Abstract
Introduction
Platelet-rich fibrin (PRF) is formed by an autologous blood concentrate, with properties that promote cell proliferation and regenerationof bone, gingival and epithelial tissue.
Objective
To compare four different procedures for processing as well as obtaining PRF, and analyzing their formation through laboratory techniques. The purpose of the study is to validate a method that produces higher quality PRF for oral surgery use in different branches of dentistry.
Material and method
The experiment consisted of collecting blood from 12 volunteers, and processing each patient’s sample in 4 different ways. In the following two-stage process analysis, the first, quantitative, step was to analyze the samples' platelet-poor plasma (PPP),with a Neubauer Hemocytometer to count blood components. In the second, qualitative step, the sample’s PRF were analyzed under microscopy using histological slides.
Result
The statistical analysis of the leukocyte, red blood cell and platelet count did not show any significant distinction when comparing different processes. Histological analysis of the PRF showed fibrin network with platelet aggregation, several leukocytes and presence of red blood cells, with double centrifuged samples presenting more white blood cells than the others. Conclusion: Among the analyzed procedures, the sample centrifuged once at 3000 RPM (1900 G) for 10 min showed the best quality PRF.
Descriptors:
Processing; sample; blood concentrate; leukocytes; dental procedures
Resumo
Introdução
A fibrina rica em plaquetas (FRP) é formada por um concentrado sanguíneo autólogo, com propriedades de promover a proliferação celular, regeneração de tecido, dentre eles tecido ósseo, gengival e epitelial.
Objetivo
Comparar quatro tipos diferentes de processamento e obtenção de FRP, analisando a sua formação através de técnicas laboratoriais. A finalidade do estudo é validar um método que obtenha maior qualidade para o uso em cirurgias nas diversas áreas da odontologia.
Material e método
O experimento foi constituído de uma coleta de sangue de 12 voluntários, onde a amostra de cada paciente foi processada de 4 formas. A análise dos processamentos se deu em duas etapas, onde na primeira, quantitativa, foi analisado o plasma pobre em plaquetas (PPP), através de contagem de constituintes sanguíneos em câmara de Neubauer. Na segunda etapa, qualitativa, foram analisadas as FRP em microscopia através de lâminas histológicas.
Resultado
A análise estatística da contagem de leucócitos, hemácias e plaquetas não mostraram diferença significativa entre os distintos processamentos. As análises histológicas do FRP mostraram redes de fibrina com agregação plaquetária, inúmeros leucócitos e presença de hemácias, sendo que as amostras de dupla centrifugação apresentaram mais leucócitos que nas demais.
Conclusão
Dos procedimentos analisados, a amostra centrifugada a 1 única vez a 3000 RPM (1900 G) por 10 min mostrou-se a melhor qualidade de FRP.
Descritores:
Processamento; amostra; concentrado sanguíneo; leucócitos; procedimentos odontológicos
INTRODUCTION
Platelet-rich fibrin (PRF) is essential for the proliferation of multiple cells, such as monocytes, macrophages, fibroblasts, osteoblasts, epithelial, endothelial cells, and so forth11 Alves R, Botelho J, Machado V, Rua J, Delgado A, Mendes JJ. Fibrina rica em plaquetas (prf) - aplicações em periodontologia e implantologia. Rev OMD. 2019;2:31-9.
2 Salgado-Peralvo A, Sánchez-Linares S, Salgado García A. Revisión del uso de la malla de fibrina autóloga en la regeneración de los tejidos bucales. Gaceta Dental. 2015 Feb;266:114-23.-33 Costa PA, Santos P. Plasma rico em plaquetas: uma revisão sobre seu uso terapêutico. RBAC. 2016;48(4):311-9..
PRF is obtained from an autologous blood sample (collected from the patients), and is mainly composed of a serum containing a large number of platelets, leukocytes, fibrin, among other blood components, with no addition of other external elements44 Amaral RG, Dietrich L, Gontijo GR, Parsia Gontijo JM, Costa MDMA. Benefícios da utilização da fibrina rica em plaquetas na implantodontia. Rev Odontol Contemp. 2018 Maio;1(2):37..
While in the blood vessels, fibrin has a tridimensional architecture and plays a crucial part in the coagulation process, being responsible for directing angiogenesis and guiding epithelial cells towards the injured surface33 Costa PA, Santos P. Plasma rico em plaquetas: uma revisão sobre seu uso terapêutico. RBAC. 2016;48(4):311-9..
Platelet-rich fibrin has been largely employed in procedures related to dental implants and treatment of osteitis, as healing complement for procedures such as gum graft surgery and for corrections of bone deformity, as well as for alveolar crest preservation in extractions and surgeries for oncology patients’ bone regeneration4-6. It can also be used in dental surgeries in association with demineralized bone powder to substitute bone grafting, providing an adequate and less invasive bone formation55 Vasconcellos AVB, Teixeira APF, Cruz PV. Plaqueta rica em fibrina: um novo conceito em reparação tecidual. Innov Implant J Biomater Esthet. 2008 Set;3(6):27-31.
