Abstract
Fungi of Rhizoctonia complex are mycorrhizal of orchids and may to germinate yours seeds and development the seedlings. In this context, our objective was to select a fungal isolate to promote seed germination and seedling development of Cyrtopodium saintlegerianum. Pelotons were found in the roots and three mycorrhizal fungi were isolated. We tested mycorrhizal isolates obtained from C. saintlegerianum roots and six mycorrhizal fungi from other orchids as well three pathogenic isolates (of rice and bean) to germinate the seeds in oatmeal-agar medium. Seeds not inoculated were used as control. The isolates En07 (Waitea circinata), Cs10 (Tulasnella sp.) and Ro88 (Rhizoctonia oryzae) were efficient to promote seed germination, but only En07 differing statistically of the control. The non-specific isolate En07 promoted germination in 81% of seeds and the specific isolate (Cs10) promoted 60%, evidencing the non-specificity mycorrhizal association in this orchid during germination. Axenic seedlings were inoculated with four mycorrhizal fungi (non-inoculated seedlings - control). After six months, the isolates En07 and Cs10 were efficient in the interaction with the seedlings, but did not differ to the control. Therefore, our results suggested that fungi of the Rhizoctonia complex can be used in the germination and seedling development of C. saintlegerianum.
Key words:
mycorrhizal association; non-specific fungi; Orchidaceae; plant pathogen; Rhizoctonia complex
Resumo
Fungos do complexo Rhizoctonia são micorrízicos de orquídeas e podem germinar suas sementes e desenvolver as plântulas. Neste contexto, nosso objetivo foi selecionar um isolado fúngico para promover a germinação e o desenvolvimento de plântulas de Cyrtopodium saintlegerianum. Foram encontrados pelotons nas raízes desta orquídea e foram identificados três fungos micorrízicos. Nós testamos os isolados obtidos das raízes de C. saintlegerianum e seis outros isolados micorrízicos assim como três isolados patogênicos (de arroz e feijão) para germinar as sementes em meio de aveia-agar. Sementes não inoculadas foram usadas como controle. Os isolados En07 (Waitea circinata), Cs10 (Tulasnella sp.) e Ro88 (Rhizoctonia oryzae) foram eficientes para promover a germinação, mas somente o En07diferiu estatisticamente do controle. O isolado não específico En07 promoveu 81% de germinação e o isolado específico (Cs10) promoveu 60%, evidenciando a associação micorrízica não específica durante a germinação. Plântulas axênicas foram inoculadas com quatro fungos micorrízicos (plântulas não inoculadas - controle). Após seis meses, os isolados En07 e Cs10 foram eficientes na interação com as plântulas, mas não diferiram do controle. Portanto, nossos resultados sugerem que fungos do complexo Rhizoctonia podem ser usados na germinação e no desenvolvimento de plântulas de C. saintlegerianum.
Palavras chave:
associação micorrízica; fungos não específicos; Orchidaceae; patógenos de plantas; complexo Rhizoctonia
Introduction
Mycorrhizal associations are essential to the life cycle of orchids in natural habitat. The interaction begins during seed germination when the mycorrhizal fungi infect basal cells in embryo. The hyphal coils formed into these cells are digested by the orchid to obtain carbon and nutrients necessary for its developmental initial phase. The seed produces a heterotrophic structure called the protocorm, which then forms the seedling. The seedling produces its first root and fungi can colonize its cortical cells. From this phase, symbiosis with mycorrhizal fungi facilitates the acquisition of nutrients from the substrate (Peterson et al. 2004Peterson RL, Massicotte HB & Melville LH (2004) Mycorrhizas: anatomy and cell biology. NRC Research Press, Ottawa. 182p.; Rasmussen & Rasmussen 2009Rasmussen HN & Rasmussen FN (2009) Orchid mycorrhiza: implications of a mycophagous life style. Oikos 118: 334-345.; Dearnaley et al. 2012Dearnaley JDW, Martos F & Selosse MA (2012) Orchid mycorrhizas: molecular ecology, physiology, evolution and conservation aspects. In: Hock B (ed.) The Mycota IX: fungal associations. 2nd ed. Springer, Berlin. Pp. 207-230.).
Orchids Mycorrhizal Fungi (OMF) verified in Brazilian orchids belong to the Rhizoctonia complex and were identified as Ceratobasidium D.P. Rogers, Thanathephorus Donk and Tulasnella Schroet teleomorphic genera (Nogueira et al. 2005Nogueira RE, Pereira OL, Kasuya MCM, Lanna MCS & Mendonça M (2005) Fungos micorrízicos associados a orquídeas em campos rupestres na região do quadrilátero ferrífero, MG, Brasil. Acta Botanica Brasilica 3: 417-424.; Pereira et al. 2011Pereira MC, Torres DP, Guimarães FAR, Pereira OL & Kasuya MCM (2011) Germinação de sementes e desenvolvimento de protocormos de Epidendrum secundum Jacq. (Orchidaceae) em associação com fungos micorrízicos do gênero Epulorhiza. Acta Botanica Brasilica 25: 534-541., 2015Pereira MC, Rocha DI, Veloso TGR, Pereira OL, Francino DMT, Meira RMSA & Kasuya MCM (2015) Characterization of seed germination and protocorm development of Cyrtopodium glutiniferum (Orchidaceae) promoted by mycorrhizal fungi Epulorhiza spp. Acta Botanica Brasilica 29: 567-574.; Silva et al. 2016Silva CS, Araújo LG, Sousa KCI, Carvalho JCB, Gonçalves LA & Carneiro LL (2016) Cultivo in vitro de Epidendrum nocturnum Jacq. (Orchidaceae) ocorrente no Cerrado da Região Centro-Oeste. Rodriguésia 67: 1083-1091.). And studies have shown that mycorrhizal isolates promote in vitro seed germination and seedling development better than axenic commercial media for orchid propagation (Pereira et al. 2011Pereira MC, Torres DP, Guimarães FAR, Pereira OL & Kasuya MCM (2011) Germinação de sementes e desenvolvimento de protocormos de Epidendrum secundum Jacq. (Orchidaceae) em associação com fungos micorrízicos do gênero Epulorhiza. Acta Botanica Brasilica 25: 534-541.; Guimarães et al. 2013Guimarães FAR, Pereira MC, Felicio CS, Torres DP, Oliveira SF, Veloso TGR & Kasuya MCM (2013) Symbiotic propagation of seedlings of Cyrtopodium glutiniferum Raddi (Orchidaceae). Acta Botanica Brasilica 27: 590-596.; Jiang et al. 2015Jiang J, Lee Y, Cubeta MA & Chen L (2015) Characterization and colonization of endomycorrhizal Rhizoctonia fungi in the medicinal herb Anoectochilus formosanus (Orchidaceae). Mycorrhiza 25: 431-445.). In this way, the mycorrhizal fungi inoculation has been highlighted as a promising strategy to improve orchid seedling production (Cribb et al. 2003Cribb PJ, Kell SP, Dixon KW, Barrett RL (2003) Orchid conservation: a global perspective. In: Dixon KW, Kell SP, Barrett RL & Cribb PJ (eds.) Orchid conservation. Natural History Publications, Kota Kinabalu. Pp. 1-25.).
