Plant Biology 10: 165-177.). This study also showed that less shoot number was produced in media with BA or KIN without NAA. In addition, the results confirmed that BA (especially 1.00 mg l–1) is more effective than KIN for shoot regeneration when those were applied singularly (without NAA). Taha et al. (2009)Taha AM, Wagiran A, Ghazali H, Huyop F & Parveez GKA (2009) Optimization and transformation of garden balsam, Impatiens balsamina, mediated by microprojectile bombardment. Biotechnology 8: 1-12. showed superiority of BAP than TDZ for shoot induction of Impatiens balsamina. In another study on two interspecific Impatiens hybrids, BA was most effective for stimulating shoot multiplication in ‘T63-1’ and 2-iP in ‘Star Fire’ (Han & Stephens 1987Han K & Stephens LC (1987) Growth regulators affect in vitro propagation of two interspecifc Impatiens hybrids. Scientia Horticulturae 32: 307-313.). BA and 2-iP were more effective in stimulating multiple shoot formation than KIN (Stephens et al. 1985Stephens LC, Krell SL & Weigle JL (1985) In vitro propagation of Java, New Guinea, and Java X New Guinea Impatiens. HortScience 20: 362-363.). Also, Balakrishnan et al. (2009)Balakrishnan V, Ravindran KC & Philip Robinson JP (2009) In vitro regeneration of Impatiens campanulata wight an important grass land plant. Botanical Research International 2: 123-130. showed that BAP is better than other used CKs (i.e. KIN, 2-iP and zeatin) in Impatiens campanulata. One possibility could be the higher absorption of BA and 2-iP than KIN by Impatiens explants. Another possibility could be the less breakdowns of BA and 2-iP than KIN (Stephens et al. 1985Stephens LC, Krell SL & Weigle JL (1985) In vitro propagation of Java, New Guinea, and Java X New Guinea Impatiens. HortScience 20: 362-363.) in the media. Furthermore, differential endogenous and exogenous levels of cytokinins and type of explants could be the other contributor in a better effect of BA in the media.
This study revealed that supplementation of CKs at middle concentration level helped to induce higher number of leaves. From the different CKs concentrations (0.10-1.00 mg l–1), the largest number of leaves per explant was obtained in the media with higher concentration of BA and KIN (Tab. 2). These media led to the formation of leaves up to 50 per explant that was approximately five times more than some other treatments and control with about 10 leaves per explant. Dan et al. (2010)Dan Y, Baxter A, Zhang S, Pantazis CJ & Veilleux RE (2010) Development of effcient plant regeneration and transformation system for Impatiens using Agrobacterium tumefaciens and multiple bud cultures as explants. BMC Plant Biology 10: 165-177. in Impatiens walleriana cv. Accent Red showed that TDZ at 1, 3 and 7 µM had significantly greater frequencies of explants producing multiple shoots than 5 µM. For Impatiens walleriana cv. Salmon Picote, the frequency of explants producing multiple shoots was significantly greater when using 1 µM TDZ than the other concentrations tested. Han & Stephens (1987)Han K & Stephens LC (1987) Growth regulators affect in vitro propagation of two interspecifc Impatiens hybrids. Scientia Horticulturae 32: 307-313. reported that BA at 10 µM was most effective for stimulating shoot multiplication using shoot tips explants. Also, KIN at 12 or 20 mg dm–3 induced multiple shoots from shoot tip explants of Impatiens walleriana.
MS medium without PGRs produced longer shoots than other treatments. Presence of endogenous PGRs may be induced shoot length for control culture (hormone-free medium). Long shoots was produced in medium containing 0.10 mg l–1 NAA without BA, as well. Taha et al. (2009)Taha AM, Wagiran A, Ghazali H, Huyop F & Parveez GKA (2009) Optimization and transformation of garden balsam, Impatiens balsamina, mediated by microprojectile bombardment. Biotechnology 8: 1-12. found that NAA stimulated shoot elongation at the lowest concentration in Impatiens cv. T63-1 but inhibited at all concentrations in Impatiens cv. Star Fire. Contrary to our finding, maximum shoot length (13.00 cm) was produced on medium containing 2.00 mg l–1 BAP along with 1.00 mg l–1 2-iP (Balakrishnan et al. 2009)Balakrishnan V, Ravindran KC & Philip Robinson JP (2009) In vitro regeneration of Impatiens campanulata wight an important grass land plant. Botanical Research International 2: 123-130.. The highest fresh weight of leaves was calculated in the media with higher concentration of BA and KIN.