6 Khayat AIF, Miranda JES, Andrade WA, Khayat YF, Santos JLB. Aplicabilidade da fibrina rica em plaquetas em implantodontia. Anais do V Congresso de Educação em Saúde da Amazônia; 2016 nov; Universidade Federal do Pará. 2016.-77 Camargo GACG, Oliveira RLB, Fortes TMV, Santos TS. Utilização do plasma rico em plaquetas na odontologia. Odontol Clín-Cient. 2012 Jul-Set;11(3):187-90. .
A number of nomenclatures other than PRF have been previously used in several works, such as platelet-rich plasma (PRP) and platelet-poor plasma (PPP). Both are also obtained from autologous blood samples and processed with centrifugation, however, PRP is a serum sample containing numerous free platelets with no fibrin network structure. PPP, in turn, refers to residual plasma after formation of PRF and PRP, as a supernatant in the test tube77 Camargo GACG, Oliveira RLB, Fortes TMV, Santos TS. Utilização do plasma rico em plaquetas na odontologia. Odontol Clín-Cient. 2012 Jul-Set;11(3):187-90.
8 Lima MVF, Soares JA, Rocha LEA, Egawa WY, Negreiros WA. O uso da fibrina rica em plaqueta como potencializador do reparo ósseo em lesões intra-ósseas na maxila: relato de caso. Arch Health Invest. 2018;7(5):135.
9 Porto GCC, Reis MS, Kataoka TH, Soledade KR. Uso de plasma rico em plaquetas na Odontologia: revisão integrativa. Revista Textura. 2018;12(20):162-70. http://dx.doi.org/10.22479/desenreg2018v12n20p162-170.
http://dx.doi.org/10.22479/desenreg2018v...
10 Schneider KVM, Silva RBB. Plasma rico em plaquetas (PRP): classificação, mecanismos de ação e métodos de obtenção. REAS. 2020;(47):e3184.-1111 Miron RJ, Chai J, Fujioka-Kobayashi M, Sculean A, Zhang Y. Evaluation of 24 protocols for the production of platelet-rich fibrin. BMC Oral Health. 2020 Nov;20(1):310. http://dx.doi.org/10.1186/s12903-020-01299-w. PMid:33160335.
http://dx.doi.org/10.1186/s12903-020-012...
.
The literature consulted for this research report the use of centrifugation between 100G1212 Alissa R, Esposito M, Horner K, Oliver R. The influence of platelet-rich plasma on the healing of extraction sockets: an explorative randomised clinical trial. Eur J Oral Implantol. 2010;3(2):121-34. PMid:20623037. and 2000G1313 Oliveira LA, Borges TK, Soares RO, Buzzi M, Kuckelhaus SAS. Methodological variations affect the release of VEGF in vitro and fibrinolysis’ time from platelet concentrates. PLoS One. 2020 Oct;15(10):e0240134. http://dx.doi.org/10.1371/journal.pone.0240134. PMid:33027285.
http://dx.doi.org/10.1371/journal.pone.0...
. Variation in centrifugation time, g-force, rotor type, centrifuge model and collection tube type are factors that may cause platelet concentrates with variability in the fibrinous matrix organization. Different biochemical and morphological properties, as well as changes in the kinetics of release of cell growth factors, may directly interfere with the quality of the patient’s response to PRF use1414 Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária – ANVISA. Resolução RDC nº 302 de 13 de outubro de 2005. Dispõe sobre Regulamento Técnico para funcionamento de Laboratórios Clínicos. Diário Oficial da União. Brasília; 2005.,1515 Choukroun J, Adda F, Schoeffler C, Vervelle A. Une opportunité en paro-implantologie: le PRF. Implantodontie. 2001;42(55):e62..
Despite of the consensus regarding centrifugation at low g-force, the proper conditions to formalize the laboratory processing are not sufficiently clear as to allow for a better response and clinical standardization.
Taking into consideration the methods’ standardization and reproducibility, mindful of patient’s safety and meeting of clinical laboratory standards in accordance with ANVISA’s (The Brazilian Health Surveillance Agency)1616 Andriolo A, Ballarati CAF, Galoro CAO, Mendes ME, Melo MR, Sumita NM. Recomendações da Sociedade Brasileira de Patologia Clínica/Medicina Laboratorial (SBPC/ML): coleta e preparo da amostra biológica. Barueri: Manole; 2014. Board Resolution (RDC) No. 302, of October 13th, 2005 (regarding validation, conservation, and quality control), this study aims to analyze four types of blood processing to obtain PRF. For this purpose, we assessed the advantages and disadvantages of each technique by comparing the differences among them, using variables like time and speed of centrifugation to standardize the procedure of obtaining PRF for further clinical application.
MATERIAL AND METHOD
Design
This is basic research, of experimental, exploratory, descriptive, and analytical nature, in order to validate a blood sample processing method to obtain platelet-rich fibrin (PRF) for further use in dental procedures and minor outpatient surgeries.
Casuistic
Twelve healthy volunteer patients aged between 18 and 40 years, both male and female, were included in the study. None of the individuals used medication at the moment of the analysis. The following exclusion criteria were considered: patients with chronic use of antiplatelet and anticoagulant drugs, with a chronic condition (diabetes, thrombophilia, hypertension, dyslipidemia, inflammatory/infectious condition), with history of hemorrhages or blood disorders.