Due to differences in specificity observed during mycorrhizal orchid interactions, fungal isolation has been required to select the suitable isolate to promote seed germination and seedling development (Dearnaley et al. 2012Dearnaley JDW, Martos F & Selosse MA (2012) Orchid mycorrhizas: molecular ecology, physiology, evolution and conservation aspects. In: Hock B (ed.) The Mycota IX: fungal associations. 2nd ed. Springer, Berlin. Pp. 207-230.). Some orchids have a narrow specificity with some fungal genera. During seed germination experiments, Coppensia doniana (Batem. ex W. Baxter) Campacci and Oncidium flexuosum Sims demonstrated preference for the mycorrhizal fungi Ceratorhiza sp., anamorphic form of Ceratobasidium genera (Pereira et al. 2005Pereira OL, Kasuya MCM, Rollemberg CL & Chaer GM (2005) Indução in vitro da germinação de sementes de Oncidium flexuosum (Orchidaceae) por fungos micorrízicos rizoctonioides. Revista Brasileira de Ciência do Solo 29: 199-206.). In contrast, Epidendrum secundum Jacq. and Cyrtopodium glutiniferum Raddi preferred fungi Epulorhiza sp., mycorrhizal anamorphic form of Tulasnella genera (Pereira et al. 2009Pereira MC, Pereira OL, Costa MD, Rocha RB & Kasuya MCM (2009) Diversidade de fungos micorrízicos Epulorhiza sp. isolados de Epidendrum secundum (Orchidaceae). Revista Brasileira de Ciência do Solo 33: 1187-1197., 2015Pereira MC, Rocha DI, Veloso TGR, Pereira OL, Francino DMT, Meira RMSA & Kasuya MCM (2015) Characterization of seed germination and protocorm development of Cyrtopodium glutiniferum (Orchidaceae) promoted by mycorrhizal fungi Epulorhiza spp. Acta Botanica Brasilica 29: 567-574.). Understanding such specificities is valuable for selection of the symbiont that assure propagation and commercialization of healthy orchid seedlings.
Some mycorrhizal fungi also associate with non-host orchids. For example, the seeds of Tolumnia variegata (Sw.) Braem associated with the mycorrhizal isolate of Ionopsis utricularioides (Sw.) Lindl. (Otero et al. 2004Otero JT, Ackerman JD & Bayman P (2004) Differences in mycorrhizal preferences between two tropical orchids. Molecular Ecology 13: 2393-2404.); seeds of Epidendrum nocturnum Jacq. interacted with Spiranthes brevialabris Lind. mycorrhizal fungi (Zettler et al. 2007Zettler LW, Poulter SB, Mcdonald KI & Stewart SL (2007) Conservation-driven propagation of an epiphytic orchid (Epidendrum nocturnum) with a mycorrhizal fungus. HortScience 42: 135-139.); and seeds of Spathoglottis plicata Blume had better germination with two mycorrhizal isolates of Dendrobium anosmum Lind. and Paphiopedilum sukhakulii Schoser & Senghas (Aewsakul et al. 2013Aewsakul N, Maneesorn D, Serivichyaswat P, Taluengjit A & Nontachaiyapoom S (2013) Ex vitro symbiotic germination of Spathoglotis plicata Blume on common orchid cultivation substrates. Scientia Horticulturae 160: 238-242.). The performance of non-specific mycorrhizal fungi may be evaluated to select the suitable fungi for propagating of some orchids (Zettler et al. 2007Zettler LW, Poulter SB, Mcdonald KI & Stewart SL (2007) Conservation-driven propagation of an epiphytic orchid (Epidendrum nocturnum) with a mycorrhizal fungus. HortScience 42: 135-139.).
Fifty terrestrial and epiphytic species of Cyrtopodium Rchb. f. have been reported from South America, thirty of which occur in area the Cerrado (Batista & Bianchetti 2005Batista JAN & Bianchetti LB (2005) Two new taxa in Cyrtopodium (Orchidaceae) from southern Brazil. Darwiniana 43: 74-83.). Some species of the genus Cyrtopodium have been widely explored as sources for raw material for small medical industries and ornamental gardens (Barreto & Parente 2006Barreto DW & Parente JP (2006) Chemical properties and biological activity of a polysaccharide from Cyrtopodium cardiochilum. Carbohydrate Polymers 64: 287-291.; Dutra et al. 2009Dutra D, Kane ME & Rhichardson L (2009) Asymbiotic seed germination and in vitro seedling development of Cyrtopodium punctatum: a propagation protocol for a endangered Florida native orchid. Plant Cell, Tissue and Organ Culture 96: 235-243.; Vogel & Macedo 2011Vogel IN & Macedo AF (2011) Influence of IAA, TDZ, and light quality on asymbiotic germination, protocorm formation, and plantlet development of Cyrtopodium glutiniferum Raddi., a medicinal orchid. Plant Cell, Tissue and Organ Culture 104: 147-155.; Pereira et al. 2015Pereira MC, Rocha DI, Veloso TGR, Pereira OL, Francino DMT, Meira RMSA & Kasuya MCM (2015) Characterization of seed germination and protocorm development of Cyrtopodium glutiniferum (Orchidaceae) promoted by mycorrhizal fungi Epulorhiza spp. Acta Botanica Brasilica 29: 567-574.). Cyrtopodium saintlegerianum Rchb. f. occurs as an epiphyte on species of palm in the Brazilian Cerrado (Batista & Bianchetti 2006Batista JAN & Bianchetti LB (2006) A New Species of Cyrtopodium (Orchidaceae) from the Cerrado of Central Brazil. Novon 16: 17-22.; Romero-Gonzáles et al. 2008Romero-González GA, Batista JAN & Bianchetti LB (2008) A synopsis of the genus Cyrtopodium (Catasetinae: Orchidaceae). Harvard Papers in Botany 13: 189-206.; BFG 2018BFG - The Brazil Flora Group (2018) Brazilian Flora 2020: Innovation and collaboration to meet Target 1 of the Global Strategy for Plant Conservation (GSPC). Rodriguésia 69: 1513-1527.), and has been propagated in media axenic containing phytoregulators (Rodrigues et al. 2015Rodrigues LA, Paiva Neto VB, Boaretto AG, Oliveira JF, Torrezan MA, Lima SF & Otoni WC (2015) In vitro propagation of Cyrtopodium saintlegerianum Rchb. f. (Orchidaceae), a native orchid of the Brazilian savannah. Crop Breeding and Applied Biotechonoly 15: 10-17.; Silva et al. 2017Silva CS, Araújo LG, Sousa KCI, Silva DM, Sibov ST & Faria PR (2017) Germinação e desenvolvimento in vitro de orquídea epífita do Cerrado. Ornamental Horticulturae 23: 96-100.). This species has been used in the ornamentation of gardens as well as to prepare dermatological plasters.
Little information has been reported about mycorrhizal association in these genera orchids. Recently, seeds and seedlings of C. glutiniferum exhibited satisfactory development when inoculated with mycorrhizal fungi of the genus Tulasnella (Guimarães et al. 2013Guimarães FAR, Pereira MC, Felicio CS, Torres DP, Oliveira SF, Veloso TGR & Kasuya MCM (2013) Symbiotic propagation of seedlings of Cyrtopodium glutiniferum Raddi (Orchidaceae). Acta Botanica Brasilica 27: 590-596.; Pereira et al. 2015Pereira MC, Rocha DI, Veloso TGR, Pereira OL, Francino DMT, Meira RMSA & Kasuya MCM (2015) Characterization of seed germination and protocorm development of Cyrtopodium glutiniferum (Orchidaceae) promoted by mycorrhizal fungi Epulorhiza spp. Acta Botanica Brasilica 29: 567-574.). However, more studies are required to determine the presence of mycorrhizal fungi in situ, their isolation and to evaluate fungi potential during in vitro symbiotic seed germination. In view of this aspect, the objective in present study was to select a suitable fungal to promote seed germination and seedling development of C. saintlegerianum. To achieve this aim, we first confirm root mycorrhizal colonization of C. saintlegerianum throw root anatomical analysis. Its mycorrhizal fungi were isolated and identified morphologically. The seed germination test was performed co-inoculating C. saintlegerianum seeds with different isolates: its own mycorrhizal fungi, others mycorrhizal isolates and some pathogenic Rhizoctonia-like fungi. Isolates that promoted the development of the embryo as well as other specific isolates were inoculated in axenic seedlings of C. saintlegerianum to test their potential to support the ex vitro development.