Root induction and growth
The number and the length of roots varied significantly between treatments (Tab. 2). Root induction and growth efficiency was high, as 100% of shoots produced roots, allowing plant regeneration within approximately 30 days. In the present study, 0.10 mg l–1 NAA was more effective in root induction and growth, mainly promoting higher root. The largest number (29.13 per shoot) and the highest length (33.80 mm per shoot) of roots were induced on media enriched with 0.10 mg l–1 NAA (Tab. 2). Least root length (less than 10 mm) was produced in media without NAA. Acclimatized plants with well-developed leaves and roots (Fig. 2a) were transplanted and transferred to a greenhouse. These plantlets hardened in the ex vitro condition and recorded 100% survival rate after 30 days in pots filled with perlite: peat moss (1:1) (Fig. 2b). There were no visual morphological abnormalities in the micropropagated plantlets.
a-b. Hardening process of impatiens (Impatiens hawkeri cv. Sweeties Blue Star) – a. acclimatized and well-rooted plants with well expanded leaves ready for transfer to pots; b. hardened plant growing in pot containing perlite: peat moss (1:1) in greenhouse.
Similar to our findings, Nikolova et al. (1996)Nikolova RV, Lall N & Bosa AJN (1996) An assessment of the conditions for the rapid propagation of Impatiens flanaganae in-vivo and in-vitro. Acta Horticulture 440: 633-638. showed that rooting in Impatiens flanaganae was induced on MS medium containing 1 mg l–1 IAA. Root induction in Impatiens balsamina was obtained using all auxins (IAA, IBA and NAA) with the highest percentage of rooting (96%) obtained with 0.5 mg l–1 IBA followed by 0.1 mg l–1 IAA and 0.5 and 1 mg l–1 NAA (Taha et al. 2009Taha AM, Wagiran A, Ghazali H, Huyop F & Parveez GKA (2009) Optimization and transformation of garden balsam, Impatiens balsamina, mediated by microprojectile bombardment. Biotechnology 8: 1-12.). Rooting in control plantlets was 90%. In present study, rooting was relatively high in control medium. Presence of endogenous auxins may be induced rooting for control cultures (Kyungkul 1993Kyungkul H (1993) In vitro shoot regeneration from cotyledons of immature ovules of Impatiens pelatypelata Lindl. In vitro cellular and developmental biology. Plant 30: 108-112.; Taha et al. 2009Taha AM, Wagiran A, Ghazali H, Huyop F & Parveez GKA (2009) Optimization and transformation of garden balsam, Impatiens balsamina, mediated by microprojectile bombardment. Biotechnology 8: 1-12.). Optimum concentration of auxins for root induction and growth is different between species. Some investigations demonstrated that optimum root initiation and development was obtained on medium enriched with high concentration of auxins (Juras et al. 2019Juras MCR, Jorge J, Pescador R, Ferreira WM, Tamaki V & Suzuki RM (2019) In vitro culture and acclimatization of Cattleya xanthina (Orchidaceae), an endangered orchid of the Brazilian Atlantic Rainforest. Rodriguésia 70: 1-11.), in contrast, in some other species, optimum root induction was achieved by low concentrations of auxins (Winhelmann et al. 2019Winhelmann MC, Tedesco M, Lucchese JR, Fior CS & Schafer G (2019) In vitro propagation of Angelonia integerrima. Rodriguésia 70: 1-12.; Kaviani & Negahdar 2017Kaviani B & Negahdar N (2017) Propagation, micropropagation and cryopreservation of Buxus hyrcana Pojark., an endangered ornamental shrub. South African Journal of Botany 111: 326-335.). Type of species and the content of endogenous PGRs in each species are the most important factors for these differences. The data presented in Table 2 show that the maximum fresh weight of roots (0.65 g per plantlet) was obtained in medium containing 0.10 mg l–1 NAA.