Ethical Factors
This research was developed according to the following declarations and guidelines on research involving human beings: the Nuremberg Code, the Declaration of Helsinki, and the Resolution No. 466, of December 12nd, 2012, of the National Council of Health, the present study was approved and regulated by the Ethics Committee of the University of Passo Fundo – RS – Brazil, based on CAAE (Proof of Application for Ethical Review) certificate No. 31336320,1,0000,5342.
Methodological Factors
After selection and interview of the volunteers that met the inclusion and exclusion criteria, we collected 10 mL of blood from the antecubital fossa and aliquoted it in 2.5ml, to be stored in 4 PVC test tubes (BD®) without adding anticoagulant for obtaining PRF. The following processing variables were analyzed: number of centrifugations, time, and speed of centrifugation. For the quantitative assessment, we proceeded with the counting of leukocytes, RBCs, and platelets of the PPP with a Neubauer Hemocytometer. As for the qualitative assessment, the PRF samples were embedded in paraffin and the histological slides were colored with Hematoxylin and Eosin.
Sample Processing
After the aliquot procedure, the collected blood samples were incubated at a temperature of 37ºC for 30 min, for blood clot formation. Subsequently, the samples were processed according to the procedures described below.
Processing step A: Samples centrifuged only once at 1500 RPM (950 G) for 15 min.
Processing step B: Samples centrifuged only once at 3000 RPM (1900 G) for 10 min.
Processing step C: Samples centrifuged once at 1500 RPM (950 G) for 15 min. PRF transferred to another clean tube and centrifuged again at 1500 RPM (950 G) for 15 min.
Processing step D: Samples centrifuged once at 3000 RPM (1900 G) for 10 min. PRF transferred to another clean tube and centrifuged again at 1500 RPM (950 G) for 10 min.
The aforementioned protocols were performed at a speed, g-force, and longer time than the proposed by Choukroun et al.1515 Choukroun J, Adda F, Schoeffler C, Vervelle A. Une opportunité en paro-implantologie: le PRF. Implantodontie. 2001;42(55):e62. (2001), 750G for 12 min aiming at the formation of a fibrin network with lower number of RBCs, more fibrin and leukocytes, and consequently, better healing quality.
We used the benchtop centrifuge model Fanem Excelsa Baby I, with centrifugation speed rates based on relative centrifuge force (g-force) calculated by the following equation:
Where R is the value of the rotor centrifuge radius1616 Andriolo A, Ballarati CAF, Galoro CAO, Mendes ME, Melo MR, Sumita NM. Recomendações da Sociedade Brasileira de Patologia Clínica/Medicina Laboratorial (SBPC/ML): coleta e preparo da amostra biológica. Barueri: Manole; 2014..
There are several sizes and models of centrifuges available on the market, always expressing centrifugation rates in rotations per minute (RPM). However, when considering a centrifuge’s g-force, centrifugation at 2000 RPMs on an equipment with a 5cm rotor radius (Figure 1) provides a 223.60Gs g-force, while a 10cm benchtop centrifuge provides a 447.20Gs g-force at the same RPM speed, thus demonstrating the need of standardizing speed with g-force, not with RPMs.
For the qualitative analysis of the PRF, after conducting the processing procedures under different conditions, we performed a histological analysis of the samples by sorting them in a vial containing 10% formaldehyde, and forwarding it to a pathology laboratory for the preparation of the slides through microtome sectioning of the PRF samples. All analysis were performed qualitatively through optical microscopy.
In order to prepare the slides, the material was firstly inserted in a clean test tube containing 10% formaldehyde. For dehydration, the samples were stored in vials containing alcohol for 20 minutes each, where the alcohol content was increased on each flask. Samples were subsequently stored in two vials containing xylol for 30 min each, placed in two flasks containing paraffin for 30 min per vial, embedded in paraffin and beeswax. After cooling, the material was cut with the microtome and fixed on the slides, which were sent to the oven to remove the paraffin from the material, and were then colored with Hematoxylin and Eosin.
For the quantitative analysis, centrifugation was followed by the counting of RBCs, leukocytes, and platelets in the PPP. We chose to carry out the analysis in platelet-poor plasma (PPP) (Figure2), since the gelatinous matrix of the PRF hampers the performance of the Neubauer Hemocytometer (Figure 3). Despite the electronic counting of cells being more reproductible and reliable, the constituent matrix of the PRF and the PPP may obstruct the aspiration probe and counting cell chamber in the flow cytometry; therefore, cell counting was conducted in duplicate by two analysts with the Neubauer Hemocytometer, counting in quadruplicate.
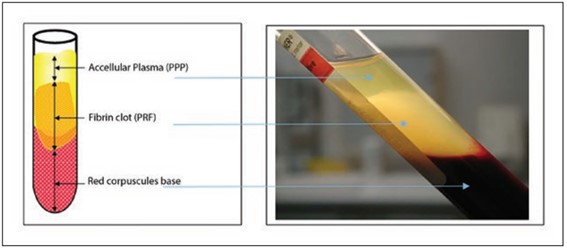
Formation of fibrin after centrifugation. Source: Adapted from Miron et al.1717 Miron RJ, Bishara M, Choukroun J. Course number: 208 basics of platelet-rich fibrin therapy. Dent Today. 2017 Apr;36(4):74-6. PMid:29235312..