Material and Methods
Capsule and root collection
Capsules and roots of C. saintlegerianum (Fig. 1a) were collected during March 2010 to August 2011 from three different plants growing on three palms in pasture areas in the Brazilian Cerrado (16o07’66.6’’S and 50o10’04.4’’W). The biological material was transported to the Laboratório de Genética de Microrganismos (LGM) at the Universidade Federal de Goiás (UFG) Brazil. Some root fragments were fixed in FAA70 (Formaldeyde - Acetic acid - Alcohol 70%) (Johansen 1940Johansen DA (1940) Botanical microtechnique. McGraw-Hill, New York. 523p.) for two days and stored in ethanol solution (70%) until anatomical characterization of mycorrhizae. Others fragments were reserved to mycorrhizal fungi isolation. The capsules were stored into flask containing silica gel and kept at 4 °C until germination experiments.
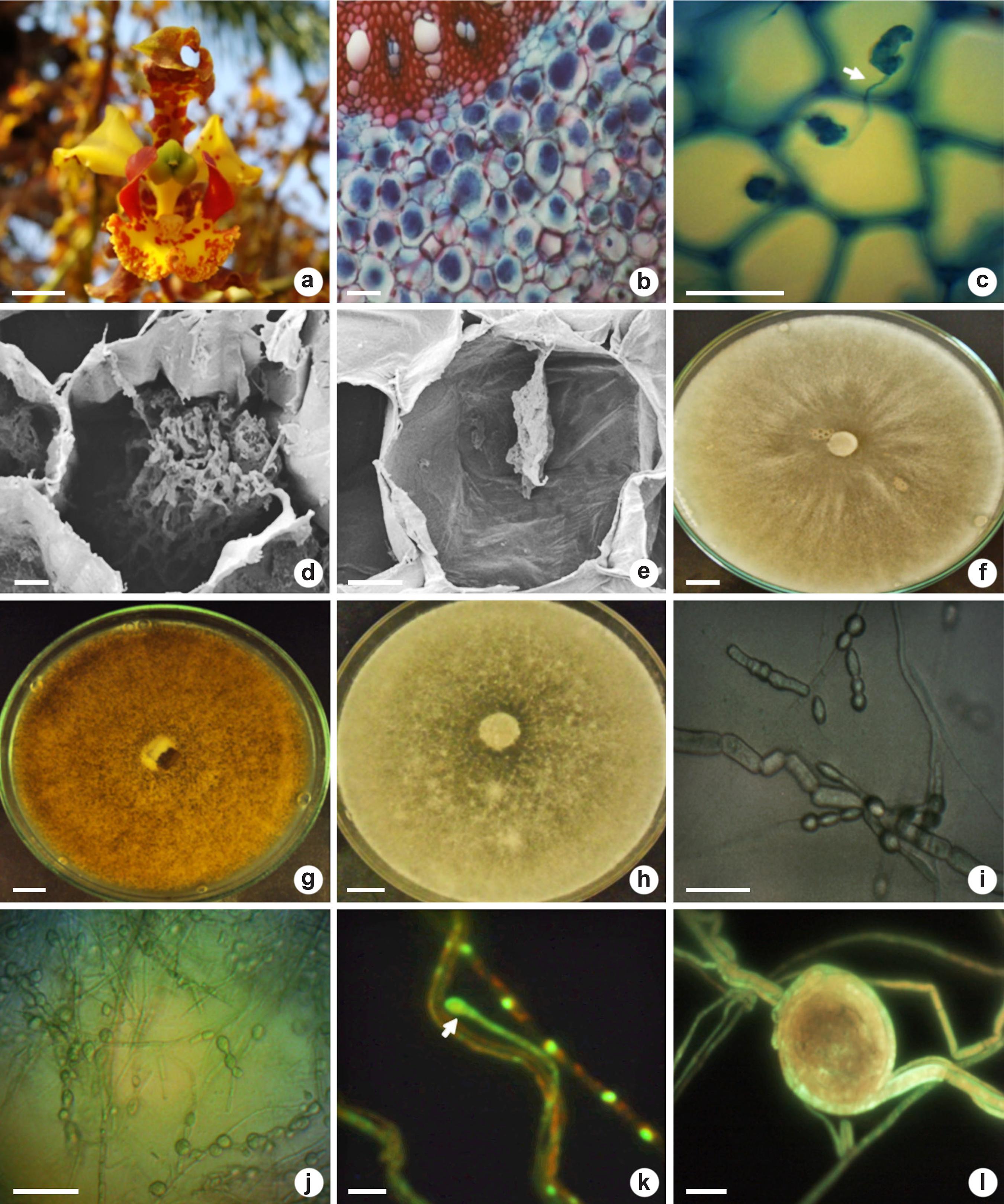
Flower (in the natural habitat), mycorrhizal colonization and fungal identification (in laboratory) of Cyrtopodium saintlegerianum - a. flower with capsule formation; b. overview of the parenchymatous cortex roots colonized by pelotons (intact and degraded); c. pelotons in neighboring cortical cells connected by hypha (arrow); d. intact peloton inside cortical cell; e. peloton in degradation; f. isolate Cs02 after 336 hours in PDA; g. isolate Cs10 after 336 hours in PDA showing brown-colored mycelium; h. isolate Cs21 in PDA; i. chain of monilioid cells from isolate Cs10; j. chain of monilioid cells (Cs21); k. young monilioid binucleate cell of Cs02 (arrow); l. peloton formed of Cs10 in microculture (PDA). Bars = 1 cm (a,f-h), 100 µm (b), 50 µm (c,i-k), 20 µm (e), 10 µm (d,l).
Mycorrhizae microscopy characterization, fungal isolation and identification
Root fragments of C. saintlegerianum were sectioned by freehand for optical microscopy (OM) observation. The sections were cleared and subjected to 1% aqueous safranin and 0.3% astra blue (Krauss & Arduin 1997Krauss JE & Arduin M (1997) Manual básico de métodos em morfologia vegetal. Universidade Rural do Rio de Janeiro, Seropédica. 198p.) for cell roots and fungal structures coloring. Root sections were prepared to Scanning Eletronic Microscope (SEM) observation according to Silva et al. (2016)Silva CS, Araújo LG, Sousa KCI, Carvalho JCB, Gonçalves LA & Carneiro LL (2016) Cultivo in vitro de Epidendrum nocturnum Jacq. (Orchidaceae) ocorrente no Cerrado da Região Centro-Oeste. Rodriguésia 67: 1083-1091. in Laboratório Multiusuário de Microscopia de Alta Resolução - LabMic, Physics Institute, UFG.
The mycorrhizal fungi were isolated according to Gonçalves et al. (2014)Gonçalves FJ, Nunes CMC, Filippi MC, Araújo LG, Gonçalves LA & Sibov ST (2014) Isolation and characterization of mycorrhizal fungi of Cyrtopodium vernum Rchb. F. & Warm (Orchidaceae). Revista de Ciências Agrárias 57: 244-249. using the PDA medium (Potato Dextrose Agar, composed of 200 g of potato, 20 g of dextrose, 20 g of agar and 1 L of water - Otero et al. 2004Otero JT, Ackerman JD & Bayman P (2004) Differences in mycorrhizal preferences between two tropical orchids. Molecular Ecology 13: 2393-2404.). Fungal isolates were cultivated on PDA plates under continuous fluorescent light for five days at room temperature (26±2 °C). Isolates with morphological characteristics of OMF (Currah & Zelmer 1992Currah RS & Zelmer CD (1992) A key and notes for the genera of fungi with orchids and a new species in the genus Epulorhiza. Reports of the Tottori Mycological Institute 30: 43-59.) were maintained in a growth chamber at 26±2 °C with a 16 h photoperiod.