In conclusion, current findings presents successfully a simple, fast and efficient protocol for in vitro propagation of Impatiens hawkeri Bull. cv. Sweeties Blue Star during 45–50 days. Best results were obtained for shoot multiplication in terms of shoot and leaf number with application of 1.00 mg l–1 BA in combination with 0.10 mg l–1 NAA. Rooting of shoots can be obtained with the addition of 0.10 mg l–1 NAA alone to root induction medium. Rooting is a crucial step to the success of micropropagation and acclimatization. Regenerated plantlets were properly acclimatized due to the suitable rooting.
Acknowledgements
Authors thank Islamic Azad University, Rasht Branch.
References
- Balakrishnan V, Ravindran KC & Philip Robinson JP (2009) In vitro regeneration of Impatiens campanulata wight an important grass land plant. Botanical Research International 2: 123-130.
- Dan Y, Baxter A, Zhang S, Pantazis CJ & Veilleux RE (2010) Development of effcient plant regeneration and transformation system for Impatiens using Agrobacterium tumefaciens and multiple bud cultures as explants. BMC Plant Biology 10: 165-177.
- Grey-Wilson C (1980) Impatiens of Africa: morphology, pollination and pollinators, ecology, phytogeography, hybridisation, keys and a systematic treatment of all African species: with a note on collecting and cultivation. A.A. Balkema, Rotterdam. 248p.
- Hamrick D (2005) Ornamental bedding plant industry and plug production. Flower seed: biology and technology. EE. UU, Cambridge. Pp. 27-38.
- Han K & Stephens LC (1987) Growth regulators affect in vitro propagation of two interspecifc Impatiens hybrids. Scientia Horticulturae 32: 307-313.
- Jain SM & Ochatt SJ (2010) Protocols for in vitro propagation of ornamental plants. Springer Protocols, Humana Press, New York. 400p.
- Juras MCR, Jorge J, Pescador R, Ferreira WM, Tamaki V & Suzuki RM (2019) In vitro culture and acclimatization of Cattleya xanthina (Orchidaceae), an endangered orchid of the Brazilian Atlantic Rainforest. Rodriguésia 70: 1-11.
- Kaviani B & Negahdar N (2017) Propagation, micropropagation and cryopreservation of Buxus hyrcana Pojark., an endangered ornamental shrub. South African Journal of Botany 111: 326-335.
- Kaviani B (2015) Some useful information about micropropagation. Journal of Ornamental Plants 5: 29-40.
- Kyungkul H & Stephens LC (1987) Growth regulators affect in vitro propagation two interspecific Impatiens hybrids. Scientia Horticulturae 32: 307-313.
- Kyungkul H (1993) In vitro shoot regeneration from cotyledons of immature ovules of Impatiens pelatypelata Lindl. In vitro cellular and developmental biology. Plant 30: 108-112.
- Murashige T & Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15: 473-497.
- Nikolova RV, Lall N & Bosa AJN (1996) An assessment of the conditions for the rapid propagation of Impatiens flanaganae in-vivo and in-vitro Acta Horticulture 440: 633-638.
- Stephens LC, Krell SL & Weigle JL (1985) In vitro propagation of Java, New Guinea, and Java X New Guinea Impatiens. HortScience 20: 362-363.
- Taha AM, Wagiran A, Ghazali H, Huyop F & Parveez GKA (2009) Optimization and transformation of garden balsam, Impatiens balsamina, mediated by microprojectile bombardment. Biotechnology 8: 1-12.
- Winhelmann MC, Tedesco M, Lucchese JR, Fior CS & Schafer G (2019) In vitro propagation of Angelonia integerrima Rodriguésia 70: 1-12.
Edited by
Publication Dates
-
Publication in this collection
09 July 2020 -
Date of issue
2021
History
-
Received
08 Feb 2020 -
Accepted
07 May 2020