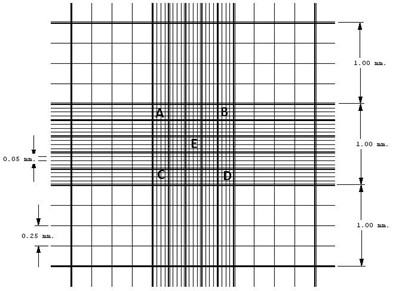
Reticles in the Neubauer Hemocytometer. Four external quadrants (4 lines and 4 columns) where the leukocytes are counted. Central quadrant (5 lines and 5 columns, subdivided in 4 lines and 4 columns, identified with letters from A to E), where the RBCs and the platelets are counted.
The RBCs and platelets counting was performed manually with the Neubauer Hemocytometer. Using an automated pipetting system, 25μL of the PPP was mixed in a test tube at 5mL of Hayen isotonic diluent (Exodo Científica®). After homogenization of the solution, the Neubauer chamber was filled, and the erythrocytes counted. We counted all five small quadrants of the central reticle in the Hemocytometer. Considering a 1:200 dilution, we applied the formula RBCs/105/μL = No. of erythrocytes counted x 200 x 10 x 5 (where 200 is the dilution value, 10 is the correction to 1mm3 of the chamber height, and 5 is the counted area).
For the leukocyte counting, 50 μL of the PPP were added to 1mL Türk solution (acetic acid at 2% methylene blue) sorting the leukocytes at the 4 external reticles (No. of leukocytes counted in the chamber x 20 x 10) /4 (where 20 is the dilution value, 10 is the correction to 1mm3 in the chamber height, and 4 is the number of counted reticles)1818 Lima AO, Soares JB, Greco JB, Galissi JB, Cançado JR. Métodos de laboratório aplicados à clínica: técnica em interpretação. 8. ed. Rio de Janeiro: Guanabara Koogan; 2001..
Statistical Analysis
Couting of leukocytes, RBCs, and platelets was performed in duplicate by two analysts. The average of the four counting procedures generated the cell quantification of each of the 12 samples. were transcribed to a work spreadsheet for statistical analysis. Firstly, we applied a Shapiro-Wilk test for normality determination. For results comparison of different processing procedures, a Kruskal-Wallis test for non-parametric data was adopted. For analysis of stochastic dominance, we used Dunn’s post hoc test on the statistical package of the SPSS 23.0 software. Results were considered statistically significant upon p ≤ 0.05. All results were assessed and expressed as median and interquartile interval.
RESULT
After the processing procedures, most of the samples presented PRF formation characterized by a light yellow/whitish part and a portion containing the formation of blood clot with RBC presence, characterized by a red color. However, for clinical use, the part containing RBCs was discarded.
In general, a visual assessment of the samples indicated a low volume PRF due to the amount of blood used to obtain it (2.5mL). Processing step A analysis showed a more friable PRF, with some samples not even forming PRF. Processing step B presented a more malleable and voluminous PRF. In Processing steps C and D, most of the samples showed PRF with lower volume, more compact and greater rigidity, as demonstrated in the following Figure 4:
The PRF samples were analyzed qualitatively using histological analysis, since the formation of the fibrin network (gel) interferes with cell quantification, making it impossible to count platelets, leukocytes, or RBCs in fibrinous mesh.
Corroborating the macroscopic findings from the blood clot analysis, the histological examination shows a disorganized and grouped leukocyte infiltration in treatments A and C, but mainly in treatment D (Figure 5). The samples of Processing step B presented an organized structure with fibrin network, leukocytes, platelets and erythrocyte aggregation on a more organized and structured histological pattern than with the other processing steps.
For quantification of the different cell lineages in the PPP, the samples were processed right after blood clot formation. Counting cells with the PPP and not with the PRF is hence justified, as the process needs to be performed in a fluid sample and not in gelled form. Since the present study is meant to be basic research, the sample validation process in the area of laboratorial biochemistry recommends the exposure to different conditions of processing, storage, and stability, in order to insure laboratorial quality control.
The statistical analysis of the results from the Kruskal-Wallis test for non-parametric data according to Dunn’s test post hoc showed no significant distinction between the different types of sample processing. Despite each counting being performed in quadruplicate, we found a high standard deviation that seems to be loosely related to the rigorous methodology, but pertinent to the individual cell variation in leukocyte count and different variables implied in the cascading coagulation phenomenon of each individual sample.
DISCUSSION
The use of autologous platelets concentrate in clinical practice has proved to have some potential for supraphysiological doses release, with 6 to 8 times of growth factor, responsible for promoting local healing1616 Andriolo A, Ballarati CAF, Galoro CAO, Mendes ME, Melo MR, Sumita NM. Recomendações da Sociedade Brasileira de Patologia Clínica/Medicina Laboratorial (SBPC/ML): coleta e preparo da amostra biológica. Barueri: Manole; 2014.