The identification of OMF were performed using features described by Currah & Zelmer (1992)Currah RS & Zelmer CD (1992) A key and notes for the genera of fungi with orchids and a new species in the genus Epulorhiza. Reports of the Tottori Mycological Institute 30: 43-59., Nogueira et al. (2005)Nogueira RE, Pereira OL, Kasuya MCM, Lanna MCS & Mendonça M (2005) Fungos micorrízicos associados a orquídeas em campos rupestres na região do quadrilátero ferrífero, MG, Brasil. Acta Botanica Brasilica 3: 417-424., Pereira et al. (2005)Pereira OL, Kasuya MCM, Rollemberg CL & Chaer GM (2005) Indução in vitro da germinação de sementes de Oncidium flexuosum (Orchidaceae) por fungos micorrízicos rizoctonioides. Revista Brasileira de Ciência do Solo 29: 199-206. and Silva et al. (2016)Silva CS, Araújo LG, Sousa KCI, Carvalho JCB, Gonçalves LA & Carneiro LL (2016) Cultivo in vitro de Epidendrum nocturnum Jacq. (Orchidaceae) ocorrente no Cerrado da Região Centro-Oeste. Rodriguésia 67: 1083-1091.. The cultural characteristics evaluated were colony diameter, growth tax, number of nuclei per cell, hyphal diameter, width and length of monilioid cells and polyphenol oxidase (PPO) production. The cultural characteristics (color, aerial mycelium, mycelium shape and size, and colony diameter) were evaluated after 72 and 336 h on PDA or CMA medium (Corn Meal Agar, composed of 15 g agar and 1 L broth obtained from cooking 30 g of corn meal).
The number of nuclei per cell and hyphal diameter were evaluated according to Meinhardt et al. (2001)Meinhardt LW, Bellato CM & Tsai SM (2001) SYBR green I used to evaluate the nuclei number of fungal mycelia. Biotechniques 31: 42-46.. The images of hyphae were captured using a Leica DMI6000 optical microscope (OM) with an epifluorescence accessory and processed in Leica IM50 editor. The width and length of monilioid cells of each isolate were assessed in fungal colonies cultured in CMA for two months and were measured from images taken under BelPhotonics microscope cells using the BelAnalyzer MicroImage software.
The fungal PPO production was evaluated in Petri plates containing YEA medium (Yeast Extract Agar, composed of 15 g Yeast Extract 15 g, 15 g Agar 15 g in water 1 L) with addition of tannic acid (5 g), according to Zelmer & Currah (1995)Zelmer CD & Currah RS (1995) Ceratorhiza pernacatena and Epulorhiza calendulina sp. nov.: mycorrhizal fungi of terrestrial orchids. Canadian Journal of Botany 73: 1981-1985.. Petri plates without tannic acid were used as a control. The production of PPO was detected by the presence of an amber-colored halo around the colonies after five days.
In vitro symbiotic germination and ex vitro development
Seeds collected from a 12-month mature capsule were sterilized as described for Silva et al. (2016)Silva CS, Araújo LG, Sousa KCI, Carvalho JCB, Gonçalves LA & Carneiro LL (2016) Cultivo in vitro de Epidendrum nocturnum Jacq. (Orchidaceae) ocorrente no Cerrado da Região Centro-Oeste. Rodriguésia 67: 1083-1091.. After capsule opening, 50% of the seeds were used to test the viability of the embryo with triphenyltetrazolium chloride (TTC) and 50% were used for symbiotic cultivation. A TTC solution was used to evaluate seed viability adopting modifications to orchid seeds (Vujanovic et al. 2000Vujanovic V, St-Arnaud M, Barabé D & Thibeault G (2000) Viability testing of orchid seed and the promotion of colouration and germination. Annals of Botany 86: 79-86.). Ten sample (with approximately 0.001 g of seed) were transferred into microtubes containing 2 mL of 1% TTC solution and kept in a water bath at 40 °C in continuous darkness to prevent TTC precipitation. The embryos were observed for the capture of images under light microscopy (BelView software).
We tested mycorrhizal isolates obtained from C. saintlegerianum roots and six mycorrhizal fungi from other orchids (fungal isolates belonging to the LGM library). Three pathogenic isolates, taxonomically related to some mycorrhizal fungi (two of Rhizoctonia oryzae Ryker & Gooch and one of Rhizoctonia solani Kühn, pathogenics of rice and bean) were supplied by the Collection of Functional Microorganisms of Embrapa Arroz e Feijão (CNPAF) - Brazil (Tab. 1). All isolates selected were grown in plates with oatmeal-agar medium (OMA - Zettler et al. 2007Zettler LW, Poulter SB, Mcdonald KI & Stewart SL (2007) Conservation-driven propagation of an epiphytic orchid (Epidendrum nocturnum) with a mycorrhizal fungus. HortScience 42: 135-139.; Steinfort et al. 2010Steinfort U, Verdugo G, Besoain X & Cisternas MA (2010) Mycorrhizal association and symbiotic germination of the terrestrial orchid Bipinnula fimbriata (Poepp.) Johnst (Orchidaceae). Flora 205: 811-817.).
Treatments (fungi isolates and controle - without fungal) used in the germination of Cyrtopodium saintlegerianum.
A 9-mm mycelial disc was taken from the edge of each isolate colony and placed in plates with OMA and containing approximately 150 seeds. Plates containing seeds without fungi were maintained as controls. The experimental design was completely randomized with ten treatments and eight replicates (Tab. 1). The plates were incubated in a germination chamber at 26±2 °C and with a 16 h photoperiod. The data obtained from the seed viability test were normalized by square root transformation, whereas the germination data were transformed using arcsin. To compare each treatment we performed an analysis of variance and a posteriori Tukey test (at 5% probability) using the software R v 2.11.0 (Díaz & Álvarez 2009Díaz MS & Álvarez CC (2009) Plant regeneration through direct shoot formation from leaf cultures and from protocorm-like bodies derived from callus Encyclia mariae (Orchidaceae), a threatened Mexican orchid. In Vitro Cellular Developmental Biology - Plant 45: 162-170.; Steinfort et al. 2010Steinfort U, Verdugo G, Besoain X & Cisternas MA (2010) Mycorrhizal association and symbiotic germination of the terrestrial orchid Bipinnula fimbriata (Poepp.) Johnst (Orchidaceae). Flora 205: 811-817.).
Germination was assessed every two weeks, under a light microscope coupled to a digital camera, using the parameters: 0 - no germination, 1 - swelled embryo and rupture of testa (germination), 2 - continued embryo enlargement and production of rhizoid (Fig. 2) adapted of Stewart & Zettler (2002)Stewart SL, Zettler LW (2002) Symbiotic germination of three semi-aquatic rein orchids (Habenaria repens, H. quinquiseta, H. macroceratitis) from Florida. Aquatic Botany 72: 25-35.. The final evaluation occurred nine months after sowing. Seeds were observed under an optical microscope to assess the presence of pelotons, which were stained according to Chutima et al. (2010)Chutima R, Dell B, Vessabutr S, Bussaban B & Lumyong S (2010) Endophytic fungi from Pecteilis susannae (L.) Rafin (Orchidaceae), a threatened terrestrial orchid in Thailand. Mycorrhiza 21: 221-229.. Seeds at different stages of germination were collected for SEM observation. Seed preparation was performed as suggested by Chou & Chang (2004)Chou L & Chang D (2004) Asymbiotic and symbiotic seed germination of Anoectochilus formosanus and Haemaria discolor and their F1 hybrids. Botanical Bulletin of Academia Sinica 45: 143-147. and visualization was done in the same mode as the root fragments.