17 Miron RJ, Bishara M, Choukroun J. Course number: 208 basics of platelet-rich fibrin therapy. Dent Today. 2017 Apr;36(4):74-6. PMid:29235312.-1818 Lima AO, Soares JB, Greco JB, Galissi JB, Cançado JR. Métodos de laboratório aplicados à clínica: técnica em interpretação. 8. ed. Rio de Janeiro: Guanabara Koogan; 2001..
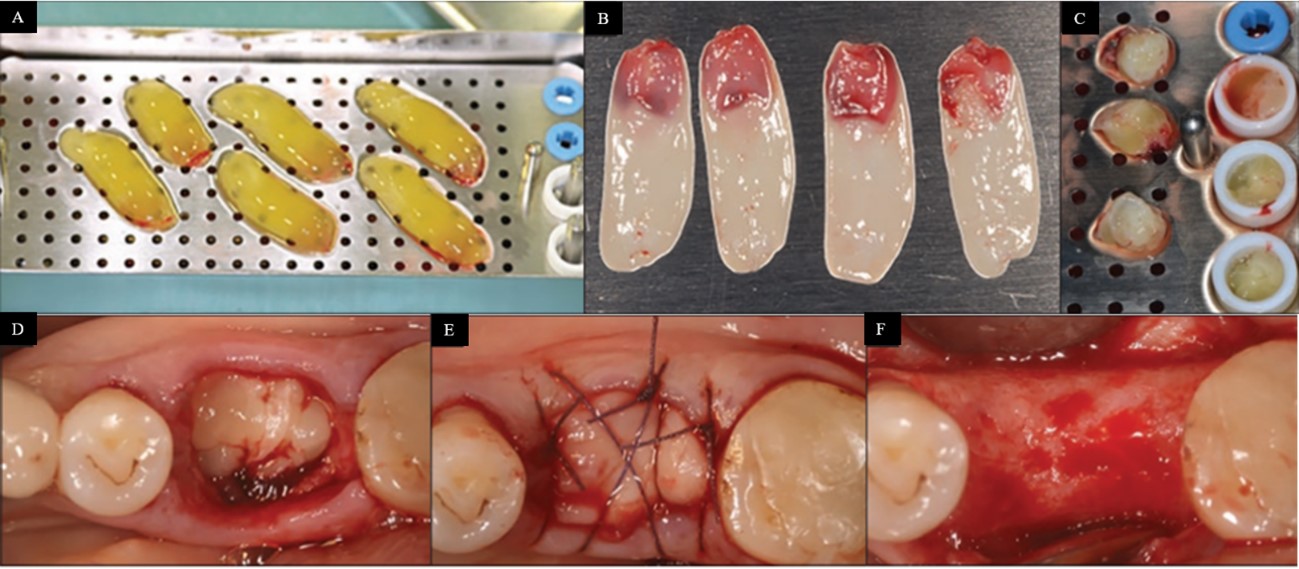
Photo A in Figure 7 shows a recently collected platelet-rich fibrin (PRF). Photo B shows the formation of PRF membranes after serum drainage, while photo C presents the elaboration of PRF “plugs”, molded during the serum drainage process. Photographs D, E, and F illustrate the clinical evolution throughout three months of use and healing of the dental alveoli after extraction filled with a PRF “plug”. Significant bone regeneration was observed without bone grafting1717 Miron RJ, Bishara M, Choukroun J. Course number: 208 basics of platelet-rich fibrin therapy. Dent Today. 2017 Apr;36(4):74-6. PMid:29235312..
Preparation of platelet-rich fibrin and its application to dentistry. Source: Adapted from Miron et al.1717 Miron RJ, Bishara M, Choukroun J. Course number: 208 basics of platelet-rich fibrin therapy. Dent Today. 2017 Apr;36(4):74-6. PMid:29235312..
This study adopted a horizontal centrifugation using mobile rotor instead of fixed/inclined rotor, since such centrifugation methodology allows to separate the elements by density (platelets < leukocytes < RBCs). In addition, horizontal centrifugation promotes no morphological deformities in cell elements, due to its lower shear force against tube walls. Cell deformity in the fixed rotor promotes a higher number of platelets and growth factors in the distal tube, when compared tothe PRF’s1111 Miron RJ, Chai J, Fujioka-Kobayashi M, Sculean A, Zhang Y. Evaluation of 24 protocols for the production of platelet-rich fibrin. BMC Oral Health. 2020 Nov;20(1):310. http://dx.doi.org/10.1186/s12903-020-01299-w. PMid:33160335.
http://dx.doi.org/10.1186/s12903-020-012...
.
Histological and qualitative analysis of the PRFs obtained through the different types of processing demonstrate that fibrin network formation with platelet aggregation constituted of numerous leukocytes and contained RBCs. Samples processed through methods A and B (both centrifuged once) presented a higher gelatinous volume of PRF, with more serum retained in the network structure. Samples processed through methods C and D (both with double centrifugation) presented a more compact PRF (±20% of the volume, when comparing it with the samples centrifuged once), less gelatinous, and with lower plasma content retained in the net. On account of being lower and less clinically manipulated, Processing steps C and D may influence the use of PRF, depending on the lesion extension. In turn, by virtue of presenting less serum, the network structure may be more compact and consistent. Based on such scenario, we also discourage double centrifugation since the change of tubes may increase the sample’s iatrogenic contamination risk.
Processing steps C and D, both with two centrifugations, showed a smaller PRF size, however, according to a microscopic analysis of the sample, such reduced size does not imply a higher concentration of essential network components – fibrin, platelets, and leukocytes. These processing procedures showed an increase in the presence of leukocytes with residual amount fibrin, thus not being recommended. Such qualitative finding is corroborated by Miron et al.1111 Miron RJ, Chai J, Fujioka-Kobayashi M, Sculean A, Zhang Y. Evaluation of 24 protocols for the production of platelet-rich fibrin. BMC Oral Health. 2020 Nov;20(1):310. http://dx.doi.org/10.1186/s12903-020-01299-w. PMid:33160335.
http://dx.doi.org/10.1186/s12903-020-012...
(2020), concluding that the higher the speed and time of centrifugation, the larger the serum volume, with lower number of platelets and higher the number of leukocytes in the PRF. Using speeds of 200G results in a higher number of platelets and growth factors, but with less leukocytes and fibrin, while the speed of 700G tends to be the opposite.
The quantitative analysis of the counting of leukocytes, platelets, and RBCs from the PPP samples with the Neubauer Hemocytometer demonstrates that Processing step A obtained a higher number of platelets and RBCs, and a lower number of leukocytes, than the other samples, corroborating Miron et al.1111 Miron RJ, Chai J, Fujioka-Kobayashi M, Sculean A, Zhang Y. Evaluation of 24 protocols for the production of platelet-rich fibrin. BMC Oral Health. 2020 Nov;20(1):310. http://dx.doi.org/10.1186/s12903-020-01299-w. PMid:33160335.
http://dx.doi.org/10.1186/s12903-020-012...
(2020). Due to the performance of only one centrifugation at lower rotation, such elements remained in the PPP, generating a lower amount in the PRF. That being said, the statistical analysis of the Kruskal-Wallis test results for non-parametric data showed no significant difference among the samples.
The analysis of the platelets counting results (Figure 6) revealed that treatments A and B led to a lower number of platelets in the PPP than with Processing steps C and D. The counting of elements in the PPP is inversely proportional to the PRF, indicating that the presence of platelets is greater in scenarios of samples centrifugated once, corroborating Miron et al.1111 Miron RJ, Chai J, Fujioka-Kobayashi M, Sculean A, Zhang Y. Evaluation of 24 protocols for the production of platelet-rich fibrin. BMC Oral Health. 2020 Nov;20(1):310. http://dx.doi.org/10.1186/s12903-020-01299-w. PMid:33160335.
http://dx.doi.org/10.1186/s12903-020-012...
(2020) and Choukroun et al.1515 Choukroun J, Adda F, Schoeffler C, Vervelle A. Une opportunité en paro-implantologie: le PRF. Implantodontie. 2001;42(55):e62. (2001). Yet, the speed proposed by both groups of authors was 700G for 8min and 700G for 12 min, respectively, contrary to the speeds of 950 and 1900G used herein.
Counting of platelets, RBCs, and leukocytes performed with different processing/treatments ensuing centrifugation and after 24h under refrigeration (4°C). Results were assessed and expressed as median and interquartile range. *P<0.05 with Kruskal-Wallis test for non-parametric data, followed by Dunn’s post hoc test.
According to Oliveira et al.1313 Oliveira LA, Borges TK, Soares RO, Buzzi M, Kuckelhaus SAS. Methodological variations affect the release of VEGF in vitro and fibrinolysis’ time from platelet concentrates. PLoS One. 2020 Oct;15(10):e0240134. http://dx.doi.org/10.1371/journal.pone.0240134. PMid:33027285.
http://dx.doi.org/10.1371/journal.pone.0...
(2020), even lower centrifugation speeds (200G) tend to promote an increase in the number of platelets and a decrease in fibrous structure, causing a higher platelet activation for producing vascular endothelial growth factors along with a more liquid PRF, which is the PRP. Such type of more fluid PRF would be better suited for skin healing processes, while a higher speed of 700G would form a higher fibrin content PRF, more indicated for surgical procedures.
The analysis of RBCs counting in the PPP (Figure 6) showed that the lowest values were found in Processing step B. Double centrifugation in Processing steps C and D presented an inconsistency due to its greater leukocyte cellularity in the PPP, while the PRF’s histological analysis (Figure 5) showed a higher number of leukocytes through microscopic assessment, probably due to the lower fibrin content found in the microscopic analysis. Lower centrifugation and speeds were characterized by a lower counting of leukocytes, but with predominance of monocytes1111 Miron RJ, Chai J, Fujioka-Kobayashi M, Sculean A, Zhang Y. Evaluation of 24 protocols for the production of platelet-rich fibrin. BMC Oral Health. 2020 Nov;20(1):310. http://dx.doi.org/10.1186/s12903-020-01299-w. PMid:33160335.
http://dx.doi.org/10.1186/s12903-020-012...