Development stages used to determine in vitro germination of Cyrtopodium saintlegerianum - a. 0: no germination (viable embryo); b. 1: swelled embryo - rupture of testa (germination); c. 2: continued embryo enlargement - production of rhizoids. Bars = 50 µm (a,b,c).
For ex vitro mycorrhization assessment, asymbiotic seedlings with thirteen months were obtained from in vitro seed germination using MS medium (Silva et al. 2017Silva CS, Araújo LG, Sousa KCI, Silva DM, Sibov ST & Faria PR (2017) Germinação e desenvolvimento in vitro de orquídea epífita do Cerrado. Ornamental Horticulturae 23: 96-100.). The mycorrhizal fungi were Tulasnella sp. isolates (Cs02, Cs10 and Cs21) obtained from C. saintlegerianum and Waitea circinata Warcup & Talbot (En07) from E. nocturnum (Tab. 1). Seedlings of C. saintlegerianum were inoculated with 0.2 g of mycelium of each isolate. The seedlings were grown in axenic substrate (Sphagnum sp.) in a complete randomized experimental design with five treatments and five replicates. Number of shoots, stem diameter (0.5 cm above the base), stem vigor in region of pseudobulb formation (1 cm above the base) and survival of the seedlings were evaluated after six months. To compare each treatment (isolate and control) we performed an analysis of variance and a posteriori Tukey test (at 5% probability) using the software R v 2.11.0 (Steinfort et al. 2010Steinfort U, Verdugo G, Besoain X & Cisternas MA (2010) Mycorrhizal association and symbiotic germination of the terrestrial orchid Bipinnula fimbriata (Poepp.) Johnst (Orchidaceae). Flora 205: 811-817.).
Results
Mycorrhizal colonization, fungal morphological characterization and identification
The pelotons were stained dark blue due to the presence of chitin in the hyphae (Fig. 1b-c). Pelotons were mostly intact with the hyphae occupying the cortical cells of the roots (Fig. 1b). Connective hyphae between pelotons within neighboring cells were found (Fig. 1c). Intact (Fig. 1d) and degraded (Fig. 1e) pelotons within cortical cells roots were observed.
From C. saintlegerianum roots were obtained three isolates (Cs02, Cs10 and Cs21 - Fig. 1f-h) with Rhizoctonia characteristics (Currah & Zelmer 1992Currah RS & Zelmer CD (1992) A key and notes for the genera of fungi with orchids and a new species in the genus Epulorhiza. Reports of the Tottori Mycological Institute 30: 43-59.). The isolate Cs02 presented faster mycelial growth than Cs10 and Cs21 in BDA medium. The mycelium of isolates Cs02 and Cs21 was white while Cs10 is brown (Fig. 1f-h). Only in CMA medium, these isolates produced round monilioid cells with chains containing up to five cells (Fig. 1i-j). The isolates presented two nuclei per cell and we registered two nuclei in one new monilioid cell of isolate Cs02 (Fig. 1k). After five days of growth on PDA microculture, the isolate Cs10 formed pelotons or hyphae bundles (Fig. 1l). None of the isolates (Cs02, Cs10 and Cs21) formed an amber-colored halo, indicating absence of PPO production. Based on morphological characterization, these isolates were identified as Tulasnella sp. (Epulorhiza sp., anamorphic phase).
In vitro germination and ex vitro development
Embryos of C. saintlegerianum seeds were stained at 3 h and the viability test demonstrated that 77% of the seeds were viable as indicated by their dark red color (Fig. 3a). The isolate non-specific En07 highlights from the other fungi providing seed tegument rupture and rhizoid formation (Stage 2) after two months (Fig. 3b). In the control (without fungus), there was no rhizoid formation (Fig. 3c). After nine months, the treatment with the En07 isolate provided 81% of germinated seeds (Stages 1 and 2). However, it was not statistically different from the isolates Cs10 (C. saintlegerianum specific) and Ro88 (pathogenic to rice), which presented 60 and 52% germinated seeds, respectively. The treatment with En07, CS10 and Ro88 showed a larger number of germinated seeds and differed statistically from the control (Tab. 2).
Cyrtopodium saintlegerianum seeds and seedlings – a. viable (v) and non-viable (nv) embryos of seeds submitted to tetrazolium test (TTC); b. seed inoculated with isolate En07 (Waitea circinata of Epidendrum nocturnum) with rhizoids after two months; c. seed without fungi (control) were swollen after nine months of growth in oatmealagar media; d. seed germinated after nine months with En07 (showed many rhizoid – Stage 2); e. seeds showed rhizoids with isolate Cs10 (Tulasnella sp.); f. rhizoid emerging from the embryo and peloton inside (seed with En07); g. swollen seed showing a crack in the seed coat, suggesting that isolate En07 colonized the embryonic cells (Stage 2); h. non-germinated seeds (0) and swollen seeds (1) derived from the association with isolate En07; i. seeds with swollen embryos, but non-germinated (control – Stage 0); j. seedling six months after of ex vitro mycorrhization with isolate En07 (non-specific); k. seedling with isolate Cs10 (specific); l. seedling without fungal (control). Bars = 1 cm (j-l), 200 μm (b-d,h), 100 μm (g,i), 50 μm (a,e), 10 μm (f).
In vitro germination of Cyrtopodium saintlegerianum seeds after inoculation with fungal isolates (after nine months of incubation).
Seeds inoculated with En07 showed many rhizoids after nine months (Fig. 3d). Hyphae of this isolate penetrate the rhizoids and formed pelotons (Fig. 3f). Seeds inoculated with isolate Cs10 too showed rhizoids and embryo swelling (Fig. 3e). The isolates Cs02 (23%) and Cs21 (21%) were specific for C. saintlegerianum, but presented germination percentages lower than control (27%). The other isolates (Cv10, Cv17, Ro89 and Rh21180) were not efficient in germinating orchid seeds (Tab. 2). In SEM we observed cracks in the coat of seeds inoculated with isolate En07, indicating seed coat rupture in consequence of embryo swelling (Fig. 3g-h). The seeds from the control showed intumescence, but did not germinate because there was no differentiation in the embryo or tegument rupture (Fig. 3i).
In the ex vitro symbiotic development, the isolates En07 and Cs10 (Fig. 3j-k) promoted thicker and vigorous stems in axenic seedlings of C. saintlegerianum after six months, but did not differ statistically from the control (Fig. 3l; Tab. 3). All isolates promoted shoot formation, but only En07 treatment presented a shoot number significantly higher than the control. Survival percentage of seedlings in association with fungi isolates was not differed from the control (Tab. 3).
The number of shoots, stem diameter, vigor and survival of Cyrtopodium saintlegerianum seedlings six months after ex vitro mycorrhization with four isolates (Control: not fungal inoculation).
Discussion
This is the first report of symbiotic germination and seedling development of C. saintlegerianum. Our study showed that embryos without fungi just exhibited intumescence, without rhizoids formation. Mycorrhizal fungi Tulasnella sp. and W. circinata promoted embryo development up to produce protocorm with rhizoids and ruptured coat, which confirm dependence of C. saintlegerianum during seed germination. Additionally, W. circinata isolate support better seedling development. Indeed, W. circinata is a potential isolate to be applied during symbiotic cultivation of C. saintlegerianum.
We observed a large number of intact and degraded pelotons in parenchyma cells of the root cortex in adult plants of C. saintlegerianum (Fig. 1b-e). These observations confirm the maintenance of the mycorrhizal association at adult phase. Thus, the presence of pelotons indicates the orchid needs fungal interaction to acquire nutrients. In addition, the pelotons degradation may be associated with the flowering period when roots were collected, in which the plant has a higher nutritional demand.