, involved in the phagocytic and scarring processes. The assessment of the remaining serum from the Processing step A indicated that after a certain centrifugation time some samples formed PRF, where the present fibrin and plateletsdeveloped a network during incubation at 37°C, followed by centrifugation. The absence of PRF formation in Processing step A may have derived from the low sampling volume that allowed platelets and coagulation factors to remain in the supernatant throughout the processing, hampering the formation of a more voluminous and consistent PRF.
Histological profile of samples of platelet-rich fibrin (PRF) in Processing steps A, B, C, and D, respectively. Caption: F – Fibrin; L - Leukocytes.
This study is innovative, on account of its analytical rigidity (testing in quadruplicate, divided between two analysts), in addition to the standardization of the samples’ processing using the g-force and the RPMs at different speeds, as well as proposing time and quantity of centrifugations for further clinical tests. Furthermore, we performed a qualitative analysis (histological analysis, through optical microscopy) combined with a quantitative analysis (cell counting with the Neubauer Hemocytometer) of blood components for PRF production.
Nevertheless, this study is limited by the number of samples due to the amount of processing procedures and replication for each analysis, making the processing rather time-consuming. The analysis of the results indicated a high standard deviation that allowed no significant statistical differences to be shown, despite the variability found along with the healthy and homogenous population selected by the exclusion criteria. In addition, the methodology was based on analytical quality control, and the individual hematological variations are very particular. The counting of platelets, leukocytes, and RBCs in the hemogram is individual, considering the particularities and complexity of the hemostasia and coagulation systems of different individuals, which would justify these variations and lack of publications on the topic.
The collected blood volume to obtain the PRF for each processing (2.5ml) served only for in vitro standardization purposes, providing the PRF with a limited structural formation according to the blood volume used to form it. For further studies targeting in vivo application, we suggest that the blood volume collected to obtain the PRF be of approximately 10mL, proportional to the number of tubes needed according to the lesion extension or surgery, considering the patient’s clinical history.
The non-standardization of centrifugation speeds is related to the purpose of the referred clinical procedure. For most cases, the literature establishes optimum speed of processing between 400G and 700G for 8 to 12 min, forming PRF with a well proportioned volume of platelets, leukocytes, and fibrin1111 Miron RJ, Chai J, Fujioka-Kobayashi M, Sculean A, Zhang Y. Evaluation of 24 protocols for the production of platelet-rich fibrin. BMC Oral Health. 2020 Nov;20(1):310. http://dx.doi.org/10.1186/s12903-020-01299-w. PMid:33160335.
http://dx.doi.org/10.1186/s12903-020-012...
, being indicated for surgical procedures, dental extractions, and implants1515 Choukroun J, Adda F, Schoeffler C, Vervelle A. Une opportunité en paro-implantologie: le PRF. Implantodontie. 2001;42(55):e62.. Conversely, lower speeds from 200 to 400G for 5 min result in a more liquid PRF with a high concentration of platelets, leukocytes, and growth factors1111 Miron RJ, Chai J, Fujioka-Kobayashi M, Sculean A, Zhang Y. Evaluation of 24 protocols for the production of platelet-rich fibrin. BMC Oral Health. 2020 Nov;20(1):310. http://dx.doi.org/10.1186/s12903-020-01299-w. PMid:33160335.
http://dx.doi.org/10.1186/s12903-020-012...
, mainly indicated for skin healing treatments1616 Andriolo A, Ballarati CAF, Galoro CAO, Mendes ME, Melo MR, Sumita NM. Recomendações da Sociedade Brasileira de Patologia Clínica/Medicina Laboratorial (SBPC/ML): coleta e preparo da amostra biológica. Barueri: Manole; 2014..
The initial hypothesis of proposing a processing method with higher speeds was not confirmed, thus suggesting that centrifugation at speeds below 700G may be more indicated depending on the clinical procedure1111 Miron RJ, Chai J, Fujioka-Kobayashi M, Sculean A, Zhang Y. Evaluation of 24 protocols for the production of platelet-rich fibrin. BMC Oral Health. 2020 Nov;20(1):310. http://dx.doi.org/10.1186/s12903-020-01299-w. PMid:33160335.
http://dx.doi.org/10.1186/s12903-020-012...
,1616 Andriolo A, Ballarati CAF, Galoro CAO, Mendes ME, Melo MR, Sumita NM. Recomendações da Sociedade Brasileira de Patologia Clínica/Medicina Laboratorial (SBPC/ML): coleta e preparo da amostra biológica. Barueri: Manole; 2014.,1717 Miron RJ, Bishara M, Choukroun J. Course number: 208 basics of platelet-rich fibrin therapy. Dent Today. 2017 Apr;36(4):74-6. PMid:29235312.. However, the results obtained herein allow us to reproduce the findings described in the literature regarding PRF histological analysis, as well as counting of platelets, RBCs, and leukocytes obtained from the PPP.
CONCLUSION
The statistical analysis intragroup of the results does not allow us to define an optimum processing technique, in view of the sensitivity of the analytical methods and patients’ interpersonal hematological variation. In spite of that, the variations found show that low-speed processing (Processing step A) presented incomplete and late formation of PRF, with high rate of missing samples. In contrast, double centrifugation in Processing steps C and D promoted a very small, compact and rigid PRF, with low flexibility and higher iatrogenic contamination risk due to the change of tubes, which may restrict its use in clinical application. Thereby, despite the reservations pointed out in the discussion, the processing procedures performed in the present study indicate that procedure B reached the best in vitro quality.
-
How to cite: Dallosto JZ, Souza MA, Prado LDS, Siqueira LO. Analysis of different platelet-rich fibrin processing. Rev Odontol UNESP. 2022;51:e20220004. https://doi.org/10.1590/1807-2577.00422
REFERENCES
-
1Alves R, Botelho J, Machado V, Rua J, Delgado A, Mendes JJ. Fibrina rica em plaquetas (prf) - aplicações em periodontologia e implantologia. Rev OMD. 2019;2:31-9.
-
2Salgado-Peralvo A, Sánchez-Linares S, Salgado García A. Revisión del uso de la malla de fibrina autóloga en la regeneración de los tejidos bucales. Gaceta Dental. 2015 Feb;266:114-23.
-
3Costa PA, Santos P. Plasma rico em plaquetas: uma revisão sobre seu uso terapêutico. RBAC. 2016;48(4):311-9.
-
4Amaral RG, Dietrich L, Gontijo GR, Parsia Gontijo JM, Costa MDMA. Benefícios da utilização da fibrina rica em plaquetas na implantodontia. Rev Odontol Contemp. 2018 Maio;1(2):37.
-
5Vasconcellos AVB, Teixeira APF, Cruz PV. Plaqueta rica em fibrina: um novo conceito em reparação tecidual. Innov Implant J Biomater Esthet. 2008 Set;3(6):27-31.
-
6Khayat AIF, Miranda JES, Andrade WA, Khayat YF, Santos JLB. Aplicabilidade da fibrina rica em plaquetas em implantodontia. Anais do V Congresso de Educação em Saúde da Amazônia; 2016 nov; Universidade Federal do Pará. 2016.
-
7Camargo GACG, Oliveira RLB, Fortes TMV, Santos TS. Utilização do plasma rico em plaquetas na odontologia. Odontol Clín-Cient. 2012 Jul-Set;11(3):187-90.
-
8Lima MVF, Soares JA, Rocha LEA, Egawa WY, Negreiros WA. O uso da fibrina rica em plaqueta como potencializador do reparo ósseo em lesões intra-ósseas na maxila: relato de caso. Arch Health Invest. 2018;7(5):135.
-
9Porto GCC, Reis MS, Kataoka TH, Soledade KR. Uso de plasma rico em plaquetas na Odontologia: revisão integrativa. Revista Textura. 2018;12(20):162-70. http://dx.doi.org/10.22479/desenreg2018v12n20p162-170
» http://dx.doi.org/10.22479/desenreg2018v12n20p162-170 -
10Schneider KVM, Silva RBB. Plasma rico em plaquetas (PRP): classificação, mecanismos de ação e métodos de obtenção. REAS. 2020;(47):e3184.
-
11Miron RJ, Chai J, Fujioka-Kobayashi M, Sculean A, Zhang Y. Evaluation of 24 protocols for the production of platelet-rich fibrin. BMC Oral Health. 2020 Nov;20(1):310. http://dx.doi.org/10.1186/s12903-020-01299-w PMid:33160335.
» http://dx.doi.org/10.1186/s12903-020-01299-w -
12Alissa R, Esposito M, Horner K, Oliver R. The influence of platelet-rich plasma on the healing of extraction sockets: an explorative randomised clinical trial. Eur J Oral Implantol. 2010;3(2):121-34. PMid:20623037.
-
13Oliveira LA, Borges TK, Soares RO, Buzzi M, Kuckelhaus SAS. Methodological variations affect the release of VEGF in vitro and fibrinolysis’ time from platelet concentrates. PLoS One. 2020 Oct;15(10):e0240134. http://dx.doi.org/10.1371/journal.pone.0240134 PMid:33027285.
» http://dx.doi.org/10.1371/journal.pone.0240134 -
14Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária – ANVISA. Resolução RDC nº 302 de 13 de outubro de 2005. Dispõe sobre Regulamento Técnico para funcionamento de Laboratórios Clínicos. Diário Oficial da União. Brasília; 2005.
-
15Choukroun J, Adda F, Schoeffler C, Vervelle A. Une opportunité en paro-implantologie: le PRF. Implantodontie. 2001;42(55):e62.
-
16Andriolo A, Ballarati CAF, Galoro CAO, Mendes ME, Melo MR, Sumita NM. Recomendações da Sociedade Brasileira de Patologia Clínica/Medicina Laboratorial (SBPC/ML): coleta e preparo da amostra biológica. Barueri: Manole; 2014.
-
17Miron RJ, Bishara M, Choukroun J. Course number: 208 basics of platelet-rich fibrin therapy. Dent Today. 2017 Apr;36(4):74-6. PMid:29235312.
-
18Lima AO, Soares JB, Greco JB, Galissi JB, Cançado JR. Métodos de laboratório aplicados à clínica: técnica em interpretação. 8. ed. Rio de Janeiro: Guanabara Koogan; 2001.
Publication Dates
-
Publication in this collection
08 June 2022 -
Date of issue
2022
History
-
Received
28 Jan 2022 -
Accepted
23 Mar 2022