The morphological characteristics and no production of PPO confirm identification of C. saintlegerianum mycorrhizal isolates as Tulasnella sp. (Currah & Zelmer 1992Currah RS & Zelmer CD (1992) A key and notes for the genera of fungi with orchids and a new species in the genus Epulorhiza. Reports of the Tottori Mycological Institute 30: 43-59.; Zelmer & Currah 1995Zelmer CD & Currah RS (1995) Ceratorhiza pernacatena and Epulorhiza calendulina sp. nov.: mycorrhizal fungi of terrestrial orchids. Canadian Journal of Botany 73: 1981-1985.; Athipunyakon et al. 2004Athipunyakon P, Manoch L & Piluek C (2004) Isolation and identification of mycorrhizal fungi from eleven terrestrial orchids. Natural Science 38: 216-228.). We observed morphological differences among our Tulasnella (Fig. 1f-l), which propel us to select the three Tulasnella isolates to in vitro seed germination experiment. These isolates presented different results during seed germination and ex vitro seedling cultivation, corroborating with Pereira et al. (2009Pereira MC, Pereira OL, Costa MD, Rocha RB & Kasuya MCM (2009) Diversidade de fungos micorrízicos Epulorhiza sp. isolados de Epidendrum secundum (Orchidaceae). Revista Brasileira de Ciência do Solo 33: 1187-1197.; 2011)Pereira MC, Torres DP, Guimarães FAR, Pereira OL & Kasuya MCM (2011) Germinação de sementes e desenvolvimento de protocormos de Epidendrum secundum Jacq. (Orchidaceae) em associação com fungos micorrízicos do gênero Epulorhiza. Acta Botanica Brasilica 25: 534-541., who observed that morphologically different Tulasnella can present divergent results in seed germination experiments.
Some species interact with mycorrhizal fungi just during seed germination, although other species maintain the interaction during the adult phase (Peterson et al. 2004Peterson RL, Massicotte HB & Melville LH (2004) Mycorrhizas: anatomy and cell biology. NRC Research Press, Ottawa. 182p.; Zettler et al. 2007Zettler LW, Poulter SB, Mcdonald KI & Stewart SL (2007) Conservation-driven propagation of an epiphytic orchid (Epidendrum nocturnum) with a mycorrhizal fungus. HortScience 42: 135-139.; Rasmussen & Rasmussen 2009Rasmussen HN & Rasmussen FN (2009) Orchid mycorrhiza: implications of a mycophagous life style. Oikos 118: 334-345.). Zettler et al. (2007)Zettler LW, Poulter SB, Mcdonald KI & Stewart SL (2007) Conservation-driven propagation of an epiphytic orchid (Epidendrum nocturnum) with a mycorrhizal fungus. HortScience 42: 135-139. reported symbiosis maintenance between Tulasnella fungi and orchid S. brevilabris from embryo stage until adult phase. In the same way, Látalová & Baláz (2010)Látalová K & Baláz M (2010) Carbon nutrition of mature green orchid Serapias strictiflora and its mycorrhizal fungus Epulorhiza sp. Biologia Plantarum 54: 97-104. and Gonçalves et al. (2014)Gonçalves FJ, Nunes CMC, Filippi MC, Araújo LG, Gonçalves LA & Sibov ST (2014) Isolation and characterization of mycorrhizal fungi of Cyrtopodium vernum Rchb. F. & Warm (Orchidaceae). Revista de Ciências Agrárias 57: 244-249. demonstrated interaction between orchid and mycorrhizal fungus from protocorm until adult stage. Silva et al. (2016)Silva CS, Araújo LG, Sousa KCI, Carvalho JCB, Gonçalves LA & Carneiro LL (2016) Cultivo in vitro de Epidendrum nocturnum Jacq. (Orchidaceae) ocorrente no Cerrado da Região Centro-Oeste. Rodriguésia 67: 1083-1091. observed that W. circinata increases the vigor of Epidendrum nocturnum seedlings in vitro and a potential interaction was observed with seeds and seedlings (in vitro and ex vitro, respectively) of C. saintlegerianum too. Thus, our isolates can support the mycotrophism of this plant during in vitro and ex vitro propagation, even in natural habitat.
Earlier investigations have shown that Tulasnella sp. is associated with many tropical orchids as C. vernun, C. glutiniferum, E. secundum, Epidendrum dendrobioides Thunb. e Sophronitis milleri (Blumenschein ex Pabst) C. Berg & M. W. (Nogueira et al. 2005Nogueira RE, Pereira OL, Kasuya MCM, Lanna MCS & Mendonça M (2005) Fungos micorrízicos associados a orquídeas em campos rupestres na região do quadrilátero ferrífero, MG, Brasil. Acta Botanica Brasilica 3: 417-424.; Pereira et al. 2005Pereira OL, Kasuya MCM, Rollemberg CL & Chaer GM (2005) Indução in vitro da germinação de sementes de Oncidium flexuosum (Orchidaceae) por fungos micorrízicos rizoctonioides. Revista Brasileira de Ciência do Solo 29: 199-206., 2009Pereira MC, Pereira OL, Costa MD, Rocha RB & Kasuya MCM (2009) Diversidade de fungos micorrízicos Epulorhiza sp. isolados de Epidendrum secundum (Orchidaceae). Revista Brasileira de Ciência do Solo 33: 1187-1197., 2015Pereira MC, Rocha DI, Veloso TGR, Pereira OL, Francino DMT, Meira RMSA & Kasuya MCM (2015) Characterization of seed germination and protocorm development of Cyrtopodium glutiniferum (Orchidaceae) promoted by mycorrhizal fungi Epulorhiza spp. Acta Botanica Brasilica 29: 567-574.; Gonçalves et al. 2014Gonçalves FJ, Nunes CMC, Filippi MC, Araújo LG, Gonçalves LA & Sibov ST (2014) Isolation and characterization of mycorrhizal fungi of Cyrtopodium vernum Rchb. F. & Warm (Orchidaceae). Revista de Ciências Agrárias 57: 244-249.). In this study, W. circinata (En07 isolate from E. nocturnum) was more efficient to support symbiotic development of C. saintlegerianum, associate in nature with Tulasnella. En07 presented germination percentage similar to seed viability, supported protocorm developed until Stage 2 (Tab. 2) and presented best results during seedling development (Tab. 3). In light of this, screening non-natural and natural mycorrhizal fungi is an important strategy to select a suitable isolate to orchid symbiotic cultivation. Some orchids present better development in association with their own OMFs. It is probably in consequence of narrow mycorrhizal specificity (Valadares et al. 2010Valadares RBS, Otero JT, Pereira MC, Steinfort U, Verdugo G, Besoain X & Cisternas MA (2010) The epiphytic orchids Ionopsis utricularioides and Psygmorchis pusilla associate with different Ceratobasidium lineages at Valle del Cauca, Colombia. Acta Botanica Brasilica 29: 40-44.; Silva et al. 2016Silva CS, Araújo LG, Sousa KCI, Carvalho JCB, Gonçalves LA & Carneiro LL (2016) Cultivo in vitro de Epidendrum nocturnum Jacq. (Orchidaceae) ocorrente no Cerrado da Região Centro-Oeste. Rodriguésia 67: 1083-1091.). Other orchids respond better to OMFs of other plants, suggesting broad mycorrhizal specificity (Otero et al. 2004Otero JT, Ackerman JD & Bayman P (2004) Differences in mycorrhizal preferences between two tropical orchids. Molecular Ecology 13: 2393-2404.), regarding with present study.
The ability of R. oryzae and R. solani isolates (Tab. 2), pathogenic fungi of rice and bean plants, to promote in vitro seed germination of C. saintlegerianum have highlighted.Masuhara et al. (1993)Masuhara G, Katsuya K & Yamaguchi K (1993) Potential for symbiosis of Rhizoctonia solani and binucleate Rhizoctonia with seeds of Spiranthes sinensis var. amoena in vitro. Mycological Research 97: 746-752. had no success in seed germination of Spiranthes sinensis var. amoena with pathogenic isolates of R. solani and R. oryzae. However, Masuhara & Katsuya (1994)Masuhara G & Katsuya K (1994) In situ and in vitro specificity between Rhizoctonia spp. and Spiranthes sinensis (Persoon) Ames. var. amoena (M. Bieberstein) Hara (Orchidaceae). New Phytologist 127: 711-718. suggested that Rhizoctonia spp. pathogens of rice (Oryza sativa) would also germinate orchid seeds. It is an evidence that orchids suppress pathogenic potential of these fungi and use them as nutrient source during embryo and seedling development.
The En07 and CS10 improved seedlings vigor, shoot number and stem diameter of C. saintlegerianum seedling (Tab. 3; Fig. 2j-l), but through the evaluations carried out it was not possible to verify statistical difference. These improvements are indispensable to plant longevity and establishment during acclimatization. Hence, in future approaches on the increases from seedlings association with fungi, more refined methods of measurement will be needed. Benefits of mycorrhization during orchid seedling establishment have been reported to C. glutiniferum (Guimarães et al. 2013Guimarães FAR, Pereira MC, Felicio CS, Torres DP, Oliveira SF, Veloso TGR & Kasuya MCM (2013) Symbiotic propagation of seedlings of Cyrtopodium glutiniferum Raddi (Orchidaceae). Acta Botanica Brasilica 27: 590-596.), Phalaenopsis sp. (Moreno et al. 2000Moreno JAE, Acuña EAG, Román AEB, Contreras DJ & López CT (2000) Fertilización química e biológica de Phalaenopsis (Orchidaceae) en condiciones de invernadero. Terra Latinoamericana 18: 125-131.; Wu et al. 2011Wu P, Huang D & Chang CN (2011) Mycorrhizal symbiosis enhances Phalaenopsis orchid’s growth and resistance to Erwinia chrysanthemi. African Journal of Biotechnology 10: 10095-10100.) e Spathoglottis plicata Blume (Aewsakul et al. 2013Aewsakul N, Maneesorn D, Serivichyaswat P, Taluengjit A & Nontachaiyapoom S (2013) Ex vitro symbiotic germination of Spathoglotis plicata Blume on common orchid cultivation substrates. Scientia Horticulturae 160: 238-242.). Additionally, mycorrhizal associations can also suppress biotic agents, such as plant pathogens (Peterson et al. 2004Peterson RL, Massicotte HB & Melville LH (2004) Mycorrhizas: anatomy and cell biology. NRC Research Press, Ottawa. 182p.; Rasmussen & Rasmussen 2009Rasmussen HN & Rasmussen FN (2009) Orchid mycorrhiza: implications of a mycophagous life style. Oikos 118: 334-345.). OMFs induced resistance in rice plants to pathogenic soil fungal (Mosquera-Espinosa et al. 2013Mosquera-Espinosa AT, Bayman P, Prado GA, Gómez-Carabalí A & Otero JT (2013) The double life os Ceratobasidium: orchid mycorrhizal fungi and their potential for biocontrol of Rhizoctonia solani sheath blight of rice. Mycologia 105: 141-150.) and our isolate En07 (W. circinata) demonstrated potential as biocontrol agent against Magnaphorte oryzae, a rice blast pathogen (Carvalho et al. 2015Carvalho JCB, Sousa KCI, Brito DC, Chaibub AA, Luzini AP, Côrtez MVCB, Filippi MCC, Kato L, Vaz BG, Costa HB, Romão W & Araújo LG (2015). Biocontrol potential of Waitea circinata, an orchid mycorrhizal fungus, against the rice blast fungus. Tropical Plant Pathology 40: 151-159.).
Our results support the use of mycorrhizal fungi in germination and development of C. saintlegerianum. This orchid has little mycorrhizal specificity, facilitating its interaction with fungi from other plants. Some Rhizoctonia isolates may supporte seed germination, plant vigor, greater longevity and resistance to environmental factors. Thereby, we advocate the use of fungi during C. saintlegerianum propagation and suggest testing the inoculation of these in other orchid seeds and seedlings. Future investigations are necessary in order to better understanding the orchid-fungal interactions as well as the evaluation of the increment of the application of the fungi in axenic seedlings.
Acknowledgments
KCI Sousa was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We thank Fundação de Amparo à Pesquisa do Estado de Goiás (FAPEG) and Laboratório Multiusuário de Microscopia de Alta Resolução - LabMic, Physics Institute, UFG.
References
- Aewsakul N, Maneesorn D, Serivichyaswat P, Taluengjit A & Nontachaiyapoom S (2013) Ex vitro symbiotic germination of Spathoglotis plicata Blume on common orchid cultivation substrates. Scientia Horticulturae 160: 238-242.
- Athipunyakon P, Manoch L & Piluek C (2004) Isolation and identification of mycorrhizal fungi from eleven terrestrial orchids. Natural Science 38: 216-228.
- Barreto DW & Parente JP (2006) Chemical properties and biological activity of a polysaccharide from Cyrtopodium cardiochilum Carbohydrate Polymers 64: 287-291.
- Batista JAN & Bianchetti LB (2005) Two new taxa in Cyrtopodium (Orchidaceae) from southern Brazil. Darwiniana 43: 74-83.
- Batista JAN & Bianchetti LB (2006) A New Species of Cyrtopodium (Orchidaceae) from the Cerrado of Central Brazil. Novon 16: 17-22.
- BFG - The Brazil Flora Group (2018) Brazilian Flora 2020: Innovation and collaboration to meet Target 1 of the Global Strategy for Plant Conservation (GSPC). Rodriguésia 69: 1513-1527.
- Carvalho JCB, Sousa KCI, Brito DC, Chaibub AA, Luzini AP, Côrtez MVCB, Filippi MCC, Kato L, Vaz BG, Costa HB, Romão W & Araújo LG (2015). Biocontrol potential of Waitea circinata, an orchid mycorrhizal fungus, against the rice blast fungus. Tropical Plant Pathology 40: 151-159.
- Chou L & Chang D (2004) Asymbiotic and symbiotic seed germination of Anoectochilus formosanus and Haemaria discolor and their F1 hybrids. Botanical Bulletin of Academia Sinica 45: 143-147.
- Chutima R, Dell B, Vessabutr S, Bussaban B & Lumyong S (2010) Endophytic fungi from Pecteilis susannae (L.) Rafin (Orchidaceae), a threatened terrestrial orchid in Thailand. Mycorrhiza 21: 221-229.
- Cribb PJ, Kell SP, Dixon KW, Barrett RL (2003) Orchid conservation: a global perspective. In: Dixon KW, Kell SP, Barrett RL & Cribb PJ (eds.) Orchid conservation. Natural History Publications, Kota Kinabalu. Pp. 1-25.
- Currah RS & Zelmer CD (1992) A key and notes for the genera of fungi with orchids and a new species in the genus Epulorhiza Reports of the Tottori Mycological Institute 30: 43-59.
- Dearnaley JDW, Martos F & Selosse MA (2012) Orchid mycorrhizas: molecular ecology, physiology, evolution and conservation aspects. In: Hock B (ed.) The Mycota IX: fungal associations. 2nd ed. Springer, Berlin. Pp. 207-230.
- Díaz MS & Álvarez CC (2009) Plant regeneration through direct shoot formation from leaf cultures and from protocorm-like bodies derived from callus Encyclia mariae (Orchidaceae), a threatened Mexican orchid. In Vitro Cellular Developmental Biology - Plant 45: 162-170.
- Dutra D, Kane ME & Rhichardson L (2009) Asymbiotic seed germination and in vitro seedling development of Cyrtopodium punctatum: a propagation protocol for a endangered Florida native orchid. Plant Cell, Tissue and Organ Culture 96: 235-243.
- Gonçalves FJ, Nunes CMC, Filippi MC, Araújo LG, Gonçalves LA & Sibov ST (2014) Isolation and characterization of mycorrhizal fungi of Cyrtopodium vernum Rchb. F. & Warm (Orchidaceae). Revista de Ciências Agrárias 57: 244-249.
- Guimarães FAR, Pereira MC, Felicio CS, Torres DP, Oliveira SF, Veloso TGR & Kasuya MCM (2013) Symbiotic propagation of seedlings of Cyrtopodium glutiniferum Raddi (Orchidaceae). Acta Botanica Brasilica 27: 590-596.
- Jiang J, Lee Y, Cubeta MA & Chen L (2015) Characterization and colonization of endomycorrhizal Rhizoctonia fungi in the medicinal herb Anoectochilus formosanus (Orchidaceae). Mycorrhiza 25: 431-445.
- Johansen DA (1940) Botanical microtechnique. McGraw-Hill, New York. 523p.
- Krauss JE & Arduin M (1997) Manual básico de métodos em morfologia vegetal. Universidade Rural do Rio de Janeiro, Seropédica. 198p.
- Látalová K & Baláz M (2010) Carbon nutrition of mature green orchid Serapias strictiflora and its mycorrhizal fungus Epulorhiza sp. Biologia Plantarum 54: 97-104.
- Masuhara G & Katsuya K (1994) In situ and in vitro specificity between Rhizoctonia spp. and Spiranthes sinensis (Persoon) Ames. var. amoena (M. Bieberstein) Hara (Orchidaceae). New Phytologist 127: 711-718.
- Masuhara G, Katsuya K & Yamaguchi K (1993) Potential for symbiosis of Rhizoctonia solani and binucleate Rhizoctonia with seeds of Spiranthes sinensis var. amoena in vitro Mycological Research 97: 746-752.
- Meinhardt LW, Bellato CM & Tsai SM (2001) SYBR green I used to evaluate the nuclei number of fungal mycelia. Biotechniques 31: 42-46.
- Moreno JAE, Acuña EAG, Román AEB, Contreras DJ & López CT (2000) Fertilización química e biológica de Phalaenopsis (Orchidaceae) en condiciones de invernadero. Terra Latinoamericana 18: 125-131.
- Mosquera-Espinosa AT, Bayman P, Prado GA, Gómez-Carabalí A & Otero JT (2013) The double life os Ceratobasidium: orchid mycorrhizal fungi and their potential for biocontrol of Rhizoctonia solani sheath blight of rice. Mycologia 105: 141-150.
- Nogueira RE, Pereira OL, Kasuya MCM, Lanna MCS & Mendonça M (2005) Fungos micorrízicos associados a orquídeas em campos rupestres na região do quadrilátero ferrífero, MG, Brasil. Acta Botanica Brasilica 3: 417-424.
- Otero JT, Ackerman JD & Bayman P (2004) Differences in mycorrhizal preferences between two tropical orchids. Molecular Ecology 13: 2393-2404.
- Pereira MC, Pereira OL, Costa MD, Rocha RB & Kasuya MCM (2009) Diversidade de fungos micorrízicos Epulorhiza sp. isolados de Epidendrum secundum (Orchidaceae). Revista Brasileira de Ciência do Solo 33: 1187-1197.
- Pereira MC, Rocha DI, Veloso TGR, Pereira OL, Francino DMT, Meira RMSA & Kasuya MCM (2015) Characterization of seed germination and protocorm development of Cyrtopodium glutiniferum (Orchidaceae) promoted by mycorrhizal fungi Epulorhiza spp. Acta Botanica Brasilica 29: 567-574.
- Pereira MC, Torres DP, Guimarães FAR, Pereira OL & Kasuya MCM (2011) Germinação de sementes e desenvolvimento de protocormos de Epidendrum secundum Jacq. (Orchidaceae) em associação com fungos micorrízicos do gênero Epulorhiza. Acta Botanica Brasilica 25: 534-541.
- Pereira OL, Kasuya MCM, Rollemberg CL & Chaer GM (2005) Indução in vitro da germinação de sementes de Oncidium flexuosum (Orchidaceae) por fungos micorrízicos rizoctonioides. Revista Brasileira de Ciência do Solo 29: 199-206.
- Peterson RL, Massicotte HB & Melville LH (2004) Mycorrhizas: anatomy and cell biology. NRC Research Press, Ottawa. 182p.
- Rasmussen HN & Rasmussen FN (2009) Orchid mycorrhiza: implications of a mycophagous life style. Oikos 118: 334-345.
- Rodrigues LA, Paiva Neto VB, Boaretto AG, Oliveira JF, Torrezan MA, Lima SF & Otoni WC (2015) In vitro propagation of Cyrtopodium saintlegerianum Rchb. f. (Orchidaceae), a native orchid of the Brazilian savannah. Crop Breeding and Applied Biotechonoly 15: 10-17.
- Romero-González GA, Batista JAN & Bianchetti LB (2008) A synopsis of the genus Cyrtopodium (Catasetinae: Orchidaceae). Harvard Papers in Botany 13: 189-206.
- Silva CS, Araújo LG, Sousa KCI, Carvalho JCB, Gonçalves LA & Carneiro LL (2016) Cultivo in vitro de Epidendrum nocturnum Jacq. (Orchidaceae) ocorrente no Cerrado da Região Centro-Oeste. Rodriguésia 67: 1083-1091.
- Silva CS, Araújo LG, Sousa KCI, Silva DM, Sibov ST & Faria PR (2017) Germinação e desenvolvimento in vitro de orquídea epífita do Cerrado. Ornamental Horticulturae 23: 96-100.
- Steinfort U, Verdugo G, Besoain X & Cisternas MA (2010) Mycorrhizal association and symbiotic germination of the terrestrial orchid Bipinnula fimbriata (Poepp.) Johnst (Orchidaceae). Flora 205: 811-817.
- Stewart SL, Zettler LW (2002) Symbiotic germination of three semi-aquatic rein orchids (Habenaria repens, H. quinquiseta, H. macroceratitis) from Florida. Aquatic Botany 72: 25-35.
- Valadares RBS, Otero JT, Pereira MC, Steinfort U, Verdugo G, Besoain X & Cisternas MA (2010) The epiphytic orchids Ionopsis utricularioides and Psygmorchis pusilla associate with different Ceratobasidium lineages at Valle del Cauca, Colombia. Acta Botanica Brasilica 29: 40-44.
- Vogel IN & Macedo AF (2011) Influence of IAA, TDZ, and light quality on asymbiotic germination, protocorm formation, and plantlet development of Cyrtopodium glutiniferum Raddi., a medicinal orchid. Plant Cell, Tissue and Organ Culture 104: 147-155.
- Vujanovic V, St-Arnaud M, Barabé D & Thibeault G (2000) Viability testing of orchid seed and the promotion of colouration and germination. Annals of Botany 86: 79-86.
- Wu P, Huang D & Chang CN (2011) Mycorrhizal symbiosis enhances Phalaenopsis orchid’s growth and resistance to Erwinia chrysanthemi African Journal of Biotechnology 10: 10095-10100.
- Zelmer CD & Currah RS (1995) Ceratorhiza pernacatena and Epulorhiza calendulina sp. nov.: mycorrhizal fungi of terrestrial orchids. Canadian Journal of Botany 73: 1981-1985.
- Zettler LW, Poulter SB, Mcdonald KI & Stewart SL (2007) Conservation-driven propagation of an epiphytic orchid (Epidendrum nocturnum) with a mycorrhizal fungus. HortScience 42: 135-139.
Edited by
Publication Dates
-
Publication in this collection
18 Mar 2019 -
Date of issue
2019
History
-
Received
01 Nov 2016 -
Accepted
19 Mar 2018